Abstract
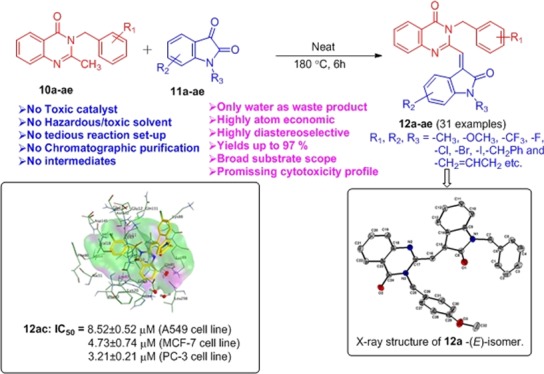
An environmentally benign highly atom-economic protocol for the construction of the C–C bond has been developed under catalyst- and solvent-free conditions. This protocol involves the efficient coupling of 2-methyl quinazolinones with isatin for the highly diastereoselective access of schizocommunin derivatives in excellent yields (up to 97%). Furthermore, the preliminary cytotoxicity screening of selected schizocommunin analogues displayed promising anticancer activity against human cancer cell lines, and the cytotoxic potential of active compound 12ac was also validated by in silico molecular docking simulation studies.
Introduction
C–H bond functionalization via direct coupling strategy and installing a new C–C bond is considered as an attractive research area in organic synthesis.1 Development of such methodologies not only facilitates the sustainable requirement of organic transformations but also found omnipresent applications in the synthesis of pharmaceuticals and complex natural products.2 Over the years, the transition-metal-catalyzed C–H bond functionalization via simple coupling reaction gained significant attention and several promising catalytic systems have been developed,3 but the apparent drawbacks cannot be ignored anymore. This includes, high cost for the catalyst synthesis, severe toxicity of the metal salts, anhydrous conditions, and toxic solvents. In contrast, an eco-friendly coupling under metal-free condition is still scarce.4
Natural products have been widely demonstrated to be the rich source of bioactive lead compounds according to their potential biological activity, good structural diversity, and compatibility with the environment and nontoxic potential.5 Because of the versatile application in medicinal chemistry as well as in allied fields, the construction of natural products and their analogues with the direct coupling strategy without using any toxic metals has always been a keen research area for organic and medicinal chemists and in great demand.5d
Additionally, there has been a paradigm shift from traditional organic chemistry to the one that assigns green chemistry values. Various simple metrics that include the principles of green chemistry such as atom economy, step economy, mass intensity, and E factors are essential parameters to the sustainable development of organic chemistry.6 Moreover, the replacement or complete removal of hazardous solvents and catalysts in chemical transformations has required urgent attention by chemists toward the synthesis of active pharmaceutical ingredients (APIs). In this context, the organic reactions without hazardous solvents, metal catalysts, and additives can eliminate the contamination of APIs as well as it can curb the environmental pollution.7 Therefore, considering the potential of the greener approach in organic synthesis, the development of new strategies via improving the atom economy of chemical processes and simple and efficient coupling under solvent- and catalyst-free approach for the construction of natural products and their analogues is a challenging task and are in great demand.
Schizocommunin is a natural product isolated from liquid culture medium of Schizophyllum commune strain and exhibits significant cytotoxic activity against murine lymphoma cells.8a The biological studies on schizocommunin and its synthetic analogues were less explored because of the lesser availability from natural resources. Chemically, the revised structure of schizocommunin 1 comprises 2-substituted quinazolinone connected by exomethylene-linked oxindole skeleton (Figure 1).8b On the other hand, the functionalized quinazolinones are present in several bio-active natural products.9 Amongst the quinazolinone class of bioactive compounds, the 2-substituted quinazolinone-based natural products such as schizocommunin,8c piriqualone,10a isaindigotone,10b CP-465022,10c ipsinesib,10d idelalisib,10e balaglitazone,10f asperlicin C,10g and benzomalvin A10h (Figure 1) have attracted considerable interest because of their broad spectrum of biological activities. The syntheses of schizocommunin and its analogues via direct functionalization at the 2-position in quinazolinones were previously reported but these methodologies have apparent drawbacks, such as acid solvents, toxic catalysts, tedious reaction setup, recovery of intermediates, and purification process.8b,8c Therefore, there is an urgent need to develop a direct, metal free, environmentally benign route to access the schizocommunin analogues along with avoidance of the aforementioned drawbacks. Herein, as a continuation of our ongoing interest in the development of a “green and sustainable” novel catalytic methodology for biologically privileged heterocycles,11 we wish to disclose an efficient, eco-friendly direct approach to access the schizocommunin analogues under catalyst- and solvent-free conditions. To the best of our knowledge, it is the first report of the environmentally benign scalable approach for the efficient construction of schizocommunin analogues under catalyst- and solvent-free simple conventional heating conditions.
Figure 1.
Schizocommunin and other 2-substituted quinazolinone-based natural products and bio-active molecules.
Results and Discussion
With an intention to obtain schizocommunin derivative 12a under the environmentally benign approach, the precursor 10a was first synthesized and screened the catalytic activity of different acid catalysts for the C–C bond formation. All the results are summarized in Table 1. In an initial attempt, 10a and 11a were used as model substrates for the screening of various Brønsted as well as Lewis acids in eco-friendly solvents EtOH, ethylene glycol, and water. However, acids used in the reaction did not give promising results and the isolated yields of desired schizocommunin derivative 12a were poor (<30%, entries 1–8, Table 1). Moving toward greener acid catalysts, that is boric acid, and greener solvents like ethylene glycol as well as water did not give promising results (entries 9–11, Table 1). We further continued our screening under catalyst- and solvent-free conditions, but the result was not improved (entry 13, Table 1). However, when we increased the temperature to 140 °C, surprisingly, we obtained the product 12a in higher amounts (53%) compared to other previous conditions (entry 14, Table 1). Therefore, we further performed a set of the reaction under solvent- and catalyst-free conditions at elevated temperatures (entries 15–18, Table 1). To our delight, on only increasing the reaction temperature and time, the better yield of desired product 12a was obtained (yield = 93%, entry 16, Table 1). Further increasing or decreasing the reaction time diminishes the yield of 12a (entries 17–18, Table 1). Therefore, the reaction of 10a with 11a at 180 °C under solvent- and catalyst-free conditions for 6 h was the optimized eco-friendly reaction condition for the synthesis of schizocommunin derivative 12a (entry 16, Table 1). It is important to note that purity of the product 12a was high enough for the direct spectroscopic analysis without any further purification. In general, reaction temperature is crucial to directly influence the molten condition and for the uniformity of the reactants under solvent-free conditions. Thus, the solvent-free coupling of 10a with 11a in an equimolar ratio under elevated temperature offers an efficient eco-friendly approach to synthesize functionalized schizocommunin analogues. It is important to note that, reaction is highly diastereoselective having exclusive formation of the (E)-isomer and this stereochemistry was well confirmed by single-crystal X-ray diffraction analysis of 12a and 12c (Figure 2).
Table 1. Optimization of Reaction Conditionsa.
| entry | catalyst (30 mol %) | solvent | temp. (°C) | time (h) | yield 12a (%)a |
|---|---|---|---|---|---|
| 1 | AcOH | EtOH | 80 | 4 | 26 |
| 2 | HCl | EtOH | 80 | 4 | 14 |
| 3 | H2SO4 | EtOH | 80 | 4 | <10 |
| 4 | PTSA | EtOH | 80 | 4 | 18 |
| 5 | CSA | EtOH | 80 | 4 | <10 |
| 6 | BF3-OEt2 | EtOH | 80 | 4 | 17 |
| 7 | TFA | EtOH | 80 | 4 | 20 |
| 8 | TFA | ethylene glycol | 80 | 4 | 13 |
| 9 | boric acid | EtOH | 80 | 4 | 25 |
| 10 | boric acid | ethylene glycol | 80 | 4 | 17 |
| 11 | boric acid | water | 80 | 4 | 34 |
| 12 | water | 100 | 4 | 13 | |
| 13 | 100 | 24 | 24 | ||
| 14 | 140 | 10 | 53 | ||
| 15 | 160 | 10 | 83 | ||
| 16 | 180 | 6 | 93 | ||
| 17 | 180 | 8 | 92 | ||
| 18 | 180 | 5 | 88 | ||
| 19b | 25 | 1 |
Yields are isolated.
Under grinding condition.
Figure 2.
Representative images of the single-crystal X-ray diffraction structures of 12a (CCDC-1902044) and 12c (CCDC-1893810) showing the diastereoselective exclusive formation of (E)-isomer.
Next, we started exploring the scope of the reaction using various substituted quinazolinones and isatins under the optimized reaction conditions (Table 2). Isatins bearing electron-withdrawing functional groups such as CN, Cl, Br, COCH3, and CF3 afforded corresponding products 12a–12ae in almost quantitative yields (up to 97%). It is notable that the iodo-functionality also survived in the optimized reaction conditions (12ab) and provides further possibilities of the diversification, which otherwise would be challenging by the transition-metal-catalyzed methodologies. The overall yields were excellent in all the cases and no column chromatography was required for the purification of the schizocommunin derivatives (12a–12ae).
Table 2. Scope of the Reactiona,b.
Yields are isolated.
E selectivity was >99% in all the synthesized compounds calculated by 1H NMR of crude reaction mixture after completion.
Yield (4.39 g of 12a) when reaction was carried out at 10 mmol scale.
Additionally, various green chemistry parameters were investigated and demonstrated by the green assessment diagram for the synthesis of schizocommunin derivatives (Figure 3). Our developed methodology has remarkable advantages in terms of several green parameters, for example, atom economy of the synthesis of 12a was reached 96.51%; carbon efficiency of the reaction was 100%; reaction mass efficiency was observed up to 89.74%; and most importantly the E-factor was reduced to 0.11 (Figure 3). Besides aforementioned improvements in the synthesis of schizocommunin derivatives, no need of the purification by column chromatography was the additional advantage associated with the developed method.
Figure 3.

Green matrix: a quick assessment of the improvement of schizocommunin derivative synthesis.
Because of the potential cytotoxicity profile of the natural product schizocommunin against murine lymphoma cells,8,9 it was anticipated that the analogues of schizocommunin would display promising cytotoxic activity. Thus, the selected synthesized Schizocommunin analogues were evaluated for their cytotoxic activity against three human cancer cell lines, that is, A549 (lung cancer), MCF-7 (breast cancer), and PC-3 (prostate cancer) using an azolium-based colorimetric cytotoxicity assay (MTT assay).12 Compounds 12i, 12p, 12ac, and 12ae exhibited above 50% cytotoxicity at 10 μM concentration. These compounds were further selected to evaluate concentration-dependent responses and displayed promising antiproliferative activity with IC50 values ranging from 3.21 ± 0.21 to 14.56 ± 0.17 μM (Table 3). The compound 12ac displayed significant antiproliferative activity against all the screened cancer cell lines. This exciting preliminary antiproliferative activity profile of this series of schizocommunin analogues further opens a new way to get a novel drug lead in cancer treatment.
Table 3. IC50 Valuesa (in μM) of Some Selected Schizocommunin Analogues in Human Cancer Cell Lines.
| no. | compounds | A549 (lung cancer) | MCF-7 (breast cancer) | PC-3 (prostate cancer) |
|---|---|---|---|---|
| 1 | 12a | >15 | >15 | >15 |
| 2 | 12i | >15 | 12.45 ± 0.33 | 14.56 ± 0.17 |
| 3 | 12p | >15 | >15 | 5.43 ± 0.64 |
| 4 | 12x | >15 | >15 | >15 |
| 5 | 12ab | >15 | >15 | >15 |
| 6 | 12ac | 8.52 ± 0.52 | 4.73 ± 0.74 | 3.21 ± 0.21 |
| 7 | 12ae | 11.24 ± 0.10 | 13.47 ± 0.18 | >15 |
| 8 | doxorubicin | 1.64 ± 0.35 | 0.14 ± 0.10 | 0.55 ± 0.32 |
50% inhibitory concentration after 48 h of drug treatment.
In order to understand the mechanism of anticancer activity of the inhibitor (12ac), molecular docking study was performed on the protein structure of cyclin-dependent kinase 2 (CDK2) enzyme (Figure 4).13a,13b Prior docking, the molecular structure of the ligand 12ac was energy minimized with the aid of the MMFF94x force field to attain the low-energy conformation. The free energy binding score of the compound 12ac was −6.99 kcal/mol and was very close to the binding energy value (−8.19 kcal/mol) of the reference drug sunitinib. An analysis of protein–ligand interactions showed that the −C=O group and nitrogen atoms in the quinazolin-4(3H)-one and oxoindolin-3-ylidene moieties are strongly interacted electrostatically with the acidic and basic amino acid domains of Glu8, Asp86, Lys88, Gln131, Lys9, Lys20, Lys89, and Gly11. The 3-chlorobenzyl group at the quinazolin-4(3H)-one ring interacted by means of hydrophobic domains of Leu134, Leu298, Val18, Phe82, Ala31, and Leu83. The benzyl moiety and 5-chlorophenyl at 2-oxoindolin-3-ylidene moieties are completely exposed to the solvent area of the protein. This indicated that, such types of projective groups are not important for the inhibition of the CDK2 enzyme. This study strongly demonstrated the key features responsible for the inhibitory activity as well as design of analogues against the CDK2 target.
Figure 4.

3D and 2D overlay illustration of inhibitor 12ac in the complex with cyclin-dependent kinase 2 (CDK2) enzyme (PDB code—3TI1).
Conclusions
In summary, we have described an environmentally benign, straightforward access for the synthesis of diastereoselective schizocommunin derivatives in the absence of catalyst and solvent in excellent yields. The advantages of the developed methodology are experimental simplicity, easy work-up, and excellent yields of products. Additionally, the product isolation does not require purification by column chromatography. This eco-friendly methodology also eliminates toxic metal catalyst-related environmental hazards and pollutions along with increase in atomic economy. Furthermore, the preliminary cytotoxic activity of selected compounds revealed that the promising cytotoxic profile in A549, MCF-7, and PC-3 human cancer cell lines. The in silico molecular docking simulation studies of compound 12ac effectively validated the in vitro experimental results and worthy of further structural optimization for the development in the anticancer drug discovery process.
Experimental Section
General
All reactions were carried out in oven- or flame-dried glassware unless otherwise noted. Except as otherwise indicated, all reactions were magnetically stirred and monitored by analytical thin-layer chromatography (TLC) using precoated silica gel glass plates (0.25 mm) with the F254 indicator. Visualization was accomplished by UV light (254 nm). Column chromatography was performed using a silica gel 100–200 mesh. Yields refer to pure compounds, unless otherwise noted. Commercial grade reagents and solvents were used without further purification. 1H and 13C NMR spectra were recorded on a Bruker 400 MHz NMR spectrometer using CDCl3/DMSO-d6 as a solvent for deuterium locking with temperature 298 K. Chemical shifts are given in ppm with TMS as an internal reference. J values are given in Hertz. High-resolution mass spectra were determined with a quadrupole time-of-flight mass spectrometer.
General Procedure for the Synthesis of Schizocommunin Derivatives (12a)
3-(4-Methoxybenzyl)-2-methylquinazolin-4(3H)-one (10a, 1 mmol, 280 mg) and 1-benzylindoline-2,3-dione (11a, 1 mmol, 237 mg) were added in a vial. The reaction mixture was heated at 180 °C. The progress of the reaction was monitored by TLC and after 6 h, the starting materials disappeared. The solidified reaction mixture was cooled down to room temperature and added ethanol (5 mL), stirred for 30 min, filtered off, which afforded the pure product 12a as a reddish orange solid (93% yield, 464 mg).
(E)-2-((1-Benzyl-2-oxoindolin-3-ylidene)methyl)-3-(4-methoxybenzyl)quinazolin-4(3H)-one (12a)
It was obtained as a reddish orange solid, yield 93%. Rf = 0.52 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.71 (s, 3H), 4.97 (s, 2H), 5.41 (s, 2H), 6.69 (d, J = 8 Hz, 1H), 6.78 (d, J = 8.8 Hz, 2H), 6.87 (td, J1 = 7.6 Hz, J2 = 0.8 Hz, 1H), 7.19 (td, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.28–7.36 (m, 7H), 7.57 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.63 (s, 1H), 7.76 (d, J = 7.2 Hz, 1H), 7.81 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.93 (d, J = 7.6 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.1, 55.2, 109.3, 114.3, 120.0, 121.4, 122.5, 125.6, 126.7, 127.3, 127.4, 127.6, 127.8, 127.9, 127.9, 128.8, 128.9, 131.7, 133.6, 134.7, 135.5, 144.7, 146.9, 150.7, 159.2, 161.9, 167.6. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H25N3O3Na, 522.1788; found, 522.1782.
(E)-2-((1-Allyl-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12b)
It was obtained as a reddish orange solid, yield 87%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.38 (dt, J1 = 5.2 Hz, J2 = 1.6 Hz, 2H), 5.23 (d, J = 1.2 Hz, 1H), 5.25 (dd, J1 = 4 Hz, J2 = 0.8 Hz, 1H), 5.45 (s, 2H), 5.79–5.89 (m, 1H), 6.80 (d, J = 7.6 Hz, 1H), 6.92 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 2H), 6.99 (dt, J1 = 9.6 Hz, J2 = 2 Hz, 1H), 7.08 (dd, J1 = 7.6 Hz, J2 = 0.4 Hz, 1H), 7.24–7.32 (m, 2H), 7.49 (s, 1H), 7.60 (td, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.78 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.84 (td, J1 = 7.2 Hz, J2 = 1.6 Hz, 1H), 7.99 (d, J = 7.6 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.4, 47.1, 109.3, 114.1, 114.4, 114.9, 115.1, 117.8, 119.8, 121.2, 122.4, 122.8, 122.8, 124.7, 126.8, 127.4, 127.7, 128.1, 130.5, 130.6, 131.0, 131.9, 134.1, 134.9, 138.1, 138.1, 144.9, 146.9, 150.4, 161.8, 164.3, 167.1; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H20FN3O2Na, 460.1432; found, 460.1424.
(E)-2-((1-Allyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12c)
It was obtained as a reddish orange solid, yield 85%. Rf = 0.62 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.35 (dt, J1 = 5.2 Hz, J2 = 1.2 Hz, 2H), 5.19–5.26 (m, 2H), 5.49 (s, 2H), 5.76–6.86 (m, 1H), 6.68 (d, J = 8.4 Hz, 1H), 6.93 (td, J1 = 8.2 Hz, J2 = 2 Hz, 1H), 7.02 (d, J = 9.6 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 7.28–7.33 (m, 1H), 7.42 (dd, J1 = 8.4 Hz, J2 = 2 Hz, 1H), 7.55 (s, 1H), 7.61 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.83 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.87 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 1.2 Hz, 1H), 8.66 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.5, 46.9, 110.5, 114.1, 114.4, 115.0, 115.2, 118.0, 121.2, 121.7, 122.8, 122.8, 126.2, 127.5, 127.5, 128.4, 130.6, 130.7, 130.7, 131.1, 133.1, 134.3, 135.1, 138.1, 138.2, 143.9, 146.6, 149.7, 161.8, 161.9, 164.3, 166.8; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H19BrFN3O2Na, 538.0537; found, 538.0534.
(E)-3-Benzyl-2-((2-oxo-1-(prop-2-yn-1-yl)indolin-3-ylidene)methyl)quinazolin-4(3H)-one (12d)
It was obtained as a reddish orange solid, yield 84%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.25 (t, J = 2.4 Hz, 1H), 4.55 (d, J = 2.8 Hz, 2H), 5.46 (s, 2H), 6.96 (td, J1 = 7.6 Hz, J2 = 0.8 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 7.25–7.31 (m, 5H), 7.35 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.54 (s, 1H), 7.59 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.77 (dd, J1 = 8 Hz, J2 = 0.4 Hz, 1H), 7.83 (td, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.99 (d, J = 7.6 Hz, 1H), 8.42 (dd, J1 = 8 Hz, J2 = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 29.3, 47.6, 72.4, 76.5, 109.3, 119.9, 121.4, 122.8, 125.7, 126.9, 127.3, 127.4, 127.6, 128.0, 128.1, 128.9, 131.9, 133.5, 134.8, 135.6, 143.7, 146.9, 150.6, 161.9, 166.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H19N3O2Na, 440.1369; found, 440.1356.
(E)-2-((1-Benzyl-2-oxoindolin-3-ylidene)methyl)-3-(2-methoxybenzyl)quinazolin-4(3H)-one (12e)
It was obtained as a reddish orange solid, yield 91%. Rf = 0.62 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.85 (s, 3H), 4.94 (s, 2H), 5.48 (s, 2H), 6.68 (d, J = 8 Hz, 1H), 6.80 (t, J = 7.2 Hz, 1H), 6.84 (d, J = 8 Hz, 1H), 6.87 (td, J1 = 7.8 Hz, J2 = 0.8 Hz, 1H), 7.08 (d, J = 7.6 Hz, 1H), 7.18–7.22 (m, 2H), 7.27–7.34 (m, 5H), 7.57 (s, 1H), 7.59 (dd, J1 = 7.6 Hz, J2 = 0.8 Hz, 1H), 7.76 (d, J = 8 Hz, 1H), 7.81 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.92 (d, J = 7.2 Hz, 1H), 8.40 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.9, 43.8, 55.4, 109.2, 110.4, 120.1, 120.7, 121.4, 122.4, 123.6, 126.1, 126.5, 127.3, 127.3, 127.5, 127.7, 127.8, 128.0, 128.8, 129.0, 131.6, 133.4, 134.6, 135.6, 144.6, 146.9, 151.1, 156.7, 162.0, 167.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H25N3O3Na, 522.1788; found, 522.1786.
(E)-2-((5-Bromo-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinolin-4(3H)-one (12f)
It was obtained as a reddish orange solid, yield 95%. Rf = 0.52 (TLC, Hex/EA, 1:4). 1H NMR (400 MHz, DMSO): δ 4.61 (S, 2H), 5.95 (d, J = 8.4 Hz, 1H), 6.21 (d, J = 7.6 Hz, 2H), 6.27 (d, J = 9.6 Hz, 1H), 6.43–4.51 (m, 2H), 6.60 (dd, J1 = 8.4 Hz, J2 = 1.6 Hz, 1H), 6.81 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 8 Hz, 1H), 7.11 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 7.6 Hz, 1H), 7.57 (s, 1H), 10.01 (s, 1H); 13C NMR (100 MHz, DMSO): δ 46.3, 112.0, 113.0, 113.5, 113.7, 114.3, 114.5, 120.9, 122.0, 122.5, 126.1, 126.9, 128.3, 130.0, 130.8, 130.9, 133.2, 134.2, 135.2, 139.2, 139.3, 143.5, 146.4, 150.3, 161.1, 163.5, 167.6; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H15BrFN3O2Na, 498.0224; found, 498.0211.
(E)-2-((1-Allyl-2-oxoindolin-3-ylidene)methyl)-3-(4-methylbenzyl)quinazolin-4(3H)-one (12g)
It was obtained as a reddish orange solid, yield 86%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.26 (s, 3H), 4.38 (dt, J1 = 5.2 Hz, J2 = 1.6 Hz, 2H), 5.22 (t, J = 1.2 Hz, 1H), 5.25 (dd, J1 = 6.4 Hz, J2 = 0.8 Hz, 1H), 5.42 (s, 2H), 5.80–5.89 (m, 1H), 6.79 (d, J = 8 Hz, 1H), 6.90 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H), 7.07 (d, J = 8 Hz, 2H), 7.20 (d, J = 8.4 Hz, 2H), 7.27 (td, J1 = 8 Hz, J2 = 1.2 Hz, 1H), 7.56 (s, 1H), 7.57 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.76 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.81 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 7.98 (d, J = 7.2 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.1, 42.4, 47.4, 109.2, 117.7, 120.0, 121.4, 122.4, 125.4, 126.8, 127.4, 127.4, 127.6, 127.9, 128.9, 129.1, 129.6, 131.1, 131.7, 132.7, 133.7, 134.7, 137.7, 144.8, 146.9, 150.8, 161.9, 167.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H23N3O2Na, 456.1682; found, 456.1676.
(E)-2-((1-Allyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(4-methylbenzyl)quinazolin-4(3H)-one (12h)
It was obtained as a reddish orange solid, yield 86%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.27 (s, 3H), 4.35 (d, J = 5.2 Hz, 2H), 5.19–5.25 (m, 2H), 5.45 (s, 2H), 5.76–5.86 (m, 1H), 6.66 (d, J = 8.4 Hz, 1H), 7.10 (d, J = 8 Hz, 2H), 7.22 (d, J = 8 Hz, 2H), 7.39 (dd, J1 = 8.4 Hz, J2 = 2 Hz, 1H), 7.58–7.63 (m, 2H), 7.81 (d, J = 7.6 Hz, 1H), 7.84 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 8.62 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.1, 42.5, 47.2, 110.4, 114.9, 117.9, 121.4, 121.8, 126.9, 127.3, 127.4, 127.5, 128.2, 129.7, 130.8, 131.0, 132.7, 132.8, 134.1, 134.9, 137.8, 143.8, 146.7, 150.1, 161.9, 166.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H22BrN3O2Na, 534.0788; found, 534.0763.
(E)-3-Benzyl-2-((5-bromo-2-oxo-1-(prop-2-yn-1-yl)indolin-3-ylidene)methyl)quinazolin-4(3H)-one (12i)
It was obtained as a reddish orange solid, yield 88%. Rf = 0.58 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.26 (t, J = 2.4 Hz, 1H), 4.53 (d, J = 2.4 Hz, 2H), 5.50 (s, 2H), 6.91 (d, J = 8 Hz, 1H), 7.24–7.27 (m, 1H), 7.32–7.34 (m, 4H), 7.49 (dd, J1 = 8.2 Hz, J2 = 2 Hz, 1H), 7.61 (s, 1H), 7.63 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.82 (dd, J1 = 8 Hz, J2 = 0.4 Hz, 1H), 7.87 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 8.42 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.64 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 29.4, 47.4, 72.8, 76.1, 110.6, 115.4, 121.4, 121.8, 127.1, 127.2, 127.5, 128.1, 129.0, 131.0, 132.5, 134.3, 135.0, 135.6, 142.6, 146.6, 149.8, 161.9, 166.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H18BrN3O2Na, 518.0475; found, 518.0458.
(E)-2-((1-Benzyl-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12j)
It was obtained as a reddish orange solid, yield 96%. Rf = 0.52 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.95 (s, 2H), 5.47 (s, 2H), 6.69 (d, J = 8 Hz, 1H), 6.88–6.94 (m, 2H), 7.00 (d, J = 9.2 Hz, 1H), 7.09 (d, J = 7.6 Hz, 1H), 7.20 (dt, J1 = 8 Hz, J2 = 0.8 Hz, 2H), 7.27–7.35 (m, 5H), 7.54 (s, 1H), 7.59 (td, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.78 (d, J = 7.2 Hz, 1H), 7.84 (td, J1 = 7.8 Hz, J2 = 1.6 Hz, 1H), 7.97 (d, J = 7.2 Hz, 1H), 8.41 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.1, 109.4, 114.2, 114.4, 114.9, 115.1, 119.9, 121.2, 122.5, 122.8, 122.8, 124.9, 126.8, 127.3, 127.4, 127.9, 127.8, 128.1, 128.8, 130.5, 130.6, 131.9, 134.0, 134.9, 135.4, 138.1, 138.2, 144.8, 146.9, 150.5, 161.8, 164.3, 167.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H22FN3O2Na, 510.1588; found, 510.1562.
(E)-3-(2-Methoxybenzyl)-2-((2-oxoindolin-3-ylidene)methyl)quinazolin-4(3H)-one (12k)
It was obtained as a reddish orange solid, yield 96%. Rf = 0.52 (TLC, Hex/EA, 1:4). 1H NMR (400 MHz, DMSO): δ 3.82 (s, 3H), 5.36 (s, 2H), 6.80 (d, J = 8 Hz, 2H), 6.91 (dd, J1 = 7.4 Hz, J2 = 1.6 Hz, 1H), 7.00 (d, J = 7.6 Hz, 1H), 7.20–7.22 (m, 1H), 7.25 (s, 1H), 7.45 (dd, J1 = 8 Hz, J2 = 2 Hz, 1H), 7.66 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.73 (d, J = 8 Hz, 1H), 7.96 (td, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 8.25 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.46 (d, J = 2 Hz, 1H), 10.87 (s, 1H); 13C NMR (100 MHz, DMSO): δ 42.3, 55.5, 110.9, 111.9, 112.9, 120.5, 120.8, 122.1, 123.7, 126.4, 126.8, 126.8, 128.2, 128.8, 130.1, 133.1, 134.2, 135.1, 143.5, 146.3, 150.5, 156.3, 161.1, 167.7; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C25H18BrN3O3Na, 510.0424; found, 510.0443.
(E)-2-((1-Allyl-2-oxoindolin-3-ylidene)methyl)-3-(2-methoxybenzyl)quinazolin-4(3H)-one (12l)
It was obtained as a reddish orange solid, yield 85%. Rf = 0.63 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.85 (s, 3H), 4.36 (d, J = 5.2 Hz, 2H), 5.21 (s, 1H), 5.24 (d, J = 5.2 Hz, 1H), 5.45 (s, 2H), 5.78–5.88 (m, 1H), 6.78–6.86 (m, 3H), 6.90 (t, J = 7.6 Hz, 1H), 7.07 (d, J = 7.2 Hz, 1H), 7.18 (t, J = 7.6 Hz, 1H), 7.28 (d, J = 7.6 Hz, 1H), 7.52 (s, 1H), 7.57 (t, J = 7.2 Hz, 1H), 7.76 (d, J = 8 Hz, 1H), 7.82 (t, J = 6.8 Hz, 1H), 7.92 (d, J = 7.6 Hz, 1H), 8.40 (d, J = 7.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.3, 42.9, 55.4, 109.1, 110.4, 117.6, 120.0, 120.7, 121.4, 122.3, 123.5, 125.9, 126.5, 127.3, 127.5, 127.8, 127.9, 129.0, 131.2, 131.6, 133.5, 134.6, 144.7, 146.9, 151.1, 156.7, 162.0, 167.1; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H23N3O3Na, 472.1624; found, 472.1613.
(E)-2-((1-Allyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(2-methoxybenzyl)quinazolin-4(3H)-one (12m)
It was obtained as a reddish orange solid, yield 88%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.87 (s, 3H), 4.33 (dt, J1 = 5.2 Hz, J2 = 1.6 Hz, 2H), 5.18–5.24 (m, 2H), 5.50 (s, 2H), 5.75–5.85 (m, 1H), 6.65 (d, J = 8.4 Hz, 1H), 6.81 (t, J = 7.6 Hz, 1H), 6.85 (d, J = 8 Hz, 1H), 7.07 (dd, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.18 (td, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H), 7.39 (dd, J1 = 8.2 Hz, J2 = 2 Hz, 1H), 7.58 (s, 1H), 7.60 (dd, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.81 (d, J = 7.2 Hz, 1H), 7.84 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H), 8.40 (dd, J1 = 8 Hz, J2 = 1.2 Hz, 1H), 8.56 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.4, 42.6, 55.5, 110.3, 110.4, 114.9, 117.8, 120.7, 121.4, 121.9, 123.7, 127.4, 127.5, 128.0, 128.1, 129.1, 130.7, 130.8, 132.6, 133.9, 134.8, 143.7, 146.7, 150.4, 156.7, 162.0, 166.8; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H22BrN3O3Na, 550.0737; found, 550.0735.
(E)-2-((5-Bromo-2-oxo-1-(prop-2-yn-1-yl)indolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12n)
It was obtained as a reddish orange solid, yield 82%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.26 (t, J = 2.4 Hz, 1H), 4.53 (d, J = 2.4 Hz, 2H), 5.48 (s, 2H), 6.92 (d, J = 8.4 Hz, 1H), 6.96 (dd, J1 = 8.4 Hz, J2 = 2 Hz, 1H), 7.02 (d, J = 9.6 Hz, 1H), 7.11 (d, J = 8 Hz, 1H), 7.29–7.34 (m, 1H), 7.50 (dd, J1 = 8.2 Hz, J2 = 2 Hz, 1H), 7.55 (s, 1H), 7.62 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.83 (d, J = 7.2 Hz, 1H), 7.88 (td, J1 = 6.8 Hz, J2 = 1.6 Hz, 1H), 8.42 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 8.68 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 29.4, 46.9, 72.8, 76.1, 110.7, 114.1, 114.3, 115.1, 115.3, 115.5, 121.2, 121.7, 122.7, 122.8, 126.6, 127.5, 127.6, 128.5, 130.6, 130.7, 131.2, 132.8, 134.5, 135.2, 138.0, 138.1, 142.7, 146.6, 149.6, 161.8, 161.9, 164.3, 166.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H17BrFN3O2Na, 536.0380; found, 536.0339.
(E)-2-((1-Benzyl-2-oxoindolin-3-ylidene)methyl)-3-(3-(trifluoromethyl)benzyl)quinazolin-4(3H)-one (12o)
It was obtained as a reddish orange solid, yield 95%. Rf = 0.52 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.95 (s, 2H), 5.52 (s, 2H), 6.68 (d, J = 7.6 Hz, 1H), 6.86 (t, J = 7.6 Hz, 1H), 7.19 (t, J = 7.6 Hz, 1H), 7.27–7.37 (m, 5H), 7.39 (d, J = 7.6 Hz, 1H), 7.46–7.52 (m, 2H), 7.53 (s, 1H), 7.56 (s, 1H), 7.60 (t, J = 6.8 Hz, 1H), 7.79 (d, J = 8 Hz, 1H), 7.85 (t, J = 8 Hz, 2H), 8.42 (d, J = 8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.1, 109.4, 119.8, 121.2, 122.5, 124.2, 124.2, 124.6, 124.9, 124.9, 126.6, 127.3, 127.5, 127.7, 127.8, 128.2, 128.8, 129.5, 130.7, 132.0, 134.2, 135.0, 135.4, 136.8, 144.8, 146.9, 150.3, 161.8, 167.4; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H22F3N3O2Na, 560.1556; found, 560.1523.
(E)-3-(2-Methoxybenzyl)-2-((2-oxoindolin-3-ylidene)methyl)quinazolin-4(3H)-one (12p)
It was obtained as a reddish orange solid, yield 91%. Rf = 0.52 (TLC, Hex/EA, 1:4). 1H NMR (400 MHz, CDCl3): δ 3.86 (s, 3H), 5.46 (s, 2H), 6.80–6.87 (m, 3H), 6.90 (td, J1 = 7.6 Hz, J2 = 0.8 Hz, 1H), 7.08 (dd, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H), 7.18 (td, J1 = 8 Hz, J2 = 1.6 Hz, 1H), 7.25 (td, J1 = 8.6 Hz, J2 = 1.2 Hz, 1H), 7.47 (s, 1H), 7.57 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.69 (s, 1H), 7.77 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.82 (td, J1 = 7.2 Hz, J2 = 1.6 Hz, 1H), 7.92 (d, J = 7.2 Hz, 1H), 8.40 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.8, 55.4, 109.9, 110.4, 120.6, 120.7, 121.4, 122.4, 123.5, 126.0, 126.9, 127.3, 127.5, 127.9, 128.0, 129.0, 131.7, 133.7, 134.7, 142.5, 146.9, 150.9, 156.7, 162.0, 168.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C25H19N3O3Na, 432.1319; found, 432.1313.
(E)-2-((1-Allyl-2-oxoindolin-3-ylidene)methyl)-3-(4-methoxybenzyl)quinazolin-4(3H)-one (12q)
It was obtained as a reddish orange solid, yield 82%. Rf = 0.55 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 3H), 4.39 (dt, J1 = 5.2 Hz, J2 = 1.6 Hz, 2H), 5.24 (s, 1H), 5.26 (dd, J1 = 8.6 Hz, J2 = 1.2 Hz, 1H), 5.40 (s, 2H), 5.81–5.91 (m, 1H), 6.80 (dd, J1 = 6.8 Hz, J2 = 2 Hz, 3H), 6.91 (td, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.28 (d, J = 8.4, 3H), 7.37 (d, J = 4.2, 1H), 7.58 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 2H), 7.77 (d, J = 7.6 Hz, 1H), 7.82 (td, J1 = 5.6 Hz, J2 = 1.6 Hz, 1H), 7.97 (d, J = 7.6 Hz, 1H), 8.42 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.5, 47.1, 55.2, 109.2, 114.3, 117.8, 120.0, 121.4, 122.4, 125.4, 126.8, 126.9, 127.4, 127.6, 127.9, 127.9, 127.9, 128.6, 128.9, 131.1, 131.7, 133.6, 134.7, 144.8, 146.9, 150.7, 159.3, 161.9, 167.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H23N3O3Na, 472.1632; found, 472.1629.
(E)-2-((1-Allyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(4-methoxybenzyl)quinazolin-4(3H)-one (12r)
It was obtained as a reddish orange solid, yield 84%. Rf = 0.58 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 3H), 4.36 (dt, J1 = 5.2 Hz, J2 = 1.6 Hz, 2H), 5.20–5.26 (m, 2H), 5.44 (s, 2H), 5.78–5.87 (m, 1H), 6.68 (d, J = 8.4 Hz, 1H), 6.82 (d, J = 8.8 Hz, 2H), 7.29 (d, J = 8.8 Hz, 2H), 7.41 (dd, J1 = 8.6 Hz, J2 = 2 Hz, 1H), 7.59 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.67 (s, 1H), 7.81 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.85 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 8.42 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.60 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.5, 46.9, 55.2, 110.5, 113.4, 114.4, 115.0, 117.9, 121.5, 121.8, 126.9, 127.5, 127.9, 128.3, 128.9, 130.8, 130.9, 132.7, 134.2, 134.9, 143.8, 146.7, 150.1, 159.3, 161.9, 166.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C28H22BrN3O3Na, 550.0737; found, 550.0734.
(E)-2-((1-Allyl-5-chloro-2-oxoindolin-3-ylidene)methyl)-3-benzylquinazolin-4(3H)-one (12s)
It was obtained as a reddish orange solid, yield 91%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (500 MHz, CDCl3): δ 4.37 (d, J = 4.5 Hz, 2H), 5.24 (d, J = 7.6 Hz, 2H), 5.46 (s, 2H), 5.78–5.86 (m, 1H), 6.73 (d, J = 8.5 Hz, 1H), 7.23–7.29 (m, 6H), 7.55 (s, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.83 (d, J = 8 Hz, 1H), 7.88 (t, J = 7.5 Hz, 1H), 8.38 (s, 1H), 8.42 (d, J = 8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 42.6, 46.9, 110.1, 117.9, 121.2, 125.4, 126.1, 127.3, 127.5, 127.6, 127.8, 127.9, 128.3, 128.4, 130.3, 130.7, 131.5, 133.2, 134.9, 135.2, 137.7, 143.4, 146.6, 149.8, 161.8, 166.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H20ClN3O2Na, 476.1136; found, 476.1123.
(E)-2-((1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(4-methoxybenzyl)quinazolin-4(3H)-one (12t)
It was obtained as a reddish orange solid, yield 95%. Rf = 0.52 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 3H), 4.94 (s, 2H), 5.45 (s, 2H), 6.56 (d, J = 8 Hz, 1H), 6.82 (d, J = 7.6 Hz, 2H), 7.26–7.32 (m, 8H), 7.59 (t, J = 6 Hz, 1H), 7.71 (s, 1H), 7.80–7.86 (m, 2H), 8.42 (d, J = 7.2 Hz, 1H), 8.56 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 46.9, 55.2, 110.5, 114.4, 115.1, 121.5, 121.9, 127.1, 127.2, 127.5, 127.5, 127.9, 128.3, 128.9, 128.9, 130.9, 132.6, 134.2, 134.9, 135.1, 143.7, 146.7, 150.1, 159.3, 161.9, 167.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H24BrN3O3Na, 600.0893; found, 600.0847.
(E)-2-((1-Allyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-benzylquinazolin-4(3H)-one (12u)
It was obtained as a reddish orange solid, yield 82%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.36 (d, J = 5.2 Hz, 2H), 5.19 (td, J1 = 8.8 Hz, J2 = 0.8 Hz, 2H), 5.51 (s, 2H), 5.76–5.86 (m, 1H), 6.68 (d, J = 8.4 Hz, 1H), 7.22–7.26 (m, 1H), 7.30–7.36 (m, 4H), 7.42 (dd, J1 = 8.2 Hz, J2 = 2 Hz, 1H), 7.61–7.65 (m, 2H), 7.82 (d, J = 8 Hz, 1H), 7.87 (td, J1 = 6.6 Hz, J2 = 1.2 Hz, 1H), 8.43 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 8.62 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.5, 47.4, 110.5, 115.0, 117.9, 121.4, 121.8, 126.7, 127.3, 127.5, 127.5, 128.0, 128.3, 129.0, 130.7, 130.9, 132.8, 134.2, 135.0, 135.7, 143.8, 146.7, 150.0, 161.9, 166.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C27H20BrN3O2Na, 520.0631; found, 520.0627.
(E)-2-((1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12v)
It was obtained as a reddish orange solid, yield 96%. Rf = 0.65 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.92 (d, J = 4.4 Hz, 2H), 5.49 (d, J = 3.6 Hz, 2H), 6.55–6.58 (m, 1H), 6.93–6.96 (m, 1H), 7.03 (d, J = 7.6 Hz, 1H), 7.12 (d, J = 6.4 Hz, 1H), 7.26–7.35 (m, 7H), 7.59–7.64 (m, 2H), 7.83–7.90 (m, 2H), 8.41 (d, J = 7.6 Hz, 1H), 8.62 (d, J = 3.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 46.9, 110.6, 114.2, 114.4, 115.0, 115.1, 115.2, 121.3, 121.8, 122.8, 126.4, 127.2, 127.5, 127.9, 128.5, 128.9, 130.6, 130.7, 131.0, 133.1, 134.4, 134.9, 135.2, 138.2, 138.2, 143.8, 146.6, 149.8, 161.8, 161.9, 164.4, 167.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H21BrFN3O2Na, 588.0693; found, 588.0678.
(E)-2-((1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(2-methoxybenzyl)quinazolin-4(3H)-one (12w)
It was obtained as a reddish orange solid, yield 95%. Rf = 0.60 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.87 (s, 3H), 4.91 (s, 2H), 5.52 (s, 2H), 6.54 (d, J = 8.4 Hz, 1H), 6.81–6.87 (m, 2H), 7.09 (d, J = 7.6 Hz, 1H), 7.18 (td, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 7.24–7.33 (m, 6H), 7.58 (t, J = 6.8 Hz, 1H), 7.63 (s, 1H), 7.80 (d, J = 7.6 Hz, 1H), 7.84 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 8.41 (d, J = 8 Hz, 1H), 8.54 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.5, 43.8, 55.5, 110.4, 114.9, 120.7, 121.4, 121.0, 123.7, 127.2, 127.4, 127.4, 127.7, 127.9, 128.1, 128.1, 128.9, 129.1, 130.7, 132.5, 133.9, 134.8, 135.1, 143.6, 146.7, 150.4, 156.7, 161.9, 167.2; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H24BrN3O3Na, 600.0893; found, 600.0884.
(E)-3-Benzyl-2-((1-benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)quinazolin-4(3H)-one (12x)
It was obtained as a reddish orange solid, yield 97%. Rf = 0.70 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.93 (s, 2H), 5.52 (s, 2H), 6.56 (d, J = 8.4 Hz, 1H), 7.23–7.36 (m, 11H), 7.61 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 7.66 (s, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.86 (td, J1 = 6.8 Hz, J2 = 1.6 Hz, 1H), 8.43 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 8.59 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.4, 110.6, 115.1, 121.4, 121.8, 126.8, 127.2, 127.3, 127.5, 127.5, 127.9, 128.0, 128.3, 128.9, 129.0, 130.9, 132.8, 134,2, 134.9, 135.0, 135.7, 143.7, 146.6, 150.0, 161.9, 167.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H22BrN3O2Na, 570.0788; found, 570.0787.
(E)-2-((1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(3-(trifluoromethyl)benzyl)quinazolin-4(3H)-one (12y)
It was obtained as a reddish orange solid, yield 92%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.92 (s, 2H), 5.56 (s, 2H), 6.55 (d, J = 8 Hz, 1H), 7.26–7.34 (m, 6H), 7.43–7.65 (m, 6H), 7.82–7.89 (m, 2H), 8.42 (d, J = 7.6 Hz, 1H), 8.56 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 46.9, 110.7, 115.1, 121.2, 121.7, 124.2, 124.3, 125.0, 125.0, 126.1, 127.2, 127.6, 127.9, 128.5, 128.9, 129.6, 130.7, 130.9, 131.2, 133.2, 134.4, 134.9, 135.2, 136.8, 143.8, 146.6, 149.7, 161.8, 167.1; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H21BrF3N3O2Na, 638.0661; found, 638.0643.
(E)-2-((1-Benzyl-5-bromo-2-oxoindolin-3-ylidene)methyl)-3-(4-methylbenzyl)quinazolin-4(3H)-one (12z)
It was obtained as a reddish orange solid, yield 84%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 2.26 (s, 3H), 4.93 (s, 2H), 5.47 (s, 2H), 6.55 (d, J = 8.4 Hz, 1H), 7.09 (d, J = 8 Hz, 2H), 7.23–7.33 (m, 8H), 7.59 (td, J1 = 7.4 Hz, J2 = 1.6 Hz, 1H), 7.68 (s, 1H), 7.80 (d, J = 7.6, 1H), 7.84 (td, J1 = 7.4 Hz, J2 = 1.6 Hz, 1H), 8.42 (dd, J1 = 8.2 Hz, J2 = 1.2 Hz, 1H), 8.58 (d, J = 2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 21.1, 43.9, 47.2, 110.5, 115.1, 121.4, 121.9, 127.0, 127.2, 127.3, 127.5, 127.5, 128.2, 128.9, 129.7, 130.9, 132.7, 132.8, 134.1, 134.9, 135.1, 137.8, 143.7, 146.7, 150.1, 161.9, 167.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H24BrN3O2Na, 584.0944; found, 584.0929.
(E)-3-Benzyl-2-((1-benzyl-2-oxo-5-phenylindolin-3-ylidene)methyl)quinazolin-4(3H)-one (12aa)
It was obtained as a reddish orange solid, yield 96%. Rf = 0.66 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.98 (s, 2H), 5.52 (s, 2H), 6.76 (d, J = 8 Hz, 1H), 7.27–7.37 (m, 14H), 7.42–7.48 (m, 3H), 7.59 (t, J = 6.8 Hz, 1H), 7.65 (s, 1H), 7.81–7.83 (m, 1H), 8.42 (d, J = 8 Hz, 1H), 8.56 (d, J = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 47.9, 47.5, 109.5, 120.5, 121.4, 125.7, 126.4, 126.5, 126.6, 127.1, 127.3, 127.4, 127.5, 127.8, 127.9, 128.0, 128.1, 128.8, 128.8, 129.0, 130.5, 133.9, 134.7, 135.4, 135.6, 135.7, 140.5, 144.1, 146.8, 150.6, 161.9, 167.7; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C37H27N3O2Na, 568.1995; found, 568.1987.
(E)-3-Benzyl-2-((1-benzyl-5-iodo-2-oxoindolin-3-ylidene)methyl)quinazolin-4(3H)-one (12ab)
It was obtained as a reddish orange solid, yield 85%. Rf = 0.55 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 4.93 (s, 2H), 5.52 (s, 2H), 6.47 (d, J = 8 Hz, 1H), 7.26–7.35 (m, 11H), 7.51–7.53 (m, 1H), 7.65 (s, 1H), 7.84–7.90 (m, 2H), 8.43 (dd, J1 = 8 Hz, J2 = 0.8 Hz, 1H), 8.85 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.4, 85.0, 111.2, 121.4, 122.2, 126.7, 127.2, 127.3, 127.5, 127.9, 128.0, 128.3, 128.9, 129.0, 132.6, 134.9, 135.0, 135.7, 136.8, 140.0, 144.3, 146.6, 150.0, 161.9, 167.1; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H22IN3O2Na, 618.0649; found, 618.0635.
(E)-2-((1-Benzyl-5-chloro-2-oxoindolin-3-ylidene)methyl)-3-(3-chlorobenzyl)quinazolin-4(3H)-one (12ac)
It was obtained as a reddish orange solid, yield 94%. Rf = 0.62 (TLC, Hex/EA, 1:1). 1H NMR (500 MHz, CDCl3): δ 4.95 (s, 2H), 5.49 (s, 2H), 6.61 (d, J = 8 Hz, 1H), 7.19–7.33 (m, 10H), 7.60 (s, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.82 (d, J = 8 Hz, 1H), 7.88 (t, J = 7.5 Hz, 1H), 8.36 (s, 1H), 8.43 (d, J = 8 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 44.0, 46.9, 110.2, 121.3, 121.3, 125.4, 126.3, 127.2, 127.4, 127.6, 127.6, 127.9, 127.9, 128.3, 128.5, 128.9, 130.3, 131.5, 133.2, 135.0, 135.2, 137.7, 143.3, 146.7, 149.8, 161.8, 161.3; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C31H21Cl2N3O2Na, 560.0903; found, 560.0895.
(E)-2-((1-Benzyl-5-methoxy-2-oxoindolin-3-ylidene)methyl)-3-(3-fluorobenzyl)quinazolin-4(3H)-one (12ad)
It was obtained as a reddish orange solid, yield 85%. Rf = 0.52 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.65 (s, 3H), 4.93 (s, 2H), 5.48 (s, 2H), 6.58 (d, J = 8.4 Hz, 1H), 6.77 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 6.92 (td, J1 = 8.4 Hz, J2 = 2 Hz, 1H), 7.02 (dt, J1 = 9.6 Hz, J2 = 1.6 Hz, 1H), 7.12 (d, J = 8 Hz, 1H), 7.26–7.33 (m, 6H), 7.55 (s, 1H), 7.59 (td, J1 = 7.6 Hz, J2 = 1.2 Hz, 1H), 7.79–7.82 (m, 2H), 7.84 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 1H), 8.42 (dd, J1 = 8 Hz, J2 = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 43.9, 47.1, 55.7, 109.8, 113.3, 114.2, 114.4, 114.9, 115,2, 117.6, 120.6, 121.2, 122.8, 122.9, 125.1, 127.2, 127.4, 127.5, 127.7, 128.2, 128.8, 130.5, 130.6, 134.6, 135.0, 135.5, 138.0, 138.1, 138.7, 146.8, 150.4, 155.4, 161.8, 161.8, 164.3, 167.4; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C32H24FN3O3Na, 540.1694; found, 540.1668.
(E)-2-((1-Benzyl-5-methoxy-2-oxoindolin-3-ylidene)methyl)-3-(2-methoxybenzyl)quinazolin-4(3H)-one (12ae)
It was obtained as a reddish orange solid, yield 92%. Rf = 0.55 (TLC, Hex/EA, 1:1). 1H NMR (400 MHz, CDCl3): δ 3.63 (s, 3H), 3.86 (s, 3H), 4.91 (s, 2H), 5.49 (s, 2H), 6.56 (d, J = 8.4 Hz, 1H), 6.75 (dd, J1 = 8.4 Hz, J2 = 2.4 Hz, 1H), 6.81 (t, J = 7.6 Hz, 1H), 6.86 (d, J = 8 Hz, 1H), 7.09 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H), 7.19 (td, J1 = 8 Hz, J2 = 1.6 Hz, 1H), 7.29–7.32 (m, 4H), 7.56 (td, J1 = 7.6 Hz, J2 = 1.6 Hz, 2H), 7.74 (d, J = 2.8 Hz, 2H), 7.78 (d, J = 7.4, 1H), 7.81 (td, J1 = 7.4 Hz, J2 = 1.2 Hz, 1H), 8.40 (dd, J1 = 7.8 Hz, J2 = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 42.9, 43.8, 55.4, 55.6, 109.6, 110.4, 112.9, 117.2, 120.7, 120.9, 120.9, 121.4, 123.5, 126.3, 126.3, 127.2, 127.4, 127.7, 127.9, 127.9, 128.8, 129.0, 134.0, 134.7, 135.6, 138.5, 146.8, 151.0, 155.3, 156.7, 161.9, 167.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H27N3O4, 552.1894; found, 552.1888.
Acknowledgments
This work was supported by grants from the DST-India in the form of INSPIRE Faculty (IFA-13, CH-116) and UGC Start-up grant from UGC-New Delhi to S.S.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01514.
Green chemistry matrix calculation for compound 12a; crystal data for 12a and 12c; and 1H NMR and 13C NMR spectra for all products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Mulla S. A. R.; Pathan M. Y.; Chavan S. S. A novel and efficient synthesis of azaarene-substituted 3-hydroxy-2-oxindoles via sp3 C-H functionalization of 2-methyl azaarenes and (2-azaaryl)methanes over a heterogeneous, reusable silica-supported dodecatungstophosphoric acid catalyst. RSC Adv. 2013, 3, 20281. 10.1039/c3ra43515f. [DOI] [Google Scholar]; b Rajeshkumar V.; Chandrasekar S.; Sekar G. An efficient route to synthesize isatins by metal-free, iodine-catalyzed sequential C(sp3)-H oxidation and intramolecular C-N bond formation of 2′-aminoacetophenones. Org. Biomol. Chem. 2014, 12, 8512. 10.1039/c4ob01564a. [DOI] [PubMed] [Google Scholar]; c Dong D.-Q.; Zhang H.; Wang Z.-L. Synthesis of benzyl esters from the commercially available alcohols catalyzed by TBAI via C(sp3)-H bond functionalization. RSC Adv. 2017, 7, 3780. 10.1039/c6ra26387a. [DOI] [Google Scholar]; d Fukumoto Y.; Hirano M.; Matsubara N.; Chatani N. Ir4(CO)12-Catalyzed Benzylic C(sp3)-H Silylation of 2-Alkylpyridines with Hydrosilanes Leading to 2-(1-Silylalkyl)pyridines. J. Org. Chem. 2017, 82, 13649. 10.1021/acs.joc.7b02375. [DOI] [PubMed] [Google Scholar]; e Venkatachalam R.; Selvaraj C.; Govindasamy S. An efficient route to synthesize isatins by metal-free, iodine-catalyzed sequential C(sp3)-H oxidation and intramolecular C-N bond formation of 2′-aminoacetophenones. Org. Biomol. Chem. 2014, 12, 8512. 10.1039/c4ob01564a. [DOI] [PubMed] [Google Scholar]; f Dong D.-Q.; Zhang H.; Wang Z.-L. Synthesis of benzyl esters from the commercially available alcohols catalyzed by TBAI via C(sp3)-H bond functionalization. RSC Adv. 2017, 7, 3780. 10.1039/c6ra26387a. [DOI] [Google Scholar]; g Chavan S. S.; Pathan M. Y.; Mulla S. A. R. Solvent free one-pot multi-component synthesis of β-azaarene substituted ketones via a Sn-catalyzed C(sp3)-H functionalization of 2-alkylazaarenes. RSC Adv. 2015, 5, 103091. 10.1039/c5ra20728b. [DOI] [Google Scholar]; h Kumar A.; Dutt Shukla R. β-Cyclodextrin catalysed C-C bond formation via C(sp3)-H functionalization of 2-methyl azaarenes with diones in aqueous medium. Green Chem. 2015, 17, 848. 10.1039/c4gc02287d. [DOI] [Google Scholar]; i Kumari K.; Allam B. K.; Singh K. N. A simple and sustainable tetrabutylammonium fluoride (TBAF)-catalyzed synthesis of azaarene-substituted 3-hydroxy-2-oxindoles through sp3 C-H functionalization. RSC Adv. 2014, 4, 19789. 10.1039/c3ra47332e. [DOI] [Google Scholar]; j Yaragorla S.; Singh G.; Dada R. “On water synthesis” of oxindoles bearing quaternary carbon center through C-H (sp3) functionalization of methyl azaarenes. Tetrahedron Lett. 2016, 57, 591. 10.1016/j.tetlet.2015.12.096. [DOI] [Google Scholar]; k Shukla R. D.; Rai B.; Kumar A. Exploration of Catalytic Activity of Trypsin for C(sp3)-H Functionalization and Consequent C-C Bond Formation. Eur. J. Org. Chem. 2019, 2019, 2864. 10.1002/ejoc.201900290. [DOI] [Google Scholar]; l Fukumoto Y.; Hirano M.; Chatani N. Iridium-Catalyzed Regioselective C(sp3)-H Silylation of 4-Alkylpyridines at the Benzylic Position with Hydrosilanes Leading to 4-(1-Silylalkyl)pyridines. ACS Catal. 2017, 7, 3152. 10.1021/acscatal.7b00539. [DOI] [PubMed] [Google Scholar]
- Abrams D. J.; Provencher P. A.; Sorensen E. J. Recent applications of C-H functionalization in complex natural product synthesis. Chem. Soc. Rev. 2018, 47, 8925–8967. , and references there cited in 10.1039/c8cs00716k. [DOI] [PubMed] [Google Scholar]
- a Giri R.; Lam J. K.; Yu J.-Q. Synthetic applications of Pd (II)-Catalyzed C-H carboxylation and mechanistic insights: expedient routes to anthranilic acids, oxazolinones, and quinazolinones. J. Am. Chem. Soc. 2010, 132, 686. 10.1021/ja9077705. [DOI] [PubMed] [Google Scholar]; b Xie J.; Huang Z.-Z. Cross-Dehydrogenative Coupling Reactions by Transition-Metal and Aminocatalysis for the Synthesis of Amino Acid Derivatives. Angew. Chem., Int. Ed. 2010, 49, 10181. 10.1002/anie.201004940. [DOI] [PubMed] [Google Scholar]; c Stowers K. J.; Fortner K. C.; Sanford M. S. Aerobic Pd-catalyzed sp3 C-H olefination: a route to both N-heterocyclic scaffolds and alkenes. J. Am. Chem. Soc. 2011, 133, 6541. 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Pitts C. R.; Bloom S.; Woltornist R.; Auvenshine D. J.; Ryzhkov L. R.; Siegler M. A.; Lectka T. Direct, Catalytic Monofluorination of sp3 C-H Bonds: A Radical-Based Mechanism with Ionic Selectivity. J. Am. Chem. Soc. 2014, 136, 9780. 10.1021/ja505136j. [DOI] [PubMed] [Google Scholar]; e Sha W.; Yu J.-T.; Jiang Y.; Yang H.; Cheng J. The benzoyl peroxide-promoted functionalization of simple alkanes with 2-aryl phenyl isonitrile. Chem. Commun. 2014, 50, 9179. 10.1039/c4cc03304c. [DOI] [PubMed] [Google Scholar]; f Wang B.; Qiu D.; Zhang Y.; Wang J. Recent advances in C(sp3)-H bond functionalization via metal-carbene insertions. Beilstein J. Org. Chem. 2016, 12, 796. 10.3762/bjoc.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Richter H.; Fröhlich R.; Daniliuc C.-G.; García Mancheño O. Mild Metal-Free Tandem α-Alkylation/Cyclization ofN-Benzyl Carbamates with Simple Olefins. Angew. Chem., Int. Ed. 2012, 51, 8656. 10.1002/anie.201202379. [DOI] [PubMed] [Google Scholar]; b Zhang B.; Cui Y.; Jiao N. Metal-free TEMPO-catalyzed oxidative C-C bond formation from Csp3-H bonds using molecular oxygen as the oxidant. Chem. Commun. 2012, 48, 4498. 10.1039/c2cc30684k. [DOI] [PubMed] [Google Scholar]; c Ochiai M.; Miyamoto K.; Kaneaki T.; Hayashi S.; Nakanishi W. Highly regioselective amination of unactivated alkanes by hypervalent sulfonylimino-λ3-bromane. Science 2011, 332, 448. 10.1126/science.1201686. [DOI] [PubMed] [Google Scholar]; d Pintr A.; Sud A.; Sureshkumar D.; Klussmann M. Autoxidative carbon-carbon bond formation from carbon-hydrogen bonds. Angew. Chem., Int. Ed. 2010, 49, 5004. 10.1002/anie.201000711. [DOI] [PubMed] [Google Scholar]
- a Herzon S. B.; Vanderwal C. D. Introduction: Natural Product Synthesis. Chem. Rev. 2017, 117, 11649–11650. , and references cited therein 10.1021/acs.chemrev.7b00520. [DOI] [PubMed] [Google Scholar]; b Li L.; Chen Z.; Zhang X.; Jia Y. Divergent Strategy in Natural Product Total Synthesis. Chem. Rev. 2018, 118, 3752–3832. 10.1021/acs.chemrev.7b00653. [DOI] [PubMed] [Google Scholar]; c Touré B. B.; Hall D. G. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009, 109, 4439–4486. 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; d Hooshmand S. E.; Heidari B.; Sedghi R.; Varma R. S. Recent advances in the Suzuki-Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. 10.1039/c8gc02860e. [DOI] [Google Scholar]
- a Lancaster M.Green Chemistry: An Introductory Text; Royal Society of Chemistry: Cambridge, U.K., 2002. [Google Scholar]; b Anastas P.; Heine L. G.; Williamson T. C.. Green Chemical Syntheses and Processes; Oxford University Press: New York. 2000. [Google Scholar]
- a Crabtree R. H.; Anastas P. T.. Handbook of Green Chemistry; Wiley-VCH, 2009. [Google Scholar]; b Horváth I. T. Solvents from nature. Green Chem. 2008, 10, 1024–1028. 10.1039/b812804a. [DOI] [Google Scholar]; c Bruckmann A.; Krebs A.; Bolm C. Organocatalytic reactions: effects of ball milling, microwave and ultrasound irradiation. Green Chem. 2008, 10, 1131. 10.1039/b812536h. [DOI] [Google Scholar]
- a Hosoe T.; Nozawa K.; Kawahara N.; Fukushima K.; Nishimura K.; Miyaji M.; Kawai K.-i. Isolation of a new potent cytotoxic pigment along with indigotin from the pathogenic basidiomycetous fungus Schizophyllum commune. Mycopathologia 1999, 146, 9–12. 10.1023/a:1007082619328. [DOI] [PubMed] [Google Scholar]; b Uehata K.; Kimura N.; Hasegawa K.; Arai S.; Nishida M.; Hosoe T.; Kawai K.-i.; Nishida A. Total synthesis of schizocommunin and revision of Its structure. J. Nat. Prod. 2013, 76, 2034–2039. 10.1021/np400263f. [DOI] [PubMed] [Google Scholar]; c Che T.; Chen S.-B.; Tu J.-L.; Wang B.; Wang Y.-Q.; Zhang Y.; Wang J.; Wang Z.-Q.; Zhang Z.-P.; Ou T.-M.; Zhao Y.; Tan J.-H.; Huang Z.-S. Discovery of novel schizocommunin derivatives as telomeric G-quadruplex ligands that trigger telomere dysfunction and the deoxyribonucleic acid (DNA) damage response. J. Med. Chem. 2018, 61, 3436–3453. 10.1021/acs.jmedchem.7b01615. [DOI] [PubMed] [Google Scholar]
- a Guedes P. M. d. M.; Urbina J. A.; de Lana M.; Afonso L. C. C.; Veloso V. M.; Tafuri W. L.; Machado-Coelho G. L. L.; Chiari E.; Bahia M. T. Activity of the new triazole derivative albaconazole against trypanosoma (Schizotrypanum) cruzi in dog hosts. Antimicrob. Agents Chemother. 2004, 48, 4286. 10.1128/aac.48.11.4286-4292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; b McLaughlin N. P.; Evans P.; Pines M. The chemistry and biology of febrifugine and halofuginone. Bioorg. Med. Chem. 2014, 22, 1993. 10.1016/j.bmc.2014.02.040. [DOI] [PubMed] [Google Scholar]; c Seger C.; Vajrodaya S.; Greger H.; Hofer O. Structure elucidation and synthesis of a new bioactive quinazolone derivative obtained from glycosmis Cf. chlorosperma. Chem. Pharm. Bull. 1998, 46, 1926. 10.1248/cpb.46.1926. [DOI] [Google Scholar]; d Kobayashi S.; Ueno M.; Suzuki R.; Ishitani H.; Kim H.-S.; Wataya Y. Catalytic asymmetric synthesis of antimalarial alkaloids febrifugine and isofebrifugine and their biological activity. J. Org. Chem. 1999, 64, 6833. 10.1021/jo990877k. [DOI] [PubMed] [Google Scholar]; e Köhne C.-H.; Thuss-Patience P.; Friedrich M.; Daniel P.; Kretzschmar A.; Benter T.; Bauer B.; Dietz R.; Dörken B. Raltitrexed (Tomudex): an alternative drug for patients with colorectal cancer and 5-fluorouracil associated cardiotoxicity. Br. J. Cancer 1998, 77, 973–977. 10.1038/bjc.1998.160. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Koohestani F.; Qiang W.; MacNeill A. L.; Druschitz S. A.; Serna V. A.; Adur M.; Kurita T.; Nowak R. A. Halofuginone suppresses growth of human uterine leiomyoma cells in a mouse xenograft model. Hum. Reprod. 2016, 31, 1540. 10.1093/humrep/dew094. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Zhang J.; Yao Q.; Liu Z. A novel synthesis of the efficient anti-coccidial drug halofuginone hydrobromide. Molecules 2017, 22, 1086. 10.3390/molecules22071086. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Kshirsagar U. A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336., references cited therein 10.1039/c5ob01379h. [DOI] [PubMed] [Google Scholar]
- a Liu J.-F.; Lee J.; Dalton A. M.; Bi G.; Yu L.; Baldino C. M.; McElory E.; Brown M. Microwave-assisted one-pot synthesis of 2,3-disubstituted 3H-quinazolin-4-ones. Tetrahedron Lett. 2005, 46, 1241–1244. 10.1016/j.tetlet.2005.01.008. [DOI] [Google Scholar]; b Yan J.-W.; Li Y.-P.; Ye W.-J.; Chen S.-B.; Hou J.-Q.; Tan J.-H.; Ou T.-M.; Li D.; Gu L.-Q.; Huang Z.-S. Design, synthesis and evaluation of isaindigotone derivatives as dual inhibitors for acetylcholinesterase and amyloid beta aggregation. Bioorg. Med. Chem. 2012, 20, 2527–2534. 10.1016/j.bmc.2012.02.061. [DOI] [PubMed] [Google Scholar]; c Lazzaro J. T.; Paternain A. V.; Lerma J.; Chenard B. L.; Ewing F. E.; Huang J.; Welch W. M.; Ganong A. H.; Menniti F. S. Functional characterization of CP-465,022, a selective, noncompetitive AMPA receptor antagonist. Neuropharmacology 2002, 42, 143–153. 10.1016/s0028-3908(01)00170-8. [DOI] [PubMed] [Google Scholar]; d Kaan H. Y. K.; Major J.; Tkocz K.; Kozielski F.; Rosenfeld S. S. “Snapshots” of Ispinesib-induced Conformational Changes in the Mitotic Kinesin Eg5. J. Biol. Chem. 2013, 288, 18588–18598. 10.1074/jbc.m113.462648. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yang Q.; Modi P.; Newcomb T.; Queva C.; Gandhi V. Idelalisib: first-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin. Cancer Res. 2015, 21, 1537–1542. 10.1158/1078-0432.ccr-14-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Agrawal R.; Jain P.; Dikshit S. N. Balaglitazone: a second generation peroxisome proliferator-activated receptor (PPAR) gamma (γ) agonist. Mini-Rev. Med. Chem. 2012, 12, 87–97. 10.2174/138955712798995048. [DOI] [PubMed] [Google Scholar]; g Haynes S. W.; Gao X.; Tang Y.; Walsh C. T. Assembly of asperlicin peptidyl alkaloids from anthranilate and tryptophan: a two-enzyme pathway generates heptacyclic scaffold complexity in asperlicin E. J. Am. Chem. Soc. 2012, 134, 17444–17447. 10.1021/ja308371z. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Clevenger K. D.; Ye R.; Bok J. W.; Thomas P. M.; Islam M. N.; Miley G. P.; Robey M. T.; Chen C.; Yang K.; Swyers M.; Wu E.; Gao P.; Wu C. C.; Keller N. P.; Kelleher N. L. Interrogation of Benzomalvin Biosynthesis Using Fungal Artificial Chromosomes with Metabolomic Scoring (FAC-MS): Discovery of a Benzodiazepine Synthase Activity. Biochemistry 2018, 57, 3237–3243. 10.1021/acs.biochem.8b00076. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Raffa D.; Daidone G.; Maggio B.; Cascioferro S.; Plescia F.; Schillaci D. Synthesis and antileukemic activity of new 3-(5-methylisoxazol-3-yl) and 3-(pyrimidin-2-yl)-2-styrylquinazolin-4(3H)-ones. Farmaco 2004, 59, 451–455. 10.1016/j.farmac.2003.10.006. [DOI] [PubMed] [Google Scholar]
- a Singh K.; Kaur A.; Mithu V. S.; Sharma S. Metal-free organocatalytic oxidative ugi reaction promoted by hypervalent iodine. J. Org. Chem. 2017, 82, 5285–5293. 10.1021/acs.joc.7b00594. [DOI] [PubMed] [Google Scholar]; b Singh K.; Malviya B. K.; Roy T. K.; Mithu V. S.; Bhardwaj V. K.; Verma V. P.; Chimni S. S.; Sharma S. Catalyst-controlled structural divergence: selective intramolecular 7-endo-dig and 6-exo-dig post-ugi cyclization for the synthesis of benzoxazepinones and benzoxazinones. J. Org. Chem. 2018, 83, 57–68. 10.1021/acs.joc.7b02123. [DOI] [PubMed] [Google Scholar]; c Singh K.; Malviya B. K.; Verma V. P.; Badsara S. S.; Bhardwaj V. K.; Sharma S. Cationic Pd(II) catalyzed regioselective intramolecular hydroarylation for the efficient synthesis of 4-aryl-2-quinolones. Tetrahedron 2019, 75, 2506–2520. 10.1016/j.tet.2019.03.026. [DOI] [Google Scholar]; d Kumar A.; Sharma S. A grinding-induced catalyst- and solvent-free synthesis of highly functionalized 1,4-dihydropyridines via a domino multicomponent reaction. Green Chem. 2011, 13, 2017–2020. 10.1039/c1gc15223h. [DOI] [Google Scholar]; e Sharma S.; Singh A. K.; Singh D.; Kim D.-P. Chemical fixation of carbon dioxide by copper catalyzed multicomponent reactions for oxazolidinedione syntheses. Green Chem. 2015, 17, 1404. 10.1039/c4gc02089h. [DOI] [Google Scholar]
- Vichai V.; Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112. 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- a Martin M. P.; Alam R.; Betzi S.; Ingles D. J.; Zhu J.-Y.; Schönbrunn E. A Novel Approach to the Discovery of Small-Molecule Ligands of CDK2. ChemBioChem 2012, 13, 2128–2136. 10.1002/cbic.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Molecular Operating Environment (MOE), 2015.10; Chemical Computing Group Inc.: 1010 Sherbrooke St. West, Suite 910, Montreal, QC, Canada, H3A 2R7, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






