Abstract

The combination of multiple physiological (swelling, porosity, mechanical, and biodegradation) and biological (cell/tissue-adhesive, cell proliferation, and hemostatic) properties on a single hydrogel has great potential for skin tissue engineering. Adhesive hydrogels based on polydopamine (PDA) have become the most popular in the biomedical field; however, integrating multiple properties on a single adhesive hydrogel remains a challenge. Here, inspired by the chemistry of mussels, we developed PDA–sodium alginate–polyacrylamide (PDA–SA–PAM)-based hydrogels with multiple physiological and biological properties for skin tissue engineering applications. The hydrogels were prepared by alkali-induced polymerization of DA followed by complexation with SA in PAM networks. The chemical composition of the hydrogels was characterized by X-ray photoelectron spectroscopy. PDA–SA complexed chains were homogeneously dispersed in the PAM network and exhibited good elasticity and excellent mechanical properties, such as a compressive stress of 0.24 MPa at a compression strain of 70% for 0.4PDA–SA–PAM. The adhesive hydrogel also maintained a highly interconnected porous structure (∼94% porosity) along with PDA microfibrils. The hydrogel possesses outstanding swelling and biodegradability properties. Owing to the presence of the PDA–SA complex in the PAM network, the hydrogels show good adhesion to various substrates (plastic, skin, glass, computer screens, and leaves); for example, the adhesive strength of the 0.4PDA–SA–PAM to porcine skin was 24.5 kPa. The adhesive component of the PDA–SA chains in the PAM network significantly improves the cell proliferation, cell attachment, cell spreading, and functional expression of human skin fibroblasts (CCD-986sk) and keratinocytes. Moreover, the PDA chains exhibited good hemostatic properties, resulting in rapid blood coagulation. Considering their excellent cell affinity, and rapid blood coagulation ability, these mussel-inspired hydrogels have substantial potential for skin tissue engineering applications.
Introduction
Hydrogels are hydrophilic interpenetrating polymeric networks (IPNs) that can absorb and retain large amounts of water.1 They are promising templates for numerous potential applications, such as drug delivery devices, tissue engineering scaffolds, sensors, and environmental pollutant filters.2−4 Furthermore, hydrogels exhibit potential as scaffolding devices for tissue engineering as they are structurally similar to the extracellular matrix (ECM) components of native tissues. The greatest challenge in tissue engineering is designing hydrogels that can adhere to biological tissue without exerting any toxic effects on the host tissue after implantation.5,6 Tissue-adhesive hydrogels are desirable for tissue engineering applications because hydrogels can enable hemostasis and adhesion between biological tissues.5 Moreover, such hydrogels can improve cell proliferation and attachment to regenerate biological tissues. The use of polysaccharide-based hydrogels has already demonstrated their potential for tissue engineering applications.7,8 However, these types of hydrogels exhibit relatively weak adhesion with biological host tissues after implantation.9 Thus, it is necessary to improve the adhesion of hydrogels that can enhance proliferation for improved interactions with biological tissue for regeneration.
Biomimetic approaches can provide an alternative and efficient method for synthesizing adhesive hydrogels to mimic nature.10 Mussel-inspired PDA has attracted considerable interest in biomedical applications because of its biocompatibility, hemostatic properties, and moderate antibacterial properties.11,12 The structural similarity between polydopamine (PDA) and mussel-secreted proteins (DOPA) has been proven to result in strong adhesion on various substrates.13 Considering its strong adhesiveness, PDA can be used to improve the cell/tissue adhesion of hydrogels in tissue engineering.14,15 PDA contains phenolic hydroxyl groups and amino groups, which aid in activating the coagulation system, preventing infections, and accelerating wound healing.16 In addition, PDA-based adhesive hydrogels have also been used in electronic skin and biosensor applications.17,18 So far, adhesive hydrogels have been prepared using DA-conjugated polymers such as hyaluronic acid (HA), SA, and CS by pendant catechol conjugation on a polymer.19−21 However, the chemical reaction between DA and the polysaccharides produces relatively few pendant catechol groups on the polymer, which generally results in poor mechanical properties for these hydrogels. The incorporation of PDA networks in the poly(acrylamide) (PAM) hydrogels further exhibited good cell adhesive and wound healing properties compared to other hydrogels.22−24
The combination of multiple biological and physiological properties is desirable in skin tissue engineering. Cell/tissue-adhesive and hemostatic biological properties are of primary importance for skin tissue engineering.25 In addition, physiological properties such as swelling, porosity, mechanical, and biodegradation further enhance skin regeneration, particularly wounded skin. Inspired by the mussel chemistry of PDA, herein, we developed cell-adhesive and hemostatic hydrogels composed of PDA–SA–PAM for tissue engineering applications. SA is a natural linear polysaccharide extracted from seaweed composed of homopolymer blocks of (1,4) crosslinked β-d-mannuronate and α-l-guluronate and widely used in well-studied alginate gels for tissue engineering applications.26 The introduction of appropriate cell adhesion polymers into SA-based three-dimensional (3D) hydrogels enables the regulation of specific interactions between cells and hydrogels, making the hydrogel more biologically suitable for the host response. Considering the cell/tissue-adhesive and hemostatic properties of PDA, we homogeneously dispersed PDA–SA in a covalently crosslinked PAM network to enhance its physicochemical and biological properties, rendering this hydrogel suitable for skin tissue engineering applications. The swelling behavior and biodegradability of the PDA–SA–PAM hydrogels were studied, and cell attachment and hemostasis were also examined in vitro on these hydrogels. Unlike previously reported PDA-based adhesive hydrogels, the developed hydrogels show multiple physiological and biological properties for potential skin tissue engineering applications.22,23
Results and Discussion
Preparation of PDA–SA–PAM Hydrogels
In this work, mussel-inspired PDA–SA–PAM hydrogels were prepared, and the cell adhesion, antibacterial, and hemostatic properties were evaluated for possible applications in tissue engineering (Scheme 1). The hydrogels were prepared via a two-step process. In the first step, the PDA–SA complex was prepared by alkali-induced polymerization of DA in a SA polymer solution (Supporting Information Figure S1). The as-formed PDA–SA complex was confirmed by Fourier-transform infrared (FTIR), UV–vis, and X-ray photoelectron spectroscopy (XPS) (Supporting Information Figure S2). In the second step, the as-prepared PDA–SA complex was homogeneously dispersed in a covalently crosslinked PAM hydrogel via redox-initiated free radical polymerization using N,N′-MBA as a crosslinker. In this study, ammonium persulfate (APS) was used as an initiator for the formation of hydrogels. However, APS is the toxic reagent and easily soluble in water compared to other initiators. Hence, APS can be easily removed from hydrogels after immersing hydrogels in DDW. The hydrogel formulations are summarized in Table S1. With the functional groups (catechol, amino, and carboxyl) of the PDA mimicking the mussel adhesion interactions, the 0.4PDA–SA–PAM hydrogels exhibited good adhesion to a wide range of surfaces. As shown in Figure 1, the 0.4PDA–SA–PAM hydrogels adhered to human skin (a), glass (b), a computer screen (c), and a leaf (d). The hydrogels also easily molded to a finger (e). Moreover, the hydrogels were transparent (c). The 0.4PDA–SA–PAM hydrogel adhered to human skin (author’s hand) without causing any irritation, damage, or pain (Supporting Information Figure S3). As demonstrated in Supporting Information Figure S4, the 0.4PDA–SA–PAM hydrogel tightly adhered to merged plastic tubes (a and b) and supported a load of 72 g. The hydrogels also displayed a highly stretchable nature (Supporting Information Figure S4c,d). Furthermore, to elucidate the adhesion performance of the 0.4PDA–SA–PAM hydrogels, a macroscopic peeling test was performed on human skin (author’s hand). As shown in Supporting Information Figure S4e,f, a stripping lag was observed during the peeling process. Moreover, the hydrogels could not be immediately removed from the skin surface. Porcine skin was used as model tissue to determine the hydrogel strength of adhesion to porcine skin using a tensile adhesion test. As shown in Figure 1f, the PDA content influenced the adhesion strength of the hydrogels. The hydrogel strength of adhesion to the porcine skin was obtained as 7.8, 14.5, and 24.5 kPa for the SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM hydrogels, respectively. The hydrogel strength of adhesion to porcine skin was strongly dependent on the PDA content. The maximum adhesion strength of the 0.4PDA–SA–PAM hydrogels led to an increased viscosity in the hydrogels, and the polymer chain can easily be diffused into the porcine skin tissue surface with a stronger connection.22 Moreover, the free catechol groups in PDA interact with amine or thiol groups on the skin tissue surface to form cation π or π–π interactions, thereby improving the adhesion strength compared to polysaccharide-based hydrogels (Figure 1g).
Scheme 1. Schematic Representation of the Formation of Adhesive Hydrogels for Tissue Engineering Applications.
Figure 1.
Photographic images of hydrogel adhesion (0.4PDA–SA–PAM) on various substrates such as (a) skin, (b) glass plate, (c) computer screen, (d) leaf, and (e) folding of hydrogel to a finger, (f) adhesive strength of hydrogels to a porcine skin (*p < 0.05). (g) Schematic representation of tissue adhesion tensile strength and its mechanism of PDA catechol groups reacted with the tissue surface.
Characterization
The functional groups and their interactions of hydrogels (SA–PAM and 0.4PDA–SA–PAM) were measured from deconvoluted high-resolution XPS spectra for carbon (C 1s) and nitrogen (N 1s) (Figure 2). Figure 2a shows the C 1s XPS spectrum for the SA–PAM hydrogels. The C 1s peaks of the SA–PAM hydrogels can be deconvoluted into three major peaks at 284.1, 284.7, and 285.7 eV, which are assigned to the C–C, C–O, and C–N or C–OH of the SA–PAM hydrogels. Additionally, the peaks at 287.4 and 288.7 eV are ascribed to H-bonded O=C of amide (O=C–NH2) and COO–, respectively. Two major peaks were observed in the N 1s spectrum of the SA–PAM hydrogels at 399.1 and 400.2 eV (Figure 2b), which are attributed to N 1s in the MBA (crosslinking agent) and H-bonded amide (−CO–NH2) in the PAM, respectively. The C 1s XPS spectrum for the 0.4PDA–SA–PAM hydrogel displayed similar peaks with lower binding energies at 285.6, 287.2, and 288.3 eV due to hydrogen-bonded C–N or C–OH, amide groups (−CO–NH2), and −COO– groups, respectively (Figure 2c). Additionally, a new peak at 286.4 eV was detected for 0.4PDA–SA–PAM due to the presence of aromatic C–OH/C–N groups in PDA. The N 1s spectrum of 0.4PDA–SA–PAM exhibited peaks at 399.1 and 400.1 eV, which were attributed to the contribution of N atoms in MBA and PAM, respectively (Figure 2d). The additional peak at 401.1 eV was attributed to the N atoms in the PDA. Hence, the XPS spectra proved the existence of functional groups of PDA in the hydrogel.
Figure 2.
XPS spectra of (a) SA–PAM (C 1s), (b) SA–PAM (N 1s), (c) 0.4PDA–SA–PAM (C 1s), and (d) 0.4PDA–SA–PAM (N 1s).
Rheology and Compressive Analysis
In general, PAM-based IPN-structured hydrogels are robust and capable of withstanding highly compressive stress without breakage.27 In the present study, the PDA–SA–PAM adhesive hydrogels appeared to be stretchable and robust. Moreover, the SA–PAM and 0.4PDA–SA–PAM hydrogels can rapidly return to the original shape after the application of a compression force (Supporting Information Figure S5). Figure 3a,b shows that the compressive stress of the SA–PAM hydrogels can reach a strength of 0.3353 MPa and a stiffness of 2479 N/m at a 70% compressive strain. Once the incorporation of PDA into hydrogels, both compressive strength and stiffness were decreased to 0.21 and 0.18 MPa and 1957 and 1393 N/m for 0.2PDA–SA–PAM and 0.4PDA–SA–PAM, respectively. The decrease in compressive strength and stiffness of the hydrogels is attributed to the increased viscosity of the hydrogels. However, the 0.4PDA–SA–PAM hydrogel displayed a compressive strength of 0.18 MPa and a stiffness of 1393 N/m at a strain of 70% with excellent stretching ability and the ability to rapidly return to its original position (Supporting Information Figure S5), which is a significant improvement compared with PAM, PDA-PAM, and other adhesive hydrogels.19−22
Figure 3.
DMA compressive properties of hydrogels, (a) stress–strain curves and their (b) compressive strength and stiffness. Rheological properties of hydrogels, (c) storage modulus and (d) loss tangent (G″/G′).
The solid-like elastic response of the hydrogels with and without PDA was evaluated via frequency-dependent rheology of the storage modulus (G″) and loss factor (tan δ = G″/G′), as shown in Figure 3c,d. In this study, the SA–PAM-based hydrogels exhibited a low, stable loss factor, suggesting that the hydrogels have good elastic recovery properties. A similar conclusion has been reported for PAM and PAM polysaccharide-based IPN-structured hydrogels.28 It is interesting to note that the 0.4PDA–SA–PAM hydrogel also exhibited a low, stable loss factor (tan δ), indicating the good elastic recovery of hydrogels.
Microstructure of Hydrogels
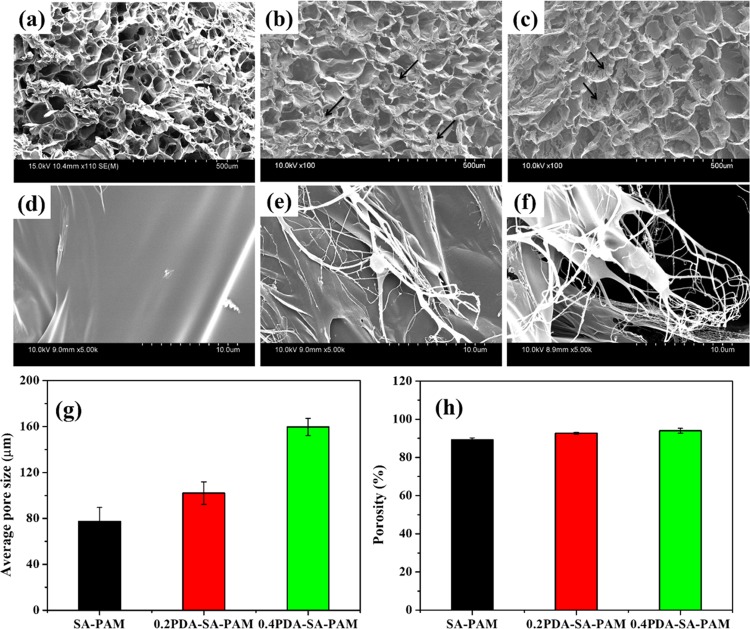
The microstructure and morphological features of the hydrogels were analyzed by scanning electron microscopy (SEM) after water was removed by freeze-drying (Figure 4). The average pore sizes of SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM were calculated to be 77.4 ± 12.0, 102.0 ± 9.8, and 159.7 ± 7.4 μm, respectively (Figure 4g). A highly interconnected porous structure with large average pore sizes was observed on the adhesive hydrogels (0.4PDA–SA–PAM, Figure 4c) because the PDA functional groups (catechol) can bind with water molecules in a regular structure, which is consistent with the measured compressive strength and G′ results. Hence, the adhesive hydrogels have great potential for tissue engineering because these interconnected porous structures enable cell recruitment to facilitate the permeation of nutrients and oxygen. Moreover, the pure SA–PAM hydrogel had a smooth surface, whereas the 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels exhibited microfibril structures (the arrow indicates the formation of PDA microfibrils on the hydrogel surface). The high-resolution field emission SEM (FE-SEM) images of SA–PAM hydrogels show a smooth surface (Figure 4d), whereas 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels had fibrous topology with microfibrils (Figure 4e,f). Microfibrils formed in the PDA incorporated SA–PAM hydrogel because of the complexation of PDA and SA, which is homogeneously dispersed in the PAM network through π–π interactions and hydrogen bonds.22,28 The scaffolds should have a porosity of at least 90% to provide a large surface area for cell–polymer interactions and to yield sufficient space for ECM regeneration during in vitro culture.29 The porosity was observed to increase with the increasing PDA content in the hydrogels, at 89.2% (SA–PAM), 92.6% (0.2PDA–SA–PAM), and 94.0% (0.4PDA–SA–PAM), respectively (Figure 4h). Overall, the microstructure results confirm that the developed PDA-based hydrogels have great potential for tissue engineering.
Figure 4.
SEM images of hydrogels at low magnification (a) SA–PAM, (b) 0.2PDA–SA–PAM, and (c) 0.4PDA–SA–PAM hydrogels and (d–f) high-magnification SEM images of their hydrogels respectively, (g) average pore sizes and (h) % porosity of hydrogels.
Swelling and Biodegradation
The water retention capacity of porous hydrogels is important for cell growth to ensure that the nutrient medium can easily penetrate the hydrogels. Thus, we explored the effect of SA and PDA–SA on hydrolytic swelling and degradation within the PAM networks in phosphate-buffered saline (PBS) (pH 7.4) at 37 °C (Figure 5a). The hydrogels are highly porous with interconnected network structures. During the swelling process, the pores inside the network rapidly fill with PBS solvent; at the same time, the polymer segments (PAM, PDA, and SA) take up PBS, the content of which depends on the attractive force between the PBS and the polymer chains. As expected, the PDA–SA–PAM hydrogels swelled significantly more than the SA–PAM hydrogels, most likely due to both the solvation of the hydrophilic segments (SA and PDA) and pore filling by the PBS solvent, since the hydrogels have high porosity. Moreover, among all samples, the 0.4PDA–SA–PAM hydrogel exhibited the highest swelling capacity because of its larger pore size, which is important for cell growth in tissue engineering.
Figure 5.
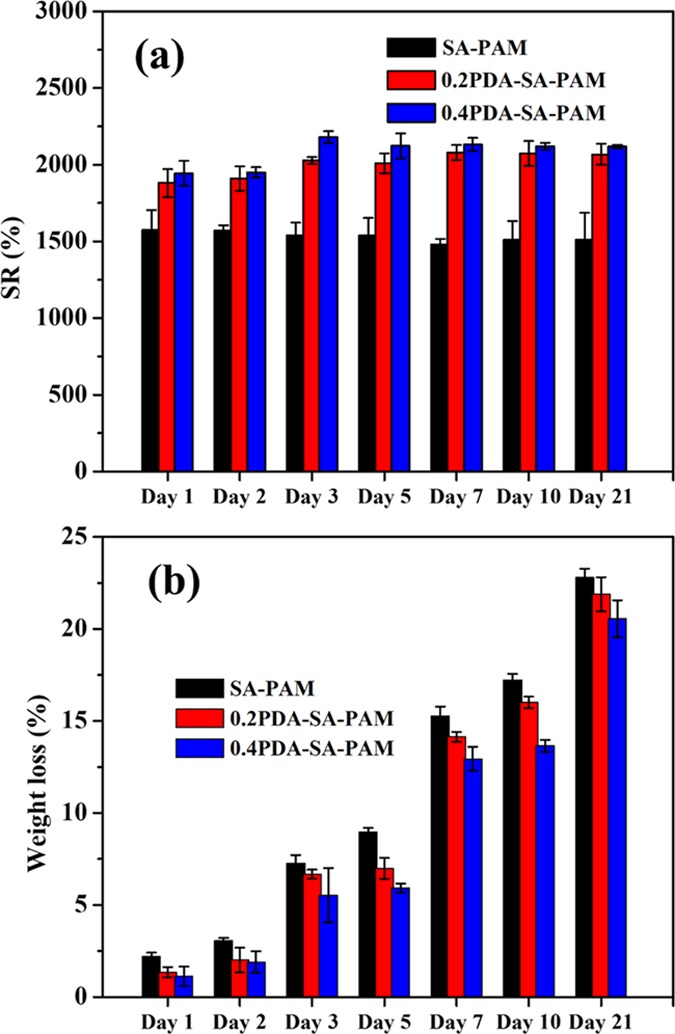
(a) Swelling (%) and (b) degradation weight loss (%) of hydrogels in PBS media.
Biodegradable hydrogels play an important role in tissue engineering applications. Ideally, the degradation rate should match the tissue regeneration rate. Biodegradable hydrogels can achieve this via polymer backbone degradation (e.g., hydrolysis, enzymatic cleavage). For this purpose, in vitro degradation of the hydrogels was investigated by monitoring the percent weight loss during incubation in PBS (pH 7.4) at 37 °C (Figure 5b). The percent weight loss of the hydrogels at different time points is shown in Figure 5b. In particular, the degradation trend obtained for the hydrogels was SA–PAM > 0.2PDA–SA–PAM > 0.4PDA–SA–PAM following a 21-day incubation with a weight loss of 22.7, 21.8, and 20.5%, respectively. In general, the PAM hydrogels exhibited the lowest degradability compared with the polysaccharide combination. The blend components used in this study (SA, PDA, and PAM) are essentially a combination of biodegradable (SA) and nonbiodegradable polymers (PDA and PAM). The SA plays an important role in improving the swelling and degradation properties; thus, the PDA–SA combination is more advantageous for improving the mechanical and biodegradation properties.
Cell Proliferation, Viability, and Attachment
PDA is an adhesive material that can better facilitate cell attachment compared with other polysaccharides and synthetic polymers.19−22,28 The combination of PDA with the SA–PAM hydrogel is expected to facilitate cell attachment compared with the SA–PAM hydrogel alone. An 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the proliferation of SFs and KTs seeded on hydrogels for 3 and 7 days (Figure 6a,b). An increase in cell proliferation (cell number) for the SFs and KTs was observed at 3 and 7 days for both the 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels compared with the SA–PAM hydrogel and tissue culture polystyrene (TCPS) as two-dimensional cell culture conditions. The proliferation capacity of both cell types (SFs and KTs) on the hydrogels was greatly improved for the hydrogels containing PDA. Overall, the adhesive hydrogels show a high cell proliferation capacity for both SF and KT cells compared with SA–PAM and TCPS (p < 0.05). Furthermore, the viability and morphology of cells (SFs and KTs) on the hydrogels (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) was evaluated via a live/dead assay using fluorescence microscopy (Figure 6c). The cells in the PDA–SA–PAM hydrogels exhibited significantly greater attachment compared with the SA–PAM hydrogels and TCPS with no dead cells observed at day 7. Hence, PDA can play an important role in improving cell attachment and proliferation due to good adhesion between the cells and the hydrogel. Overall, in vitro evaluation of the hydrogels demonstrates that the PDA-containing hydrogels are cytocompatible and promote cell proliferation and adhesion with cell spreading in the 3D environment. This evidence is supported by the recent work of Cima et al., who described the effect of adhesive PDA on cell proliferation and attachment.29 Furthermore, the adhesion of SF and KT cell lines to the hydrogels (SA–PAM and PDA–SA–PAM) was analyzed by SEM after 3 and 7 days of culture (Figure 7). SEM images showed that cells (SFs and KTs) attached more firmly to the SA–PAM hydrogels compared with the PDA–SA–PAM hydrogel. This result may be due to the adhesive component of PDA–SA. Indeed, the 0.4PDA–SA–PAM scaffold had a higher number of adherent cells (SFs and KTs) on the scaffold surface compared with the SA–PAM hydrogel, confirming that the PDA–SA-PAM hydrogel not only facilitates cell attachment but also cell spreading.
Figure 6.
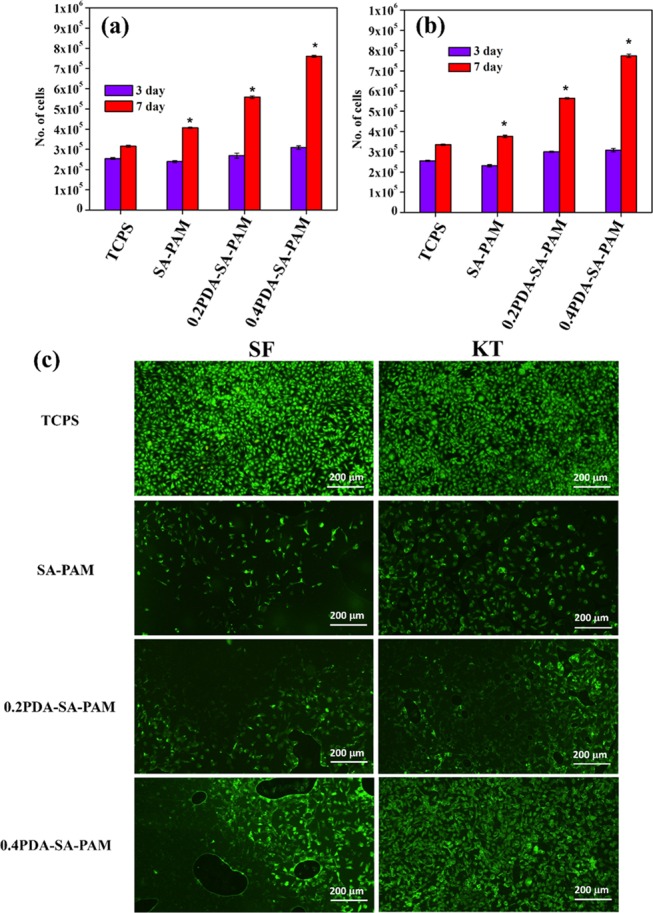
MTT assay cell proliferation of hydrogels for (a) SF and (b) KT, and (c) live/dead assay fluorescence images of SF and KT cells on hydrogels under 10× magnification (scale bar 200 μm) (*p < 0.05).
Figure 7.
SEM images of cell attachment of SF and KT cells on hydrogels in different culture conditions (3 and 7 days).
Hemostasis Performance
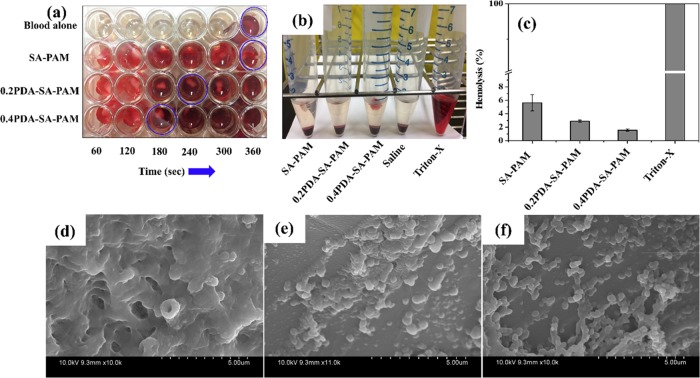
The blood clotting time was obtained by evaluating the in vitro blood clotting potential of hydrogels treated with porcine whole blood. Figure 8a shows the blood clotting time after porcine whole blood was applied to the hydrogels (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM). The blood clotting time was found to be 360 s for porcine whole blood alone, similar to blood clotting time of human whole blood reported elsewhere.30 The blood clotting time for porcine whole blood was found to be the same (360 s) on the SA–PAM hydrogel. However, the blood clotting time was 180 s for the 0.4PDA–SA–PAM hydrogel, which is much shorter than the times for 0.2PDA–SA–PAM (240 s) and SA–PAM (360 s) and blood alone (360 s). The decreased blood clotting time for the PDA-containing hydrogel is due to the hemostatic ability of PDA.
Figure 8.
Hydrogels treated with pig whole blood, (a) photographic images of a blood clot formed with respect to time, (b) photographic images showing hemolysis, (c) hemolysis (%) of different hydrogels (*p < 0.05). SEM images of platelet adhesion on (d) SA–PAM, (e) 0.2PDA–SA–PAM, and (f) 0.4PDA–SA–PAM hydrogels.
Furthermore, the hemocompatibility of the developed hydrogels was evaluated by performing a hemolysis assay (Figure 8b,c). Photographs were acquired of vials containing SA–PAM, 0.2PDA–SA–PAM, 0.4PDA–SA–PAM, Triton-X, and saline treated with red blood cells (RBCs) after centrifugation (Figure 8b). The photographs show a clear supernatant for the sample of saline-treated RBCs, revealing the hemocompatibility of the hydrogels. In contrast, the Triton-X sample was not clear because of the release of hemoglobin from the RBCs. Furthermore, the percent of hemolysis was found to be less than 5% (the allowable limit for hydrogels) for the 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels while that of the SA–PAM hydrogels was 5.5%, suggesting that high hemocompatibility is achieved when PDA is incorporated into the hydrogels (Figure 8c).
Platelet adhesion is a crucial indicator of thrombosis. To investigate the effect of PDA content on platelet adhesion to the hydrogels, we studied platelet adhesion by SEM (Figure 8). The SEM images showed low platelet adhesion to SA–PAM (Figure 8d); however, very high platelet adhesion was observed on the 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels (Figure 8e,f). In general, the fibrous topography of collagen and other nanofibrous scaffolds results in high platelet adhesion; thus, a nanofibrous topography may play an important role in platelet adhesion.31 In this study, the microfibrils formed in the hydrogel networks gave rise to a fibrous topology as observed in the hydrogel microstructure. Therefore, the platelets could sense and respond to the 0.2PDA–SA–PAM and 0.4PDA–SA–PAM hydrogels. The platelet adhesion was poor in the SA–PAM hydrogel due to its smooth surface microstructure. Thus, PDA may play an important role in facilitating platelet adhesion and accelerating blood clot formation.
Conclusions
In summary, adhesive, highly elastic hydrogels were prepared using a combination of PDA–SA–PAM for tissue engineering applications. Overall, the adhesive component of PDA–SA possesses high hydrophilicity, and its molecules can produce viscous solutions, which have a significant impact on the characteristics of PDA–SA–PAM-based hydrogels, including the morphology, cell adhesion, swelling behavior, degradation, mechanical properties, and hemostasis. Hence, the desirable pore structure, formation of adhesive microfibrils, good mechanical properties, outstanding swelling properties, controllable degradation, hemocompatibility, blood clotting ability, and favorable biocompatibility render these hydrogels promising materials for skin tissue engineering. Continuing with this basic platform, future studies will focus on in vivo evaluations to confirm the potential of these hydrogels for skin tissue engineering applications.
Experimental Section
Materials
DA, N,N′-MBA, and tetramethylenediamine (TEMED) were purchased from Sigma-Aldrich. AM and ammonium persulfate (APS) were purchased from Dae-Jung Chemical and Metal Co., Ltd., South Korea. All chemicals were used as received.
Preparation of PDA–SA–PAM Hydrogels
PDA–SA–PAM hydrogels were prepared by alkali-induced polymerization of DA in SA solution followed by free radical polymerization of AM monomers using N,N′-MBA as a crosslinker. Typically, the DA monomer was polymerized in 2% SA solution at pH 10 for 30 min under open-air atmosphere. Subsequently, AM (2.0 g), MBA (0.075 g), and APS (0.2 g) were added to the PDA–SA solution in an ice-water bath. Finally, TEMED (20 μL) was added. The reaction mixture was removed from the ice-water bath and allowed to polymerize at room temperature. After formation, the hydrogels were placed in a beaker containing distilled water for 3 days to remove unreacted monomer (AM), crosslinker (N,N′-MBA), and initiator (APS). Finally, the hydrogels were freeze-dried at −80 °C for 3 days. The hydrogels were prepared at different DA/AM ratios (0.2 and 0.4) and without PDA as summarized in Supporting Information Table S1.
Characterization
The structural and functional groups of the SA and PDA–SA complex were evaluated using Fourier-transform infrared (FTIR) spectrophotometer (PerkinElmer). The spectral data were recorded in the transmission mode over the range of 4000–500 cm–1 using 16 scans at a resolution of 4 cm–1. UV–visible spectra of the SA and PDA–SA complex were acquired using a UV–visible spectrophotometer (HITACHI U-2010) scanning between 200 and 500 nm. The rheological behavior of the hydrogels was assessed under a frequency mode range of 0.1–100 1/s using an Anton Paar rheometer (Physica MCR301; Austria) with a parallel-plate geometry at 25 °C. The mechanical properties of the hydrogels were investigated in the compression mode using a dynamic mechanical analyzer (DMA) (Q800, TA instruments, South Korea) with a 0.05 N preload force at 25 °C and a rate of 3.0 N/min. Prior to analysis, the samples were immersed in PBS solution and then cut into circular disks with a diameter of 10 mm and a height of 10 mm. The structural composition and functional group interactions of the hydrogels were analyzed by XPS using ESCALAB 250 with an Al Kα X-ray monochromatic source (hν = 1486.6 eV) and a spot size of 200 μm. The microstructure of the hydrogels (SA–PAM and PDA–SA–PAM) was analyzed by SEM using Hitachi S-4800.
Porosity
To evaluate the porosity, a known weight of freeze-dried hydrogels (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) (M1) was placed in a bottle filled with absolute ethanol, which was later degassed by supersonic treatment to infiltrate the hydrogel with ethanol.32 Then, the hydrogels were removed, the excess surface ethanol was wiped with filter paper, and then the hydrogels were immediately weighed (M2). The following equation was applied to calculate the porosity of the hydrogels (N = 3 for each time point).
where ρ is the density of the absolute ethanol and V is the volume of the hydrogel.
Swelling and Biodegradation
Before the physical properties of the hydrogels were evaluated, the dry weight (Wd) of the hydrogels (10 mm diameter) was measured. Then, the samples were immersed in PBS solution (10 mL) and incubated at 37 °C, and the PBS was replaced every week. At predetermined time intervals (1, 2, 3, 5, 7, 10, and 21 days), the hydrogel samples were removed, and the swollen hydrogel sample weights were measured (Ws). The percent swelling ratio (% SR) of the hydrogels was calculated by the following equation (N = 3 for each time point).
After the swollen hydrogel samples were weighed, the samples were washed with water, lyophilized, and then weighed (Wf) again. The biodegradation of the hydrogel samples was measured as the percent mass loss of the hydrogel samples, calculated by the following equation (N = 3 for each time point).
Strength of Hydrogel Adhesion to Porcine Skin
The adhesion strength of the SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM hydrogels to porcine skin was measured using a tensile test (Instron 5567). The hydrogels were applied to the specimen surface with a bonding area of 25 × 20 mm2. The strength of adhesion was measured as the maximum load divided by the bonded area.
Cell Studies (Cell Proliferation, Viability, and Attachment)
Human skin fibroblasts (CCD-986sk) (SFs) and keratinocytes KTs (American Type Cell Culture Collection (ATCC)) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin solution. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °Chironomus prior to the cell studies, the samples were sterilized in 70% ethanol for 3 h, repeatedly washed with PBS, and finally incubated overnight in DMEM solution.
The proliferation of SF and KT cells on the hydrogels (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) was assessed using the MTT assay. Briefly, SF and KT cells were seeded at a density of 5 × 104 cells/cm2 onto 24-well plates containing hydrogels, which were incubated in a humidified atmosphere (5% CO2 at 37 °C) for 3 and 7 days. Then, 200 μL of MTT dye solution (5 mg/mL PBS solution) was added to each well, followed by incubation in a humidified atmosphere for 4 h. After the medium was removed, the scaffolds containing dye were dissolved by the addition of acidic isopropanol solution and incubated for 30 min in the dark at room temperature. From each well, the resulting solution was transferred to a 24-well plate, and the optical density of the converted dye was screened at 570 nm using a microplate reader. The cell proliferation (cell number) was calculated based on a calibration curve that was constructed from different cell densities.
Furthermore, the effect of the hydrogels on cell viability and their cytotoxicity (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) were examined using a live/dead assay kit (Life Technology). Briefly, cells were grown at a density of 5 × 104 cells in a scaffold placed in a 24-well plate and were placed in a humidified incubator (5% CO2 at 37 °C) for 7 days. The culture medium was changed every other day. Then, the culture medium was replaced with 600 μL/well from a mixture of 10 mL of PBS containing 20 μL of ethidium homodimer-1 (red dye for the detection of dead cells) and 2 μL of calcein AM (white dye for the detection for live cells), followed by incubation for 30 min. The scaffolds containing cells were visualized by fluorescence microscopy (Nikon Eclipse Ti, Italy).
The adhesion of SFs and KTs to the hydrogels (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) was also assessed. The cells (5 × 104) were seeded onto the surface of the hydrogels, which were fitted in 12-well plates and were incubated in a humidified atmosphere for 3 or 7 days. TCPS was used for comparison of 3D cell culture with hydrogels. After incubation, the medium was removed, and the hydrogels were washed with PBS. Then, the cells were fixed by applying 4% formaldehyde solution for 10 min. Finally, the samples were washed again with PBS, followed by dehydration with an ethanol–water mixture (30, 50, 70, and 100%). The morphology of the adherent cells on the hydrogels was observed by FE-SEM (SEM, Hitachi S-4800).
In Vitro Hemostasis
Porcine whole blood was kindly received from the Lotte Foods Co., Ltd. slaughterhouse, to which sodium citrate was added (3 mg/mL blood). The hydrogel samples (SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM) were placed in 42-well plates. Then, 300 μL of recalcified porcine whole blood was added to each well containing a hydrogel sample.33 Wells without hydrogels were used as a control. At specific points in time, the hydrogel samples containing wells treated with saline solution were used to remove the remaining nonclotted blood. The blood clotting time was recorded for the formation of stable clots after the wells were washed with saline.
Hemolysis Assay and Platelet Adhesion
Porcine whole blood with sodium citrate solution (3.8%) was centrifuged to separate red blood cells (RBCs) and platelet-rich plasma (PRP) for a hemolysis assay and to evaluate platelet adhesion to the hydrogel samples.33,34 To evaluate the hemocompatibility of the hydrogels, 500 μL of diluted RBCs (1 mL of RBCs with 9 mL of saline) was added to 100 mg of SA–PAM, 0.2PDA–SA–PAM, and 0.4PDA–SA–PAM hydrogels in separate vials. In addition, 100 μL of 0.1% Triton-X and 100 μL of saline were used as positive and negative controls, respectively. The vials were incubated at 37 °C for 1 h and then centrifuged at 3500 rpm for 10 min. The obtained supernatant was analyzed to determine the optical density using a microplate reader at 540 nm. The percentage of hemolysis was determined using the following equation (N = 3 for each time point).
Before the platelet adhesion test, the hydrogel samples were immersed in PBS solution for 3 h and then placed in 24-well plates. A PRP suspension was added to the samples, which were then incubated for 1 h at 37 °C. Subsequently, the hydrogels were washed twice with PBS and immersed in 1% glutaraldehyde solution for 2 h to fix platelets on the hydrogels. Then, the hydrogels were again washed with PBS and dehydrated in gradient ethanol solutions (30, 50, 70, and 100%). Finally, the morphology of the adherent platelets was examined by FE-SEM (SEM, Hitachi S-4800).
Statistical Analysis
The data are given as the mean ± standard deviation. Statistical differences were analyzed using a one-way analysis of variance, and a value of p < 0.05 was determined to be statistically significant.
Acknowledgments
K.M.R. and S.S.H. would like to acknowledge the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Technology (NRF-2019R1I1A3A01063627 and NRF-2017R1D1A3B03031234).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01302.
Composition of preparation of hydrogels (Table S1); schematic representation of formation of possible chemistry of the PDA–SA complex (Figure S1); results of the SA–PDA complex characterized by UV–vis spectra, FTIR spectra, and XPS spectra (Figure S2); 0.4PDA–SA–PAM hydrogel adhesion on the author’s hand (Figure S3); photographic images of the 0.4PDA–SA–PAM hydrogel was tightly adhered to the merged plastic tubes and enabled to support a load of 72 g, stretchability and skin adhesion of author hand peeling off (Figure S4); photographic images of DMA loading and unloading of ability of hydrogels (Figure S5) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ahmed E. M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Votruba A. R.; Farokhzad O. C.; Langer R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. 10.1021/nl102184c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenger D.; Topuz F.; Groll J. Hydrogels in Sensing Applications. Prog. Polym. Sci. 2012, 37, 1678–1719. 10.1016/j.progpolymsci.2012.09.001. [DOI] [Google Scholar]
- Pekel N.; Güven O. Separation of Heavy Metal Ions by Complexation on Poly (N-Vinyl Imidazole) Hydrogels. Polym. Bull. 2004, 51, 307–314. 10.1007/s00289-004-0224-x. [DOI] [Google Scholar]
- Mehdizadeh M.; Yang J. Design Strategies and Applications of Tissue Bioadhesives. Macromol. Biosci. 2013, 13, 271–288. 10.1002/mabi.201200332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J. C.; Ruff G. L.; King M. W.; Dattilo P. P. Barbed, Bi-Directional Surgical Sutures. Med. Text. Biomater. Healthc. 2006, 395–403. [Google Scholar]
- Rinaudo M. Main Properties and Current Applications of Some Polysaccharides as Biomaterials. Polym. Int. 2008, 57, 397–430. 10.1002/pi.2378. [DOI] [Google Scholar]
- Malafaya P. B.; Silva G. A.; Reis R. L. Natural–origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug Delivery Rev. 2007, 59, 207–233. 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Francis Suh J.-K.; Matthew H. W. Application of Chitosan-Based Polysaccharide Biomaterials in Cartilage Tissue Engineering: A Review. Biomaterials 2000, 21, 2589–2598. 10.1016/S0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Lee B. P.; Messersmith P. B.; Israelachvili J. N.; Waite J. H. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sileika T. S.; Kim H.-D.; Maniak P.; Messersmith P. B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. 10.1021/am200978h. [DOI] [PubMed] [Google Scholar]
- Ding Y. H.; Floren M.; Tan W. Mussel-Inspired Polydopamine for Bio-Surface Functionalization. Biosurface Biotribology 2016, 2, 121–136. 10.1016/j.bsbt.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhurakkat Perikamana S. K.; Lee J.; Lee Y. B.; Shin Y. M.; Lee E. J.; Mikos A. G.; Shin H. Materials from Mussel-Inspired Chemistry for Cell and Tissue Engineering Applications. Biomacromolecules 2015, 16, 2541–2555. 10.1021/acs.biomac.5b00852. [DOI] [PubMed] [Google Scholar]
- Han L.; Zhang Y.; Lu X.; Wang K.; Wang Z.; Zhang H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. 10.1021/acsami.6b11043. [DOI] [PubMed] [Google Scholar]
- Jing X.; Mi H. Y.; Napiwocki B. N.; Peng X. F.; Turng L. S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. 10.1016/j.carbon.2017.09.071. [DOI] [Google Scholar]
- Liu C.; Yao W.; Tian M.; Wei J.; Song Q.; Qiao W. Mussel-Inspired Degradable Antibacterial Polydopamine/Silica Nanoparticle for Rapid Hemostasis. Biomaterials 2018, 179, 83–95. 10.1016/j.biomaterials.2018.06.037. [DOI] [PubMed] [Google Scholar]
- Jing X.; Mi H. Y.; Peng X. F.; Turng L. S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. 10.1016/j.carbon.2018.04.065. [DOI] [Google Scholar]
- Jing X.; Mi H. Y.; Lin Y. J.; Enriquez E.; Peng X. F.; Turng L. S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. 10.1021/acsami.8b06475. [DOI] [PubMed] [Google Scholar]
- Hou J.; Li C.; Guan Y.; Zhang Y.; Zhu X. X. Enzymatically Crosslinked Alginate Hydrogels with Improved Adhesion Properties. Polym. Chem. 2015, 6, 2204–2213. 10.1039/C4PY01757A. [DOI] [Google Scholar]
- Hong S.; Yang K.; Kang B.; Lee C.; Song I. T.; Byun E.; Park K. I.; Cho S.-W.; Lee H. Hyaluronic Acid Catechol: A Biopolymer Exhibiting a PH-Dependent Adhesive or Cohesive Property for Human Neural Stem Cell Engineering. Adv. Funct. Mater. 2013, 23, 1774–1780. 10.1002/adfm.201202365. [DOI] [Google Scholar]
- Ryu J. H.; Lee Y.; Kong W. H.; Kim T. G.; Park T. G.; Lee H. Catechol-Functionalized Chitosan/Pluronic Hydrogels for Tissue Adhesives and Hemostatic Materials. Biomacromolecules 2011, 12, 2653–2659. 10.1021/bm200464x. [DOI] [PubMed] [Google Scholar]
- Han L.; Yan L.; Wang K.; Fang L.; Zhang H.; Tang Y.; Ding Y.; Weng L.-T.; Xu J.; Weng J.; et al. Tough, Self-Healable and Tissue-Adhesive Hydrogel with Tunable Multifunctionality. NPG Asia Mater. 2017, 9, e372 10.1038/am.2017.33. [DOI] [Google Scholar]
- Han L.; Lu X.; Liu K.; Wang K.; Fang L.; Weng L.-T.; Zhang H.; Tang Y.; Ren F.; Zhao C.; et al. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano 2017, 11, 2561–2574. 10.1021/acsnano.6b05318. [DOI] [PubMed] [Google Scholar]
- Drury J. L.; Mooney D. J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337–4351. 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Shevchenko R. V.; James S. L.; James S. E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc., Interface 2010, 7, 229–258. 10.1098/rsif.2009.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidsrød O.; Skja°k-Br̵lk G. Alginate as Immobilization Matrix for Cells. Trends Biotechnol. 1990, 8, 71–78. 10.1016/0167-7799(90)90139-O. [DOI] [PubMed] [Google Scholar]
- Ohsedo Y.; Taniguchi M.; Saruhashi K.; Watanabe H. Improved Mechanical Properties of Polyacrylamide Hydrogels Created in the Presence of Low-Molecular-Weight Hydrogelators. RSC Adv. 2015, 5, 90010–90013. 10.1039/C5RA16823F. [DOI] [Google Scholar]
- Han L.; Wang M.; Li P.; Gan D.; Yan L.; Xu J.; Wang K.; Fang L.; Chan C. W.; Zhang H.; et al. Mussel-Inspired Tissue-Adhesive Hydrogel Based on the Polydopamine–Chondroitin Sulfate Complex for Growth-Factor-Free Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. 10.1021/acsami.8b05314. [DOI] [PubMed] [Google Scholar]
- Cima L. G.; Vacanti J. P.; Vacanti C.; Ingber D.; Mooney D.; Langer R. Tissue Engineering by Cell Transplantation Using Degradable Polymer Substrates. J. Biomech. Eng. 1991, 113, 143. 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- Muthiah Pillai N. S.; Eswar K.; Amirthalingam S.; Mony U.; Kerala Varma P.; Jayakumar R. Injectable Nano Whitlockite Incorporated Chitosan Hydrogel for Effective Hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. 10.1021/acsabm.8b00710. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Thomas V.; Xu Y.; Bellis S. L.; Vohra Y. K. An in Vitro Regenerated Functional Human Endothelium on a Nanofibrous Electrospun Scaffold. Biomaterials 2010, 31, 4376–4381. 10.1016/j.biomaterials.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Sornkamnerd S.; Okajima M. K.; Kaneko T. Tough and Porous Hydrogels Prepared by Simple Lyophilization of LC Gels. ACS Omega 2017, 2, 5304–5314. 10.1021/acsomega.7b00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthiah Pillai N. S.; Eswar K.; Amirthalingam S.; Mony U.; Kerala Varma P.; Jayakumar R. Injectable Nano Whitlockite Incorporated Chitosan Hydrogel for Effective Hemostasis. ACS Appl. Bio Mater. 2019, 2, 865–873. 10.1021/acsabm.8b00710. [DOI] [PubMed] [Google Scholar]
- Henkelman S.; Rakhorst G.; Blanton J.; van Oeveren W. Standardization of Incubation Conditions for Hemolysis Testing of Biomaterials. Mater. Sci. Eng., C 2009, 29, 1650–1654. 10.1016/j.msec.2009.01.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.