Abstract
The Alzheimer’s disease (AD) therapeutic research is yielding a large number of potent molecules. The nanoparticle-based therapeutics against the protein aggregation in AD is also taking a lead especially with amyloid-β as a primary target. In this work, we have screened for the first time protein-capped (PC) metal nanoparticles for their potency in inhibiting Tau aggregation in vitro. We present a novel function of PC-Fe3O4 and PC-CdS nanoparticles as potent Tau aggregation inhibitors by fluorescence spectrometry, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electron microscopy. We demonstrate that the biologically synthesized PC-metal nanoparticles, especially iron oxide do not affect the viability of neuroblastoma cells. Moreover, PC-CdS nanoparticles show dual properties of inhibition and disaggregation of Tau. Thus, the nanoparticles can take a lead as potent Tau aggregation inhibitors and can be modified for specific drug delivery due to their very small size. The current work presents unprecedented strategy to design anti-Tau aggregation drugs, which provides interesting insights to understand the role of biological nanostructures in Alzheimer’s disease.
Introduction
Protein misfolding and accumulation is a hallmark of various diseases, including neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease, etc.1,2 The pathological cascades as well as the proteins involved in them vary according to the diseases. For example, the pathological cascade of Alzheimer’s disease involves the aggregation and accumulation of two aggregation-prone proteins, amyloid-β and Tau.3,4 Amyloid-β (Aβ1-42) is an amyloidogenic peptide secreted by the neurons in response to faulty processing of the membrane-bound amyloid precursor protein by the enzymes β and γ secretases. This sticky peptide then accumulates and forms extracellular amyloid plaques in the brain.5 Tau, on the other hand, is an intracellular microtubule-associated protein, which helps in stabilizing the microtubules.6 The domain organization of Tau is depicted in Figure 1A. Tau protein aggregates in response to various factors-like genetic mutations, abnormal post-translational modifications, etc.7−9 Tau aggregates accumulate in the neurons forming paired helical filaments (PHFs) followed by neurofibrillary tangles (NFTs). Although Tau lacks rigid secondary structure in native state, it adopts a partial β-sheet structure in the pathological state. Tau is conformationally dynamic and temperature can influence its conformation in solution.10,11 The two hexapeptide motifs present in the repeat region of Tau play a crucial role in the random coil to β-sheet transition during aggregation.12,13 Since the past two decades, considerable research has been done to search for potent therapeutics against protein aggregation in neurodegenerative diseases.14 The target-based research has yielded a number of candidates against amyloid-β15 and Tau pathology but with little success in the higher clinical trials. The research has yielded therapeutics from different classes like small molecules, natural compounds, peptide inhibitors, etc.16,17 Along with these classes of therapeutics, considerable research is being done in the field of nanoparticles (NPs) with respect to protein aggregation. Several high-throughput screening methods are also being developed to determine the interactions between nanoparticles and proteins using fluorescent dyes like 8-anilino-1-naphthalenesulfonic acid and Nile Red.18 The functionality of nanoparticles is multidimensional depending on their surface charge, size, solubility, thermostability, etc.19 The nanosize in conjunction with the large surface-to-volume ratio makes the nanoparticles more efficient with respect to drug delivery and crossing the blood–brain barrier.20 The size, surface area, and hydrophobicity play a crucial role in determining the effect of nanoparticles on the protein aggregation. These properties either enhance the fibrillization process or inhibit it. For example, the copolymeric nanoparticles N-isopropylacrylamide/N-tert-butylacrylamide (NIPAM/BAM) decrease the lag times for the fibrillization process of β2-microglobulin. The hydrophobic surface enhances the association of protein monomers with nanoparticles, thus decreasing the critical concentration required for nucleation.21 Apart from the nanoparticle properties, protein stability and amino acid composition the in-built aggregation, propensity also plays a role for the interaction with nanoparticles, and this interaction determines the fate of protein fibrillation.22 This is reflected in a study with NIPAM/BAM copolymeric nanoparticles, wherein these nanoparticles lengthen the nucleation phase and thus delay the polymerization of amyloid-β protein. This effect of the nanoparticles might be attributed to the firm binding of amyloid-β monomers to the nanoparticles, thus blocking the sites for binding of the free monomers to initiate the polymerization.23
Figure 1.
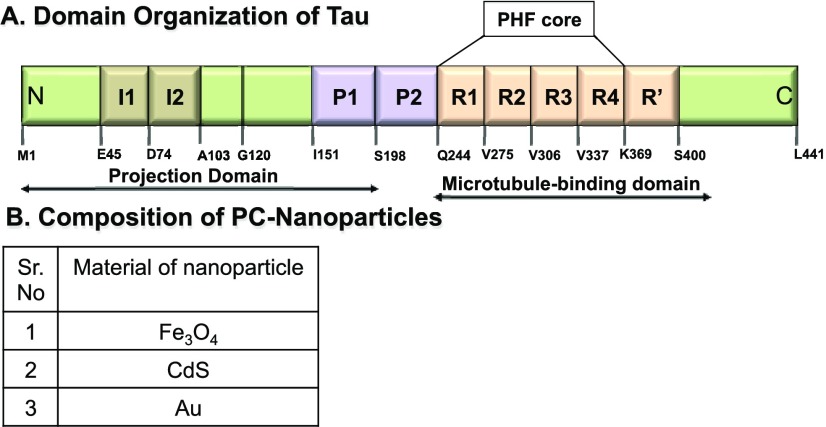
(A) Tau protein and its domains. The full-length Tau protein comprises two major domains, projection and microtubule-binding domain. The projection domain includes two inserts and a polyproline stretch. The microtubule-binding domain has four imperfect repeats of 29 amino acids each of which is mainly involved in binding to microtubules. These repeats also form the core of paired helical filaments (PHFs) that enhances the aggregation of Tau protein. (B) Table of nanoparticles with their chemical composition.
The concentration of nanoparticles or the ratio between the amyloid-β protein and nanoparticles also modulates the fibrillation process with higher particles concentration leading to abolishment of fibril formation.24 Considering all of these characteristics, we screened biologically synthesized protein-capped (PC) nanoparticles to study their function with respect to Tau protein aggregation. As already discussed above, Tau protein misfolding and aggregation is one of the major causes of neuronal death in AD, and the biologically synthesized metal nanoparticles were screened against Tau aggregation.
The important characteristics of the nanoparticles used are their complete biological synthesis using two fungal species; Fusarium oxysporum and Verticillium sp.25 The composition of capping proteins varies according to the nanoparticles synthesized. For example, the magnetite nanoparticles are capped with hydrolytic proteins from fungi, whereas the CdS nanoparticles are capped with the mixture of four different proteins probably belonging to the group of sulfate-reducing enzymes. The crystalline iron oxide nanoparticles were synthesized extracellularly by the fungal species at ambient temperature with transient ferromagnetic properties, which otherwise would require the use of toxic chemical and investment of large amount of energy.26 The fungal synthesis of these nanoparticles, which layers it with capping proteins, might play a role in their morphology. The synthesis of CdS nanoparticles was also performed by the fungal species by a complete enzymatic process. These nanoparticles are synthesized from the extracellular sulfate-reducing enzymes secreted by the fungus when provided with mixture of salts. The particles size falls under the quantum dots type and are easy to isolate due to extracellular synthesis.27 The gold nanoparticles were synthesized by Verticillium species via bioreduction of AuCl4– ions.28 These NPs were synthesized in the fungal cell walls. To observe the effect of biologically synthesized metal nanoparticles on Tau assembly, we selected a set of three nanoparticles (Figure 1B). The main aim of this study was to determine whether the protein-capped metal nanoparticles inhibit Tau fibrillization in vitro and to inhibit Tau fibrillization in vitro and to observe the morphology of nanoparticles-treated Tau aggregates.
Results and Discussion
Synthesis and Characterization of PC-Metal Nanoparticles
The synthesis of the PC-metal nanoparticles was carried out by fungal species of F. oxysporum and Verticillium sp. In brief, the synthesis of these particles was carried out by exposing the fungi to the aqueous mixtures of ferricynanide or ferrocyanide for 24 h for magnetite nanoparticles29 and Cd2+ and SO42– ions for CdS nanoparticles.27 Further, the nanoparticles were characterized by various techniques.
Transmission electron microscopy (TEM) analysis of magnetite nanoparticles was found to be nonuniform with quasi-spherical morphology. The nanoparticle size was found to be 50–60 nm (Figure S3A), which is in conjunction with the previous characterization, wherein the size was found to be within 20–60 nm. The crystalline nature of nanoparticles was deduced from selected area electron diffraction analysis, and Bragg reflection characteristic of Fe3O4 was observed in the X-ray diffraction analysis in our previous studies. Fourier transform infrared analysis demostrated prominent resonances at 522, 568, and 627 nm, which can be attributed to Fe–O–Fe stretching modes of vibrations. Two absorption bands were also observed in amide I and amide II regions, which indicates the presence of proteins in the preparation of nanoparticles. These capping proteins were further characterized and found to be 55 kDa, cationic with hydrolytic activity capable of hydrolysis of anionic iron complexes to form PC-metal nanoparticles.29
UV–vis spectra of CdS nanoparticles showed an absorption peak at 450 nm, which highlights the characteristic size of nanoparticles in the quantum regime. The presence of capping proteins was elucidated by absorption peak at 280 nm. These capping proteins probably prevent the aggregation of nanoparticles, as evidenced by TEM analysis, wherein dispersed nanoparticles with average size between 10 and 20 nm were observed (Figure S3B). Further analysis of capping proteins suggested possible presence of sulfate-reducing enzymes, which could synthesize CdS nanoparticles from Cd2+ and SO42– ions.27 Based on this previous characterization, the nanoparticles were checked for their activity against Tau aggregation and PHF dissolution.
PC-Metal Nanoparticles Inhibit Tau Fibrillization
Tau aggregation inhibition has been studied with respect to various molecules, which might have potential therapeutic application. Most of these molecules belong to different classes of synthetic or naturally occurring small molecules.30,31 The effect of PC-metal nanoparticles was studied for inhibition of Tau PHF assembly. The aggregation kinetics of full-length Tau in the presence of three PC-metal nanoparticles (Figure 1B) showed an inhibitory effect of varying degree (Figure 2A,B). The PC-CdS nanoparticles showed 63% inhibition of Tau aggregation, followed by PC-iron oxide NPs as 49% and gold nanoparticles as 18%. Recent reports suggest that the nanocomposites loaded with methylene blue efficiently reduce AD symptoms in animal models by reducing Tau hyperphosphorylation and relieving the mitochondrial dysfunction.32 Our study shows direct effect of PC-NPs on Tau aggregation inhibition and dissolution of mature fibrils.
Figure 2.
Effect of nanoparticles on Tau PHF assembly. The effect of three nanoparticles was tested for the inhibitory activity on Tau aggregation. (A) Thioflavin S (ThS) fluorescence was found to increase at the initial time points for treated as well as control samples. Further, the time-dependent decrease in intensity was observed in the treated samples. (B) Comparative analysis of the end time point fluorescence showed maximum inhibition of PHF assembly by PC-CdS NPs. (C) ThS fluorescence showed a concentration-dependent decrease with increase in the concentration of PC-Fe3O4 NPs. (D) The highest concentration of PC-Fe3O4 NPs (1 mg/mL) showed 49% inhibition of aggregation propensity of hTau40WT. (E) Concentration-dependent inhibition of Tau aggregation by PC-CdS NPs showed a rapid decrease in ThS fluorescence. (F) PC-CdS NPs (1 mg/mL) treatment shows 63% inhibition of Tau PHF assembly. (The values are mean ± standard deviation of three independent experiments.)
Iron Oxide and Cadmium Sulfide PC-Nanoparticles Inhibit Tau Polymerization Efficiently
The comparative analysis of these three PC-metal nanoparticles revealed that the iron oxide and cadmium sulfide PC-NPs were more potent in inhibiting Tau aggregation and thus were selected for further studies. The inhibitory dose for Tau aggregation was determined by treating the soluble Tau with a range of concentrations of NPs (0–1 mg/mL). The aggregation kinetics revealed a concentration-dependent decrease in ThS fluorescence with increase in incubation time (Figure 2C,E). Both the nanoparticles PC-Fe3O4 and PC-CdS showed 49 and 63% inhibition, respectively, at the highest concentration of the treatment (1 mg/mL) (Figure 2D,F). The PC-CdS NPs treatment showed an efficient inhibition compared to PC-Fe3O4 NPs as the ThS fluorescence decreased to a greater extent from day 4 onward compared to control.
The PC-Fe3O4 nanoparticles are crystalline and show a signature ferromagnetic transition.29 The size of the particles ranges from 40 to 50 nm. The PC-Fe3O4 nanoparticles show a lower rate of inhibition compared to PC-CdS NPs. The PC-CdS nanoparticles have a size ranging from 5 to 20 nm, and the enhanced rate of aggregation inhibition might be due to differential adsorption properties on these nanoparticles. The iron oxide nanoparticles find the applications in biomedical fields of imaging (magnetic resonance imaging) as they act as efficient contrasting agents.
Apart from this, the iron oxide nanoparticles are also used in the magnetic drug delivery in cancer treatment.33 The ferromagnetic iron oxide nanoparticles have recently been found to adsorb amyloid-β with the specificity for aggregates but not monomers.34 Here, we report that the biologically synthesized iron oxide nanoparticles can interfere with Tau aggregation and inhibit it. The inhibitory effect of iron oxide nanoparticles might be attributed to the size or the surface charge of nanoparticles or both. The careful analysis of the polymerization kinetics of Tau in the presence of PC-Fe3O4 nanoparticles reveals an initial increase in the ThS intensity for the higher concentration of NPs suggesting an efficient adsorption of intermediate Tau aggregates on these NPs. But the gradual drop in ThS fluorescence suggests the inhibition of Tau fibrillation in the presence of PC-NPs. The sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis also showed an initial increase in the intensity of intermediate aggregates with gradual decrease at subsequent time intervals. All of these observations suggest that the intermediate aggregates might be adsorbed on the surface of nanoparticles in such a way that they are inefficient in undergoing further fibrillation. Similar mechanism had been observed and postulated for aggregation inhibition of amyloid-β.22,35,36 The adsorption phenomenon might be due to the coat protein composition on the NPs, which could enhance the interaction of Tau and NPs, but this needs to be further investigated.
Morphological Characteristics of PC-Nanoparticles-Treated Tau
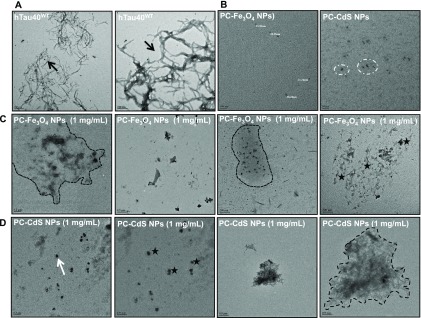
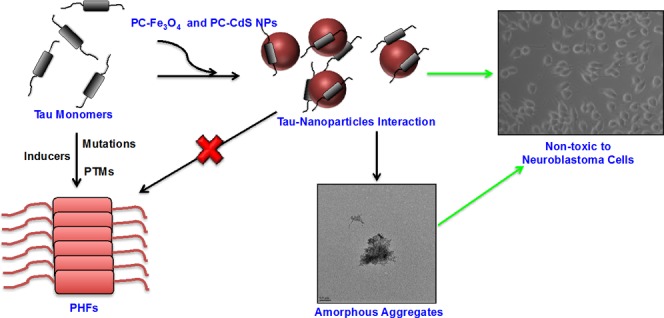
Tau protein forms the fibrillar aggregates upon assembly. The nanoparticles treatment inhibited the formation of the fibrillar aggregates (Figure 3). The control shows the presence of filamentous Tau aggregates (Figure 3A), but the nanoparticles-treated samples showed the presence of amorphous Tau aggregates (Figure 3C,D). Nanoparticles without Tau were also visualized by TEM. Thus, nanoparticles prevent Tau aggregation by leading to the formation of amorphous aggregates that cannot form potentially toxic fibrils.
Figure 3.
Effect of nanoparticles on morphology of Tau aggregates. (A) The control sample demonstrates the presence of long fibrillar aggregates (black arrow). (B) PC-Fe3O4 and PC-CdS NPs-, (C) PC-Fe3O4 NPs-, and (D) PC-CdS NPs-treated Tau show the presence of only amorphous aggregates (enclosed in dotted lines). These amorphous aggregates are seen in the presence of agglomerates of the nanoparticles (white arrow and black stars).
SDS-PAGE Analysis of PC-Nanoparticles-Mediated Tau Aggregation Inhibition
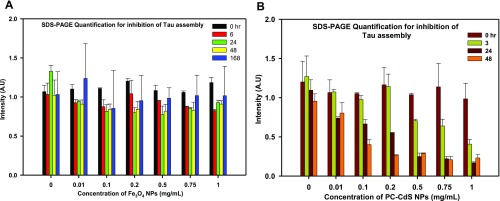
Tau aggregation leads to the formation of SDS-resistant higher-order Tau aggregates that are visualized by SDS-PAGE. The SDS-PAGE analysis of Tau inhibition by nanoparticles showed that, initially, PC-Fe3O4 NPs treatment leads to the formation of higher-order aggregates that become prominent at 24 h but reduce in intensity as the time of incubation increases (Figures 4A and S1A). At 168 h of incubation, most of the higher-order aggregates disappear compared to control. On the other hand, PC-CdS NPs show the disappearance of the higher-order aggregates in an early period of incubation (24 h). At 48 h, there is complete absence of higher-order aggregates in the highest concentration of PC-CdS NPs (Figures 4B and S1B).
Figure 4.
(A) SDS-PAGE quantification showing the time- and dose-dependent decrease in intensity, suggesting inhibition of Tau aggregation by PC-Fe3O4 NPs. (B) SDS-PAGE quantification of PC-CdS NPs-treated Tau showing a significant decrease in intensity demonstrating Tau aggregation inhibition. (The values are mean ± standard deviation of two independent experiments.)
These results confirm with the kinetics data that both types of nanoparticles prevented the formation Tau aggregates. The cadmium sulfide nanoparticles are being widely used as semiconductors in electronic devices due to their small size.37 They have a large number of biomedical applications, including probe development, cellular imaging, biomedical diagnostics, etc. The multivariate applications is due to the differential fluorescence properties of these nanoparticles owing to their size.38 In our study, to the best of our knowledge, we are reporting for the first time that PC-CdS nanoparticles can inhibit Tau aggregation in vitro in an efficient manner. The kinetics and the SDS-PAGE analysis suggest the adsorption of soluble proteins on the surface of the nanoparticles, which might prevent them from aggregation.
PC-CdS Nanoparticles Disassemble Tau PHFs
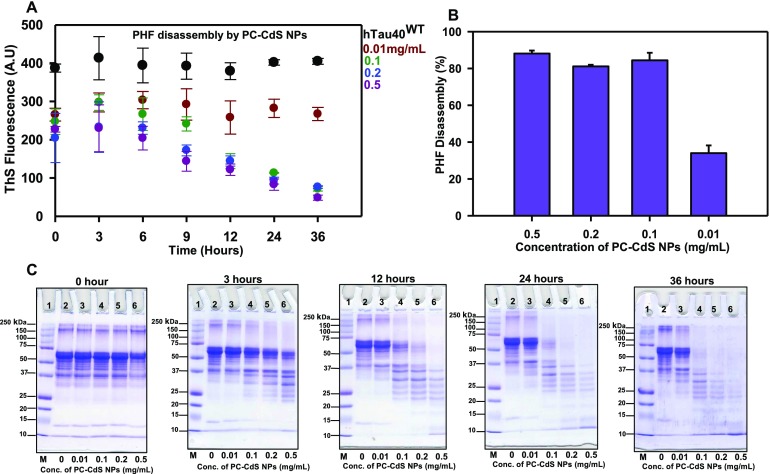
The potency of PC-CdS NPs in inhibiting Tau fibrillization led to further investigating their role in Tau disassembly. The PHF disassembly was observed in the PC-CdS NPs-treated reactions in a concentration-dependent manner. The ThS kinetics revealed the PHF disassembly even at low concentrations of 0.1 mg/mL (Figure 5A) Moreover, the highest concentration of 0.5 mg/mL showed the disassembly starting at 3 h. At the end of 36 h, the highest concentration of PC-CdS nanoparticles (0.5 mg/mL) showed 88% PHF disassembly (Figure 5B). The inhibition shown in kinetics was also confirmed by SDS-PAGE. The SDS-PAGE analysis demonstrated that nanoparticles efficiently cleared the Tau aggregates at submicrogram concentrations. At the extended incubations, complete clearance of PHFs was observed in all treated samples on the SDS-PAGE (Figure 5C). The disassembly analysis by electron microscopy showed fibrillar aggregates, which are well-separated, single fibrils (Figure S2A). The PC-CdS-treated fibrils showed the presence of broken fragments of Tau fibrils at 0 h (Figure S2B). The pieces were observed to be clumped together. At 36 h, the clumps of broken fibrils at heavily stained regions were observed (Figure S2C). This might be due to adsorption of fibrils on the agglomerated nanoparticles, which may help in Tau fibril disassembly.
Figure 5.
Disassembly of Tau PHFs by PC-CdS NPs. (A) ThS kinetics for Tau disassembly by PC-CdS NPs at different concentrations. (B) Percentage of Tau assembly by PC-CdS NPs at the end of 36 h. (C) SDS-PAGE analysis of PHF disassembly by PC-CdS NPs showing time- and concentration-dependent clearance of PHFs.
Neuronal Cell Toxicity of Nanoparticles
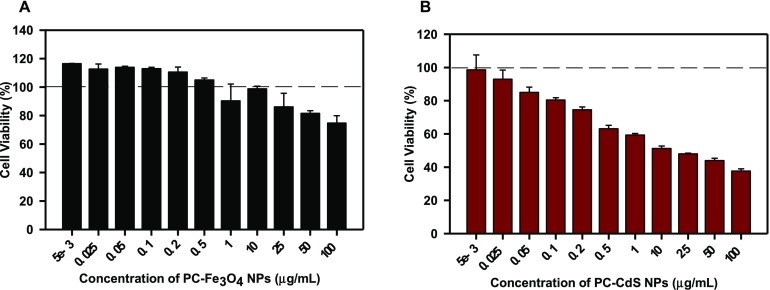
The toxicity of nanoparticles was assessed on neuroblastoma cell line neuro2a. The cells were treated with 5–100 μg/mL of nanoparticles. The PC-Fe3O4 NPs did not show toxicity and maintained the significant viability at around 80% even at higher concentration of 100 μg/mL (Figure 6A). The cell morphology was also maintained with intact neuritic extensions at all of the concentrations (Figure S4). The uncapped CdS nanoparticles are found to be toxic to bacterial as well as HeLa cells due to increased reactive oxygen species production and the resulting oxidative stress.39 But capping confers an entirely different property upon these nanoparticles, which make them more biocompatible. The PC-CdS nanoparticles showed more than 50% viability up to 10 μg/mL (Figure 6B), suggesting the role of capping in masking the toxicity of otherwise toxic CdS nanoparticles. Although the PC-CdS NPs reduced the viability of neuro2a cells beyond 10 μg/mL, the cell morphology was not significantly affected at the higher concentrations (Figure S5). The dextrin-capped CdS NPs do not show toxicity at lower concentrations on HepG2 and HeLa cell line.37 This is in agreement with our observations that the protein-capped CdS nanoparticles show no cytotoxicity in methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay for the neuronal cells at concentrations lower than 10 μg/mL when exposed for 24 h.
Figure 6.
Neuronal cell toxicity of nanoparticles. (A) Neuro2a neuroblastoma cell line treated with PC-Fe3O4 NPs does not show any toxic effects up to 100 μg/mL of dose. The concentrations up to 0.5 μg/mL rather showed enhanced viability compared to control. (B) PC-CdS NPs are not found to be cytotoxic to the neuronal cell line up to 10 μg/mL. The viability is found to decrease beyond 50% at 25–100 μg/mL dosages. (The values are mean ± standard deviation of three independent experiments.)
Thus, apart from the application of iron oxide and cadmium sulfide nanoparticles in the biomedical diagnostic devices, we report a novel role of these nanoparticles in inhibiting the aggregation of Tau protein, which can have potential therapeutic benefits. Although the nanoparticles can act as excellent delivery systems and inhibit the in vitro protein aggregation via varied specific and nonspecific interactions, the in vivo translation of this activity is challenging due to various interfering molecular species. Thus, this needs to be addressed by use of relevant animal models as well as applying the specific techniques to get the desired output.40
Materials and Methods
Tau Protein Purification
The Tau protein purification was carried out as previously described.41 In brief, the bacterial cell pellets expressing the recombinant Tau protein were homogenized and the obtained lysate was boiled at 90 °C for 15 min after addition of 0.5 M NaCl and 5 mM dithiothreitol (DTT). The lysate was centrifuged at 40 000 rpm for 50 min. The supernatant was dialyzed in Sepharose A buffer overnight. The obtained dialyzed sample was then subjected to ultracentrifugation, and the supernatant was loaded onto the cation exchange column for further purification. The bound protein was eluted using an ionic gradient. The eluted proteins were pooled and concentrated for size exclusion chromatography (SEC). Post SEC, the protein was concentrated and the concentration was determined by bicinchoninic acid assay.
Tau Aggregation Inhibition Assay
Soluble Tau protein (20 μM) was incubated in 20 mM BES buffer pH 7.4 (Sigma) with 5 μM Heparin 17 500 Da (MP Biomedicals) in the presence of 25 mM NaCl (MP Biomedicals), 1 mM DTT (Calbiochem), protease inhibitor cocktail (Roche), 0.01% sodium azide (MP Biomedicals), and different concentrations of nanoparticles ranging from 0 to 1 mg/mL. The reaction mixtures were incubated at 37 °C.
ThS Fluorescence Assay
ThS fluorescence assay monitored Tau aggregates formation as follows. The reaction mixtures (5 μL) were diluted in 50 mM ammonium acetate pH 7.0 containing 8 μM ThS to a final volume of 50 μL. The ratio of protein to ThS was maintained as 1:4 for the fluorescence measurement. Fluorescence measurements were carried out using Tecan Infinite 200 Pro series plate reader. The excitation and emission wavelengths were set at 440 and 521 nm, respectively. The readings were carried out in triplicate for each sample at 25 °C. The fluorescence was normalized for background by subtracting the buffer blank fluorescence.
Disaggregation of Tau PHFs
The PHFs for Tau were prepared by incubating 100 μM protein with 25 μM Heparin 17 500 Da in 20 mM BES buffer pH 7.4 with 25 mM NaCl. Other additives like protease inhibitor cocktail, sodium azide, and DTT were added as already mentioned in the respective ratios. The reaction tubes were incubated at 37 °C for 8 days. The PHF formation was confirmed by ThS fluorescence and SDS-PAGE analysis. These PHFs were incubated with a range of concentrations of nanoparticles at 37 °C. The disaggregation of Tau was monitored by ThS fluorescence as well as SDS-PAGE analysis.
Electron Microscopy Analysis
For electron microscopy, 2 μM reaction mixtures were applied to 400 mesh carbon-coated copper grids for 45 s followed by two washes with ultrapure water for 45 s each. The samples were negatively stained for 1 min using 2% uranyl acetate. The dried grids were analyzed using a Tecnai T20 transmission electron microscope.
SDS-PAGE Analysis
The inhibition of PHF assembly by nanoparticles was confirmed by loading the reaction mixtures at different time intervals on 10% SDS-PAGE. The SDS-PAGE gel quantification was carried out using Image Lab software (Bio-Rad). The quantification was carried out for two sets of independent assay gels, and the obtained intensities were plotted as bar graphs.
MTT Cell Toxicity Assay
The neuro2a cells were seeded in 96-well culture plates at the cell density of 10 000 cells/well. The cells were grown in Dulbecco’s modified Eagle’s medium F12 media containing 10% fetal bovine serum (FBS) and antibiotic (Pen-Strep) for 24 h. The cells were treated with nanoparticles (0–100 μg/mL) in serum-starved media (0.5% FBS) for 24 h. Methylthiazolyldiphenyl-tetrazolium bromide (MTT, 0.5 mg/mL) was added to each well and incubated for 3 h at 37 °C. The formazan crystals formed after reduction of MTT by viable cells enzymes were dissolved in 100 μL of 100% dimethyl sulfoxide (DMSO). The purple color developed was measured at 570 nm in TECAN plate reader.
Statistical Analysis
All of the graphical representation and standard deviation calculations were carried out using SigmaPlot 10.2.
Acknowledgments
This project was supported in part by grants from the Department of Biotechnology from Neuroscience Task Force (Medical Biotechnology-Human Development & Disease Biology (DBT-HDDB))-BT/PR/15780/MED/30/1629/2015 and in-house CSIR-National Chemical Laboratory grant MLP029526 and CSIR-network project BSC0115. Tau constructs were kindly gifted by Prof. Roland Brandt from University of Osnabruck, Germany. S.K.S. acknowledges the fellowship from Department of Biotechnology (DBT), India.
Glossary
Abbreviations
- AD
Alzheimer’s disease
- PHFs
paired helical filaments
- ThS
thioflavin S
- MTT
methylthiazolyldiphenyl-tetrazolium bromide
- PC
protein-capped
- NPs
nanoparticles
- Fe3O4
iron oxide
- CdS
cadmium sulfide
- SEC
size exclusion chromatography
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01411.
SDS-PAGE analysis of PC-NPs-mediated Tau aggregation inhibition, TEM images for dissolution of Tau fibrils by PC-NPs, and cell images for PC-NPs-treated neuro2a cells (PDF)
Author Contributions
S.C. and S.K.S. designed and carried out the experiments. A.A. synthesized the nanoparticles. S.C and S.K.S. analyzed the data. S.C. and S.K.S. wrote the article. S.C. conceived the idea of the project.
The authors declare no competing financial interest.
Supplementary Material
References
- Forman M. S.; Trojanowski J. Q.; Lee V. M. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat. Med. 2004, 10, 1055–1063. 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- Chiti F.; Dobson C. M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A.; Frosch M. P.; Masliah E.; Hyman B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect. Med. 2011, 1, a006189 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J.; Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2015, 22–35. 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Coppola G.; Chinnathambi S.; Lee J. J.; Dombroski B. A.; Baker M. C.; Soto-Ortolaza A. I.; Lee S. E.; Klein E.; Huang A. Y.; Sears R.; et al. Evidence for a role of the rare p. A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum. Mol. Genet. 2012, 21, 3500–3512. 10.1093/hmg/dds161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K.; Liu F.; Gong C.-X. Tau and neurodegenerative disease: the story so far. Nat. Rev. Neurol. 2016, 12, 15–27. 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- Gong C.-X.; Liu F.; Grundke-Iqbal I.; Iqbal K. Post-translational modifications of Tau protein in Alzheimer’s disease. J. Neural Transm. 2005, 112, 813–838. 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- Gorantla N. V.; Khandelwal P.; Poddar P.; Chinnathambi S.. Global Conformation of Tau Protein Mapped by Raman Spectroscopy. In Tau Protein; Smet-Nocca C., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, 2017; Vol. 1523, p 21. [DOI] [PubMed] [Google Scholar]
- Gorantla N.; Shkumatov A.; Chinnathambi S.. Conformational Dynamics of Intracellular Tau Protein Revealed by CD and SAXS. In Tau Protein; Smet-Nocca C., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, 2017; Vol. 1523, p 3–20. [DOI] [PubMed] [Google Scholar]
- Von Bergen M.; Barghorn S.; Biernat J.; Mandelkow E.-M.; Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim. Biophys. Acta, Mol. Basis Dis. 2005, 1739, 158–166. 10.1016/j.bbadis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Von Bergen M.; Friedhoff P.; Biernat J.; Heberle J.; Mandelkow E.-M.; Mandelkow E. Assembly of Tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming β structure. Proc. Natl. Acad. Sci. 2000, 97, 5129–5134. 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M.; Mandelkow E.; Selkoe D. J. Alzheimer disease in 2020. Cold Spring Harbor Perspect. Med. 2012, 2, a011585 10.1101/cshperspect.a011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E.; Mercken M.; De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discovery 2011, 10, 698–712. 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Cummings J. L.; Morstorf T.; Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M.; Avila J. New perspectives on the role of Tau in Alzheimer’s disease. Implications for therapy. Biochem. Pharmacol. 2014, 88, 540–547. 10.1016/j.bcp.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Nasir I.; Fatih W.; Svensson A.; Radu D.; Linse S.; Lago C. C.; Lundqvist M. High Throughput Screening Method to Explore Protein Interactions with Nanoparticles. PLoS One 2015, 10, e0136687 10.1371/journal.pone.0136687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman M.; Ahmad E.; Qadeer A.; Rabbani G.; Khan R. H. Nanoparticles in relation to peptide and protein aggregation. Int. J. Nanomed. 2014, 9, 899–912. 10.2147/IJN.S54171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva C.; Praça C.; Ferreira R.; Santos T.; Ferreira L.; Bernardino L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Controlled Release 2016, 235, 34–47. 10.1016/j.jconrel.2016.05.044. [DOI] [PubMed] [Google Scholar]
- Linse S.; Cabaleiro-Lago C.; Xue W.-F.; Lynch I.; Lindman S.; Thulin E.; Radford S. E.; Dawson K. A. Nucleation of protein fibrillation by nanoparticles. Proc. Natl. Acad. Sci. 2007, 104, 8691–8696. 10.1073/pnas.0701250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro-Lago C.; Szczepankiewicz O.; Linse S. The effect of nanoparticles on amyloid aggregation depends on the protein stability and intrinsic aggregation rate. Langmuir 2012, 28, 1852. 10.1021/la203078w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaleiro-Lago C.; Quinlan-Pluck F.; Lynch I.; Lindman S.; Minogue A. M.; Thulin E.; Walsh D. M.; Dawson K. A.; Linse S. Inhibition of amyloid β protein fibrillation by polymeric nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. 10.1021/ja8041806. [DOI] [PubMed] [Google Scholar]
- Cabaleiro-Lago C.; Quinlan-Pluck F.; Lynch I.; Dawson K. A.; Linse S. Dual effect of amino modified polystyrene nanoparticles on amyloid β protein fibrillation. ACS Chem. Neurosci. 2010, 1, 279. 10.1021/cn900027u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry M.; Ahmad A.; Khan M. I.; Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Hyeon T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 8, 927–934. 10.1039/b207789b. [DOI] [PubMed] [Google Scholar]
- Ahmad A.; Mukherjee P.; Mandal D.; Senapati S.; Khan M. I.; Kumar R.; Sastry M. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J. Am. Chem. Soc. 2002, 124, 12108–12109. 10.1021/ja027296o. [DOI] [PubMed] [Google Scholar]
- Mukherjee P.; Ahmad A.; Mandal D.; Senapati S.; Sainkar S. R.; Khan M. I.; Ramani R.; Parischa R.; Ajayakumar P.; Alam M. Bioreduction of AuCl4– ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem., Int. Ed. 2001, 40, 3585–3588. . [DOI] [PubMed] [Google Scholar]
- Bharde A.; Rautaray D.; Bansal V.; Ahmad A.; Sarkar I.; Yusuf S. M.; Sanyal M.; Sastry M. Extracellular biosynthesis of magnetite using fungi. Small 2006, 2, 135–141. 10.1002/smll.200500180. [DOI] [PubMed] [Google Scholar]
- Bulic B.; Pickhardt M.; Mandelkow E. Progress and developments in Tau aggregation inhibitors for Alzheimer disease. J. Med. Chem 2013, 56, 4135–4155. 10.1021/jm3017317. [DOI] [PubMed] [Google Scholar]
- Blair L. J.; Zhang B.; Dickey C. A. Potential synergy between Tau aggregation inhibitors and Tau chaperonemodulators. Alzheimer’s Res. Ther. 2013, 5, 41. 10.1186/alzrt207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Du Y.; Zhang K.; Liang Z.; Li J.; Yu H.; Ren R.; Feng J.; Jin Z.; Li F.; et al. Tau-Targeted Multifunctional Nanocomposite for Combinational Therapy of Alzheimer’s Disease. ACS Nano 2018, 12, 1321–1338. 10.1021/acsnano.7b07625. [DOI] [PubMed] [Google Scholar]
- Berry C. C.; Curtis A. S. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl. Phys. 2003, 36, R198. 10.1088/0022-3727/36/13/203. [DOI] [Google Scholar]
- Bu T.; Zako T.; Zeltner M.; Sörgjerd K. M.; Schumacher C. M.; Hofer C. J.; Stark W. J.; Maeda M. Adsorption and separation of amyloid beta aggregates using ferromagnetic nanoparticles coated with charged polymer brushes. J. Mater. Chem. B 2015, 3, 3351–3357. 10.1039/C4TB02029D. [DOI] [PubMed] [Google Scholar]
- Xiong N.; Dong X.-Y.; Zheng J.; Liu F.-F.; Sun Y. Design of LVFFARK and LVFFARK-functionalized nanoparticles for inhibiting amyloid β-protein fibrillation and cytotoxicity. ACS Appl. Mater. Interfaces 2015, 7, 5650–5662. 10.1021/acsami.5b00915. [DOI] [PubMed] [Google Scholar]
- Peng E.; Choo E. S. G.; Tan C. S. H.; Tang X.; Sheng Y.; Xue J. Multifunctional PEGylated nanoclusters for biomedical applications. Nanoscale 2013, 5, 5994–6005. 10.1039/c3nr00774j. [DOI] [PubMed] [Google Scholar]
- Reyes-Esparza J.; Martínez-Mena A.; Gutiérrez-Sancha I.; Rodríguez-Fragoso P.; Cruz G. G.; Mondragón R.; Rodríguez-Fragoso L. Synthesis, characterization and biocompatibility of cadmium sulfide nanoparticles capped with dextrin for in vivo and in vitro imaging application. J. Nanobiotechnol. 2015, 13, 83 10.1186/s12951-015-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairdolf B. A.; Smith A. M.; Stokes T. H.; Wang M. D.; Young A. N.; Nie S. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu. Rev. Anal. Chem. 2013, 6, 143–162. 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain S. T.; Mukherjee S. K. Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J. Hazard. Mater. 2013, 260, 1073–1082. 10.1016/j.jhazmat.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Ke P. C.; Pilkington E. H.; Sun Y.; Javed I.; Kakinen A.; Peng G.; Ding F.; Davis T. P. Mitigation of Amyloidosis with Nanomaterials. Adv. Mater. 2019, 1901690 10.1002/adma.201901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S.; Biernat J.; Mandelkow E.. Purification of Recombinant Tau Protein and Preparation of Alzheimer-Paired Helical Filaments In Vitro. In Amyloid Proteins; Sigurdsson E. M., Ed.; Methods in Molecular Biology; Humana Press, 2005; pp 35–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.