ABSTRACT
The intracellular peptide pump TAP feeds antigenic peptides for loading onto HLA class I molecules, and its down-modulation is a frequent immune evasion mechanism in human cancers. Two recent papers describe which ‘hidden‘ antigens we might exploit to target these escaped cancer variants by CD8 T cells. These unTAPped peptides are now ready for clinical testing.
KEYWORDS: T cells, HLA class I, immunotherapy, cancer antigens
Introduction
Loss of essential components in the antigen-processing machinery is frequently observed in human cancers, including the peptide pump TAP. This results in low HLA class I antigen surface expression and facilitates immune escape from CD8 T cells. Interestingly, a novel category of non-mutated neoantigens (called ‘TEIPP’- T cell epitopes associated with impaired peptide processing) is presented by these cancers and might function as salvage antigens. Two recent papers shed new light on clinical translation of TEIPP antigens. Marijt et al. described a hybrid forward-reverse immunological screen, which led to the identification of 16 novel human TEIPP peptides1, and Durgeau et al. demonstrated that a pooled peptide vaccination strategy, which included a TEIPP peptide, was able to control the outgrowth of TAP-deficient lung carcinoma in a humanized mouse model.2 Together, these papers show the way for clinical exploration of these unTAPped cancer antigens.
Background of TEIPP research
The last year's much effort has been put in the identification of many point-mutated neoantigens in human cancer, aiming at personalized immunotherapeutic strategies like T cell receptor gene transfer and peptide or mRNA vaccination therapies.3 However, cancers can acquire resistance to CD8 T cell attack by immuno-editing, especially in cases of high immune pressure due to successful immunotherapy.4 Acquired resistance in checkpoint therapy-refractory cancers has been ascribed to loss-of-function mutations in the IFNy signaling pathway, as well as to lost expression of antigen processing genes, including the peptide pump TAP and other chaperones.5 Consequently, this results in low HLA class I (HLA-I) antigen presentation, immune evasion, and cancer progression. The prevalence of cancers that down modulate gene expression TAP1 and TAP2 in a cohort non-small-cell lung carcinomas (NSCLCs) reached up to 71% when compared to levels in adjacent normal tissue.2 Down modulation of TAP and other components of the antigen processing machinery is observed in many other cancer types, including melanoma, colon carcinoma, and cervical carcinoma, especially in progressed cancers.6 Although these tumor cell variants might fail to present conventional tumor-specific cancer antigens, we reported back in 2006 that the residual presenting molecules accommodate antigenic peptides that selectively emerge on such immune-escaped cancers. We named these TEIPP (T cell Epitopes associated with Impaired Peptide Processing).7 TEIPPs are non-mutated “self” antigens derived from housekeeping proteins, but exclusively presented by HLA-I on TAP-deficient cancer cells (Figure 1). Fundamental questions on the characteristics of TEIPP antigen processing, the thymus education of their cognate T cell repertoire and therapeutic potential, were all studied in mouse tumor models. These studies have led to a great understanding of TEIPPs and TEIPP-specific CD8 T cells and were recently summarized in a review paper.8 The hunt for human TEIPPs was rewarded when the group of Fathia Mami-Chouaib in Paris discovered the identity of the peptide-epitope of CD8 T cells recognizing TAP-deficient lung carcinomas.9 The leader peptide of the preprocalcitonin protein was liberated by the proteolytic enzyme SPP (signal peptide peptidase) and loaded unto HLA-A2 in a TAP-independent way. Actually, restoration of the peptide pump TAP decreased recognition by the CD8 T cell clone, exactly as predicted by results obtained from the mouse tumor models.10 Subsequently, we reported in 2010 that TEIPP-specific T cells are not rare, but can rather be readily isolated from blood of healthy donors, although their identity remained unknown.8
Figure 1.
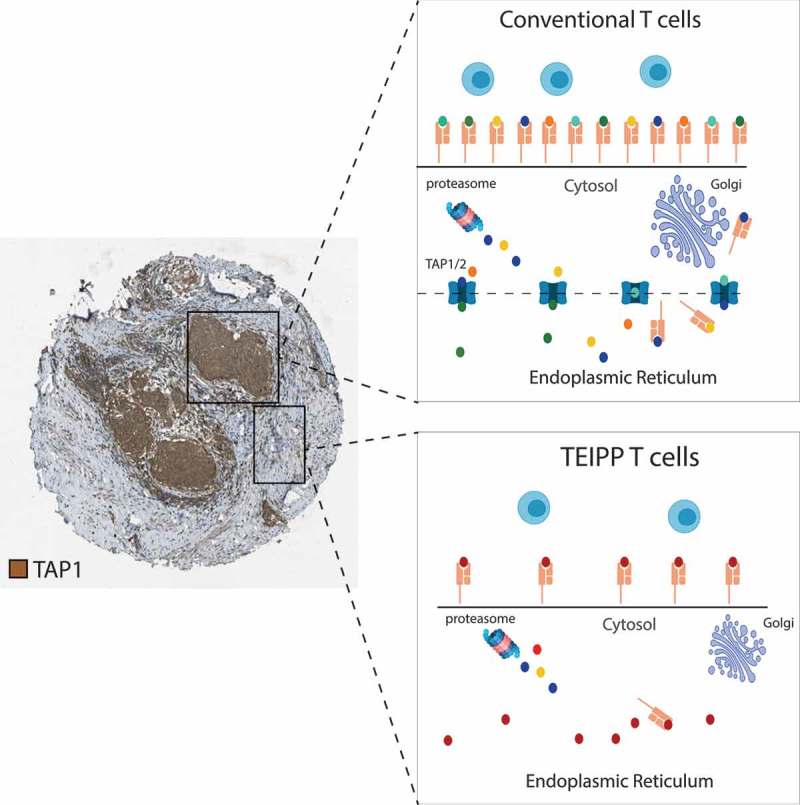
TAP1 protein expression on cervical carcinoma tissue visualized by immunohistochemistry (http://proteinatlas.org).
High expression of TAP1 facilitates the transport of cytosolic peptides into the endoplasmic reticulum (ER) and loading of those antigenic peptides onto HLA class I (HLA-I) molecules. When cancer cells down modulate the peptide pump TAP, it gives rise to the presentation of TEIPP antigens.
Recent characterization of 16 human TEIPP antigens and their efficacy in vivo
Since immune escape often limits the success of T cell mediated therapies, we wanted to investigate the broadness of TEIPPs in humans, and identify more TEIPP antigens for therapeutic purposes against immune-escaped cancers. Therefore, we developed a unique proteome-wide hybrid forward-reverse immunological screen1 Based on antigen processing rules learned from our mouse TEIPP models, protein topology, algorithms for HLA-I binding affinity and large databases of peptides purified from HLA-I molecules of cancers, we created a shortlist of 40 potential HLA-A2 restricted TEIPP candidates for further investigation. First, we examined the existence of a specific CD8 T cell repertoire in the blood of healthy donors by performed HLA-I tetramer pull-down assays succeeded with in vitro peptide stimulations. T cells were detected against 16 out of 40 TEIPP peptide candidates in multiple donors. Second, we observed that T cell receptors of several clones behaved as intermediate-to-high affinity against titrated concentrations of peptide. Interestingly, CD8 T cells against some TEIPP peptides could only be detected in the naïve repertoire of these healthy donors, indicating they were never recruited during an immune response in these persons and ready for exploitation. In-depth analysis of one TEIPP antigen (p14) revealed that TEIPP specific T cell clones were able to target a broad range of TAP-deficient cancer types, including lymphoma, renal cell carcinoma, melanoma, and colon carcinoma, without affecting their TAP-positive counterparts or healthy tissue. All these data involved in vitro culture systems. To assess the therapeutic efficacy of human TEIPP antigens, Durgeau et al. developed a combinatorial peptide vaccination strategy, in which the TEIPP antigen from preprocalcitonin was included. Immunodeficient NSG mice were engrafted with a TAP-deficient human lung carcinoma and treated with blood cells and the peptide vaccine. Excitingly, this approach resulted in increased T cell infiltration of the tumor and improved survival duration of the animals, indicating that TEIPP antigens are capable of targeting immune-escaped tumor variants which are otherwise resistant to conventional antitumor T cells.
Future prospects of TEIPPs
These two recent studies strongly warrant the step forward. The molecular identification of TEIPP antigens enables vaccine applications in different formats, whether it be synthetic peptides, mRNA or DNA. In addition, cloned TEIPP-specific T cell receptors are formidable tools for ‘off-the-shelf’ therapeutics. TEIPPs are non-mutated and expressed by a large diversity of cancer types, so broadly applicable. We argue that targeting of TEIPP antigens might be combined with other immunotherapy modalities that target more conventional cancer antigens in order to prevent immune escape.
Funding Statement
This work was supported by the KWF Cancer Society [2013–6142].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Marijt KA, Blijleven L, Verdegaal EME, Kester MG, Kowalewski DJ, Rammensee H-G, Stevanović S, Heemskerk MHM, van der Burg SH, van Hall T.. Identification of non-mutated neoantigens presented by TAP-deficient tumors. J Exp Med. 2018;215(2325–2337). doi: 10.1084/jem.20180577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durgeau A, Virk Y, Gros G, Voilin E, Corgnac S, Djenidi F, Salmon J, Adam J, de Montpréville V, Validire P, et al. Human preprocalcitonin self-antigen generates TAP-dependent and -independent epitopes triggering optimised T-cell responses toward immune-escaped tumours. Nat Commun. 2018;9(5097). doi: 10.1038/s41467-018-07603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vormehr M, Tureci O, Sahin U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu Rev Med. 2019;70(395–407). doi: 10.1146/annurev-med-042617-101816. [DOI] [PubMed] [Google Scholar]
- 4.Verdegaal EM, de Miranda NFCC, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536(91–95). doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 5.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(707–723). doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leone P, Shin E-C, Perosa F, Vacca A, Dammacco F, Racanelli V. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumor cells. J Natl Cancer Inst. 2013;105(1172–1187). doi: 10.1093/jnci/djt184. [DOI] [PubMed] [Google Scholar]
- 7.van Hall T, Wolpert EZ, van Veelen P, Laban S, van der Veer M, Roseboom M, Bres S, Grufman P, de Ru A, Meiring H, et al. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12(417–424). doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]
- 8.Marijt KA, Doorduijn EM, van Hall T. TEIPP antigens for T-cell based immunotherapy of immune-edited HLA class I(low) cancers. Mol Immunol. 2018. doi: 10.1016/j.molimm.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 9.El Hage F, Stroobant V, Vergnon I, Baurain J-F, Echchakir H, Lazar V, Chouaib S, Coulie PG, Mami-Chouaib F. Preprocalcitonin signal peptide generates a cytotoxic T lymphocyte-defined tumor epitope processed by a proteasome-independent pathway. Proc Natl Acad Sci U S A. 2008;105(10119–10124). doi: 10.1073/pnas.0802753105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira CC, Querido B, Sluijter M, Derbinski J, van der Burg SH, van Hall T. Peptide transporter TAP mediates between competing antigen sources generating distinct surface MHC class I peptide repertoires. Eur J Immunol. 2011;41(3114–3124). doi: 10.1002/eji.201141836. [DOI] [PubMed] [Google Scholar]


