There has been a transformation in the power and throughput of analytic methods delivered by large-scale facilities, such as synchrotrons. This has placed an increasing demand on the supply of high-quality samples (purified proteins and protein crystals) for structural studies. To meet this requirement, protein production has been streamlined to improve efficiency and throughput. Nonetheless, delivering recombinant proteins in sufficient quantity and quality remains a significant bottleneck in the gene to structure pipeline for a variety of reasons including low levels of expression, poor solubility, and toxicity when over-expressed. Many of these issues had been encountered by participants on the START workshop on “Biophysics & Structural Biology at Synchrotrons,” during the course of their projects. Therefore, our aim over the first day of the workshop was to share our experience of setting up and running protein production workflows that address the challenge of producing a wide variety of proteins for structural analysis. Lessons learnt were explained and some examples of how to clone, express, and purify different proteins were discussed with the participants in some “virtual” practical work and case studies.
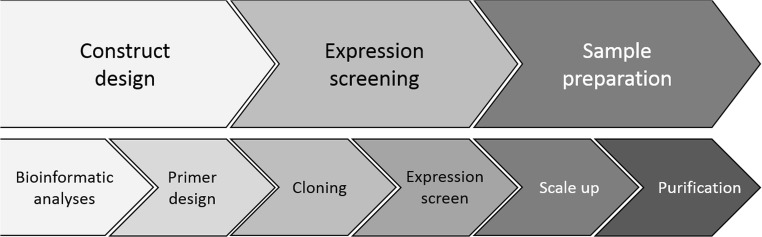
A typical protein production workflow can be broadly divided into three stages: (1) design, (2) screening expression, and (3) sample preparation (Fig. 1). Within each stage, there are two essential operations, beginning with the design of expression vectors. For this step, prior knowledge from related proteins in the Protein Data Bank and bioinformatics analyses using machine learning to predict structural features in a sequence are combined to define the gene segment that will be cloned and expressed. Many of the projects of the students involved making a relatively small number of vectors and expressing them in just one host, typically, E. coli. However, by designing and constructing multiple vectors in parallel and expressing them in more than one host (e.g., insect and E. coli), the optimum sequence and expression host for producing a target protein can be more quickly identified. Suites of plasmid vectors have been developed that are adapted for ligation-independent cloning and enable expression testing in E. coli, mammalian, and insect cells (Nettleship et al. 2019). Inclusion of a short histidine tag comprising 6–10 residues in all expression vectors provides a facile way of detecting and purifying the expressed protein.
Fig. 1.
Overview of the protein production pipeline (from Nettleship et al. 2019 reproduced with permission from Springer Protocols)
Expression screening of multiple constructs is carried out at small scale in either 24- or 96-well plate formats reducing experimental costs by ensuring that subsequent scale-up and purification is only carried using optimized construct/host cell combinations.
E. coli has been used successfully to produce a large variety of recombinant proteins and remains the host of choice for prokaryotic and some eukaryotic proteins, particularly structural domains (Gräslund et al. 2008). By using insect and mammalian cell systems as well, expression of eukaryotic proteins that fail to be produced in E. coli can often be rescued (Savitsky et al. 2010).
Given that automation and/or the use of multichannel pipettes can be used to carry out the cloning and screening steps efficiently, this stage of the process is not rate-limiting. Whereas, the scale-up and purification of selected proteins still require some form of batch culture of cells typically in shake flasks and column-based chromatography steps, to isolate the protein sample, all of which are relatively low throughput operations. However, having pre-screened for expression, a simple two-stage purification strategy combining an affinity capture step, typically immobilized metal affinity chromatography (IMAC) and size exclusion chromatography, is often sufficient to produce samples of > 95% purity (by SDS-PAGE). Structural biology techniques generally require the purified protein sample to be monodisperse, and a size fractionation step during purification provides the first indication of protein behavior. Analysis of the purified product by intact protein mass spectrometry can then be used to authenticate the sample and identify any post-translation modifications that change the expected mass of the protein. It is a truism that the protein is the key variable and therefore quality assessment steps are necessary to both characterize the sample and to minimize batch to batch variation.
In summary, the main take-home points from our session were as follows:
Construct design is critical and should be informed by all available information from the literature and bioinformatic analyses.
Protein production throughput can be increased by using standard operating procedures and parallel processing of vector construction and expression screening.
Incorporating quality assessment of sample purity and homogeneity into the workflow is essential to characterize the protein and ensure consistency of batch to batch preparation.
Footnotes
This article is part of a Special Issue on “Biophysics & Structural Biology at Synchrotrons” edited by Trevor Sewell.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Gräslund S, Nordlund P, Weigelt J, et al. Protein production and purification. Nat Methods. 2008;5:135–146. doi: 10.1038/nmeth.f.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleship JE, Rada H, Owens RJ (2019) Overview of a high throughput pipeline for streamlining the production of recombinant proteins. Methods Mol Biol 2025 (in press) [DOI] [PubMed]
- Savitsky Pavel, Bray James, Cooper Christopher D.O., Marsden Brian D., Mahajan Pravin, Burgess-Brown Nicola A., Gileadi Opher. High-throughput production of human proteins for crystallization: The SGC experience. Journal of Structural Biology. 2010;172(1):3–13. doi: 10.1016/j.jsb.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]