Abstract

Owing to the rise in antimicrobial and chemotherapeutic drug resistance, there is a desperate need to formulate newer as well as more effective agents. With this perspective, here we outline the synthesis of two novel gemini surfactants with different substitutions at the nitrogen atom of the benzimidazolium ring. Both the compounds induced significant reductions in Candida growth in various yeast strains. The reduction in Candida growth seemed likely through the reduction in ergosterol biosynthesis: a sterol constituent of yeast cell membranes. Different concentrations of both compounds were used to determine the cellular ergosterol content which indicates an important disordering of the ergosterol biosynthetic pathway. Cytotoxic studies were carried out using HEK 293 (human embryonic-kidney cells) and Galleria mellonella larvae (an in vivo model of antimicrobial studies). Administration of both the compounds to G. mellonella larvae diseased by the yeast Candida albicans resulted in increased survival indicating their in vivo activity.
1. Introduction
One of the serious problems people currently face in daily life is their inexorable exposure to several bacteria, fungi, and many other micro-organisms. The emergence of resistant micro-organisms against available antimicrobial agents is a matter of serious concern.1 Persistent fungal infections are connected with higher death/mortality rates in immuno-compromised hosts, such as cancer patients, organ transplantation, and acquired immuno deficiency syndrome.2 The range of effective and safer antifungal agents is limited, despite thehigh death rate associated with fungal infections.3 Conventional antifungal agents, that is, fluconazole (FLC), caspofungin, and amphotericin B have some therapeutic limits like related drug toxicity, uneven bioavailability, and absence of oral/intravenous preparations. More significantly, increasing resistance to the majority of the clinically used antifungal agents has considerably minimized the outcome rate of antifungal therapy leading to higher mortality.4 Thus, the identification of harmless and effective antifungal agents to gear up challenges of increasing frequency/incidences of fungal infections is immediately required.4,5 Gemini surfactants are novel surfactants which have drawn much attention from both academic and industrial research communities and possess effective antimicrobial activity.6,7 These are amphiphilic in nature having two hydrophilic moieties as well as two hydrophobic tails joined by a spacer who may be stiff or stretchy.8−10 Gemini surfactants have improved physicochemical features like lesser critical micelle concentration (cmc), high efficiency of adsorption, better wetting and foaming ability, solubilizing power, as well as soap lime-dispersing properties,11−15 wetting agent, emulsifier, fabric softener, and foaming agents.16−19 Gemini surfactants are more efficient in reducing surface tension and form micelles comparatively at lower concentrations as compared to monomeric surfactants.20,21
The surfactants with the quaternary ammonium head group have been extensively used as antimicrobial agents over the decades.22,23 These surfactants kill the microorganisms mostly because of electrostatic and hydrophobic interactions on the cell surfaces24 by intercalating the cell membrane and changing its molecular organization, which increases membrane permeability and thus results in cytoplasmic diffusion and cell lysis.25 The cytotoxicity and green behavior of the gemini surfactants are of immense importance ahead of their use in any potential field.26−28 Although various reports are centered on their antimicrobial activities,29−31 the exact means of action is not yet completely understood. Consequently, the main aim of this study was to design and synthesize new gemini surfactants and evaluate their antifungal activity. Therefore, in this work, we have synthesized two novel benzimidazolium-based gemini surfactants 1,3-bis(1,1-dialkyl benzimidazolium)propane bromide expressed as BG8 and BG10, respectively, and characterized by Fourier transform infrared (FT-IR), NMR, and mass spectroscopy (Scheme S1). The purity of these surfactants was checked by high performance liquid chromatography. The physicochemical characterization was also performed using tensiometry given in the Supporting Information. The anti-candida studies for these compounds against three strains of Candida (Candida albicans, Candida glabrata, and Candida tropicalis) along with one fluconazole (FLC) resistant C. albicans strain were performed. The following Scheme 1 shows the probable mechanism of action of gemini benzimidazolium surfactants with yeast membrane.
Scheme 1. Probable Mechanism of Action of Gemini Benzimidazolium Surfactants with a Yeast Membrane.

2. Results and Discussion
2.1. Anti-candida Studies
2.1.1. In Vitro Anti-candida Activity
The anti-candida studies for these compounds against three strains of Candida (C. albicans, C. glabrata, and C. tropicalis) along with one FLC resistant C. albicans strain were performed and the results suggested that gemini benzimidazolium surfactants showed better anti-candida potential as compared to the reference drug FLC. Both surfactants, BG8 and BG10 also exhibited potent inhibitory activity against the resistant strain with minimum inhibitory concentration (MIC) value of 23.7 and 10.9 μM, respectively, indicating that BG10 is more effective against the resistant C. albicans strain compared with standard drug FLC (Table 1). BG8 exhibited a MIC value of 23.7 μM against all the three sensitive strains. Moreover, BG10 was observed to be more effective than that of BG8 and reference drug FLC 51 μM against C. tropicalis strains with a value of MIC 10.9 μM, respectively. Because it is renowned that C. albicans is to blame for 50–60% cases of the pathological condition of fungus infection (candidiasis),30,32 we concentrate our studies on the C. albicans strain. Based on the results obtained with standard Candida strains, these surfactants were further investigated against FLC-resistant C. albicans. Similar results were also reported by Ruiz et al.32 They reported that green cationic gemini surfactants Nα,Nω-bis(Nα,caproylarginine)α,ω-propyldiamide (C3(CA)2) and lichenysin exhibited antimicrobial synergies against C. albicans, Bacillus subtilis, Yersinia enterocolitica, and Escherichia coli, and observed that C3(CA)2-lichenysin exhibited lesser MIC values than the individual ones. Zhang et al. has also reported the antibacterial as well as antifungal activities of gemini surfactant monomers and copolymers with better MICs than the conventional surfactants.33
Table 1. MIC Values (in μM) of BG8 and BG10 against Different Strains of Candidaa.
| strains | BG8 | BG10 | FLC |
|---|---|---|---|
| C. albicans ATCC90028 | 23.7 | 21.8 | 51.0 |
| C. glabrata ATCC90030 | 23.7 | 10.9 | 25.5 |
| C. tropicalis ATCC750 | 23.7 | 10.9 | 51.0 |
| FLC-resistant C. albicans | 23.7 | 10.9 | 3265 |
C. albicans resistant = clinical isolates of C. albicans (FLC resistant); FLC = fluconazole.
2.1.2. Growth Curve
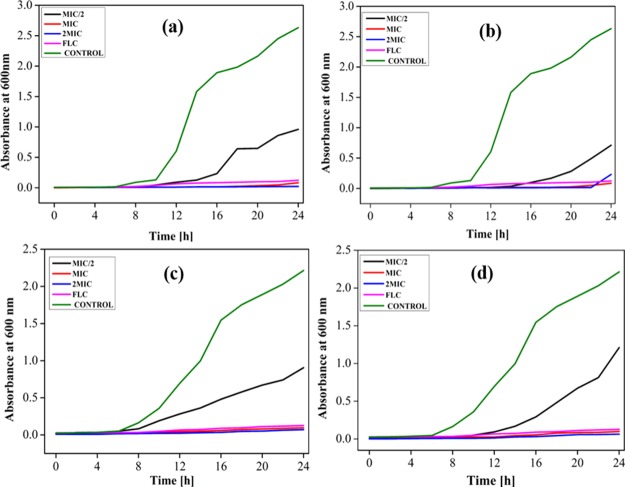
To investigate the impact of those potent surfactants (BG8 and BG10) on the expansion of Candida cells, growth curve studies were performed. Completely different concentrations of the test compounds (2MIC, MIC, and MIC/2) were exposed on the Candida cells (Figure 1a,b). The positive and negative controls were taken as FLC-treated cells and untreated cells, respectively. The Candida cells do not show any noteworthy growth once exposed to MIC and 2MIC concentrations of these surfactants with the incessant lag stage of 24 h. But, at sub-MIC concentrations of BG8 and BG10, growth has been found after 7 and 12 h, respectively, for a standard C. albicans strain. In the case of the FLC-resistant Candida strain, growth has been observed after 10 and 14 h when exposed to sub-MIC concentrations of BG8 and BG10, respectively. Moreover, FLC exhibited less efficacy in inhibiting the growth of FLC-resistant Candida cells than BG8 and BG10. The above results reveal that BG10 was observed to be a good inhibitor against Candida cell growth as compared to BG8 as shown in Figure 1c,d.
Figure 1.
Dose-dependent growth curve of C. albicans ATCC-90028 in the presence of (a) BG8 and (b) BG10, FLC-resistant C. albicans D15.9 in the presence of (c) BG8 and (d) BG10.
2.1.3. Synergistic Study
The in vitro synergistic activity of anti-candida in grouping with FLC was also performed against standard and FLC-resistant C. albicans strains. The outcomes revealed that anti-candida activity of each surfactant BG8 and BG10 against the standard C. albicans strain was significantly improved when used in a mixture with FLC. The fractional inhibitory concentration index (FICI) values of surfactants BG8 and BG10 were 0.253 and 0.127 against the standard C. albicans strain indicating moderate synergy of BG8 and a highly synergistic effect of BG10 (Table 2). Moreover, BG10 also exhibited potential synergistic activity with a FICI value of 0.250 against the FLC-resistant C. albicans strain while BG8 also shows synergy in combination with FLC in the same strain (Tables 2 and 3).35,36
Table 2. In Vitro Synergistic Anti-candida Activity of BG8.
| MIC alone
(μM) |
MIC in combination (μM) |
|||||
|---|---|---|---|---|---|---|
| compound | BG8 | FLC | BG8 | FLC | FICIa | mode of interaction |
| C. albicans (ATCC 90028) | 23.7 | 51.0 | 5.29 | 12.7 | 0.253 | synergy |
| C. albicans D15.9a | 23.7 | 3265 | 11.86 | 816 | 0.250 | synergy |
FICI = fractional inhibitory concentration index; synergy and antagonism were defined by FICI ≤ 0.5 and >4, respectively. Indifference was defined by 0.5 < FICI ≤ 4.
Table 3. In Vitro Synergistic Anti-candida Activity of BG10.
| MIC alone (μM) |
MIC in combination (μM) |
|||||
|---|---|---|---|---|---|---|
| compound | BG10 | FLC | BG10 | FLC | FICIa | mode of interaction |
| C. albicans (ATCC 90028) | 21.8 | 51.0 | 2.73 | 6.36 | 0.127 | synergy |
| C. albicans D15.9a | 10.9 | 3265 | 1.36 | 816 | 0.250 | synergy |
FICI = fractional inhibitory concentration index; synergy and antagonism were defined by FICI ≤ 0.5 and >4, respectively. Indifference was defined by 0.5 < FICI ≤ 4.
2.1.4. Ergosterol Biosynthesis in C. albicans
The effect of these compounds on ergosterol biosynthesis in C. albicans was examined in order to determine whether they affected cell membrane biosynthesis. The quantitative total sterol profiling in C. albicans ATCC 90028 was determined as described by an earlier stated method.37 FLC was taken as the positive control. The results clearly demonstrated a decrease in dose-dependent ergosterol content as cells were grown in variable concentrations of BG compounds. It can be inferred from the results that the exposure of cells to BG compounds with MIC and MIC/2 levels expressively inhibited ergosterol biosynthesis (Figure 2a,b). However, these compounds are comparatively less effective at low concentrations (MIC/4). Moreover, BG8 and BG10 inhibited the total ergosterol synthesis content more effectively than FLC at all the tested concentrations.
Figure 2.
UV spectrophotometric sterol profiles of C. albicans ATCC-90028 after treatment with various concentrations (MIC/4, MIC/2, and MIC) of test compounds (a) BG8 and (b) BG10.
2.2. In Vivo Studies
2.2.1. Toxicity of BG8 and BG10 Gemini Surfactants in Galleria mellonella Larvae
Further, to evaluate the in vivo toxicity of these compounds, larvae of Galleria mellonella were inoculated with 20 μL of BG8 and BG10, and viability was assessed over 96 h. At concentrations ranging from 3030 to 70 μM, these compounds were nontoxic in the G. mellonella larvae, yielding 100 ± 0% viability after 96 h (Figure 3a,c).
Figure 3.
Toxicity of BG8 (a) and BG10 (b) gemini surfactants in G. Mellonella larvae and antifungal efficacy of BG8 (c) and BG10 (d) gemini surfactants in G. Mellonella larvae.
2.2.2. Antifungal Efficacy of BG Compounds in the G. mellonella Larvae
To estimate the in vivo anti-candida efficiency of BG compounds, the G. mellonella larvae was infected by an inoculum of C. albicans (5 × 105 per 20 μL). One hour post infection, the larvae were administered with 20 μL of BG compounds (3030–70 μM) or 20 μL of dimethyl sulphoxide (DMSO) (10% v/v) control, and viability was assessed over 96 h. BG8 at 76 μM increased the viability of the G. mellonella larvae to 76.66 ± 3.33% at 48 h and 53.00 ± 3.33% compared to the respective DMSO-injected controls (48 h; 66.66 ± 3.33%, 72 h; 33.33 ± 6.66%). BG8 at concentrations ranging from 70 to 3030 μM significantly increased the viability of the G. mellonella larvae following with C. albicans infection. Concentrations of BG10 of 70–3030 μM significantly increased larval viability at 48 h (100 ± 0%, p > 0.01). A concentration of 151 μM increased larval viability at 72 h (76.66 ± 3.33%, p > 0.05), as compared to the relative DMSO control (33.33 ± 6.66%). BG10 was effective in blocking C. albicans-induced larval mortality at 48 h (100% ± 0%, p > 0.01) Figure 3b,d. The above outcomes indicate that administration of BG8 and BG10 to the larvae produced no adverse effects (e.g., melanisation) or death which is a good indication that they may be nontoxic in mammals. Administration of compounds to larvae infected with C. albicans resulted in increased survival, indicating in vivo activity. This has been reported previously with novel antimicrobial compounds38,39 and a strong relationship among the in vivo activity of compounds in larvae and rats has previously been demonstrated.40
2.3. In Silico Studies
The effect of these compounds on ergosterol biosynthesis was further confirmed by in silico studies in which both gemini surfactants were docked with an enzyme lanosterol 14-α demethylase (CYP51) member of the cytochrome P-450 super family that catalyzes the oxidative elimination of the 14-α alkyl group from lanosterol in the ergosterol biosynthesis pathway. The inhibition of CYP51 directly depleted ergosterol followed by the inhibition of yeast growth and acts as a primary target of azoles as antifungal drugs. The CYP51 crystal structure from pathogenic yeast C. albicans was recently published by the group of Keniya et al. and submitted to RCSB protein data bank (PDB ID: 5v5z).41 The interaction and binding energy of inhibitors BG8 and BG10 with CYP51 were identified using molecular docking (Figure 4), and the docked results were compared with reference drug itraconazole. Both gemini surfactants contain the benzimidazolium group flanked by long alkyl hydrophobic side chains (C8H17 in BG8 and C10H21 in BG10). The docking studies revealed that BG8 was bound close to the heme group in the active site of CYP51 interacting with active site residues such as Phe-449, Ile-471, Gln-142, Tyr-132, Leu-376, and forms a hydrogen bond with His-468 with a binding affinity of −7.7 kcal mol–1. While as BG10 was bound to residues Tyr-447, Phe-105, Glu-115, Arg-469, His-310, and a hydrogen bond with GLY-303 with a binding affinity of −8.3 kcal mol–1 as compared to itraconazole shows −9.6 kcal mol–1.34 Thus, the docking results support BG8, BG10 binding with the 14-α demethylase enzyme (CYP51) and inhibiting ergosterol biosynthesis. The probable mechanism of action for gemini benzimidazolium surfactants with the yeast membrane is shown in Scheme 1.
Figure 4.

Cartoon model, 2D and ligplot of (A) CYP51–BG8 and (B) CYP51–BG10 complexes.
2.4. Toxicity
2.4.1. Hemolytic Assay
The toxicity of surfactants BG8 and BG10 on human red blood cells (hRBCs) were observed to evaluate the hemolytic assay. At 1510 μM concentration, BG8 and BG10 showed reasonable toxicity triggering only 28 and 33% hemolysis, respectively, as compared to the reference drug FLC with 78% hemolysis. However, at 152 μM concentration, these surfactants show low toxicity triggering only 13% (BG8) and 16% (BG10) cell lysis. At 38 μM concentration, BG8 and BG10 are totally harmless causing only 4 and 8% cell lysis, respectively. Thus, these potent anti-candida geminis exhibited less toxicity than the reference drug FLC at each tested concentration extending from 38 to 1510 μM showing an extra protection index, as compared to reference drug FLC (Figure 5a).
Figure 5.

Hemolytic assays (a) and cell viability assay (b) of BG8 and BG10.
2.4.2. Cytotoxicity Assay
The selected compounds were further evaluated for cytotoxicity by the (3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay on the noncancerous human cell line (HEK293), which is a specific cell line initially resulting from the cells of the human embryonic kidney grown by a tissue culture. HEK 293 cells are widely used in cell biological research over decades, because of their consistent growth and inclination for transfection.42−44 The selected compounds were screened in the concentration range of 0–160 μM, and it was found that even at 160 μM, compounds BG8 and BG10 do not disrupt the feasibility of HEK293 in a significant manner. These results clearly advocated that the selected compounds are noncytotoxic to HEK293 cells in the tested concentration range. Further, these results speculate that these compounds might be used as potential antifungal molecules, as in the tested sub-micromolar concentration range, these exhibited a noncytotoxic nature towards normal human cells (Figure 5b).30
3. Conclusions
Two benzimidazolium-based gemini surfactants BG8 and BG10 have been synthesized and were well characterized by FT-IR, 1H NMR, 13C NMR, and mass spectroscopy. Moreover, their physicochemical characterization was also done using a surface tension method to evaluate various interfacial parameters viz: Γmax, pC20, Amin, and P (packing parameter). The anti-candida studies for these compounds along with reference drug, FLC against three strains of Candida (C. albicans, C. glabrata, and C. tropicalis) were performed and the results suggested that gemini BG surfactants showed better anti-candida potential as compared to the reference drug. BG8 and BG10 exhibit potent inhibitory activity against the resistant strain with MIC values of 23.7 and 10.9 μM, respectively, indicating that BG10 is more effective against the resistant C. albicans strain compared with standard drug FLC. Administration of these compounds to larvae infected with C. albicans resulted in increased survival indicating in vivo activity. The consequence of these compounds on ergosterol biosynthesis in C. albicans was examined to determine affected cell membrane biosynthesis and the results confirmed a decrease in dose-dependent ergosterol content once the cells had been grown in variable concentrations of test compounds. Our docking studies further confirmed the ergosterol biosynthesis in C. albicans, revealing that both BG8 and BG10 were bound close to the heme group in the active site of CYP51 with high binding affinities. The MTT assay with noncancerous human cell line (HEK293) results indicated that the selected compounds are noncytotoxic to the HEK293 cells in the tested concentration range.
4. Experimental Section
4.1. Materials
Benzimidazole (purity 99% Sigma-Aldrich USA), 1-bromooctane, and 1-bromodecane (purity 99% Sigma-Aldrich USA). The reagents were used without any extra purification. Milli-Q H2O with specific conductivity <1.4 μS cm–1 was used throughout the experiments.
4.2. Synthesis
1,3-Bis(1-octylbenzimidazolium)propane bromide (C34H53Br2N4) simply represented as BG8 and 1,3-bis(1-decylbenzimidazolium)propane bromide (C38H63Br2N4) represented as BG10 were synthesized by the procedure given in the Supporting Information. The FT-IR, NMR and mass spectra were used to confirm the structures of all the products.
The spectral results of FT-IR, 1H NMR, 13CNMR, and mass are given below. (Figures S1–S6)
4.2.1. BG8 Gemini 1,3-Bis(1-octylbenzimidazolium)propane Bromide (C34H53Br2N4)
FT-IR (4000–600 cm–1 ATR tablet) 3050 cm–1 [υ(Ar-H)], 2915 cm–1 [υ(CH3)], 2848 cm–1 [υ(CH2)], 2031 cm–1 [υ(π)] that is exocyclic C=C vibration, 1410 cm–1 [υ(C=N)], 1349 cm–1 [υ(C=C bending)], 1192 cm–1 [υ(C–C bending)], 888 cm–1 [υ(C–H bending)] and at 3324 cm–1 is due to the absorbed water.
1H NMR (CDCl3-δ = 7.5 ppm) δ = 0.75 ppm (t, 6H), 1.25 (s, 4H), 1.6 (m, 16H), 3.6 (q, 4H), 4.5 (t, 4H), 5.8 (d, 4H), 7.8 (m, 4H), 9.2 (d, 4H), 10.2 (s, 2H), 10.9(t, 4H).
13C NMR (CDCl3-δ = 80 ppm) δ = 13.94 ppm (CH3), 22.4 (CH2), 28.90 (CH2), 30.10 (CH2), 32.0 (CH2), 50 (CH2), 115.9 (CH), 127.2 (C attached at the bridged position), 131.2 (CH of the six-membered ring), 139.9 (CH2), 140 (CH=).
ESI-MS (LCMS): C33H50N4Br2 found 649, calcd 660; C26H35N4Br2 539, calcd 557; C7H15 112, calcd 99.
4.2.2. BG10 Gemini 1,3-Bis(1-decylbenzimidazolium)propane Bromide (C38H63Br2N4)
FT-IR (4000–600 cm–1 ATR tablet) 3022 cm–1 [υ(Ar-H)] 2922 cm–1 [υ(CH3)]2853 cm–1 [υ(CH2)] 1870 cm–1 [υ(π)] that is exocyclic C=C vibration, 1559 cm–1 [υ(C=N)], 1452 cm–1 [υ(C=C bending)], 1209 cm–1 [υ(C–C bending)], 752 cm–1 [υ(C–H bending)] and 3401 and 1616 cm–1 is due to the O–H stretching and bending of absorbed water.
1H NMR (CDCl3-δ = 7.5 ppm) δ = 0.75 ppm (t, 6H), 1.5 (m, 4H), 2.0 (m, 20H), 3.6 (q, 4H), 4.5 (t, 4H), 5.2 (s, 2H), 7.5 (q, 4H), 8.6 (d, 2H), 10.2 (d, 2H), 10.75 (t, 4H).
13C NMR (CDCl3-δ = 80 ppm) δ = 13.97 ppm (CH3), 22.51 (CH2), 29.23 (CH2), 30.1 (CH2), 45.73 (CH2), 47.83 (CH2), 114.79 (CH2 attached to positively charged nitrogen), 115.28 (CH), 127.6 (CH), 130.82 (C attached at the bridged position), 141.52 (CH=).
ESI-MS (LCMS): C37H58N4Br2 found 721, calcd 718; C28H39N4Br2 557, calcd 585; C7H15 110, calcd 110.7.
4.3. Physicochemical Characterization
The physicochemical characterization of two newly synthesized gemini surfactants was done by using surface tensiometry (more details are given in the Supporting Information). The different interfacial parameters viz cmc, πcmc, pC20, Γmax, Amin, and P were evaluated by using different equations and are given in Table 4.10,16,17 In addition, the cmc values were also confirmed by UV-visible and pyrene probe fluorescence spectroscopy and given in the Supporting Information (Figure S9).
Table 4. Surface Active Parameters of BG8 and BG10.
| surfactants | cmc (mM) | γcmc (mN m–1) | πcmc (mN m–1) | pC20 | Γmax (103 mol m–2) | Amin (nm2) | P |
|---|---|---|---|---|---|---|---|
| BG8 | 13 | 35.75 | 39.6 | 1.57 | 2.22 | 0.74 | 0.45 |
| BG10 | 1.5 | 32.4 | 40.4 | 3.35 | 1.58 | 1.05 | 0.32 |
4.4. In Vitro Anti-candida Activity
Both the surfactants, BG8 and BG10 were investigated for their in vitro anti-candida activity against C. albicans (ATCC 90028), C. glabrata (ATCC 90030), C. tropicalis (ATCC 750), and FLC resistant C. albicans D15.9 strains by using the broth dilution procedure according to the quality protocol for antifungal assessment by NCCLS (National Committee for Clinical Laboratory Standards).45 FLC (3.26 μM) was used as a positive control for the anti-candida study. The surfactant solutions were made in DMSO and diluted serially in a broth medium to attain the desired concentration of DMSO less than 1%. Variable concentrations of surfactants (1500–10.9 μM) were distributed into a 96 well plate in a Sabouraud dextrose (SD) broth medium in a 100 μL final volume. Further, 100 μL of yeast cells (approximate 2.5 × 103 cells/mL using (McFarland standard)45 were distributed into the 96 well plate (Tarson) with incubation at 37 °C for 24 h. During the incubated period each well was investigated for the attendance or absence of visual growth of yeast cells. The lowermost concentration of the tested compound on which no observable growth occurs characterizes its MIC value. Moreover, the growth was observed turbidometrically at 600 nm using a ThermoMultiskan Go spectrophotometer after incubation.
4.5. Growth Curve Studies
The cells of C. albicans were revived freshly by a subculture on a SD agar plate. A loopful of inoculum was introduced into the SD broth, finally the cells were grown for 16 h at 37 °C before use. About 2 × 103 cells/mL were injected into the freshly prepared 50 mL sterile SD medium. Various concentrations equal to MIC, MIC/2, and 2MIC of the surfactants were separately added into the conical flasks comprising of the inoculated medium, which was incubated at 37 °C and 160 rpm. Strain specific concentration of FLC was used as positive control of 102 μM for C. albicans ATCC90028 and 3260 μM C. albicans D15.9 (FLC resistant), respectively. At prearranged time periods (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 after incubation with agitation at 37 °C), 1 mL aliquot from each sample in a conical flask was removed and growth was observed turbidometrically at 600 nm using a Thermo Multiskan spectrophotometer. The optical densities were recorded against each concentration with time (hour).
4.6. FIC Index/Synergistic Activity
The fractional inhibitory concentration (FIC) index or synergistic activity of BG8 and BG10 with FLC was determined against C. albicans. Synergy and antagonism were defined by FIC indices of <0.5 and >4, respectively. The FIC index result of > 0.5, but < 4 was considered indifferent. Both surfactants BG8 and BG10 were serially diluted in growth medium with concentration ranging from 95 to 0.04 μM against C. albicans ATCC90028 and 62.5–0.03 μM of FLC, respectively. The MIC values of compound alone and in combination with FLC were determined by broth micro dilution method in 96 well plate. FIC index was calculated by using the following eq 1(34)
| 1 |
4.7. Ergosterol Biosynthesis in C. albicans
The whole intracellular sterols were estimated as reported earlier using standard C. albicans ATCC 90028. Three distinct conical flasks containing compounds BG8 and BG10 at MIC, MIC/2, and MIC/4 in the SD broth injected with freshly cultured cells of C. albicans ATCC 90028 were used. FLC (130 μM) was taken as the positive control and untreated cells were taken as the negative control for comparison and conical flasks were incubated at 35 °C for 16 h. The cells were harvested at their stationary phase after incubation and the weight of the pellet was determined. The pellet was treated with KOH solution (25% alcoholic potassium hydroxide) followed by incubation at 85 °C for 1 h. After incubation, sterol was removed by the addition of n-heptane/distilled water (1:3 ratio). The layers of heptane were moved into the fresh test tubes diluted five-fold in 100% ethanol and observed spectrophotometrically in the range of 240–300 nm. The existence of ergosterol and late-sterol intermediate DHE in the extracted samples shows a characteristic four peak curve.46,47
4.8. Assessment of Anti-candida Compound Toxicity and Efficacy in G. mellonella
Sixth instar larvae of the G. mellonella greater wax: moth (Live foods Direct Ltd. Sheffield, England) were kept in the dark at 15 °C to avoid pupation. Larvae which weigh 0.22 ± 0.03 g were selected and used within 2 weeks of receiving. 10 healthy larvae per treatment and controls (n = 3) were positioned in sterile 9 cm Petri dishes creased with Whatman filter paper with some wood shavings. Larvae were adjusted to 30 °C for 1 h prior to all experiments and incubated at 30 °C for all studies. All experiments were done independently at three distinct times. For toxicity studies, larvae were injected with 20 μL of BG8 and BG10 (3030–76 μM) by last left pro-leg, and reaction to stimuli was measured every 24 h. To assess the antifungal efficacy of BG8 and BG10 a culture of Candida albicans were grown to the immobile phase (nearly 2 × 108/mL) in the YEPD broth (2% w/v glucose, 2% w/v bactopptone, 1% w/v yeast extract) at 30 °C and 200 rpm. By centrifugation, (2056g for 5 min) the cells were harvested by using a GS-6 Beckmann bench-centrifuge and washed in phosphate-buffered saline (PBS) three times and resuspended again in PBS at 5 × 105/20 μL. The larvae were injected with yeast cells via the last left pro-leg into the hemocoel with a Myjector U-100 insulin syringe (Terumo. Europe. N.V., Belgium) and positioned at 30 °C in the dark. One hour post injection, larvae were injected with compounds BG8 and BG10 at varying concentrations resuspended in PBS added with 10% DMSO (v/v) through the last right pro-leg. Infected larvae were injected with 20 μL PBS supplemented with 10% DMSO (v/v). To assess larval viability, larvae which did not respond to touch stimulus were considered dead.
4.9. Hemolytic Assay
The haemolytic activity of the surfactants BG8 and BG10 and the conventional antifungal drug FLC were determined on human red blood cells (hRBCs). Human erythrocytes from healthy individuals were collected in tubes containing EDTA as an anticoagulant. The erythrocytes were harvested by centrifugation for 10 min at 2000 rpm and 20°C, and washed three times in phosphate buffered saline (PBS). To the pellet, PBS was added to yield a 10% (v/v) erythrocytes/PBS suspension. The 10% suspension of erythrocytes was then further diluted with PBS at a 1:10 ratio. 100 μL of the final diluted erythrocytes was added to 100 μL of PBS having a previously determined concentration gradient (38 μM to 1.51 mM) of the test compounds in microcentrifuge tubes. Total hemolysis was attained in 1% Triton X-100. The tubes were incubated for 1 h at 37 °C and then centrifuged for 10 min at 2000 rpm at room temperature. From the supernatant fluid, 150 μL was transferred to a flat bottomed microtiter plate (Tarson), and the absorbance was measured spectrophotometrically at 450 nm by using a Thermo Multiskan spectrophotometer. The hemolysis percentage was calculated by following eq 2(37)
| 2 |
4.10. Cytotoxicity by MTT Assay
HEK293 cell lines were procured from the National Centre for Cell Sciences (NCCS) Pune, India. Dulbecco’s modified Eagle’s medium (DMEM), antibiotic cocktail, and fetal bovine serum (FBS) were procured from Gibco Life Technologies. Thermo Fisher Scientific (USA). MTT and trypsin-EDTA solution has been purchased from Sigma (St. Louis, MO). HEK293 cells has been cultured and maintained in DMEM media enriched with 10% heat inactivated FBS and 1% penicillin, streptomycin solution at 37 °C in a humidified atmosphere of 5% CO2. Cell cultures have been routinely preserved and trypsinized not beyond 30 passages. HEK293 cells were broadcasted in triplicate in 96-well plate containing a cell count of approximately 9000–10 000 cells/well and incubated in a CO2 incubator for 24 h. Then, the cells were incubated with increasing concentrations of test compounds (5–250 μM) in a final volume of 200 μL for 48 h at 37 °C in a CO2 incubator. The mixture of the culture medium and compounds were removed after 48 h of incubation at 37 °C and cells have been washed two times with a PBS (pH 7.4) solution. After that, freshly prepared 20 μL MTT at a concentration of 48.27 mM in PBS and 100 μL of DMEM has been added to each well and the plates were incubated for 4–5 h at 37 °C in the CO2 incubator. DMSO (150 μL/well) was added to solubilize the formazan crystals, the metabolized MTT product, and was allowed a short incubation of 10 min at 27 °C. The absorbance was noted at 570 nm on the multi-plate ELISA reader (Bio-Rad, USA). % viability was taken as the comparative absorbance of treated versus untreated control cells and plotted as a function of concentration of compounds. All the results were obtained in triplicate.
4.11. Statistical Analysis
The data were evaluated as one-way analysis of variance (AVOVA) to detect the mean values observed for control and after treatment with compounds. Dunnett’s test was used to compare the treatment, control and statistical significance was set at P ≤ 0.01.
Acknowledgments
R.P. thanks the Science and Engineering Research Board (SERB), New Delhi for providing research grant with Sanction order nos. (SB/EMEQ-097/2013 and EEQ/2016/000339). The authors also thank DST for providing the FIST grant with Sanction order no. (SR/FIST/LS-541/2012).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01056.
Experimental protocol of physicochemical characterization and docking studies (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Samosorn S.; Tanwirat B.; Muhamad N.; Casadei G.; Tomkiewicz D.; Lewis K.; Suksamrarn A.; Prammananan T.; Gornall K. C.; Beck J. L.; Bremner J. B. Antibacterial activity of berberine-NorA pump inhibitor hybrids with a methylene ether linking group. Bioorg. Med. Chem. 2009, 17, 3866–3872. 10.1016/j.bmc.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. D.; Denning D. W.; Levitz S. M.. Tackling Human Fungal Infections; American Association for the Advancement of Science, 2012. [DOI] [PubMed] [Google Scholar]
- Lai C.-C.; Tan C.-K.; Huang Y.-T.; Shao P.-L.; Hsueh P.-R. Current challenges in the management of invasive fungal infections. J. Infect. Chemother. 2008, 14, 77. 10.1007/s10156-007-0595-7. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A.; Diekema D. J.; Rinaldi M. G.; Barnes R.; Hu B.; Veselov A. V.; Tiraboschi N.; Nagy E.; Gibbs D. L. Results from the ARTEMIS DISK Global Antifungal Surveillance Study: a 6.5-year analysis of susceptibilities of Candida and other yeast species to fluconazole and voriconazole by standardized disk diffusion testing. J. Clin. Microbiol. 2005, 43, 5848–5859. 10.1128/jcm.43.12.5848-5859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva J. A.; Pereira J. M. New antifungal antibiotics. Curr. Opin. Infect. Dis. 2013, 26, 168–174. 10.1097/qco.0b013e32835ebcb7. [DOI] [PubMed] [Google Scholar]
- Patel R.; Mir M. U. H.; Maurya J. K.; Singh U. K.; Maurya N.; Parray M. u. d.; Khan A. B.; Ali A. Spectroscopic and molecular modelling analysis of the interaction between ethane-1,2-diyl bis(N,N-dimethyl-N-hexadecylammoniumacetoxy)dichloride and bovine serum albumin. Luminescence 2015, 30, 1233–1241. 10.1002/bio.2886. [DOI] [PubMed] [Google Scholar]
- Sharma T.; Dohare N.; Kumari M.; Singh U. K.; Khan A. B.; Borse M. S.; Patel R. Comparative effect of cationic gemini surfactant and its monomeric counterpart on the conformational stability and activity of lysozyme. RSC Adv. 2017, 7, 16763–16776. 10.1039/c7ra00172j. [DOI] [Google Scholar]
- Bhadani A.; Singh S. Synthesis and properties of thioether spacer containing gemini imidazolium surfactants. Langmuir 2011, 27, 14033–14044. 10.1021/la202201r. [DOI] [PubMed] [Google Scholar]
- Menger F. M.; Keiper J. S. Gemini surfactants. Angew. Chem., Int. Ed. 2000, 39, 1906–1920. . [DOI] [PubMed] [Google Scholar]
- Patel R.; Khan A. B.; Dohare N.; Maroof Ali M.; Rajor H. K. Mixed micellization and interfacial properties of ionic liquid-type imidazolium gemini surfactant with amphiphilic drug amitriptyline hydrochloride and its thermodynamics. J. Surfactants Deterg. 2015, 18, 719–728. 10.1007/s11743-015-1709-3. [DOI] [Google Scholar]
- Leclercq L.; Noujeim N.; Schmitzer A. R. Development ofN,N′-Diaromatic Diimidazolium Cations: Arene Interactions for Highly Organized Crystalline Materials. Cryst. Growth Des. 2009, 9, 4784–4792. 10.1021/cg900630a. [DOI] [Google Scholar]
- Kim S. S.; Zhang W.; Pinnavaia T. J. Ultrastable mesostructured silica vesicles. Science 1998, 282, 1302–1305. 10.1126/science.282.5392.1302. [DOI] [PubMed] [Google Scholar]
- Tieke B. Polymerisation of styrene in microemulsion with catanionic surfactant mixtures. Colloid Polym. Sci. 2005, 283, 421–430. 10.1007/s00396-004-1168-2. [DOI] [Google Scholar]
- Zana R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv. Colloid Interface Sci. 2002, 97, 205–253. 10.1016/s0001-8686(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Kamboj R.; Singh S.; Bhadani A.; Kataria H.; Kaur G. Gemini imidazolium surfactants: synthesis and their biophysiochemical study. Langmuir 2012, 28, 11969–11978. 10.1021/la300920p. [DOI] [PubMed] [Google Scholar]
- Maurya J. K.; Mir M. U. H.; Singh U. K.; Maurya N.; Dohare N.; Patel S.; Ali A.; Patel R. Molecular investigation of the interaction between ionic liquid type gemini surfactant and lysozyme: A spectroscopic and computational approach. Biopolymers 2015, 103, 406–415. 10.1002/bip.22647. [DOI] [PubMed] [Google Scholar]
- Wani F. A.; Khan A. B.; Alshehri A. A.; Malik M. A.; Ahmad R.; Patel R. Synthesis, characterization and mixed micellization study of benzene sulphonate based gemini surfactant with sodium dodecyl sulphate. J. Mol. Liq. 2019, 285, 270–278. 10.1016/j.molliq.2019.04.057. [DOI] [Google Scholar]
- Patel R.; Mir M. U. H.; Singh U. K.; Beg I.; Islam A.; Khan A. B. Refolding of urea denatured cytochrome c : Role of hydrophobic tail of the cationic gemini surfactants. J. Colloid Interface Sci. 2016, 484, 205–212. 10.1016/j.jcis.2016.09.004. [DOI] [PubMed] [Google Scholar]
- ud din Parray M.; Maurya N.; Wani F.; Ahmad Wani M. S.; Arfin N.; Ahmad Malik M.; Patel R. Comparative effect of cationic gemini surfactant and its monomeric counterpart on the conformational stability of phospholipase A2. J. Mol. Struct. 2019, 1175, 49–55. 10.1016/j.molstruc.2018.07.078. [DOI] [Google Scholar]
- Matsuoka K.; Chiba N.; Yoshimura T.; Takeuchi E. Effect of double quaternary ammonium groups on micelle formation of partially fluorinated surfactant. J. Colloid Interface Sci. 2011, 356, 624–629. 10.1016/j.jcis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Pei X.; Zhao J.; Wei X. Wormlike micelles formed by mixed cationic and anionic gemini surfactants in aqueous solution. J. Colloid Interface Sci. 2011, 356, 176–181. 10.1016/j.jcis.2010.12.065. [DOI] [PubMed] [Google Scholar]
- Martins L. M. S.; Mamizuka E. M.; Carmona-Ribeiro A. M. Cationic vesicles as bactericides. Langmuir 1997, 13, 5583–5587. 10.1021/la970353k. [DOI] [Google Scholar]
- Wong Y.-L.; Hubieki M. P.; Curfman C. L.; Doncel G. F.; Dudding T. C.; Savle P. S.; Gandour R. D. A structure-activity study of spermicidal and anti-HIV properties of hydroxylated cationic surfactants. Bioorg. Med. Chem. 2002, 10, 3599–3608. 10.1016/s0968-0896(02)00245-6. [DOI] [PubMed] [Google Scholar]
- Maisuria B. B.; Actis M. L.; Hardrict S. N.; Falkinham J. O.; Cole M. F.; Cihlar R. L.; Peters S. M.; Macri R. V.; Sugandhi E. W.; Williams A. A.; Poppe M. A.; Esker A. R.; Gandour R. D. Comparing micellar, hemolytic, and antibacterial properties of di- and tricarboxyl dendritic amphiphiles. Bioorg. Med. Chem. 2011, 19, 2918–2926. 10.1016/j.bmc.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Ding M.; He X.; Wang Z.; Li J.; Tan H.; Deng H.; Fu Q.; Gu Q. Cellular uptake of polyurethane nanocarriers mediated by gemini quaternary ammonium. Biomaterials 2011, 32, 9515–9524. 10.1016/j.biomaterials.2011.08.074. [DOI] [PubMed] [Google Scholar]
- Falsini S.; Ristori S.; Ciani L.; Di Cola E.; Supuran CT; Arcangeli A.; In M. Time resolved SAXS to study the complexation of siRNA with cationic micelles of divalent surfactants. Soft Matter 2014, 10, 2226–33. 10.1039/c3sm52429a. [DOI] [PubMed] [Google Scholar]
- In S.; Ciani L.; Candiani G.; Battistini C.; Frati A.; Grillo I.; In M. Complexing a small interfering RNA with divalent cationic surfactants. Soft Matter 2012, 8, 749–756. 10.1039/c1sm06470c. [DOI] [Google Scholar]
- Muzzalupo R.; Infante M. R.; Pérez L.; Pinazo A.; Marques E. F.; Antonelli M. L.; Strinati C.; La Mesa C. Interactions between gemini surfactants and polymers: Thermodynamic studies. Langmuir 2007, 23, 5963–5970. 10.1021/la063244r. [DOI] [PubMed] [Google Scholar]
- Brycki B.; Szulc A. Gemini alkyldeoxy-D-glucitolammonium salts as modern surfactants and microbiocides: synthesis, antimicrobial and surface activity, biodegradation. PLoS One 2014, 9, e84936 10.1371/journal.pone.0084936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obłąk E.; Piecuch A.; Krasowska A.; Łuczyński J. Antifungal activity of gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. 10.1016/j.micres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Tan H.; Xiao H. Synthesis and antimicrobial characterization of novel l-lysine gemini surfactants pended with reactive groups. Tetrahedron Lett. 2008, 49, 1759–1761. 10.1016/j.tetlet.2008.01.079. [DOI] [Google Scholar]
- Ruiz A.; Pinazo A.; Pérez L.; Manresa A.; Marqués A. M. Green Catanionic Gemini Surfactant-Lichenysin Mixture: Improved Surface, Antimicrobial, and Physiological Properties. ACS Appl. Mater. Interfaces 2017, 9, 22121–22131. 10.1021/acsami.7b03348. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Ding M.; Zhou L.; Tan H.; Li J.; Xiao H.; Li J.; Snow J. Synthesis and antibacterial characterization of gemini surfactant monomers and copolymers. Polym. Chem. 2012, 3, 907–913. 10.1039/c2py00558a. [DOI] [Google Scholar]
- Paluch E.; Piecuch A.; Obłąk E.; Lamch Ł.; Wilk K. A. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surf., B 2018, 164, 34–41. 10.1016/j.colsurfb.2018.01.020. [DOI] [PubMed] [Google Scholar]
- Piecuch A.; Obłąk E.; Guz-Regner K. Antibacterial Activity of Alanine-Derived Gemini Quaternary Ammonium Compounds. J. Surfactants Deterg. 2016, 19, 275–282. 10.1007/s11743-015-1778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood M. M.; Irfan M.; Khan P.; Alajmi M. F.; Hussain A.; Garrison J.; Rehman M. T.; Abid M. 1,2,3-Triazole-quinazolin-4(3H)-one conjugates: evolution of ergosterol inhibitor as anticandidal agent. RSC Adv. 2018, 8, 39611–39625. 10.1039/c8ra08426b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja B.; Azam M.; Alam S.; Perwez A.; Maguire R.; Yadava U.; Kavanagh K.; Daniliuc C. G.; Rizvi M. M. A.; Haq Q. M. R.; Abid M. Natural Product-Based 1,2,3-Triazole/Sulfonate Analogues as Potential Chemotherapeutic Agents for Bacterial Infections. ACS Omega 2018, 3, 6912–6930. 10.1021/acsomega.8b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R.; Moran C.; McCann M.; Kavanagh K. Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. BioMetals 2009, 22, 461. 10.1007/s10534-008-9182-3. [DOI] [PubMed] [Google Scholar]
- Browne N.; Hackenberg F.; Streciwilk W.; Tacke M.; Kavanagh K. Assessment of in vivo antimicrobial activity of the carbene silver(I) acetate derivative SBC3 using Galleria mellonella larvae. BioMetals 2014, 27, 745–752. 10.1007/s10534-014-9766-z. [DOI] [PubMed] [Google Scholar]
- McCann M.; Santos A. L. S.; Da Silva B. A.; Romanos M. T. V.; Pyrrho A. S.; Devereux M.; Kavanagh K.; Fichtner I.; Kellett A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(ii) and silver(i) complexes. Toxicol. Res. 2012, 1, 47–54. 10.1039/c2tx00010e. [DOI] [Google Scholar]
- Irfan M.; Alam S.; Manzoor N.; Abid M. Effect of quinoline based 1,2,3-triazole and its structural analogues on growth and virulence attributes of Candida albicans. PLoS One 2017, 12, e0175710 10.1371/journal.pone.0175710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. R. N.; Reddy Y. N.; Krishna D. R.; Himabindu V. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology 2010, 272, 11–16. 10.1016/j.tox.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Su Y.; Hu M.; Fan C.; He Y.; Li Q.; Li W.; Wang L.-h.; Shen P.; Huang Q. The cytotoxicity of CdTe quantum dots and the relative contributions from released cadmium ions and nanoparticle properties. Biomaterials 2010, 31, 4829–4834. 10.1016/j.biomaterials.2010.02.074. [DOI] [PubMed] [Google Scholar]
- Selvaraj V.; Bodapati S.; Murray E.; Blough E.; Winston N.; Rice K.; Shokufar T.; Zhao Y. Cytotoxicity and genotoxicity caused by yttrium oxide nanoparticles in HEK293 cells. Int. J. Nanomed. 2014, 9, 1379. 10.2147/ijn.s52625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne P.Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard; CLSI document M27-A2, 2002.
- Aneja B.; Irfan M.; Kapil C.; Jairajpuri M. A.; Maguire R.; Kavanagh K.; Rizvi M. M. A.; Manzoor N.; Azam A.; Abid M. Effect of novel triazole-amino acid hybrids on growth and virulence of Candida species: in vitro and in vivo studies. Org. Biomol. Chem. 2016, 14, 10599–10619. 10.1039/c6ob01718e. [DOI] [PubMed] [Google Scholar]
- Arthington-Skaggs B. A.; Warnock D. W.; Morrison C. J. Quantitation of Candida albicans Ergosterol Content Improves the Correlation between In Vitro Antifungal Susceptibility Test Results and In Vivo Outcome after Fluconazole Treatment in a Murine Model of Invasive Candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2081–2085. 10.1128/aac.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.