Glioblastoma (GB) is the most common and most aggressive form of diffuse glioma, with a median overall survival of 15 months after standard of care.1 Young GB patients (<40 years old) may carry isocitrate dehydrogenase 1 or 2 (IDH1/2) mutations or, in rare instances, harbor H3F3A or HIST1H3B mutations. Other cases are wild-type for those genes and harbor gain of chromosome (chr) 7, loss of chr 10, and epidermal growth factor receptor (EGFR) amplification (amp). IDH1/2 mutations are associated with longer survival (24 mo), whereas histone H3F3A/HIST1H3B gene mutations are associated with shorter survival (9–12 mo).1 Novel reliable prognostic factors are needed for IDH and H3 wild-type GB.
Mitochondria are responsible for the production of ATP through oxidative phosphorylation (oxphos). The 16 569 nucleotide long mitochondrial DNA (mtDNA), present in hundreds to thousands of copies per cell, is circular, intron-less and exclusively maternally inherited. It encodes, among others, 13 essential polypeptides required for oxphos.2 High mtDNA copy number has been associated with variable clinical outcomes in different cancer types.3
The mtDNA copy number was assessed by real-time quantitative PCR as described elsewhere4 in 67 primary GB developed in patients aged between 18 and 40 years. Clinical, molecular, and survival data were available for all patients. In addition, whole mitochondrial genome next-generation sequencing (NGS) was performed in 18/67 GB. Written informed consent was obtained from all individual participants with approval of the research ethics committee of Angers University Hospital.
In the overall cohort, the sex ratio (M/F) was 1.3; the median age at diagnosis was 35 years, and the median overall survival was 14.3 months. Seventeen of 67 (25.3%) cases harbored IDH1-R132H mutation; 30/67 (44.8%) had chr 7 gain and chr 10 loss and/or EGFR amp (identified by single nucleotide polymorphism arrays); and 4/67 (6%) harbored H3F3A or HIST1H3B mutations. The remaining 16 cases (23.9%) had none of the above-mentioned genetic alterations.
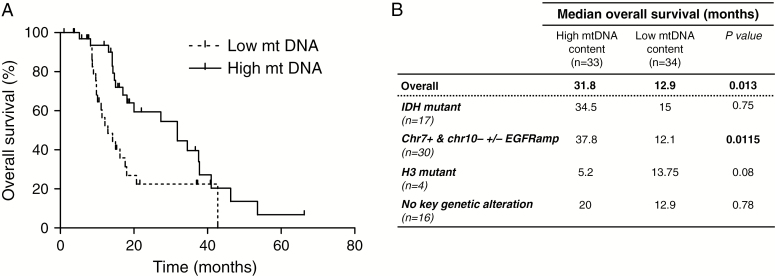
The mtDNA/nuclear DNA ratio ranged from 25.6 to 3882.4 and was subdivided into 2 groups: low and high according to the median of the mtDNA copy number (median 194.9). Thirty-four patients (50.7%) displayed a low ratio (ratio ≤ median), whereas 33 (49.3%) displayed a high ratio (> median). A log-rank test was performed: the overall survival in the high vs low group was significantly longer: 31.8 months vs 12.9 months (P = 0.013; hazard ratio = 2.338; 95% CI [1.196; 4.571]) (Fig. 1A). Overall survival was also significantly longer for the high versus low GB patients within the genetic subgroup chr7+/chr10−/EGFR amp (37.8 mo vs 12.1 mo; P = 0.0115) (Fig. 1B). There was still a trend toward longer survival in the high vs low groups within the other genetic subgroups (IDH-mutant and no genetic alteration) except for the H3-mutant GB (only 4 patients in this subgroup) (Fig. 1B). Of note, IDH status and mtDNA copy number were 2 independent variables (P = 0.36, Pearson’s chi-square test). In addition, NGS analysis did not reveal the accumulation of somatic mtDNA variants in 18/67 GB (data not shown), as opposed to what has been previously reported in GB.3
Fig. 1.
Overall survival of young GB patients according to the mtDNA copy number level (high vs low). (A) Kaplan–Meier survival curves in young adult GB stratified according to the tumor mtDNA content. (B) Median overall survival according to the tumor mtDNA content (high vs low) in the whole cohort and according to the genetic subgroups. In the high group, there were 10 IDH-mutant GB (30.3%), one H3-mutant GB (3%), 12 GB with chr7+/chr10−/EGFR amp (36.4%) and 10 cases without key genetic alteration (30.3%). The low group comprised 7 IDH-mutant GB (20.6%), 3 H3-mutant GB (8.8%), 18 GB with chr7+/chr10−/EGFR amp (53%), and 6 cases with no key genetic alteration (17.6%). Of note, the genetic subgroups were equally distributed between both groups (high vs low). Within the IDH-mutant subgroup, there was a trend toward longer survival in the high vs low GB but the difference did not reach statistical significance.
The present study revealed that higher mtDNA copy number was significantly correlated with better overall survival in young adult GB. Of great interest, high mtDNA level is linked to oxidative metabolism, which is known to decrease tumor aggressiveness, and to cell differentiation.5 The modulation of mtDNA copy number in a model of GB was recently shown to induce changes to DNA methylation and gene expression of the nuclear genome, and thus to modulate the tumorigenic potential of GB cells in vivo.6 Therapeutics to increase mtDNA copy number might induce tumor cell differentiation and slow disease progression. Further analyses on larger cohorts of GB patients of all ages are needed, especially in the older population who is the most affected by the disease. In conclusion, our study indicates that mtDNA copy number may be a novel prognostic biomarker in GB patients.
Funding
None.
Acknowledgments
We thank the Public Assistance–Hospital of Marseille (AP-HM) Tumor Bank (authorization number: AC-2018–31053; CRB BB-0033-00097) and the French Glioblastoma Biobank for providing samples.
Conflict of interest statement. None declared.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.. WHO Classification of Tumours of the Central Nervous System. IARC Lyon; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol. 2010;5:297–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larman TC, DePalma SR, Hadjipanayis AG, et al. ; Cancer Genome Atlas Research Network Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci U S A. 2012;109(35):14087–14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boucret L, Bris C, Seegers V, et al. Deep sequencing shows that oocytes are not prone to accumulate mtDNA heteroplasmic mutations during ovarian ageing. Hum Reprod. 2017;32(10):2101–2109. [DOI] [PubMed] [Google Scholar]
- 5. Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun X, St John JC. Modulation of mitochondrial DNA copy number in a model of glioblastoma induces changes to DNA methylation and gene expression of the nuclear genome in tumours. Epigenetics Chromatin. 2018;11(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]