Abstract
Critical cytotoxicity evaluation of pharmaceuticals is necessary for the clinical practice of chemotherapy. To quantitatively evaluate cell viability, currently there are two main types of sensitive methods including real-time cell analysis (RTCA) and CCK-8 assay, in which RTCA records electrochemical signal changes around an incubated cell, whereas CCK-8 is based on the colorimetric method. Despite the different detection principles adopted for the cytotoxicity assessment, the comparison of the two methods in terms of the application scope is lacking. In this study, comparison studies were conducted between the RTCA and CCK-8 assays using anticancer drugs including doxorubicin hydrochloride, curcumin, irinotecan (CPT-11), taxol, and oxaliplatin, which are classified into two groups of drug molecules in the absence and presence of additives. The cytotoxicity evaluation of these drugs on cancer cells revealed that the physicochemical properties of drug formulations such as optical and electrochemical properties are closely linked with the readout of cytotoxic methods. The experimental results suggested that the preselection of cytotoxic assay is critical for the quantitative measurement of cytotoxicity of anticancer drugs, which is of clinical importance for their therapeutic usage.
1. Introduction
Nowadays, with the fast development and technical breakthrough in the pharmaceutical industry, there is an urgent need for the drug screening and toxicity tests. Although animal experiments are required for such purposes, the in vitro cell-based assays as a promising alternative are becoming widely used.1 According to the analyzed signals, cell-based assays, in principle, can be classified into colorimetric assays,2−4 luminogenic assays,2,3 electrochemical methods,5,6 cell counting methods,2 and so forth. Among these methods, two conventional assays are usually applied for in vitro cellular cytotoxicity evaluation because of their easy operation and standardized readout: electrochemical methods that record impedance related to the physiological status of incubated cells on the gold microchips and optical methods that measure the absorbance of cell viability-sensitive dyes in the tested solution as typified by the real-time cell analysis (RTCA) and cell counting kit-8 (CCK-8) assay, respectively.
On the basis of dye labels, the conventional colorimetric assays such as CCK-8 and MTT assay were historically established for the cytotoxic evaluation of drugs7−11 because of the remarkable sensitivity and operational convenience. By the use of highly water-soluble tetrazolium salt, CCK-8 assay exhibits superior detection sensitivity than other tetrazolium salts-based assays like MTT.12 In the CCK-8 measurement, the dye of WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4disulfophenyl)-2H-tetrazolium, monosodium salt] was reduced by dehydrogenase in cells to form a water-soluble orange-colored product (formazan). The amount of the produced formazan dye by cellular dehydrogenases is correlated with the number of living cells. Therefore, the cell viability can be simply estimated by recording the optical density (OD) of formazan at 450 nm using a microplate reader. Although CCK-8 assay as a representative end point method allows convenient colorimetric readout, it limits in that each measurement can be conducted at a single time point. Additionally, when colored drugs were used, precautions should be taken as it may cause spectroscopic interference to the determination.
Recently, RTCA holds promising potential in cellular assays because of the advantages of recording signals in a dynamic and label-free mode. In a typical run, upon cells’ incubation on the arrayed gold microchips, the produced electrical impendence reflecting the physiological status of cells such as cell proliferation and viability was continuously monitored.13−22 Without the use of labeled dyes, RTCA permits a direct and continuous measurement of cells under physiological conditions.23 Notably, as electrochemical signals were obtained, the cell number and morphology are the only determining factors in the RTCA assay, whereas other physicochemical properties like the spectroscopic absorbance of cellular components exert no influences on the analysis. Nevertheless, in the cytotoxic assay by RTCA, attention should be paid to the drug formulations that may contain electrically conductive additives.
Till now, there are sparse reports on the comparison of two types of cytotoxic methods. In this study, we have directly calculated and compared half-maximal inhibitory concentrations (IC50) of doxorubicin hydrochloride (DOX), curcumin (CCM), irinotecan (CPT-11), taxol, and oxaliplatin from the cytotoxicity evaluations by both CCK-8 and RTCA assays.
2. Results and Discussion
First, the spectroscopic absorbance of ∼50 μg/mL tested drug molecules in the dimethyl sulfoxide (DMSO) solution was measured on a UV–vis spectrophotometer. DOX showed the absorbance maximized at 480 nm, whereas CCM was at 425 nm. Note that both the absorbance peaks of DOX and CCM were close to that of formazan at 450 nm (Figure 1). CPT-11 displayed the absorbance band around 360 nm without the observable absorbance at 450 nm. By comparison, both taxol and oxaliplatin virtually showed no absorbance in the wavelength range of 300–600 nm.
Figure 1.
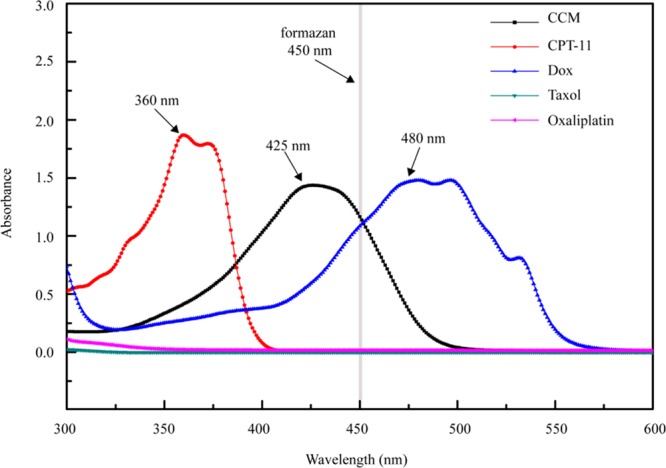
UV–vis spectra of 50 μg/mL CCM, irinotecan hydrochloride (CPT-11), DOX, taxol, and oxaliplatin.
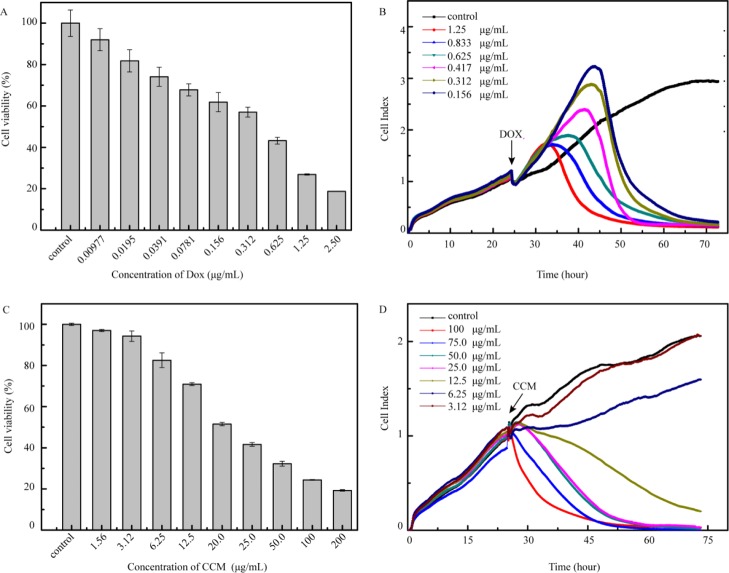
To calculate IC50 of the drugs for the cytotoxicity evaluation, cancer cells were exposed to different concentrations of the tested drugs. We speculated that DOX and CCM with an absorbance peak in the vicinity of that of formazan may cause spectral interference to the analysis results. Hence, we first tested the cytotoxicity of these two drugs. In the CCK-8 assay using HeLa cells, different concentrations of DOX of double dilution were prepared for the cytotoxicity evaluation. As calculated by Statistical Product and Service Solutions (SPSS), the IC50 value was 0.311 μg/mL (Figure 2A). In RTCA, DOX of different concentrations at 0.156, 0.312, 0.417, 0.625, 0.833, and 1.25 μg/mL were chosen for recording the cell growth curves, and the IC50 value of 0.364 μg/mL was obtained, which is close to the result of the CCK-8 assay (Figure 2B). As for CCM that is not water-soluble, CCM was first dissolved in pure DMSO and then diluted with the culture medium prior to the analysis. Note that CCM was orange-colored. To avoid the spectral interference of CCM in the tested solution to a great extent, we have pipetted out the CCM-added culture medium and rinsed the plate quickly with the fresh medium before adding the CCK-8 reagent. The IC50 value of CCM obtained from the CCK-8 assay was 27.513 μg/mL (Figure 2C) in comparison to 10.7 μg/mL obtained from RTCA (Figure 2D), indicating a threefold difference in the cytotoxicity results between the two methods. Despite a possible spectral interference of both CCM and DOX, the comparison studies between CCK-8 and RTCA suggested the amount of colored drugs is the determining factor to cause the bias to the experimental results of cytotoxicity assessment. In the case of DOX of relatively high pharmaceutical effect, less amounts of drug molecules were used so that the drug absorption into cancer cells was even less, which contributed to no observable difference in the cytotoxicity evaluation results. As for CCM, to achieve the anticancer effect, more drug molecules were used; hence, virtually, a color change to the tested solution was observed (Figure S1A). Although a further rinsing step was applied to reduce such color interference of CCM (Figure S1B), the calculated IC50 by CCK-8 was still largely different from that of RTCA, which may point to the interference from the remaining drug molecules adsorbed into the cells. To further rule out the possible interference from the cell itself, the cytotoxicity evaluation of CCM was carried out by the use of A549 cells. Similar to the experimental results of HeLa, the IC50 value obtained was 24.0 and 9.61 μg/mL in CCK-8 (Figure S2A) and RTCA assay (Figure S2B), respectively, suggesting the spectral interference of CCM is the main factor to cause bias in the colorimetric method. Note that the rinsing of cells with the medium inevitably causes a loss of incubated cells (Figure S1C,D). Therefore, we concluded that the CCK-8 assay may not be suitable for the cytotoxicity evaluation of colored drugs with a less pronounced pharmaceutical effect. Considering that the concentrations of DMSO were different for the gradient dilution of the CCM solution, the cytotoxicity of DMSO was also evaluated by the CCK-8 assay, and the concentrations chosen were in accord with those in the CCM series (Figure 2C). Among all the groups, over 96% cells survived after 24 h (Figure S3A), indicating that DMSO caused almost no influence.
Figure 2.
Cell viability of HeLa induced by DOX (A) and CCM (C) after 24 h by CCK-8 assay; cell growth curve of HeLa induced by DOX (B) and CCM (D) by RTCA.
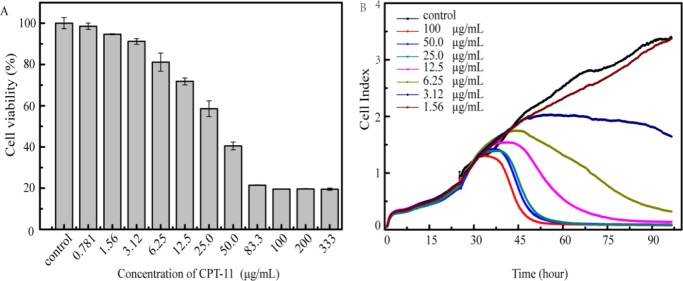
In clinical applications, the anticancer drug formulation often contains additives. The presence of additives functions in several aspects such as to improve the therapeutic effect, pharmacokinetics, and stability of the main drug/drug formulations. To investigate the possible influence of additives on the analysis results, HeLa cells were exposed to CPT-11 of varied concentrations in RTCA and CCK-8 assays. As shown in Figure 3, the IC50 value was calculated to be 33.6 and 17.2 μg/mL by CCK-8 and RTCA, respectively (Figure 3). As there is virtually no absorbance of CPT-11 at 450 nm of formazan used for CCK-8 assay, we reasoned that the additives of drugs such as sorbitol, lactate, and sodium hydroxide may cause some interference to the RTCA determination. To test this hypothesis, the dielectric properties of CPT-11-added cells were studied by electrochemical impedance spectroscopy (EIS) using a glassy carbon electrode (GCE). As shown in Figure 3C, the impedance spectra of the electrode modified with cells or drug-added cells were recorded. The diameter of the semicircle extrapolated in the obtained Nyquist diagram showed a correlation of electron-transfer resistance of the redox probe on the electrode surface with the CPT-11 concentration. At 50.0 μg/mL of CPT-11, there is a significant change of the recorded semicircle in the Nyquist diagram. This observation suggested that the additive in CPT-11 may alter the impedance of the drug formulation used in RTCA. Therefore, RTCA appeared to be suitable for the cytotoxicity evaluation of anticancer drugs without any electroactive components or pure drug molecules.
Figure 3.
(A) Cell viability of HeLa induced by CPT-11 after 48 h incubation by CCK-8 assay; (B) cell growth curve of HeLa induced by CPT-11 monitored by RTCA; (C) electrochemical impendence spectra of GCEs modified with HeLa cells only, the cells containing 12.5 and 50.0 μg/mL CPT-11, respectively.
As for the anticancer drugs of taxol and oxaliplatin, both of them showed no observable absorbance in the wavelength range of 300–600 nm. However, compared with the water-soluble oxaliplatin, taxol exhibited poor water solubility. To test the cytotoxicity in solution, we dissolved taxol in DMSO to make a stock solution, which was further diluted with the medium to make a series of drug solutions to treat HeLa cells in CCK-8 assay (Figure 4A) and RTCA (Figure 4B), respectively. The IC50 value obtained by CCK-8 was 1.08 μg/mL, which was comparable with that of 1.07 μg/mL obtained by RTCA. The cell viability of HeLa cells after incubation with DMSO of different concentrations for 48 h was measured by CCK-8 assay to evaluate its cytotoxicity of DMSO and influence. The concentrations of DMSO in Dulbecco’s modified Eagle’s medium (DMEM) were the same with those in the solution of taxol used in the CCK-8 assay above. All the groups showed cell viability over 96% (Figure S3B), which suggested that the DMSO utilized caused almost no interference. In the case of oxaliplatin, the tested solution was made by directly dissolving the drug with the medium because of its superior water solubility. The drug concentrations of 0.781, 1.56, 3.12, 9.38, 12.5, 18.8, 25.0, 37.5, 50.0, 75.0, and 200 μg/mL were used in the CCK-8 assay (Figure 4C) and 3.12, 6.25, 12.5, 25.0, 50.00, 100, and 200 μg/mL in RTCA (Figure 4D), respectively. The obtained IC50 was 16.9 μg/mL in CCK-8 assay that accorded well with that of 17.1 μg/mL in RTCA. These experimental results using taxol and oxaliplatin indicated a consistency of both the CCK-8 and RTCA methods when there was not any spectral or dielectric interference from the tested drugs as well as the components.
Figure 4.
Cell viability of HeLa induced by taxol (A) and oxaliplatin (C) after 48 h in CCK-8 assay; cell growth curve of HeLa induced by taxol (B) and oxaliplatin (D) in RTCA.
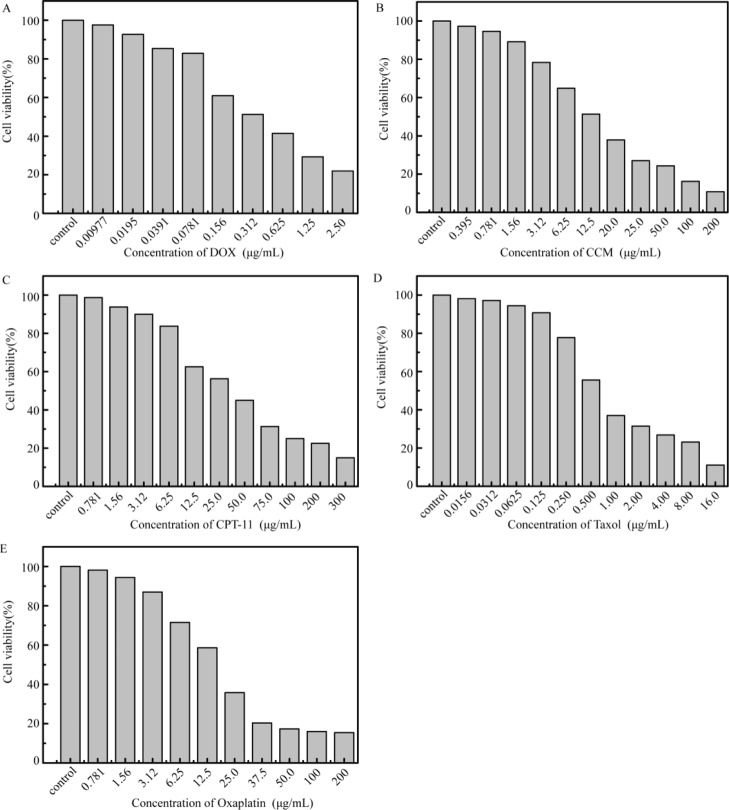
Considering that the value of IC50 was method-dependent, distinct results could be obtained among different assays even as there are no detection bias. Therefore, cell counting assays were conducted to further validate the measured results by the CCK-8 and RTCA assays. The IC50 value of CCM by cell counting was 13.04 μg/mL (Figure 5B) close to that of RTCA, which suggested the possible interferences involved in the colorimetric determination. Nevertheless, in accordance with the result of the CCK-8 assay, the value of IC50 of CPT-11 derived from manual counting was 34.6 μg/mL (Figure 5C), which indicated that the CCK-8 assay was relatively applicable for the cytotoxicity evaluation of drug formulation with the excipients. Additionally, the other IC50 values obtained by manual cell counting assays were 0.39 μg/mL for DOX (Figure 5A), 1.01 μg/mL for taxol (Figure 5D), and 16.26 μg/mL for oxaliplatin (Figure 5E). These results were consistent with those of both the CCK-8 and RTCA assays, which also accord well with those reported previously.24−26 Taken together, the data obtained by manual cell counting supported the comparison results between the CCK-8 and RTCA assays, which suggested that the applicability of these two methods should be taken into consideration in terms of cytotoxicity evaluation of the drugs.
Figure 5.
Cell viability of HeLa cells induced by DOX (A) and CCM (B) after 24 h and treated with CPT-11 (C), taxol (D), and oxaliplatin (E) after 48 h in cell counting assays.
3. Conclusions
In this work, we have compared the cytotoxicity evaluation results of several anticancer drugs between the CCK-8 and RTCA assays. The IC50 values obtained by the two methods are outlined in Table 1. The results indicated that the CCK-8 assay as an end point method measured the optical intensity of dyes in the cell at a specific time point, of which the readout interpretation can be interfered by the colored drugs used, especially those with the absorbance peak close to 450 nm of dyes used for the assay. By comparison, RTCA measured the drug-added cells on the basis of electrochemical impedance and hence can give more reliable cytotoxicity evaluation results. Nevertheless, when electroactive ingredients are used for the drug formulation, CCK-8 may give the validated assessment of cytotoxicity of the anticancer drugs. Therefore, the comparison results toward different samples should be based on the methodology. In the long run, with the fast development of new drug formulation, our studies may indicate that the preselection of methods is necessary for the drug cytotoxicity evaluation and screening to avoid the misleading results, which will be meaningful for later clinical practice.
Table 1. Cytotoxicity of Anticancer Drugs (IC50) Obtained by CCK-8 and RTCA Assays.
| IC50 (μg/mL) |
||||
|---|---|---|---|---|
| HeLa |
A549 |
|||
| drug | CCK-8a | RTCA | CCK-8 | RTCA |
| doxorubicin | 0.368 ± 0.059 | 0.364 | ||
| CCM | 27.0 ± 2.40 | 10.6 | 24.0 | 9.61 |
| CPT-11 | 35.5 ± 6.51 | 17.2 | ||
| taxol | 1.08 ± 0.016 | 1.07 | ||
| oxaliplatin | 16.5 ± 0.334 | 17.1 | ||
CCK-8 assay was conducted in triplicate to ensure the accuracy. CPT-11: irinotecan hydrochloride.
4. Experimental Section
4.1. Cell Culture
HeLa cells were cultured in DMEM (Gibco by Thermo Fisher Scientific) supplemented with 10% (v/v) fetal bovine serum (FBS, ExCell Biology) and 1% (v/v) penicillin–streptomycin (Gibco by Thermo Fisher Scientific). A549 cells were cultured in Roswell Park Memorial Institute Medium 1640 (Gibco by Thermo Fisher Scientific) supplemented with 10% (v/v) FBS and 1% (v/v) penicillin–streptomycin (Gibco by Thermo Fisher Scientific). All the cells were incubated at 37 °C in 5% CO2 atmosphere. Before each experiment, the cultures were washed with phosphate-buffered saline (PBS, pH 7.4, Gibco by Thermo Fisher Scientific), detached with 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) solution (Beyotime Biotechnology), and then centrifuged at 1000 rpm for 5 min, followed by resuspension.
4.2. CCK-8 Assay
Cancer cells were first counted, and approximately 4000 cells per well were seeded in a 96-well cell culture plate (Corning Inc.). Then, after incubation at 37 °C in a humidified atmosphere with 5% CO2 for 24 h, the culture medium was replaced by a series of concentrations of drugs diluted with the corresponding culture fluid. Five replicates were made for each measurement, and the time of co-incubation was determined by the efficiency of each drug. In this study, DOX (MedChemExpress Co., Ltd.) and CCM (Sinopharm Chemical Reagent Co., Ltd.) were co-incubated with the cells for 24 h at 37 °C under the same conditions as described above, whereas irinotecan hydrochloride injection (20 mg/mL, Qilu Pharmaceutical), taxol (Aladdin), and oxaliplatin (Aladdin) were co-incubated for 48 h. Finally, 10 μL of the CCK-8 reagent (MedChemExpress Ltd.) was added into each well, and OD at 450 nm was measured using a multifunction microplate reader (Infinite M200 Pro, Tecan) after incubation for 2 h at 37 °C. The percentage each concentration accounted for of the control was presented as cell viability. The IC50 value was calculated using SPSS.
4.3. Real-Time Cell Analysis
To monitor the cellular growth and responses to the drugs, we utilized the “xCELLigence” system (Roche Applied Sciences and ACEA Biosciences) including 16-well E-plates for continuous and label-free detection. After setting up the program, 50 μL of the culture medium per well was dropped into the E-plate to plot the baseline, followed by seeding 8000 cells in each well. The cells were placed at room temperature for 30 min to attach to the E-plate before subsequent detection. The culture medium was pipetted out, and a series of concentrations of the drugs were added after a 24 h incubation at 37 °C with 5% CO2.
4.4. Cell Counting Assays
A total of 4000 HeLa cells per well were seeded in 96-well cell culture plates. After incubation at 37 °C in a humidified atmosphere with 5% CO2 for 24 h, solutions of the anticancer drugs in different concentrations were added to the cells. After incubation for a certain time at 37 °C, the culture medium was removed and the cells were washed with PBS. Then, 100 μL trypsin–EDTA solution was added to each well, followed by 3 min incubation at 37 °C until the cells were thoroughly dissociated from the bottom of the plates. Equal volume of fresh culture medium was added to stop the reaction, and living cells were counted manually with a Neubauer cell counting chamber. The survival rate of the cells was determined by comparing the results with that of the untreated control group.
4.5. Electrochemical and Spectroscopic Measurement
EIS was obtained using an integrated electrochemical analyzer (CHI760D, Shanghai Chenhua Instrument Co., Ltd.). The system utilized a saturated calomel electrode as a reference electrode, a platinum wire as an auxiliary electrode, and a GCE as a working electrode. The GCEs were polished with aluminum oxide nanoparticles followed by ultrasonication in deionized water before use. 10 μL aliquots of cells in DMEMor in DMEM containing 12.5 and 50 μg/mL irinotecan hydrochloride respectively were then dropped on the polished GCE and dried at room temperature overnight. The solution above was freshly prepared with the same amount of HeLa cells before modification. The UV–vis spectrum was obtained on a spectrophotometer (UH5300, Hitachi). All drugs were dissolved with DMSO (Solarbio Life Sciences) using DMSO as the blank control for calibration.
Acknowledgments
The work was supported by National Natural Science Foundation of China (U1703118), Natural Science Foundation of Jiangsu Province (no. BK20181364), Scientific Research Foundation of Jiangsu Health and Health Committee (no. H2018087), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Shuangchuang Program, Open project of the National Laboratory of Biomacromolecules (2017kf05), the cooperative project between Southeast university and Nanjing Medical University (2018DN0004), and Jiangsu Specially-Appointed Professor project, China.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01142.
Images of CCM-treated HeLa cells; cytotoxicity results of CCM on A549 cells; and cell viability of HeLa cells treated with DMSO (PDF)
Author Contributions
¶ Equal contribution.
The authors declare no competing financial interest.
Supplementary Material
References
- Kirstein S. L.; Atienza J. M.; Xi B.; Zhu J.; Yu N.; Wang X.; Xu X.; Abassi Y. A. Live cell quality control and utility of real-time cell electronic sensing for assay development. Assay Drug Dev. Technol. 2006, 4, 545–553. 10.1089/adt.2006.4.545. [DOI] [PubMed] [Google Scholar]
- Braun K.; Stürzel C. M.; Biskupek J.; Kaiser U.; Kirchhoff F.; Lindén M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. in Vitro 2018, 52, 214–221. 10.1016/j.tiv.2018.06.019. [DOI] [PubMed] [Google Scholar]
- Elisia I.; Popovich D. G.; Hu C.; Kitts D. D. Evaluation of Viability Assays for Anthocyanins in Cultured Cells. Phytochem. Anal. 2008, 19, 479–486. 10.1002/pca.1069. [DOI] [PubMed] [Google Scholar]
- Fischer D.; Li Y.; Ahlemeyer B.; Krieglstein J.; Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- Zhou S.; Guo P.; Li J.; Meng L.; Gao H.; Yuan X.; Wu D. An electrochemical method for evaluation the cytotoxicity of fluorene on reduced graphene oxide quantum dots modified electrode. Sens. Actuators, B 2018, 255, 2595–2600. 10.1016/j.snb.2017.09.066. [DOI] [Google Scholar]
- Qin H.; Liu J.; Zhang Z.; Li J.; Gao G.; Yang Y.; Yuan X.; Wu D. In situ electrochemical assessment of cytotoxicity of chlorophenols in MCF-7 and HeLa cells. Anal. Biochem. 2014, 462, 60–66. 10.1016/j.ab.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Liao J.; Zheng H.; Fei Z.; Lu B.; Zheng H.; Li D.; Xiong X.; Yi Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. 10.1016/j.ijbiomac.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Li G.; Long C.; Xu J.; Cen J.; Yang X. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. 10.1016/j.foodchem.2018.01.074. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang H.; Bai M.; Ning T.; Ge S.; Deng T.; Liu R.; Zhang L.; Ying G.; Ba Y. Exosomes Serve as Nanoparticles to Deliver Anti-miR-214 to Reverse Chemoresistance to Cisplatin in Gastric Cancer. Mol. Ther. 2018, 26, 774–783. 10.1016/j.ymthe.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T.; Ahmad R.; Azad I.; Raza S.; Joshi S.; Khan A. R. Computer-aided drug design and virtual screening of targeted combinatorial libraries of mixed-ligand transition metal complexes of 2-butanone thiosemicarbazone. Comput. Biol. Chem. 2018, 75, 178–195. 10.1016/j.compbiolchem.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Wang Y.-J.; Zhou S.-M.; Xu G.; Gao Y.-Q. Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay. Molecules 2015, 20, 8060–8071. 10.3390/molecules20058060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Li W.-Y.; Lan R.; Wang J.-Y. Quality Monitoring of Porous Zein Scaffolds: A Novel Biomaterial. Engineering 2017, 3, 130–135. 10.1016/j.eng.2017.01.001. [DOI] [Google Scholar]
- Kaschula C. H.; Hunter R.; Stellenboom N.; Caira M. R.; Winks S.; Ogunleye T.; Richards P.; Cotton J.; Zilbeyaz K.; Wang Y.; Siyo V.; Ngarande E.; Parker M. I. Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012, 50, 236–254. 10.1016/j.ejmech.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Vosjan M. J. W. D.; Vercammen J.; Kolkman J. A.; Stigter-van Walsum M.; Revets H.; van Dongen G. A. M. S. Nanobodies Targeting the Hepatocyte Growth Factor: Potential New Drugs for Molecular Cancer Therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. 10.1158/1535-7163.mct-11-0891. [DOI] [PubMed] [Google Scholar]
- Choi Y.; Kang D.; Han I.-O.; Oh E.-S. Hierarchy between the transmembrane and cytoplasmic domains in the regulation of syndecan-4 functions. Cell. Signalling 2012, 24, 1522–1530. 10.1016/j.cellsig.2012.03.020. [DOI] [PubMed] [Google Scholar]
- Ohta S.; Misawa A.; Fukaya R.; Inoue S.; Kanemura Y.; Okano H.; Kawakami Y.; Toda M. Macrophage migration inhibitory factor (MIF) promotes cell survival and proliferation of neural stem/progenitor cells. J. Cell Sci. 2012, 125, 3210–3220. 10.1242/jcs.102210. [DOI] [PubMed] [Google Scholar]
- Rammah M.; Dandachi F.; Salman R.; Shihadeh A.; El-Sabban M. In vitro cytotoxicity and mutagenicity of mainstream waterpipe smoke and its functional consequences on alveolar type II derived cells. Toxicol. Lett. 2012, 211, 220–231. 10.1016/j.toxlet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurs U.; Lajkó E.; Mező G.; Orbán E.; Öhlschläger P.; Marquardt A.; Kőhidai L.; Manea M. GnRH-III based multifunctional drug delivery systems containing daunorubicin and methotrexate. Eur. J. Med. Chem. 2012, 52, 173–183. 10.1016/j.ejmech.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Böhmert L.; Niemann B.; Thuenemann A. F.; Lampen A. Cytotoxicity of peptide-coated silver nanoparticles on the human intestinal cell line Caco-2. Arch. Toxicol. 2012, 86, 1107–1115. 10.1007/s00204-012-0840-4. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Shi X.; Jiang H.; Song Y.; Zhang L.; Wang F.; Du S.; Chen J. A general method to regenerate arrayed gold microelectrodes for label-free cell assay. Anal. Biochem. 2017, 516, 57–60. 10.1016/j.ab.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Xu Z.; Song Y.; Jiang H.; Kong Y.; Li X.; Chen J.; Wu Y. Regeneration of Arrayed Gold Microelectrodes Equipped for a Real-Time Cell Analyzer. J. Visualized Exp. 2018, 133, e56250 10.3791/56250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Cai L.; Jiang H.; Wen Y.; Peng L.; Wu Y.; Chen J. Real-time cell analysis of the cytotoxicity of a pH-responsive drug-delivery matrix based on mesoporous silica materials functionalized with ferrocenecarboxylic acid. Anal. Chim. Acta 2019, 1051, 138–146. 10.1016/j.aca.2018.11.017. [DOI] [PubMed] [Google Scholar]
- Otero-González L.; Sierra-Alvarez R.; Boitano S.; Field J. A. Application and Validation of an Impedance-Based Real Time Cell Analyzer to Measure the Toxicity of Nanoparticles Impacting Human Bronchial Epithelial Cells. Environ. Sci. Technol. 2012, 46, 10271. 10.1021/es301599f. [DOI] [PubMed] [Google Scholar]
- Liu M.; Chang Y.; Yang J.; You Y.; He R.; Chen T.; Zhou C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. 10.1039/c5tb02725j. [DOI] [PubMed] [Google Scholar]
- Sriraman S. K.; Pan J.; Sarisozen C.; Luther E.; Torchilin V. Enhanced Cytotoxicity of Folic Acid-Targeted Liposomes Co-Loaded with C6 Ceramide and Doxorubicin: In Vitro Evaluation on HeLa, A2780-ADR, and H69-AR Cells. Mol. Pharm. 2016, 13, 428–437. 10.1021/acs.molpharmaceut.5b00663. [DOI] [PubMed] [Google Scholar]
- Rezaei S. J. T.; Sarbaz L.; Niknejad H. Folate-decorated redox/pH dual-responsive degradable prodrug micelles for tumor triggered targeted drug delivery. RSC Adv. 2016, 6, 62630–62639. 10.1039/c6ra11824k. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.