ABSTRACT
The gut microbiota can play a role in pancreatitis and, likely, in the development of type 1 diabetes (T1D). Anti-microbial peptides and secretory proteins are important mediators of the innate immune response against bacteria but their expression in the human pancreas is not fully known. In this study, immunohistochemistry was used to analyze the expression of seven anti-microbial peptides (Defensin α1, α4, β1-4 and Cathelicidin) and two secretory proteins with known antimicrobial properties (REG3A and GP2) in pancreatic and duodenal biopsies from 10 non-diabetic organ donors and one organ donor that died at onset of T1D. Immunohistochemical data was compared with previously published whole-transcriptome data sets. Seven (Defensin α1, β2, β3, α4, GP2, Cathelicidin, and REG3A) host defense molecules showed positive staining patterns in most non-diabetic organ donors, whereas two (Defensin β1 and β4) were negative in all non-diabetic donors. Two molecules (Defensin α1 and GP2) were restricted to the exocrine pancreas whereas two (Defensin β3, α4) were only expressed in islet tissue. Cathelicidin, β2, and REG3A were expressed in both islets and exocrine tissue. The donor that died at onset of T1D had generally less positivity for the host defense molecules, but, notably, this pancreas was the only one where defensin β1 was found. Neither donor age, immune-cell infiltration, nor duodenal expression correlated to the pancreatic expression of host defense molecules. In conclusion, these findings could have important implications for the inflammatory processes in diabetes and pancreatitis as we find several host defense molecules expressed by the pancreatic tissue.
KEYWORDS: Islet of Langerhans, beta cell, defensin, bacteria, diabetes, pancreas
Background
The defensin molecules are a family of anti-microbial peptides (AMPs) that play important roles in the innate and adaptive immune system. They are evolutionarily highly conserved, and exist in numerous multi-cellular organisms such as fungi, plants and mammals.12
The defensins are divided into three different categories – alpha, beta and theta – based on their molecular topology. The alpha and beta defensins are expressed in human subjects.3
The alpha defensins exert their antimicrobial effects in vitro by permeabilizing the cell membranes of bacteriae and monocellular pathogens, and by causing viruses to agglutinate, facilitating their phagocytosis as reviewed by Lehrer et al.4 The beta defensins act in a similar way, and have been found to inhibit bacterial cell-wall synthesis and to exert their anti-viral effects by interacting directly with viruses and indirectly with their target cells.2 Another member of the AMP superfamily is Cathelicidin, which has a similar anti-microbial spectrum as the defensin molecules as well as immunomodulatory effects, as reviewed by Agier et al. Cathelicidin was first characterized in neutrophil granulocytes, but are known to be expressed and inducible in a number of cell types including colonic enterocytes and epithelial cells.5
The defensins are not limited to antimicrobial effects. In 1999, Yang et al discovered pro-inflammatory properties of beta defensins, inducing chemo-attraction of CD4 + T cells and immature dendritic cells by binding to the chemokine receptor CCR6.6 Beta defensins have also been shown to attract cell types that do not express CCR6, such as macrophages.7 The defensins have also been linked to several other functions, such as wound healing, reproduction and the host defense against carcinogenesis, as further reviewed by Semple et al.2
The defensin genes are susceptible to a high variation of copy numbers between individuals, and copy number variations (CNV) have been associated with psoriasis, where individuals with more copies are at higher risk of developing the disease. The risk of developing Crohns disease, on the other hand, is associated with lower expression of beta defensins in the paneth cells of the ileum.1
Besides classic AMP molecules, many other proteins have attracted attention in gastrointestinal inflammation research. Regenerating islet-derived protein 3-alpha (REG3A), formerly known as HIP/PAP in humans, is a secretory protein with antimicrobial properties.8 It is expressed in several tissue types including the Paneth cells of the small intestine and in pancreatic tissue.
Glycoprotein 2 (GP2) was initially characterized as a secretory granule protein expressed exclusively in the pancreas. Further research has shown that GP2 is expressed in epithelial mucosal cells and on several other cell types, and that it is involved in the intestinal anti-microbial defense and exerts immunomodulary effects.9
The intestinal bacterial flora and leakage in the intestinal epithelium have been linked to type 1 diabetes (T1D).10,11 Also, the close communication between the duodenum and the pancreas, through the papilla Vateri and the pancreatic duct, could allow translocation of duodenal bacteria, viruses and bile to the pancreas, which has been hypothesized to initiate the innate inflammatory response in the pancreas that contributes to the development of T1D.12-14 The installation of bacteriae in the pancreatic ducts is in fact a model for T1D in rats.12 Tentatively, anti-microbial peptides play important roles in the pancreatic defense against refluxed bacteria but also in mediating a potentially harmful beta-cell cytotoxic inflammatory response. The aim of this study was to characterize the expression of these peptides in the pancreas from non-diabetic individuals and from an organ donor that died at acute onset of T1D.
Materials and methods
Ethics statement
The consent to use tissue from deceased organ donors for research purposes was obtained verbally from the deceased’s next of kin by the physician in charge or obtained from an online database and fully documented in accordance with Swedish law and regional standard practices. All tissue included in the study was procured, stored and analyzed as approved by the Regional Ethics Committee in Uppsala (Dnr: 2015/444).
Human tissue
Pancreatic tissue from ten non-diabetic multi-organ donors aged 1 to 27 years (mean 11.7) and one donor that died at acute onset of T1D were included in the study (Table 1). The donor with T1D was a 29 year-old man who died as a result of a series of complications associated with onset of the disease and his clinical history has been described in detail in a previous publication.12 One tissue sample from the body region of the pancreas from each of the non-diabetic donors was included. From the donor with T1D, two tissue samples from separate pancreatic regions were included; one of these (D1CD45hi) was known to have intense immune cell (CD45+) infiltration while the other (D1CD45lo) came from a part of the pancreas with less inflammation. All pancreases were shipped to Uppsala in cold preservation solution optimized for transplantation purposes where tissue biopsies were immediately taken and fixed in formalin. Duodenal and spleen samples were dissected from the retrieved organ and fixed in formalin the same way.
Table 1.
Characteristics of the donors included in the morphological analysis.
| Donor | Diabetes | Age, years | Sex | BMI, kg/m2 | ISO BMI*, kg/m2 | HbA1c, % (mmol/mol) |
|---|---|---|---|---|---|---|
| ND1 | no | 1 | M | 14.8 | N/A | n.d. |
| ND2 | no | 5 | M | 13.9 | <25 | 5.2 (33) |
| ND3 | no | 5 | F | 21.9 | >30 | n.d. |
| ND4 | no | 8 | M | 20.4 | 25–30 | 5.2 (33) |
| ND5 | no | 12 | M | 14.9 | <25 | 5.4 (36) |
| ND6 | no | 12 | F | 18.4 | <25 | n.d. |
| ND7 | no | 13 | M | 19.7 | <25 | 5.2 (33) |
| ND8 | no | 13 | F | 30.5 | >30 | 4.6 (27) |
| ND9 | no | 21 | M | 20.1 | N/A | 5.2 (33) |
| ND10 | no | 27 | M | 26 | N/A | 5.7 (39) |
| D1 | T1D | 29 | M | 24.2 | N/A | 10.4 (90) |
*ISO BMI according to the international cut off points for body mass index for overweight and obesity by sex between 2 and 18 years15 N/A; not applicable, n.d.; not determined
Immunohistochemistry and histological analysis
Formalin-fixed and paraffin-embedded tissue samples were cut into consecutive 6 μm sections and processed and labelled using a standard immunoperoxidase technique, described before, using Dako Autostainer Plus system. All antigens were unmasked by heat-induced epitope retrieval using pH 6.0 or pH 9.0 and labelled with specific primary antibodies (Abcam) (Table 2) visualized using the DAKO EnVision+ System-HRP (DAB) with species-specific secondary antibody. Double stainings were performed with the same antibody clones and protocols as described earlier for synaptophysin/CD45 and insulin/CD3 on sections consecutive to those stained for host defense molecules.16 These were visualized using EnVision G/2 Doublestain System, Rabbit/Mouse (DAB+/Permanent Red) (DAKO). Sections were counterstained with hematoxylin and analyzed by light microscopy. Isotype controls were included for all primary antibodies. Duodenal, tonsil and spleen tissue were used as positive controls (Table 2). The staining patterns for each analyzed host defense molecule was evaluated and the intensity graded as (–) for negative and from 1 (+) to 4 (++++) with (+) being the least intensive positive staining and (++++) being the most intense positive staining. The positive grading corresponds to the overall intensity of the positive staining taking into account roughly the number of positive cells per field of view and the intensity of the positivity. For duodenal tissue, the positivity score accounts for positivity in the duodenal mucosa.
Table 2.
Antibodies used for immunohistochemistry.
| Antibody | Clone | pH | Dilution | Pos. Control |
|---|---|---|---|---|
| Anti-Alpha defensin 1 | Goat polyclonal | 6.0 & 9.0 | 1:100 | Spleen |
| Anti-Alpha defensin 4 | Rabbit polyclonal | 6.0 | 1:100 | Spleen |
| Anti-Reg3a | Rabbit polyclonal | 6.0 | 1:100 | Spleen |
| Anti-Cathelicidin | Rabbit polyclonal | 6.0 | 1:100 | Spleen |
| Anti-Glycoprotein 2 | GP2/1712 | 9.0 | 1:200 | Pancreas |
| Anti-Beta defensin 1 | M11-14b-D10 | 9.0 | 1:50 | Spleen |
| Anti-Beta defensin 2 | Rabbit polyclonal | 9.0 | 1:500 | Duodenum |
| Anti-Beta defensin 3 | Rabbit polyclonal | 6.0 & 9.0 | 1:200 | Spleen |
| Anti-Beta defensin 4 | L13-10-D1 | 9.0 | 1:100 | Tonsil |
Analysis of gene expression data
Datasets generated in other studies of gene expression in 1) isolated human islets and exocrine cell clusters17 and 2) gene expression in laser-captured islets from organ donors of different age were used to analyze the expression of antimicrobial peptides on a transcriptional level. The latter included data from five of the ten donors used also for IHC in the present study (donors ND1 ND2, ND4, ND5, ND6, and ND10). The data were analyzed using Omics Explorer version 3.3 software with an interface to R (Qlucore, Lund, Sweden). Differences in expression of antimicrobial peptides in isolated islets and exocrine cell clusters were analyzed with Student’s T-test and age related differences in the expression in islets were analyzed using a simple linear regression model. The false discovery rate (FDR) was calculated using the Benjamini-Hochberg method.
Results
Expression of anti-microbial peptides in the pancreas of non-diabetic donors
IHC stainings specific for the antimicrobial peptides in non-diabetic organ donors showed positive expression patterns in some or all donors for seven (Cathelicidin, defensin α1, defensin β2, defensin β3, GP2, defensin α4 and REG3A) of the peptides analyzed in the study, while two peptides (defensin β1 and defensin β4) showed no positivity at all in any of the non-diabetic donors. Five (Cathelicidin, defensin α1, defensin β2, GP2 and REG3A) peptides showed positive staining patterns in the exocrine niche of some or all non-diabetic donors, and five peptides (Cathelicidin, defensin β2, defensin β3, defensin α4, REG3A) showed positively stained cells in the Islets of Langerhans in some or all none-diabetic donors. For three (Cathelicidin, defensin β2 and REG3A) peptides, cells were stained positively in both the exocrine and the endocrine niche of the pancreas. (Table 3, Figure 1). Representative examples of staining patterns for each anti-microbial peptide are shown in Figure 2 (Cathelicidin, defensin α1, defensin β2), Figure 3 (defensin β3, GP2, defensin α4) and Figure 4 (Reg 3A, defensin β1) as well as Suppl. Figure 1–2. The age of the donor did not correlate to the positivity score of any of the analyzed peptides in exocrine (Suppl. Figure 4) or endocrine (Suppl. Figure 5) pancreatic tissue.
Table 3.
Expression of antimicrobial peptides in pancreatic tissue and duodenal mucosa in 10 non-diabetic organ donors aged between 1 and 27 years and expression data of the encoding gene in previously published data.
| Molecule | IHC Positivity score# Exocrine tissue |
IHC Positivity score# Endocrine Tissue |
IHC Positivity score# Duodenum |
Staining patterns | Encoding gene and its expression in previously published datasets*, Median (range) |
|---|---|---|---|---|---|
| Cathelicidin | -/+ | -/+/++ | - | Varies between donors, one donor completely negative. Exocrine parenchyma generally negative, singular cells and cell clusters positive. Islet parenchyma negative, singular positive cells. Duodenal mucosa negative. |
CAMP LCM islets: 0 (0–0.13) Isolated islets: 0.1 (0–0.5) Isolated exocrine: 0.5 (0.2–0.5) |
| Defensin Alpha-1 | +/++/+++ | - | -/+ | Exocrine acinar cells largely positive but varies between donors. Some donors show negative acinar cells. Islet parenchyma negative. Dodenal mucosa positive in a few donors. |
DEFA1B LCM islets 0 (0–0.26) Isolated islets: 4.4 (0.0–8.4) Isolated exocrine: 9.4 (8.8–11.5) |
| Defensin Beta-1 | - | - | - | - |
DEFB1 LCM islets: 4.4 (0–244) Isolated islets: 20.4 (13–85) Isolated exocrine: 215 (206–235) |
| Defensin Beta-2 | ++ | ++/+++ | ++/+++/++++ | Exocrine acinar cells largely positive. Islet parenchyma strongly positive Duodenal mucosa shows varying positivity between donors. |
DEFB4A LCM islets: 0 (0–1.13) Isolated islets: 0 (0–3.7) Isolated exocrine: 0.2 (0–7.4) |
| Defensin Beta-3 | + | - | Exocrine parenchyma negative. Islet cell cytoplasms partly positive. Duodenal mucosa negative. |
DEFB103A LCM islets: 0 (0–0.87) Isolated islets: 0 (0–0) Isolated exocrine: 0 (0–0.2) |
|
| Defensin Beta-4 | - | - | - | - |
DEFB4B LCM islets: 0 (0–0.13) Isolated islets: 0 (0–0.6) Isolated exocrine: 0 (0–1.3) |
| GP2 | +/++/+++/++++ | - | - | Exocrine acinar cells shows varying degrees of positivity between cells. Islet parenchyma negative. Duodenal mucosa negative. |
GP2 LCM islets: 382 (46–5774) Isolated islets: 56 (33–172) Isolated exocrine: 966 (603–1515) |
| Defensin Alpha-4 | - | +/++ | +/++/+++ | Exocrine parenchyma negative. Islet cells generally positive. Variation between donors. Duodenal mucosa shows varying positivity between donors |
DEFA4 LCM islets: 0 (0–0) Isolated islets: 0 (0–0.4) Isolated exocrine: 0 (0–0) |
| REG3A | -/+/++ | -/+/++ | +/++/+++ | Two completely negative donors. Exocrine parenchyma shows singular positive cells and clusters in most donors, completely negative in some. Several donors show positive Islet cells. Duodenal mucosa shows varying degrees of positivity. |
REG3A LCM islets: 14 (0.15–1356) Isolated islets: 47 (5.3–1762) Isolated exocrine: 800 (717– 1564) |
#;IHC staining was graded from (–, negative) to (++++, strongly positive)
*Data from previously published datasets17 of expression of the encoding gene in islets extracted by laser capture microdissection, isolated islets and isolated exocrine cell clusters. Data from LCM islets had been acquired by AmpliSeq are expressed as normalized counts whereas data from isolated islets and exocrine tissue are expressed as fpkm (fragments per kilo base per million mapped reads).
Figure 1.
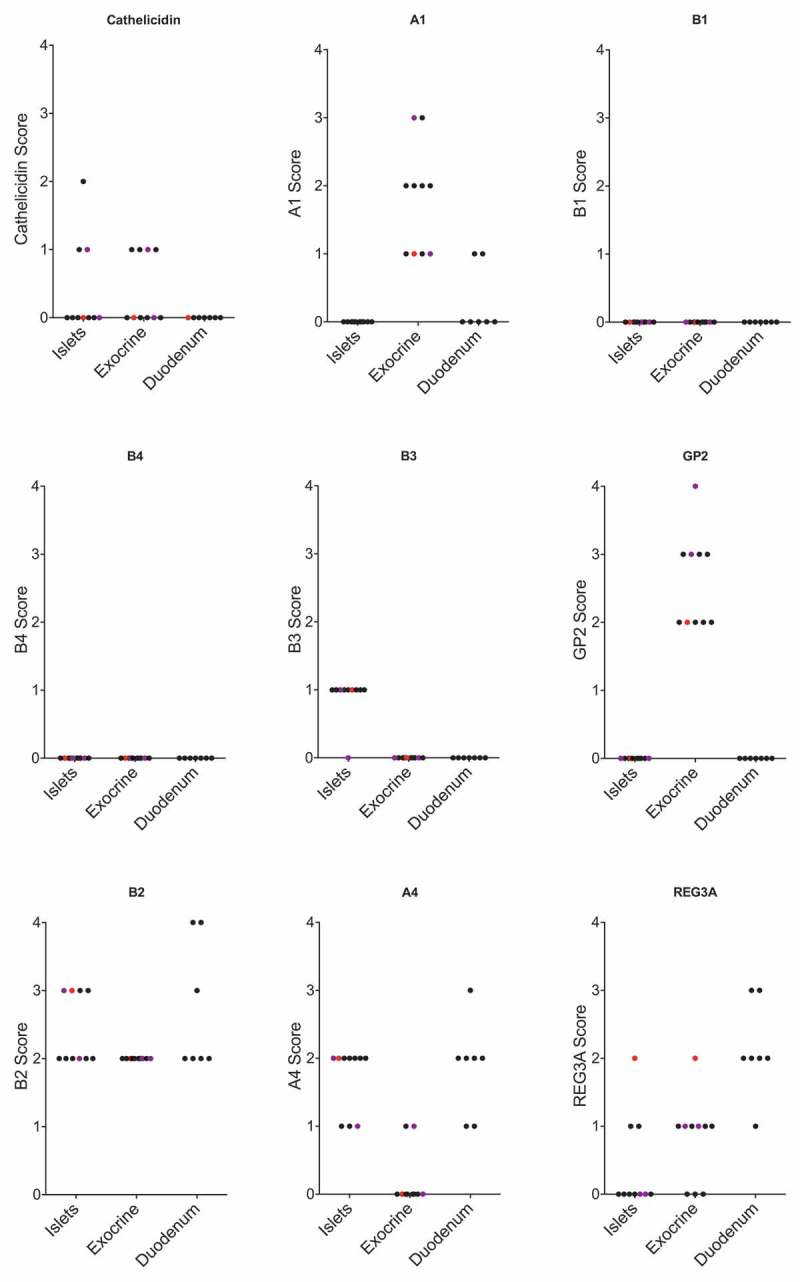
Positivity score for IHC staining of each antimicrobial peptide in islets, exocrine tissue and duodenal mucosa. Each dot represents a pancreatic biopsy from one ND donor. One biopsy had relatively high pancreatic infiltration by immune cells (CD45+) and is marked in red. Two biopsies with moderate infiltration by immune cells are marked in purple. The remaining biopsies had only few infiltrating immune cells.
Figure 2.
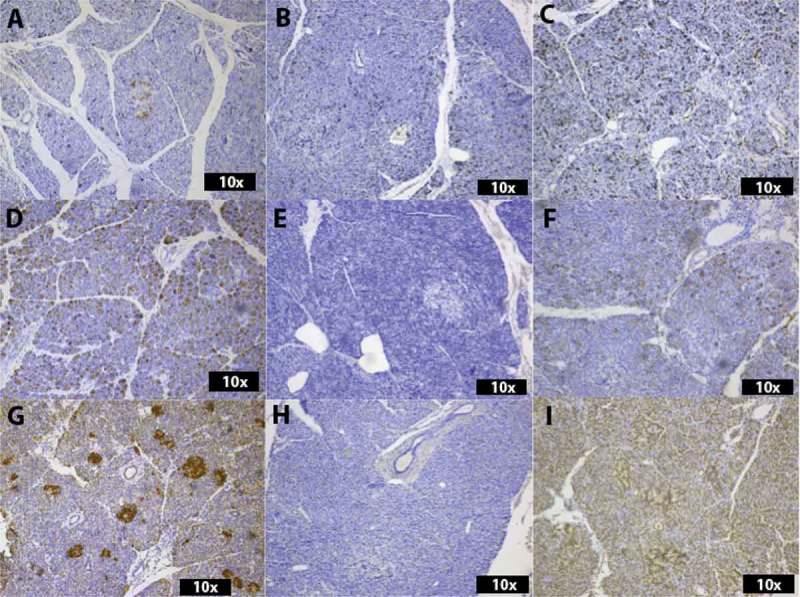
Histological analysis of the expression of Cathelicidin (A-C), Alpha 1 (D-F) and Beta 2 (G-I). In (A), an islet with positive cells is visible in an ND donor. Singular positive cells are scattered throughout the exocrine tissue. The donor with recent onset T1D shows no Cathelicidin-positive cells in the biopsy with low grade immune-cell infiltration (D1CD45lo) (B), whereas the biopsy from the same T1D donor with intense infiltration by immune cells (D1CD45hi) shows multiple positive cells throughout the exocrine parenchyma and in endocrine tissue (C). In (D), Alpha 1-positive cells are spread throughout the exocrine parenchyma of an ND donor, whereas the parenchyma of D1CD45lo is completely negative (E). Notably, D1CD45hi (F) is similar to the ND donor (D). In (G), cells in the exocrine parenchyma as well as multiple islets of an ND donor show strong positivity for Beta 2. Only, singular Beta 2-positive cells can be seen in the exocrine parenchyma of D1CD45lo (H), whereas D1CD45hi shows strong positivity in exocrine and endocrine tissue (I).
Figure 3.
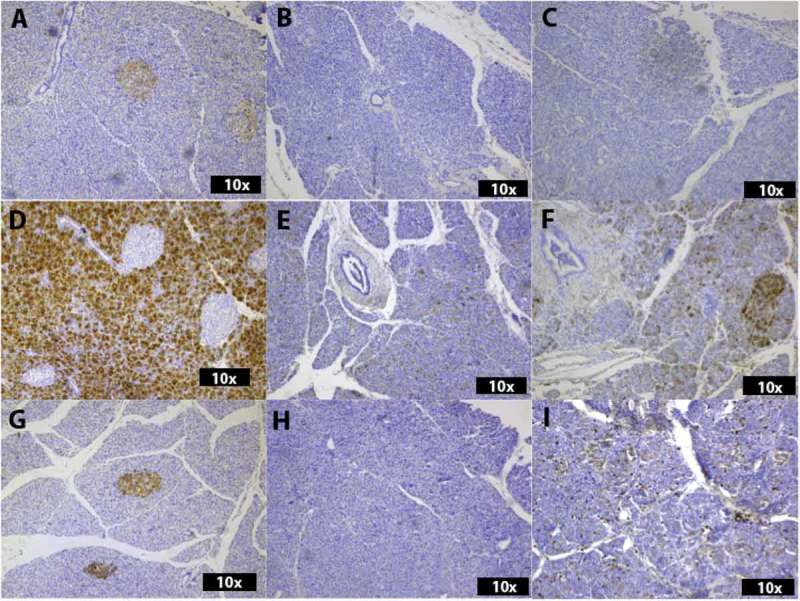
Histological analysis of the expression of Beta 3 (A-C), GP2 (D-F) and Alpha 4 (G-I). In A, islet cell cytoplasm show positivity Beta 3 in an ND donor. Exocrine parenchyma is negative. The two biopsies from a donor with recent onset T1D (B and C) show no positivity for Beta 3. GP2 is highly positive in exocrine parenchyma but negative in islets in an ND donor (D). Singular GP2-positive cells can be seen in the exocrine parenchyma of a T1D biopsy (D1CD45lo)(E) and a stronger positivity is present in the exocrine parenchyma of D1CD45hi (F). In (G), the islet parenchyma is positive for Alpha 4 in an ND donor. D1CD45lo (H) is negative, while D1CD45hi shows singular Alpha 4-positive cells throughout the exocrine parenchyma (I).
Figure 4.
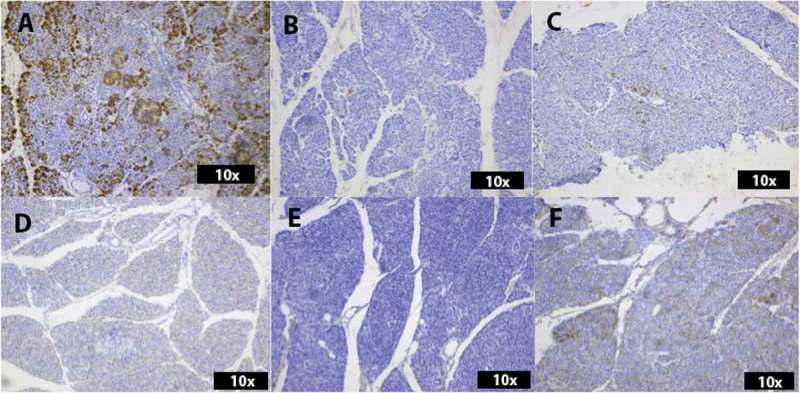
Histological analysis of the expression of REG3A (A-C) and Beta 1 (D-F). Numerous REG3A-positive cells are spread through the exocrine parenchyma and the islets in an ND donor (A). Only singular REG3A-positive cells are present in the exocrine parenchyma of both biopsies from the donor with recent onset T1D (B and C). ND donors and D1CD45lo show no positivity for Beta 1 (D-E), whereas the immune-cell infiltrated T1D biopsy, D1CD45hi, shows Beta 1-positive cells spread through the exocrine tissue (F).
Figure 5.
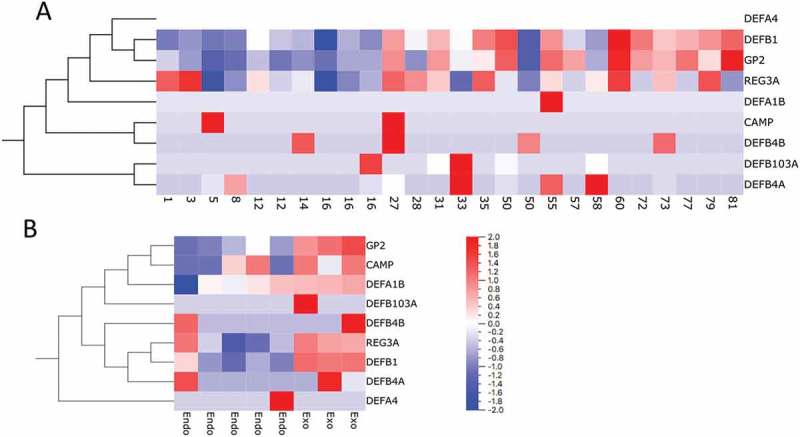
Analysis of the expression of genes encoding antimicrobial peptides in previously published transcriptome data sets from laser-captured islets from donors of different age (range 1-81 years)(A)(Stenwall et al. Submitted) and isolated endocrine and exocrine pancreatic tissue (B) (Danielsson et al., 2014). Genes are ordered by hierarchical clustering.
Analysis of transcriptome data generated in other studies showed that, on the RNA level, the expression of most genes encoding antimicrobial peptides was low both in endocrine and exocrine pancreatic tissue. Three of the genes (DEFB1 encoding defensin β1, GP2 encoding Glycoprotein 2, and REG3A encoding regenerating islet-derived protein 3 α) were highly expressed, especially in exocrine pancreatic tissue but also in islets (Table 3). Moderate expression of DEFA1B, encoding defensin α1, was also present in isolated pancreatic tissue. By linear regression analysis, it was found that the expression of GP2 and DEFB1 correlated positively with increasing age (FDR <0.05) (Figure 5(A)). Heat-maps of the expression levels in laser-captured islets from donors of different age and in isolated endocrine and exocrine tissue are shown in Figure 5(A, B), respectively.
Expression of anti-microbial peptides in the pancreas of a donor with acute onset T1D
IHC staining for the antimicrobial peptide products in the pancreas from an organ donor that died at onset of T1D show positive staining for seven (Cathelicidin, defensin α1, defensin β1, defensin β2, GP2, defensin α4 and REG3A) of the antimicrobial peptides, with two (defensin β3 and defensin β4) showing no positivity (Table 4). Generally, the part of the T1D pancreas with intense immune-cell infiltration (D1CD45hi) was more positively stained for antimicrobial peptides than the part of the same pancreas with little inflammation (D1CD45lo). However, even in the inflamed part, the T1D donor had generally less positivity for the host defense molecules than pancreata from non-diabetic control donors. Cathelicidin, defensin α1, defensin β1, and defensin α4 staining was negative in D1CD45lo but positive in D1CD45hi. Interestingly, defensin β1, which was not expressed in any of the ND pancreata, was clearly positive in scattered cells within the infiltrated pancreatic parenchyma of D1CD45hi (Figure 4(F)). Three (Cathelicidin, defensin β2 and defensin α4) peptides are positively stained in the endocrine niche in one of the biopsies, with no positive cells in the islets in the other biopsy.
Table 4.
IHC positivity score of antimicrobial peptide expression in a 29-year-old subject (D1) that died at onset of type 1 diabetes. Sections are taken from two different parts of the pancreas; one with few infiltrating immune cells (D1CD45lo) and one with intense cellular infiltration (D1CD45hi). The morphological characteristics of the sections from the donor with type 1 diabetes are compared to the characteristics of the non-diabetic (ND) donors. Generally, the staining for antimicrobial peptides is more pronounced in the part of the pancreas with intense infiltration by immune cells (D1CD45hi).
| Molecule | D1CD45lo | D1CD45hi | Comment and comparison with ND donors |
|---|---|---|---|
| Cathelicidin | - | + | D1CD45lo shows no positivity. D1CD45hi shows singular positive cells spread throughout the tissue. Similar morphology as ND cases. |
| Alpha 1 | - | + | D1CD45lo shows no positivity. D1CD45hi shows singular positive cells throughout the tissue but compared with ND cases, the exocrine parenchyma is partially negative. |
| Beta 1 | - | + | D1CD45lo shows no positivity. D1CD45hi shows singular positive cells in the exocrine tissue. ND donors negative. |
| Beta 2 | + | ++/+++ | D1CD45lo shows singular positive cells in exocrine tissue. D1CD45hi shows positivity in exocrine parenchyma and islets, but slightly less compared with ND donors. |
| Beta 3 | - | - | D1CD45lo and D1CD45hi show no positivity, ND cases show positivity in islets. |
| Beta 4 | - | - | - |
| GP2 | + | +/++ | D1CD45lo and D1CD45hi show singular positive cells in exocrine tissue. Less exocrine positivity compared with ND cases. |
| Alpha 4 | - | ++ | D1CD45lo shows no positivity, D1CD45hi show many positive singular cells scattered throughout the tissue. ND donors show positive islets surrounded by negative exocrine tissue. |
| REG3A | + | + | D1CD45lo and D1CD45hi show singular positive cells. ND donors varies, with singular positive cells in exocrine tissue and a few donors with positive islet cells. |
Expression of anti-microbial peptides in the duodenum
Staining for host defense peptides in duodenal mucosa show positivity for 4 (Alpha 1, Alpha 4, Beta 2, and REG3A) peptides, with inter-individual variation in positivity score (Figure 1). We find no correlation between the expression levels in duodenal mucosa and the expression of host defense peptides in the pancreas of ND donors except for Beta 1 and Beta 4, which are negative in both pancreatic and duodenal tissue in all ND donors.
No correlation between immune cell infiltration and the expression of anti-microbial peptides
Morphological analysis of the presence of CD3 and CD45 positive cells in the pancreas show varying degrees of inflammation in the ND donors (Table 5). We find the levels of infiltration generally low, but with one donor having a relatively high level of CD45 positive cell infiltration and two donors having moderate infiltration. We find no apparent correlation between the level of CD45 positive cell infiltration and the positivity score of host defense peptides (Figure 1).
Table 5.
Evaluation of the number of infiltrating CD3 positive cells and CD45 positive cells in the pancreas of 10 non-diabetic donors (ND 1–10) and two biopsies from a donor with recent onset T1D (D1).
| Donor | CD3 | CD45 |
|---|---|---|
| ND1 | + | +++ (Suppl. Fig 3) |
| ND2 | - | + |
| ND3 | + | ++ |
| ND4 | - | + |
| ND5 | - | ++ |
| ND6 | - | + |
| ND7 | - | + |
| ND8 | - | + |
| ND9 | - | + |
| ND10 | - | + |
| D1CD45hi | ++ | ++++ |
| D1CD45lo | + | + |
Discussion
The expression of defensins and other host defense peptides in the human pancreas has not yet been fully characterized. We report IHC findings showing pancreatic exocrine and/or endocrine expression of seven host defense molecules. Several of these showed varying expression patterns between ND donors, suggesting inter-individual differences in host defense peptide expression levels. We find no apparent correlation between the age of the donors, the duodenal expression of host defense molecules or the presence of CD3 or CD45 positive cells in the pancreas, and the pancreatic expression of host defense molecules. However, in a donor that died at onset of T1D, the expression of antimicrobial defense molecules is more pronounced in a part of the pancreas with intense immune-cell infiltration than in a part of the same pancreas with low presence of immune cells. Overall, the pancreas from the donor with T1D had less pronounced expression of host defense molecules compared with ND control tissue. Interestingly, defensin β3, which was detected in the islets from 9/10 ND donors in our study but not in the donors with T1D, has an ortholog in mice that has been shown to protect from diabetes in the NOD mouse when expressed by islet cells.18 Also, cathelicidin antimicrobial peptide has been suggested to have beta-cell protective properties in rodents.19-21
The alpha- and beta defensins are hitherto known to be expressed both constitutively and inductively in various types of tissue, as reviewed by Ramasundara et al. The alpha defensins 1–4 are expressed in neutrophils in the lamina propria of the GI tract, and their expression is induced in intestinal epithelial cells in an inflammatory setting. Beta defensin 1 is constitutively expressed in colonic epithelial while the expression of beta defensins 2–4 is induced by bacteria and IL-1α.22,23 The expression of REG3A is induced by injury and inflammation in the colon.24 When considering inter-individual differences in the expression of defensins in the light of constitutive and induced expression, we hypothesized that the inter-individual differences in our material could be due to varying exposure to inflammatory and infectious stimuli as well as varying sensitivity and capacity to respond to these. The presence of bacteria in pancreases retrieved for organ donation is high and varies between donors.25-32 This is indeed expected due to the direct communication between the duodenum and the pancreas. However, we find no correlation to host defense expression levels and the levels of CD3 or CD45 positive cell infiltrates in the tissue. An observation tentatively explained by the fact that the pancreases were obtained from organ donors. However, since autolysis of the pancreas occurs rapidly after death, biopsies without severe artifacts cannot be obtained from autopsies.
The differences in expression levels seen between individuals could also be a naturally occurring variance, which in turn implies the question of the importance of the constitutive expression levels of host defense peptides in disease susceptibility. The consequences of an absence of, or an abnormally high, constitutive expression level could be related to individually different outcomes in an inflammatory setting.
The importance of REG3A in health and disease has been the subject of several studies. The expression levels of REG3A is increased in the intestinal mucosa of patients with IBD, and high expression levels of other members of the REG family (REG1A and REG4) has been linked to poor outcome in colorectal cancer as reviewed by Granlund et al.33 GP2 has been the focus of much scientific effort in the context of inflammatory bowel disease. Evidence has shown an immunomodulatory role for GP2, as reviewed by Roggenbuck,34 but perhaps most important in a diabetes and pancreatitis context is its ability to bind pathogenic and commensal enterobacteriae, and to be involved in the humoral immune response in the intestinal niche.35 It has also been shown that epithelial cells stimulated with GP2 are able to attract T cells.9 Interestingly, GP2 has been identified as a pancreatic auto-antigen specific to Crohns disease,36 though its mechanistic role in the disease remains unclear.9 Our demonstration of varying degree of REG3A and GP2 positivity in pancreatic tissue from ND donors calls for further studies of the role of these proteins in pancreatic disease.
The gene expression levels of REG3A, GP2, Cathelicidin and human defensin genes were extracted from AmpliSeq expression data from Islets of Langerhans from organ donors aged between 1 and 81 years of age and from RNA sequencing data from isolated islets and endocrine tissue from organ donors.16 Interestingly, the expression levels of genes encoding host defense molecules show inter-individual variation as well, confirming the varying expression patterns demonstrated by IHC. The expression data from isolated endocrine and exocrine tissue show inter-individual differences in the expression levels of several host defense peptides both in islets and exocrine tissue, with higher expression levels of most genes encoding anti-microbial peptides in exocrine tissue compared to islets (Figure 5). The expression level characteristics of Cathelicidin, Alpha 1 and REG3A show similar patterns as in the morphological analysis with an inter-individual variation in positivity in the material. The expression pattern of GP2 is also similar to that of the morphological analysis, with low expression levels in isolated endocrine tissue and low expression in endocrine tissue from the younger donors (age <17 years) in the LCM material.
When analyzing the expression data from islets obtained through laser capture microdissection from non-diabetic organ donors of different ages, Beta 1 and GP2 show expression patterns that vary significantly with advancing age (Figure 5). This is largely analogous to the results from the morphological analysis where GP2 and Beta 1 is not expressed in islets, as all non-diabetic donors included in the present study are 29 years old or younger. Characterizing peptide expression on an RNA expression level is somewhat difficult however, as it does not necessarily equate the amount of final protein or peptide product synthesized by the cells. Also, careful interpretation of the expression data is advised since the RNA expression data analyzed in the study is not from the same biopsies as the morphological analysis and the sample size of the present study is relatively small.
The expression of pancreatic host defense peptides is highly relevant in the context of diabetes and pancreatitis. T1D is in general regarded as an autoimmune disease with specific T-cell mediated beta-cell destruction. There is however a growing body of evidence suggesting that T1D is a disease affecting the entire pancreas, and that it depends on external triggering factors, such as environment and gut microbiota.12,13 As GP2, REG3A and the defensin peptides show antimicrobial properties, it is reasonable to speculate that their presence or absence in the pancreas could result in an adverse immune reaction, where a local bacterial infection from the gut either is not completely eradicated or where an immune response is dysregulated resulting in a destructive immune reaction. While we acknowledge that our material is too small for statistically significant conclusions, we do report a difference in the expression of some of the host defense peptides included in the study between ND donors and one donor with recent-onset T1D as well as marked regional differences in the donor with recent onset T1D. This is especially intriguing as T1D is a lobular disease with anatomically heterogeneous loss of beta cells.13
In pancreatitis, an inflammatory process is triggered within the pancreas and a complex immune reaction results in fulminant disease, as reviewed by Habtezion.37 Gallstones and high alcohol intake are the two most common causes, but bacterial and viral infections are also known to trigger pancreatitis. The importance of defensins in the setting of pancreatitis has been the focus of highly relevant research, and several important findings point towards a role of host defense molecules in the immunological theatre of pancreatic inflammation. In 2010 Tiszlavicz showed that certain polymorphisms of defensin Beta 2 is associated with higher risk of developing acute pancreatitis,38 and in 2014 Cunha showed that the expression levels of antimicrobial peptides belonging to the α defensin family is increased in rats with acute pancreatic injury,39 suggesting the importance of antimicrobial peptides in pancreatic inflammation and pancreatitis.
Several studies have tentatively explored the connection between defensin expression and diabetes. In a recent paper, Németh et al utilized ELISA based protocols to show a significantly higher expression of circulating levels of alpha defensins in subjects with type 1 and type 2 diabetes compared with non-diabetic blood donors.40 Also, decreased circulating levels of cathelicidin and defensin β1 have been demonstrated in subjects with diabetes.41 Similarly, patients with diabetic nephropathy and macroalbuminuria have been shown to have higher concentrations of circulating alpha defensins, when compared to patients with micro- or normoalbuminuria.42 Interestingly, high glucose levels have been found to affect the expression of beta defensins in keratinocytes and in amniotic epithelial cells,43,44 implicating the possibility of defensin expression being somewhat dependent on the metabolic state of the organism. These observations cannot be evaluated in our material, but the possible metabolic regulation of the expression of host defense molecules is highly interesting in the context of diabetes, where an induction of defensin expression from high blood sugar levels could possibly be a protective factor in an early stage of the disease.
We conclude that these findings could have important implications for the inflammatory processes in diabetes and pancreatitis as we find several host defense molecules expressed by the pancreatic tissue. We report both inter-individual and inter-lobular differences, and as the presence of host defense molecules could be induced by a pathogenic stimuli or be constitutive, we find these results particularly interesting as they may be indicative of inter-individual variations in the pancreatic inflammatory repertoire. We do acknowledge however, that the sample size of the present study is small, and careful interpretation of the IHC analysis and the expression data is advised. Further research is needed to unravel the role of antibacterial peptides in the regulation of pancreatic inflammation and of possible connections between the expression of host defense molecules and disease susceptibility.
Funding Statement
The project was funded by the Swedish Medical Research Council (K2015-54X-12219-19-4, 921-2014-7054), the Sten A Olssons Foundation, the Ernfors Family Fund, Barndiabetesfonden, the Swedish Diabetes Association, the Novo Nordisk Foundation, the Diabetes Wellness foundation, the Åke Wiberg foundation, the Tore Nilsson foundation, the South-Eastern Norway Regional Health Authority, EUFP7-Health 2010 PEVNET 261441. Human pancreatic biopsies were obtained from the Nordic Network for Clinical Islet Transplantation, supported by the Swedish national strategic research initiative Excellence of Diabetes Research in Sweden (EXODIAB) and the JDRF.
Acknowledgments
We wish to thank everyone in the Nordic Network for Clinical Islet Transplantation involved in the procurement of pancreatic tissue. We also give our deepest gratitude to all organ donors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Machado LR, Ottolini B.. An evolutionary history of defensins: a role for copy number variation in maximizing host innate and adaptive immune responses. Front Immunol. 2015;6:115. doi: 10.3389/fimmu.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Lu W. Defensins in innate immunity. Curr Opin Hematol. 2014;21:37–42. doi: 10.1097/MOH.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 4.Lehrer RI, Lu W. α-Defensins in human innate immunity. Immunol Rev. 2012;245:84–112. doi: 10.1111/j.1600-065X.2011.01082.x. [DOI] [PubMed] [Google Scholar]
- 5.Agier J, Efenberger M, Brzezinska-Blaszczyk E. Cathelicidin impact on inflammatory cells. Cent Eur J Immunol. 2015;40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Hoover DM, Yang D, Boulegue C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner L, Paclik D, Fritz C, Reinhold D, Roggenbuck D, Sturm A. Identification of pancreatic glycoprotein 2 as an endogenous immunomodulator of innate and adaptive immune responses. J Immunol. 2012;189:2774–2783. doi: 10.4049/jimmunol.1103190. [DOI] [PubMed] [Google Scholar]
- 10.de Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238–1244. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, Piemonti L, Pastore MR, Paroni R. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 12.Korsgren S, Molin Y, Salmela K, Lundgren T, Melhus A, Korsgren O. On the etiology of type 1 diabetes: a new animal model signifying a decisive role for bacteria eliciting an adverse innate immunity response. Am J Pathol. 2012;181:1735–1748. doi: 10.1016/j.ajpath.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skog O, Korsgren S, Melhus A, Korsgren O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes. 2013;20:118–123. doi: 10.1097/MED.0b013e32835edb89. [DOI] [PubMed] [Google Scholar]
- 14.Skog O, Korsgren O. Aetiology of type 1 diabetes: physiological growth in children affects disease progression. Diabetes Obes Metab. 2018;20:775–785. doi: 10.1111/dom.2018.20.issue-4. [DOI] [PubMed] [Google Scholar]
- 15.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundberg M, Seiron P, Ingvast S, Korsgren O, Skog O. Insulitis in human diabetes: a histological evaluation of donor pancreases. Diabetologia. 2017;60:346–353. doi: 10.1007/s00125-016-4140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danielsson A, Ponten F, Fagerberg L, Hallstrom BM, Schwenk JM, Uhlen M, Korsgren O, Lindskog C, Real FX. The human pancreas proteome defined by transcriptomics and antibody-based profiling. PLoS One. 2014;9:e115421. doi: 10.1371/journal.pone.0115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miani M, Le Naour J, Waeckel-Enee E, Verma SC, Straube M, Emond P, Ryffel B, van Endert P, Sokol H, Diana J. Gut microbiota-stimulated innate lymphoid cells support beta-defensin 14 expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 2018;28:557–72 e6. doi: 10.1016/j.cmet.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Pound LD, Patrick C, Eberhard CE, Mottawea W, Wang GS, Abujamel T, Vandenbeek R, Stintzi A, Scott FW. Cathelicidin antimicrobial peptide: a novel regulator of islet function, islet regeneration, and selected gut bacteria. Diabetes. 2015;64:4135–4147. doi: 10.2337/db15-0788. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity. 2015;43:304–317. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Xu M, Ortsäter H, Lundeberg E, Juntti-Berggren L, Chen YQ, Haeggström JZ, Gudmundsson GH, Diana J, Agerberth B. Cathelicidins positively regulate pancreatic β-cell functions. FASEB J. 2016;30:884–894. doi: 10.1096/fj.15-275826. [DOI] [PubMed] [Google Scholar]
- 22.Ramasundara M, Leach ST, Lemberg DA, Day AS. Defensins and inflammation: the role of defensins in inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24:202–208. doi: 10.1111/j.1440-1746.2008.05772.x. [DOI] [PubMed] [Google Scholar]
- 23.Habil N, Abate W, Beal J, Foey AD. Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: dependence on inflammatory cytokines. Benef Microbes. 2014;5:483–495. doi: 10.3920/BM2013.0061. [DOI] [PubMed] [Google Scholar]
- 24.Granlund A, Beisvag V, Torp SH, Flatberg A, Kleveland PM, Ostvik AE, Waldum HL, Sandvik AK. Activation of REG family proteins in colitis. Scand J Gastroenterol. 2011;46:1316–1323. doi: 10.3109/00365521.2011.605463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolic DM. Effects of bacterial infection on insulin secretory capacity of human adult pancreatic islets. Br J Biomed Sci. 2011;68:181–184. [DOI] [PubMed] [Google Scholar]
- 26.Kin T, Rosichuk S, Shapiro AM, Lakey JR. Detection of microbial contamination during human islet isolation. Cell Transplant. 2007;16:9–13. doi: 10.3727/000000007783464498. [DOI] [PubMed] [Google Scholar]
- 27.Bucher P, Oberholzer J, Bosco D, Mathe Z, Toso C, Buhler LH, Berney T, Morel P. Microbial surveillance during human pancreatic islet isolation. Transpl Int. 2005;18:584–589. doi: 10.1111/j.1432-2277.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 28.Bucher P, Mathe Z, Bosco D, Oberholzer J, Toso C, Andres A, Buhler L, Morel P, Berney T. Microbial surveillance during human pancreatic islet isolation. Transplant Proc. 2004;36:1147–1148. doi: 10.1016/j.transproceed.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Lakey JR, Rajotte RV, Warnock GL. Microbial surveillance of human islet isolation, in vitro culture, and cryopreservation. Clin Invest Med. 1995;18:168–176. [PubMed] [Google Scholar]
- 30.Taylor GD, Kirkland T, Lakey J, Rajotte R, Warnock GL. Bacteremia due to transplantation of contaminated cryopreserved pancreatic islets. Cell Transplant. 1994;3:103–106. [DOI] [PubMed] [Google Scholar]
- 31.Scharp DW, Lacy PE, McLear M, Longwith J, Olack B. The bioburden of 590 consecutive human pancreata for islet transplant research. Transplant Proc. 1992;24:974–975. [PubMed] [Google Scholar]
- 32.Carroll PB, Ricordi C, Fontes P, Rilo HR, Phipps J, Tzakis AG, Fung JJ, Starzl TE. Microbiologic surveillance as part of human islet transplantation: results of the first 26 patients. Transplant Proc. 1992;24:2798–2799. [PMC free article] [PubMed] [Google Scholar]
- 33.van Beelen Granlund A, Ostvik AE, Brenna O, Torp SH, Gustafsson BI, Sandvik AK. REG gene expression in inflamed and healthy colon mucosa explored by in situ hybridisation. Cell Tissue Res. 2013;352:639–646. doi: 10.1007/s00441-013-1592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roggenbuck D, Reinhold D, Schierack P, Bogdanos DP, Conrad K, Laass MW. Crohn’s disease specific pancreatic antibodies: clinical and pathophysiological challenges. Clin Chem Lab Med. 2014;52:483–494. doi: 10.1515/cclm-2013-0801. [DOI] [PubMed] [Google Scholar]
- 35.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 36.Roggenbuck D, Reinhold D, Wex T, Goihl A, von Arnim U, Malfertheiner P, Büttner T, Porstmann T, Porstmann S, Liedvogel B, et al. Autoantibodies to GP2, the major zymogen granule membrane glycoprotein, are new markers in Crohn’s disease. Clin Chim Acta. 2011;412:718–724. doi: 10.1016/j.cca.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Habtezion A. Inflammation in acute and chronic pancreatitis. Curr Opin Gastroenterol. 2015;31:395–399. doi: 10.1097/MOG.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiszlavicz Z, Szabolcs A, Takacs T, Farkas G, Kovacs-Nagy R, Szantai E, Sasvári-Székely M, Mándi Y. Polymorphisms of beta defensins are associated with the risk of severe acute pancreatitis. Pancreatology. 2010;10:483–490. doi: 10.1159/000276987. [DOI] [PubMed] [Google Scholar]
- 39.Cunha DM, Koike MK, Barbeiro DF, Barbeiro HV, Hamasaki MY, Coelho Neto GT, Machado MCC, Da Silva FP. Increased intestinal production of α-defensins in aged rats with acute pancreatic injury. Exp Gerontol. 2014;60:215–219. doi: 10.1016/j.exger.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth BC, Varkonyi T, Somogyvari F, Lengyel C, Fehertemplomi K, Nyiraty S, Kempler P, Mándi Y. Relevance of alpha-defensins (HNP1-3) and defensin beta-1 in diabetes. World J Gastroenterol. 2014;20:9128–9137. doi: 10.3748/wjg.v20.i27.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brauner H, Luthje P, Grunler J, Ekberg NR, Dallner G, Brismar K, Brauner A. Markers of innate immune activity in patients with type 1 and type 2 diabetes mellitus and the effect of the anti-oxidant coenzyme Q10 on inflammatory activity. Clin Exp Immunol. 2014;177:478–482. doi: 10.1111/cei.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraheimo M, Forsblom C, Pettersson-Fernholm K, Flyvbjerg A, Groop PH, Frystyk J. Increased levels of alpha-defensin (−1, −2 and −3) in type 1 diabetic patients with nephropathy. Nephrol Dial Transplant. 2008;23:914–918. doi: 10.1093/ndt/gfm711. [DOI] [PubMed] [Google Scholar]
- 43.Lan CC, Wu CS, Huang SM, Kuo HY, Wu IH, Liang CW, Chen G-S. High-glucose environment reduces human beta-defensin-2 expression in human keratinocytes: implications for poor diabetic wound healing. Br J Dermatol. 2012;166:1221–1229. doi: 10.1111/j.1365-2133.2012.10847.x. [DOI] [PubMed] [Google Scholar]
- 44.Szukiewicz D, Alkhalayla H, Pyzlak M, Watroba M, Szewczyk G, Wejman J. Human beta-defensin 1, 2 and 3 production by amniotic epithelial cells with respect to human papillomavirus (HPV) infection, HPV oncogenic potential and the mode of delivery. Microb Pathog. 2016;97:154–165. doi: 10.1016/j.micpath.2016.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


