Abstract
Withania coagulans is an Indian medicinal herb, the natural extracts of which are purported to have health-benefiting properties. In this study, the extract was encapsulated in nature-derived polymers with the aim of enhancing its bioavailability. The aqueous extract obtained from the plant W. coagulans was found to elicit the glucose-lowering effect by means of promoting insulin secretion from pancreatic β cells. The cells treated with the extract showed a nearly 2-fold increase in insulin secretion compared to untreated cells. A delivery system for the extract was developed based on electrosprayed chitosan nanoparticles coated with food-based starch. The enteric starch coating retarded (by 2.5 times) the release of the extract in the stomach. The bioactivity of the encapsulated extract was subsequently tested in vitro on mouse-derived pancreatic β cells, whereby the delivery system was found to promote insulin secretion. Finally, the extract-encapsulated oral delivery system was tested on diabetic mice and was validated to decrease blood glucose levels by 60%. In summary, it could be inferred that food-grade enteric-coated polysaccharide-based particles increase the bioavailability of the extracted compounds from the plant W. coagulans.
1. Introduction
There is an ever-increasing interest in complementary and alternative medicine to combat chronic illnesses. The impetus to exploit such alternative therapies lies in the intent to identify new molecules that may potentially be more efficient than existing drugs, with possibilities of reduced side effects.1 Moreover, the advantage of using the traditional system of medicine lies in the fact that many of these naturally obtained extracts offer an early intervention and health maintenance approach in alleviating diseases.2 Such naturally obtained extracts could also serve as leads to the chemical synthesis of new drugs.3 While there are numerous natural, plant-based compounds used in traditional medicines, extracted nutrients from the food that possess therapeutic properties are classified as nutraceuticals.4 Owing to their food-based origin, they are generally considered less toxic and are thus more widely accepted, although dosage-related toxicity or toxicity due to cross-reactivity with synthetic drugs cannot be ruled out.5 In fact, with the increasing focus on health and well-being globally, the demand for nutraceuticals, especially from developing economies, i.e., Brazil, China, India, etc., is on the rise, whereby recent estimates reflect that the global nutraceutical market will reach USD 578.23 billion by 2025.6 In view of this growing interest in nutraceuticals, rigorous and systematic scientific-based investigations are imperative to understand their therapeutic effects, toxic doses, and mechanisms of action.
Diabetes, a chronic metabolic disorder, is manifested mainly as high-blood-glucose levels, which when left untreated leads to complications in other organs such as kidney, heart, eyes, and the nervous system.7 Lately, diabetes has become a global epidemic, affecting people in both poor and affluent countries. Type 2 diabetes, predisposed by obesity and physical inactivity, is the most prevalent type of diabetes.8 According to a recent 2017 report, about 451 million people are affected by diabetes. Of the total, type I diabetes accounts for only a small percentage of the total estimates. Global estimates for type 1 diabetes in children and adolescents aged less than 20 years was 1 106 200 in 2017. While patients with type I diabetes need daily insulin injections to survive, for type II diabetes, the first line of treatment is mainly lifestyle changes, followed by single or combination therapies with oral hyperglycemic drugs. When oral medications fail to control blood glucose levels, insulin injections are prescribed.9 In addition to the health setback experienced by the patient, diabetes also poses a considerable financial burden, with the requirement for life-long medication. The mean cost for the management of the disease can range from 1000 to 4000 international dollars per person, depending on the economic region.9,10 This is reflected from the projected treatment market size of USD 64 billion by 2026.11
In the recent decade, there have been reports on plant extracts exhibiting antidiabetic properties.12,13Withania coagulans (common name: Paneer dodi) is one such plant, whose extracts have traditionally been used in the coagulation of milk to make Indian cheese14 and have also been used in the traditional Indian system of medicine due to its numerous therapeutic properties.15−17 The use of such extracts in the management of diabetes may be preferred over existing drugs, especially if proven to be more efficacious and safer. While the consumption of the raw extract is traditionally practiced by immersing the fruit overnight in water, its bitter taste and strong odor deter consumption of the necessary quantities of it to elicit a health-benefiting effect. To overcome this, such naturally derived extracts can be encapsulated into concentrated forms to function as nutraceuticals. The validation of these extracts against diabetes therefore warrants a rigorous and thorough investigation through scientific approaches. Another distinct challenge in the consumption of the extracts is that, since the extracts are consumed orally, they have to encounter the low-pH conditions in the stomach and various enzymes throughout the gastrointestinal tract (GIT). Plant-based extracts are usually sensitive to such adverse conditions, which may render them inactive, thus reducing their bioavailability.18,19
Based on the above discourse, it was hypothesized that the encapsulation of the extract of W. coagulans into food-based particles, with specific small-intestine-targeting functionality, would provide sustained glucose-lowering capabilities. To test this hypothesis, the active compound was solvent-extracted and encapsulated into a delivery system that could retain its bioactivity until it reaches the intended site of adsorption along the GIT. Subsequently, its efficacy was evaluated through in vitro and in vivo studies. This study is a primary initiative to encapsulate extracts from W. coagulans and to evaluate their efficacy on diabetic mice.
2. Experimental Section
2.1. Materials
Low-molecular-weight chitosan (≥75% deacetylated, viscosity 20–300 cps, and molecular weight 50–190 kDa), sodium tripolyphosphate (TPP), sodium trimetaphosphate (STMP), starch, pepsin, and pancreatin were purchased from Sigma-Aldrich, Singapore. Dulbecco’s minimum essential medium (DMEM)/high glucose with l-glutamine and sodium pyruvate (DMEM–GE Hyclone) used for MIN6 culture was obtained from GE Healthcare Pte. Ltd. Fetal bovine serum (FBS) (Gibco), 1% penicillin streptomycin solution (Gibco), and 0.25% trypsin-ethylenediaminetetraacetic acid (Gibco) were procured from Life Technologies Holdings Pte. Ltd. The cell counting kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. The mouse insulin ELISA assay kit was purchased from Mercodia. Blood glucose levels in mice were determined using Terumo Blood Glucose Test Strips with Terumo, Medisafe Ex Blood Glucose Meter (Terumo Corp., Tokyo, Japan). W. coagulans was sourced from a herb supplier (Haridass Aggarwal & Sons, Mumbai, India).
2.2. Methods
2.2.1. Extraction of Coagulans
Withanolides were extracted from the plant W. coagulans sourced from a herb supplier in India. The extraction process was performed according to a previously reported procedure with slight modifications.20 The berries of known weight from the plant were crushed mechanically using a pestle and mortar, and the coarse powder obtained was soaked in a 1:1 mixture of water and ethanol for 24 h. The extract was collected by filtering out the coarse powder using a Whatman filter paper. The hydroalcoholic extract was concentrated using a rotary evaporator for 1 h. An aliquot of the concentrated extract was mixed with a 1:1 mixture of water and ethyl acetate and extracted to obtain a water-soluble fraction and an ethyl acetate-soluble fraction. The water-soluble fraction was concentrated using a rotary evaporator and subjected to column chromatography over silica gel (230–400 mesh size) using a mixture of water and methanol (1:1) as the mobile phase. A glass Pasteur pipette filled up to 2.5 in. high with silica gel was first eluted with the mobile phase to wet the silica. The concentrated water-soluble fraction (5 mL) was added to the column and eluted using 10 mL of the mobile phase. This eluent was then subjected to rotary evaporation to remove the methanol and freeze-dried to obtain a dry powder. This freeze-dried fraction, referred to as P4 hereafter, was evaluated for its glucose-lowering effect.
2.2.2. Characterization of P4
P4 was characterized using Fourier transform infrared spectroscopy (FTIR) to confirm the presence of compounds with the steroidal lactone backbone. The dry powder of P4 was mixed with potassium bromide (KBr) in the ratio of 1:100 and pelletized using a hydraulic press and analyzed using a PerkinElmer Frontier FTIR spectrophotometer. The raw data was plotted on Origin to obtain the peaks. P4 was also characterized using nuclear magnetic resonance (NMR) spectroscopy. For this, P4 was dissolved in deuterated water (D2O) and NMR spectra were recorded on a Bruker AV 400 MHz spectrometer, with tetramethylsilane as the internal reference. For molecular weight evaluation using matrix-assisted laser desorption ionization–time-of-flight (MALDI–TOF) mass spectrometry, P4 dissolved in water was crystallized along with the matrix and sinapic acid and analyzed using a Shimadzu Axima performance MALDI–TOF mass spectrometer.
2.2.3. In Vitro Studies
Mouse pancreatic β cells, MIN6 (CRL-11506), were used as the β cell model to study insulin secretion. The cells were cultured in DMEM, supplemented with 10% FBS, 100 U mL–1 penicillin, 100 μg mL–1 streptomycin, and 0.001% β-mercaptoethanol. Cells were passaged once they reached 80% confluency. All experiments were done using cells with passage numbers 40–47. For toxicity studies, the cells were seeded on 96-well plates at a density of 2 × 104 cells per well. Cells reached confluency after 24 h; different concentrations of P4 (1, 2, 5, 10, and 25 μM) were added to the cells, and cell viability was evaluated after 2 h using the CCK-8 assay kit. For the insulin release study, cells were seeded on 6-well plates at the density of 1 × 105 cells per well and studied using the glucose-stimulated insulin secretion assay (GSIS).21,22 Briefly, once the cells reach confluency after 48 h, DMEM was removed and the cells were washed and starved for 2 h by incubating in Ca-10 buffer with 0.5 mM glucose. This initial 2 h incubation is the standard for depriving the cells of glucose to limit the insulin secretion. After 2 h starvation, the buffer was removed and the cells were incubated for another 30 min in Ca-10 buffer with 0.5 mM glucose. The samples were collected and were used to measure for the baseline insulin at nonstimulated condition by ELISA. After this 30 min starvation, the cells were stimulated by treating with Ca-10 buffer containing 16 mM glucose and the P4 treatments were done. P4 was dissolved in water at a concentration of 1 mM and was diluted accordingly to reach 1, 2, 5, 10, and 25 μM with Ca-10 buffer containing 16 mM glucose before adding 200 μL to each of the wells. After a treatment time of 30 min, the samples from the wells were collected to be analyzed by ELISA for insulin secretion under the glucose-stimulated condition in the presence or absence of P4. The insulin index was calculated using the equation below. Ca-10 buffer was prepared as follows: 125 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES, and 0.2% BSA with a desired concentration of glucose added for the low- and high-glucose conditions.
| 1 |
2.2.4. Fabrication and Encapsulation of P4 in the Carrier
The fabrication and characterization of chitosan nanoparticles (chnp) and chnp coated with starch (C+S) were conducted similar to what had previously been reported by the authors.23 Briefly, chnp were fabricated using electrospraying with the optimized parameters as follows: the concentration of chitosan dissolved in 50% acetic acid, 0.1% W/V; the concentration of TPP dissolved in water, 1% W/V with 0.5% (V/V) Tween 80; voltage, 25 kV; flow rate, 0.2 mL h–1; and working distance, 7 cm. The particles were electrosprayed directly into the cross-linking TPP solution and collected by centrifugation at an RCF of 13 000g. For coating with starch, the nanoparticle suspension was added to gelatinized soluble starch, 1:1 ratio of chnp and starch (Sigma S7965, 5 mg mL–1, gelatinized by heating starch granules in water to 90 °C), and stirred for 1 h for electrostatic interactions between starch and chnp. Starch was then cross-linked using 1% (W/V) STMP dissolved in water. For encapsulation of P4, it was mixed directly into the chitosan solution and electrospraying was carried out as detailed above. The encapsulation efficiency (EE) of P4 was calculated using the equation below.
| 2 |
2.2.5. In Vitro Release Studies
Release studies of P4 were carried out sequentially in simulated gastric fluid (SGF) (pH 1.2), simulated intestinal fluid (SIF) (pH 6.8), and pH 4.4 medium to mimic the path of the particles in the GIT.23 Release studies were performed by suspending the particles in the release media at 37 °C in an incubator fitted with a rotating wheel at a speed of 20 rpm over a period of 48 h. The time frame in each simulated fluid was chosen to mimic the residence times of food in each region of the GIT: stomach (2 h), small intestine (3 h), and pH 4.4 buffer (PBS adjusted to pH 4.4) (43 h). At fixed time points, the medium was removed by centrifugation and replaced with fresh medium. The release of P4 was quantified using high-performance liquid chromatography (HPLC) (Shimadzu Prominence UFLC) with a photodiode array detector SPD-M20A, Ascentis C18 column (10 cm, 2.1 cm I.D., and 5 μm) with methanol–water (1:1) as the mobile phase at a flow rate of 0.2 mL min–1, maintained at 30 °C. The dominant peak for P4 was observed at a retention time of 11.1 min. Suitable standards of P4 were prepared in methanol–water (1:1) to obtain a calibration curve.
2.2.6. Bioactivity Testing of P4 Encapsulated in Particles
To investigate the bioactivity of P4 encapsulated in particles, both starch-coated and uncoated chnp, encapsulated with P4, were subjected to sequential GIT conditions: SGF, SIF, and pH 4.4 medium. The concentration of P4 in the collected release media at each time point was kept at 1 μM after dilution with Ca-10 buffer containing 16 mM glucose. This diluted release medium containing P4 was added to MIN6 cells (200 μL well–1), and GSIS assay was carried out as mentioned in Section 2.2.3.
2.2.7. In Vivo Studies
The ability of the aqueous extract P4 in lowering blood glucose was further evaluated in mice using an alloxan-induced diabetes model. Male ICR mice, aged 5 weeks, weighing 28–30 g, were obtained from Nomura Siam International, Thailand. On arrival, the animals were housed in stainless steel cages (2 mice per cage) at the Laboratory Animal Facility, Suranaree University of Technology. After acclimatization for a week, the mice were induced with diabetes by peritoneal injection of alloxan (120 mg kg–1), based on the protocol adapted from Alam et al.24 Food [Smartheart Hamster Food, Perfect Companion (Taiwan) Co., Ltd] and water were given ad libitum. Fasting blood glucose (FBG) levels were monitored after 3 days of alloxan injection. Diabetic animals with FBG levels ≥250 mg dL–1 were used for the study. To keep the initial FBG levels same across the groups, the animals were grouped such that the average of the FBG in each group was not statistically different across the groups. For the initial study, the animals were divided into two treatment groups, with N = 3, and were treated with P4 (50 mg kg–1)20 or deionized (DI) water (negative control) for 5 days. P4 was dissolved in water and was fed by oral gavage every day for the entire experiment period. The body weight and FBG was monitored on day 0 at the start of the experiment and at day 5 at the end of the experiment. A set of control animals that were not induced with diabetes also received similar treatments over 15 days to assess for any toxicity of the fraction.
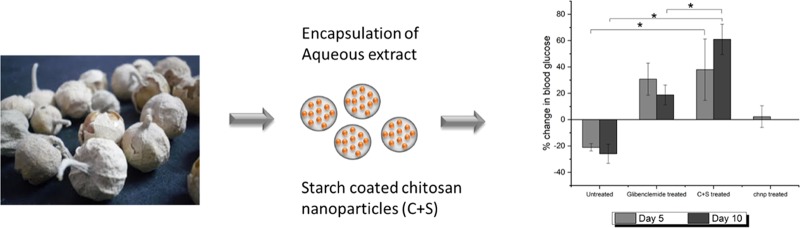
The effect of the extract-loaded delivery system was studied using the P4-encapsulated chnp and C+S particles. The particles were prepared as outlined in Section 2.2.4, and based on the EE, the amount of particles was matched to 50 mg kg–1 of P4 before feeding to the animals. Each animal was fed 8.25 mg of chnp and 16.5 mg of C+S every day (the weight of C+S is double due to the starch coating; similar amounts of chnp was used in both cases: 1 mg of chnp contains 0.2 mg of P4). The particles were suspended in DI water and fed to the animals by oral gavage. Four test groups of diabetic animals, with N = 3, were fed P4-encapsulated chnp, P4-encapsulated C+S, or DI water (negative control) for 5 days. After 5 days, the treatment was stopped and the FBG level was monitored for another 5 days. The fourth test group was treated with glibenclamide (antidiabetic drug as a positive control at 20 mg kg–1) for 10 days continuously. Data is represented as the percentage change in FBG, calculated on the basis of the equation below. All procedures were approved and conducted following the guidelines of the Institutional Animal Care and Use Committee, Suranaree University of Technology [Accreditation number: B 2559/00020.001 (SUT Laboratory Animal Facility), Permission number: U1-01433-2558 (Investigator)].
3. Results
3.1. Characterization of P4
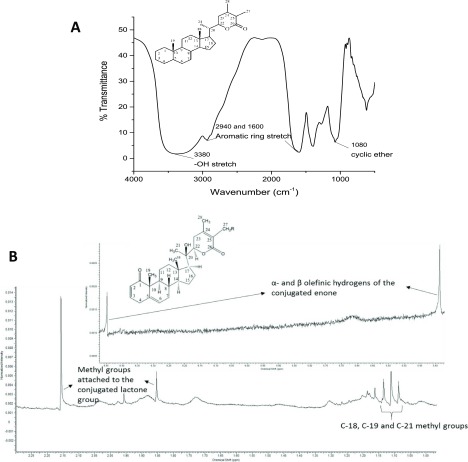
P4 obtained from W. coagulans was characterized using FTIR, NMR, and MALDI–TOF spectroscopies. For compounds previously extracted from the Withania species plants, a steroidal lactone backbone has been commonly reported. From the FTIR spectra of P4, represented in Figure 1a, the presence of the characteristic cyclic ether of the α–β unsaturated ketone peak at 1080 cm–1 and the peak for δ lactone at 1660 cm–1 confirmed the steroidal lactone backbone. Other notable peaks confirming this structure were observed at 3380 cm–1 attributed to the −OH stretch and at 1600 and 2940 cm–1 that correspond to the aromatic ring stretch. The presence of these characteristic peaks provides a preliminary quantitative confirmation of the molecules present in the fractions.25
Figure 1.
(a) FTIR spectrum of P4 showing the characteristic peaks of the steroidal lactone backbone and (b) NMR spectrum of P4 showing the peaks characteristic of the steroidal lactone backbone. The inset shows the peaks from 5.4 to 6.5 chemical shifts and the steroidal lactone structure. Structural formula in (a) is the reprinted from (26). Structural formula in (b) is reprinted with permission from ref (27). Copyright 2003 Elsevier.
Further confirmation of the similarity of P4 to that of the reported analytes25 was done using 1H NMR spectroscopy. Based on the NMR peaks obtained (Figure 1b), the peaks at 1.87 and 2.15 ppm can again be attributed to the steroidal lactone ring of P4. The α- and β-olefinic hydrogens of the conjugated enone were clearly evident at 5.39 and 6.45 ppm, respectively (see the inset). The C-18, C-19, and C-21 methyl groups were also prominently evident at 1.09, 1.12, and 1.14 ppm, respectively, as indicated in Figure 1b.
Mass spectrometric analysis was performed using MALDI–TOF–MS, as a qualitative analysis, to identify the molecular weight of the dominant compound. Three dominant peaks were observed at 491.06 m/z, 530.53 m/z, and 560.99 m/z. This led to the conclusion that there is more than one compound present in the fraction and that the higher-molecular-weight compounds in P4 might be due to a glycoside attached to the steroidal lactone rings, as commonly observed for some of the withanolides such as coagulin L, as identified in the extracts of W. coagulans.(28)
3.2. Effect of P4 on Insulin-Secreting MIN6 Cells and Diabetic Mice
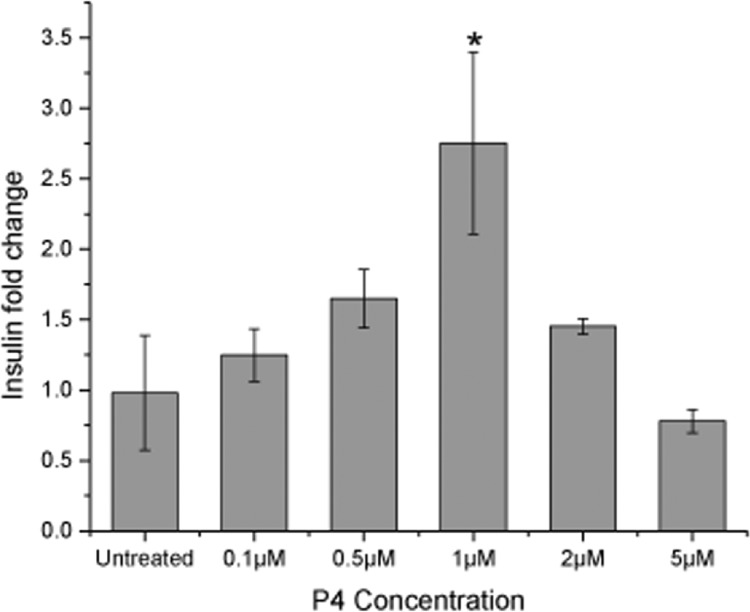
P4 was evaluated to assess its glucose-lowering effect in vitro. Cytotoxicity studies of P4 on MIN6 cells revealed that P4 was relatively benign to the cells up to 1 μM concentration (Figure S1). At concentrations of 2 and 5 μM, approximately 70% of the cells survived, which was a significant reduction in cell viability in comparison to that at lower concentrations (p < 0.05). Using the same five concentrations (0.1, 0.5, 1, 2, and 5 μM), the insulin secretion ability of MIN6 cells was evaluated using the GSIS assay. Here, the amount of insulin released is represented as a fold change, whereby the fold change for the cells treated with P4 was compared to that of the untreated cells. In Figure 2, four of the five concentrations chosen showed better insulin fold change than the untreated. A concentration-dependent increase in insulin secretion was observed up to 1 μM, beyond which the insulin secretion began to decline. At 1 μM P4, MIN6 cells showed a significant surge in insulin secretion (p < 0.05). Based on the cytotoxicity studies (Figure S1), given that cell viability reduced to ∼70% at 2 and 5 μM, it is unsurprising that the insulin fold change decreased correspondingly. Since insulin secretion from β cells is a tightly regulated pathway,29 cell survivability might have an impact on one or more processes regulating the insulin-secreting activity. Hence, this further emphasizes the importance of encapsulating this compound into delivery systems that would provide controlled release at regulated concentrations. Based on the results, the concentration of 1 μM was chosen for subsequent in vitro bioactivity tests using P4-encapsulated chnp and C+S. The effect of P4 on pancreatic β cells has not been reported despite traditional beliefs on the antidiabetic effect of the plant. Such investigations would help assess the mechanism by which the plant extracts lower blood glucose.
Figure 2.
Insulin fold change observed for the mouse pancreatic β cells (MIN6) on treatment with different concentrations of P4. *p < 0.05 (one-way analysis of variance (ANOVA) and post-hoc Tukey test, n = 3). Data are expressed as mean and standard deviation.
The aqueous fraction, P4, was further evaluated in vivo to validate its effectiveness in reducing blood glucose levels in a diabetic mouse model induced with alloxan. In a preliminary experiment to evaluate the toxicity of P4, the animals in the nondiabetic control group were fed DI water or P4 at 50 mg kg–1 for 15 days and their body weight and FBG levels were monitored over this time period (Figure S2). There was no significant difference observed in the body weight and blood glucose levels in the animals that were fed either DI water or P4. This suggested that P4 did not elicit any harmful or toxic responses in the animals, confirming its safety up to the tested concentration.
3.3. Encapsulation and Release Kinetics of P4 from chnp and Starch-Coated chnp (C+S)
P4 was encapsulated into a food-grade carrier system (EE of 39 ± 8%), and its release kinetics from chnp and C+S particles was studied in vitro by mimicking the different environments the particles would encounter in the GIT, i.e., a GI simulator. As observed in Figure 3, the uncoated chnp (control) released 38% of P4 in SGF within 2 h, whereas the C+S particles released 16% of P4 (∼2.5 times reduction), which was significantly different from the control (p < 0.05). This indicates that the starch layer was able to retard the release of P4 in SGF, mimicking the low-pH condition in the stomach. When transferred to SIF, the swollen, control (uncoated chnp) continued to release P4, achieving a cumulative release of 56% at 5 h. For the C+S particles, while the starch coating was digested in SIF, there were no enzymes or conducive acidic pH environment to trigger the release of P4 from chnp; hence, only 14% was released in SIF. To mimic cellular uptake, the particles were next transferred to the pH 4.4 medium. Here, the slightly acidic pH promotes the swelling of chnp and a rapid release of P4, as observed within the first few hours. It is shown that the uncoated chnp released less than 7% of P4 in the first 2 h, whereas more than 20% of release was observed from C+S particles during the same period. This can be attributed to the burst release due to the swelling of chitosan, from the now uncoated C+S particles, under an acidic pH environment. Subsequently, P4 continued to release progressively from the C+S particles, with a total of 60% during this period. This demonstrates the protection starch rendered to the C+S particles, as ∼80% of the encapsulated P4 remained even after being exposed to SGF. It was hypothesized that a delayed release of P4 in the SGF as such would increase the bioavailability of P4, which will potentially enhance its glucose-lowering effects.
Figure 3.
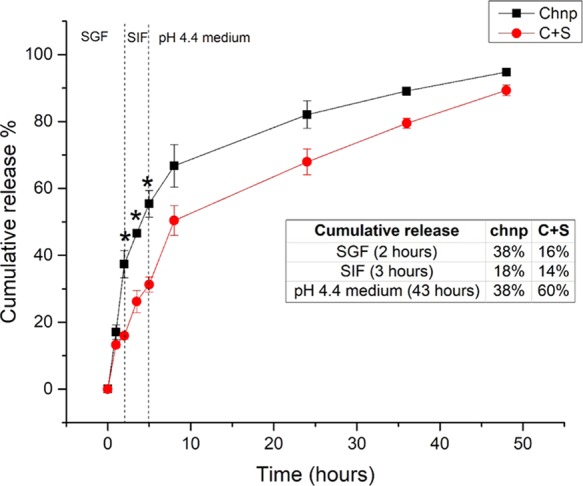
Release profile of P4 from chnp and C+S sequentially in SGF, SIF, and pH 4.4 medium. *Significantly different (p < 0.05) from C+S at the corresponding time point, one-way ANOVA, post-hoc, Dunn–Sidak test, n = 3. Data are expressed as mean and standard deviation.
3.4. Bioactivity Testing of P4 Encapsulated in Particles on MIN6 Cells
With the knowledge that the starch coating is able to retard the release of P4 in SGF and might facilitate the release within the enterocytes itself, it is still imperative to ensure and prove that P4 remains bioactive to elicit its health-benefiting effects. For this purpose, the GSIS assay was carried out by measuring insulin secretion of MIN6 cells exposed to the release media collected from the particles subjected to a sequence of SGF, SIF, and pH 4.4 medium.
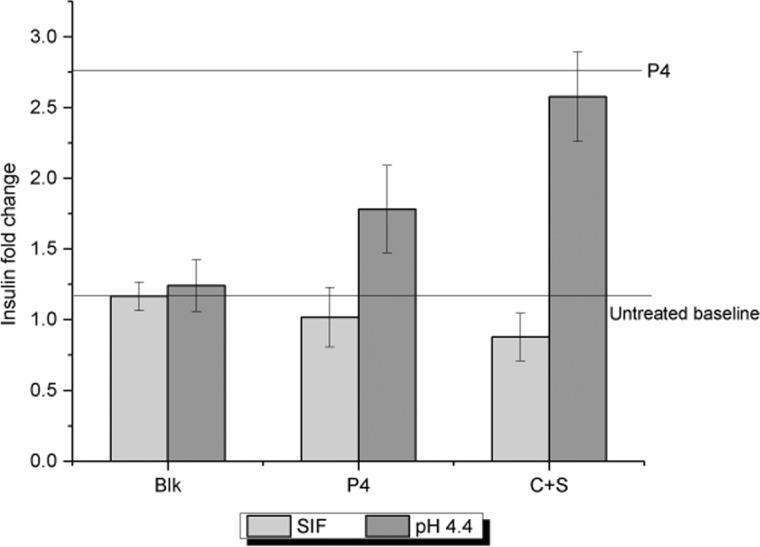
From the results shown in Figure 4, it is evident that the blank release medium itself does not have any effect on the insulin secretion from MIN6 cells, achieving the same insulin levels as the basal insulin secretion. The fold change in insulin secretion observed on treatment with 1 μM P4 was 2.7, and this was kept as the upper limit. For free P4 (without encapsulation), there was no increase in insulin secretion when added to SIF, whereas P4 in pH 4.4 media promotes insulin secretion. There are two possibilities for this observation: (1) the enzymes in SIF alter P4, causing it to lose its bioactivity and (2) since P4 is free, there is a partial loss of bioactivity at pH 4.4. The former is again verified as a similar trend of bioactivity loss in SIF was observed for the P4 released in SIF from C+S particles. P4 that was released in pH 4.4 from C+S had its bioactivity completely preserved because of the encapsulation within C+S particles. This proves that P4 retains its bioactivity when encapsulated in C+S particles, even after passing through the GIT.
Figure 4.
Insulin fold change observed in MIN6 cells on treatment with free P4 and P4 encapsulated in C+S after sequential release in SGF, SIF, and pH 4.4 medium. The blank release medium was used as a negative control. Data are expressed as mean and standard deviation.
3.5. Blood-Glucose-Lowering Effect of P4-Loaded Particles on Diabetic Mice
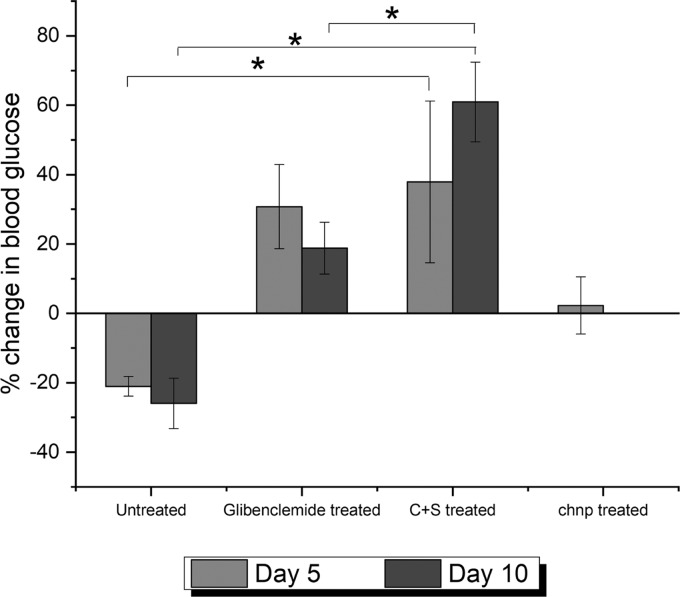
We further validated the above in vitro observation with in vivo study using diabetic mice. Diabetic mice were fed by oral gavage the formulations and control for 5 days and monitored for another 5 days for their FBG. Glibenclamide treatment for the positive control group had to be carried on continuously for the entire 10 days of the study period, as there was a high risk of mortality due to severe diabetes in the absence of treatment. In Figure 5, after 5 days of treatment with C+S and chnp particles, it was observed that the animals that received the chnp treatment did not show any difference in the FBG levels, consistent with the fact that chnp releases P4 preemptively in the stomach, where its effect cannot be manifested. Hence, this group was ruled out for the subsequent day 10 study. On the other hand, P4 encapsulated within C+S was able to significantly lower the FBG values compared to the untreated animals (p < 0.05). Interestingly, even after stopping the treatment for 5 days, the animals in the C+S treatment group continued to show a decrease in FBG values. This was significantly different (p < 0.05) compared to those in the positive control group (glibenclamide treated) and negative control (DI water treated) group, suggesting the ability of the delivery system to prolong the release of P4 over an extended period of time. In addition, the result also proves that the C+S delivery system not only retards the premature release of the drug but also enables P4 to be absorbed systemically to exert an effect.
Figure 5.
Percentage change in FBG levels of diabetic mice, treated with P4-encapsulated chnp and C+S, compared against positive and negative controls. A positive change indicates a decrease in FBG and a negative change indicates an increase in FBG compared to the FBG values at day 0. *p < 0.05 using one-way ANOVA and post-hoc Tukey test, n = 3. Data are expressed as mean and standard deviation.
4. Discussion
In an effort to combine the alternative system of medicine using plant extracts with that of the burgeoning field of encapsulation technology, the aqueous extract (P4) from the berries of W. coagulans has been encapsulated into a food-based polymeric carrier, with two converging aims: one is to provide a suitable delivery system for P4 whereby no such nanocarrier system currently exists and the other is to prove the small-intestine-targeting ability of the developed food-grade carrier in vivo.
There is a lack of information when it comes to the testing of the extracts from W. coagulans in vitro, which could largely help in the understanding of their mechanism of action. Since most of the plant extracts that exert an antidiabetic effect act by promoting insulin secretion from β cells, it was first hypothesized that W. coagulans promotes insulin secretion by sensitizing or stimulating the β cells through activation of Ca2+ channels and, in turn, exocytosis of insulin from insulin-containing granules.30 This ability of P4 to promote insulin secretion at a certain concentration was confirmed in vitro using pancreatic β cells (MIN6) (Figure 2). Different organic compounds such as saponins, flavonoids, tannins, alkaloids, terpenoids, and sterols have been previously shown to be present in the extracts of W. coagulans.(31) Such bioactive components have been associated with hypoglycemic activity.32 Based on the characterization of P4, since the structural backbone is similar to that of a sterol, it is hypothesized that the presence of a sterol could be responsible for the glucose-lowering effect observed for P4.
P4 was encapsulated into a chitosan particle delivery system using electrospraying. This fabrication methodology does not use any toxic solvents that could degrade the plant extracts. The successful encapsulation of P4 within the delivery system demonstrates the feasibility of loading the compounds extracted from W. coagulans into a carrier, which has not been previously reported. The results shown for the encapsulation of P4 open the possibility of using different materials to encapsulate the extract, depending on the desired applications. The targeted delivery of P4 to the small intestine to achieve enhanced absorption was tested in vitro using different simulated GIT fluids. The starch coating on chnp for C+S particles was able to retard the burst release of P4 in SGF. The chnp were designed in a way to be taken up by the enterocytes lining the small intestine to increase the bioavailability of the encapsulated compound. Having previously shown the uptake of chnp by Caco2 cells,23 the release kinetics study evaluated the ability of the particles to release P4 inside the lysosome of the cells during the cell uptake. This was simulated using the pH 4.4 medium to mimic the condition inside the lysosome. It is worth noting that toxicity studies are needed to assess the potential toxicity of the proposed particle delivery system. Such studies need to include the interactions with food and potential physicochemical transformations across the GIT in tandem with a robust cell culture model to mimic the intestinal epithelium.33−36
The main aim of encapsulating P4 was to protect it from degradation during transit through the GIT and preserve its activity until it reaches the site of action. Although the release kinetics studies indicated that the delivery system was able to perform this role, the preservation of the activity of P4 was tested by assessing the insulin secretion ability using the GSIS assay on MIN6 cells. As observed in Figure 4, an interesting finding of this study was that not only the low pH of SGF but also the enzymes in SIF could also destroy the bioactivity of such labile compounds. The results from this study further strengthened the claim that encapsulating P4 protects its activity and the delivery system served to overcome this undesired loss of activity.
To prove the targeting and uptake ability of the carrier in animal models, in vivo studies were carried out. This was investigated by comparing the effect of uncoated chnp encapsulated with P4 and starch-coated chnp encapsulated with P4 on diabetic mice. Uncoated chnp were unable to lower the FBG, as P4 needs to be released and absorbed in the small intestine to lower blood glucose. C+S particles, on the other hand, were able to lower FBG, indicating the targeting ability of the coated starch layer and the ability of chnp, thus released, to have been taken up by the enterocytes, which could have led to the systemic response of lowering blood glucose.
As an extension of the above study, the ability of the C+S particles to produce a sustained effect in the absence of treatment was also investigated. An important observation in Figure 5 is the increase in FBG after day 5, indicated as a decrease in the percentage change of FBG for the glibenclamide-treated group. Glibenclamide is a drug that induces insulin secretion from β cells, and the alloxan-induced diabetic mice used in the study have a reduced number of β cells. The increase in FBG on day 10 after a momentary decrease on day 5 with glibenclamide treatment could be due to the long-term toxicity of the drug, leading to β-cell apoptosis. This is also in agreement with the results reported previously for the toxicity of such sulfonylurea class of drugs against β cells.37 On the other hand, the progressive decrease of FBG for the C+S-treated group could be indicative of the protective effect of P4 on β cells. With the ability of P4 to promote insulin secretion, as suggested from the in vitro studies on MIN6 cells and the in vivo studies indicating the possible ability of P4 to promote regeneration of β cells, the encapsulation of P4 further helped prolong its effect, even in the absence of treatment, beyond 5 days, as observed in Figure 5. This observation strongly emphasized not only the need for a delivery system to protect the labile compounds extracted from plants but also the significant role such a delivery system can play in targeting and sustaining the effect of an orally administered active ingredient.
In summary, the hypothesis that a food-grade delivery system could achieve small-intestine-specific delivery was validated using starch-coated electrosprayed chnp encapsulated with P4. P4 was found to promote insulin secretion in MIN6 cells. It was also verified that the delivery system, with its starch enteric coating, was able to preserve the bioactivity of P4. A glucose-lowering effect was observed in the diabetic mice model for the P4-encapsulated delivery system, which continued even in the absence of the treatment. This could be due to the sustained effect of the P4-encapsulated delivery system, demonstrating the advantages of the food-grade delivery system in small-intestine-targeted delivery.
Acknowledgments
The authors would like to acknowledge the financial support from the Singapore Centre for Environmental Life Sciences Engineering (SCELSE) (MOE/RCE: M4330019.C70), the Ministry of Education AcRF-Tier 1 grant (RG19/18), the NTU-HSPH grant (NTU-HSPH 17002), the Bill and Melinda Gates Foundation (OPP1199116), and the Office of the Higher Education Commission under NRU Project of Thailand and Suranaree University of Technology. The authors would also like to thank Dr Gu Peiyang (School of Materials Science and Engineering, Nanyang Technological University) for his assistance in carrying out NMR.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00823.
In vitro and in vivo toxicity analyses data for P4; cytotoxicity effect of P4 on MIN6 cells; body weight and FBG values of normal control mice fed DI water or P4 for 15 days; calibration standards for insulin using the ELISA assay; calibration standards for P4 using HPLC (PDF)
Author Contributions
K.S. co-designed the experiments with S.C.J.L., performed the experiments and data analysis, and also drafted the manuscript. S.R. together with N.C. carried out the in vivo experiments. C.K.T. performed the extraction of P4 from Withania coagulans and its characterization using FTIR. S.C.J.L. provided the direction for the study and edited the manuscript drafts. P.D. reviewed the manuscript drafts.
The authors declare no competing financial interest.
Supplementary Material
References
- Yang N.; Sampathkumar K.; Loo S. C. J. Recent advances in complementary and replacement therapy with nutraceuticals in combating gastrointestinal illnesses. Clin. Nutr. 2017, 36, 968–979. 10.1016/j.clnu.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Sun H.; Wang P.; Han Y.; Wang X. Future perspectives of personalized medicine in traditional Chinese medicine: A systems biology approach. Complementary Ther. Med. 2012, 20, 93–99. 10.1016/j.ctim.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Dias D. A.; Urban S.; Roessner U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Sarrías A.; Larrosa M.; García-Conesa M. T.; Tomás-Barberán F. A.; Espín J. C. Nutraceuticals for older people: Facts, fictions and gaps in knowledge. Maturitas 2013, 75, 313–334. 10.1016/j.maturitas.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Ruth A.; Izzo A. A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177–1194. 10.1111/bph.13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand View Research Nutraceuticals Market Analysis By Product (Dietary Supplements, Functional Food, Functional Beverage), By Region (North America, Asia Pacific, Europe, CSA, MEA), And Segment Forecasts, 2018–2025. https://www.grandviewresearch.com/industry-analysis/nutraceuticals-market (accessed 30 July).
- Blair M. Diabetes Mellitus Review. Urologic Nurs. 2016, 36, 27–36. 10.7257/1053-816X.2016.36.1.27. [DOI] [PubMed] [Google Scholar]
- Kharroubi A. T.; Darwish H. M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. H.; Shaw J. E.; Karuranga S.; Huang Y.; da Rocha Fernandes J. D.; Ohlrogge A. W.; Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- GlobalData Type 2 diabetes market to more than double, to $64 billion by 2026. https://www.globaldata.com/type-2-diabetes-market-double-64-billion-2026/ (accessed 30 July).
- El-Tantawy W. H.; Temraz A. Management of diabetes using herbal extracts: review. Arch. Physiol. Biochem. 2018, 124, 383–389. 10.1080/13813455.2017.1419493. [DOI] [PubMed] [Google Scholar]
- Grover J. K.; Yadav S.; Vats V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. 10.1016/S0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Lea S. On a ‘Rennet’ Ferment Contained in the Seeds of Withania coagulans. Proc. R. Soc. London 1883, 36, 55–58. 10.1098/rspl.1883.0081. [DOI] [Google Scholar]
- Machin R. P.; Veleiro A. S.; Nicotra V. E.; Oberti J. C.; Padron J. M. Antiproliferative activity of withanolides against human breast cancer cell lines. J. Nat. Prod. 2010, 73, 966–968. 10.1021/np9006734. [DOI] [PubMed] [Google Scholar]
- Hemalatha S.; Wahi A.; Singh P.; Chansouria J. Hypoglycemic activity of Withania coagulans Dunal in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2004, 93, 261–264. 10.1016/j.jep.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Prasad S.; Kumar R.; Patel D.; Hemalatha S. Wound healing activity of Withania coagulans in streptozotocin-induced diabetic rats. Pharm. Biol. 2010, 48, 1397–1404. 10.3109/13880209.2010.486837. [DOI] [PubMed] [Google Scholar]
- Vermaak I.; Viljoen A. M.; Hamman J. H.; Van Vuuren S. F. The effect of simulated gastrointestinal conditions on the antimicrobial activity and chemical composition of indigenous South African plant extracts. S. Afr. J. Bot. 2009, 75, 594–599. 10.1016/j.sajb.2009.02.180. [DOI] [Google Scholar]
- Bhattarai S.; Tran V. H.; Duke C. C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001, 90, 1658–1664. 10.1002/jps.1116. [DOI] [PubMed] [Google Scholar]
- Maurya R.; Singh A. B.; Srivastava A. K. Coagulanolide, a withanolide from Withania coagulans fruits and antihyperglycemic activity. Bioorg. Med. Chem. Lett. 2008, 18, 6534–6537. 10.1016/j.bmcl.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Stanford J. C.; Morris A. J.; Sunkara M.; Popa G. J.; Larson K. L.; Ozcan S. Sphingosine 1-phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells. J. Biol. Chem. 2012, 287, 13457–13464. 10.1074/jbc.M111.268185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagpulinsa D. A.; Cao J. J. L.; Driscoll R. K.; Sîrbulescu R. F.; Penson M. F. E.; Sremac M.; Engquist E. N.; Brauns T. A.; Markmann J. F.; Melton D. A.; Poznansky M. C. Alginate-microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2019, 1930–1940. 10.1111/ajt.15308. [DOI] [PubMed] [Google Scholar]
- Sampathkumar K.; Loo S. C. J. Targeted Gastrointestinal Delivery of Nutraceuticals with Polysaccharide-Based Coatings. Macromol. Biosci. 2018, 18, 1700363 10.1002/mabi.201700363. [DOI] [PubMed] [Google Scholar]
- Alam M. M.; Meerza D.; Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014, 109, 8–14. 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Yousaf M.; Gul W.; Qureshi S.; Choudhary M. I.; Voelter W.; Hoff A.; Jens F.; Naz A. Five new withanolides from Withania coagulans. Heterocycles 1998, 9, 1801–1811. 10.3987/COM-98-8208. [DOI] [Google Scholar]
- Mirjalili M. H.; Moyano E.; Bonfill M.; Cusido R. M.; Palazon J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ur-Rahman A.; e-Shahwar D.; Naz A.; Choudhary M. I. Withanolides from Withania coagulans. Phytochemistry 2003, 63, 387–390. 10.1016/S0031-9422(02)00727-6. [DOI] [PubMed] [Google Scholar]
- Maurya R. Chemistry and pharmacology of Withania coagulans: an Ayurvedic remedy. J. Pharm. Pharmacol. 2010, 62, 153–160. 10.1211/jpp.62.02.0001. [DOI] [PubMed] [Google Scholar]
- Fu Z.; Gilbert E. R.; Liu D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2013, 9, 25–53. 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K.; Dikshit P.; Shukla R.; Gambhir J. K. The aqueous extract of Withania coagulans fruit partially reverses nicotinamide/streptozotocin-induced diabetes mellitus in rats. J. Med. Food 2012, 15, 718–725. 10.1089/jmf.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D.; Agrawal R.; Shrivastava V. Phytochemical screening and determination of antioxidant potential of fruits extracts of Withania coagulans. Recent Res. Sci. Technol. 2011, 3, 26–29. [Google Scholar]
- Arika W.; Nyamai D.; Agyirifo D.; Ngugi M.; Njagi E. In vivo antidiabetic effect of aqueous leaf extract of Azardirachta indica, A. juss in alloxan induced diabetic mice. J. Diabetic Complications Med. 2016, 1, 106 10.4172/jdcm.1000.106. [DOI] [Google Scholar]
- DeLoid G. M.; Wang Y.; Kapronezai K.; Lorente L. R.; Zhang R.; Pyrgiotakis G.; Konduru N. V.; Ericsson M.; White J. C.; De La Torre-Roche R.; Xiao H.; McClements D. J.; Demokritou P. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol. 2017, 14, 40. 10.1186/s12989-017-0221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoid G. M.; Sohal I. S.; Lorente L. R.; Molina R. M.; Pyrgiotakis G.; Stevanovic A.; Zhang R.; McClements D. J.; Geitner N. K.; Bousfield D. W.; Ng K. W.; Loo S. C. J.; Bell D. C.; Brain J.; Demokritou P. Reducing Intestinal Digestion and Absorption of Fat Using a Nature-Derived Biopolymer: Interference of Triglyceride Hydrolysis by Nanocellulose. ACS Nano 2018, 12, 6469–6479. 10.1021/acsnano.8b03074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements D. J.; DeLoid G.; Pyrgiotakis G.; Shatkin J. A.; Xiao H.; Demokritou P. The role of the food matrix and gastrointestinal tract in the assessment of biological properties of ingested engineered nanomaterials (iENMs): State of the science and knowledge gaps. NanoImpact 2016, 3–4, 47–57. 10.1016/j.impact.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Zhang R.; Xiao H.; Bhattacharya K.; Bitounis D.; Demokritou P.; McClements D. J. Development of a standardized food model for studying the impact of food matrix effects on the gastrointestinal fate and toxicity of ingested nanomaterials. NanoImpact 2019, 13, 13–25. 10.1016/j.impact.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maedler K.; Carr R. D.; Bosco D.; Zuellig R. A.; Berney T.; Donath M. Y. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J. Clin. Endocrinol. Metab. 2005, 90, 501–506. 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.