ABSTRACT
Immune checkpoint blockade (ICB) therapy has achieved remarkable clinical benefit in melanoma. However, our understanding of biomarkers that predict response to ICB remained obscure. Here we systematically analyzed the association between somatic mutations profile and clinicopathologic information from 336 melanoma patients treated by ICB (CTLA-4/PD-1). We identified eight new significantly mutated genes including COL5A1, SEMA3E, COL28A1, DGKG, RAPGEF5, GLDN, NCF2 and RCAN2. A mutational signature featured by enrichment of T > C mutations was identified to be associated with immune resistance (logistic regression model, OR, 2.59 [95%CI, 1.07 to 7.00], P = .043). High neoantigen quality was associated with prolonged immunotherapy survival (log-rank test, P = .009). This association remained significant after controlling for age, gender, stage and hypermutation (Cox proportional hazards model, HR, 0.56 [95%CI, 0.38 to 0.82], P = .003). Our findings shed new insights on biomarkers that are useful to predict melanoma patients who may benefit from ICB treatment; however, these biomarkers need to be validated in future studies.
KEYWORDS: Melanoma, immunotherapy, mutational signatures, COL5A1, neoantigen quality
Introduction
Immune checkpoint blockades (ICB) therapy such as anti-CLA4 and/or anti-PD-1 have demonstrated durable antitumor effects in treatment of melanoma and other tumors.1-4 Recent genomic studies elucidated potential genetic aberrations underlying ICB response in melanoma.5-10 The cancer genomic and transcriptomic features were shown to associate with ICB response. High tumor mutation load was associated with favorable immune response in patients who received ICB treatment.5 Mutations in genes involved in antigen presentation and interferon circuits were associated with resistance to ICB treatment.11,12
The characteristic mutational signatures are the fingerprints of endogenous and exogenous factors that have acted over the course of tumor development and progression. In melanoma, C > T transitions (correlated with ultraviolet radiation exposure) were more prominent in patients benefit from immunotherapy.13 Tumor microenvironment (TME) also associates with response to ICB therapy. Fibroblasts are major cellular component of the TME and cancer-associated fibroblasts induce specific deletion of CD8+ T cells to protect tumor cells.14,15 Baseline levels of tumor-infiltrating CD8+ T cells were shown to be correlated with the likelihood of immune response.16
Somatic mutations can cause tumor-specific neoantigen (neopeptide fragments) that could serve as T-cell targets.17 Neoantigen prediction can be exploited to identify responders to immune checkpoint inhibitors.18,19 The purposes of this study were to characterize driver genes, mutational signatures and prognosticators in melanoma patients who were treated by ICB.5-10 Findings emerged from this study may be useful for guiding immunotherapy treatment for melanoma patients.
Results
Tumor genomic characteristics and SMGs associated with immune response
Somatic mutational profiles of 336 melanoma patients from previous genomic immune studies were analyzed (Table S1 in Supplementary Material). A median of 331 mutations per sample (range from 1 to 9210) in a total of 225,691 coding somatic mutations were collected from six previously published research. Overall, we found that higher somatic tumor mutation load was significantly associated with superior immune response (Wilcoxon rank sum test, P = .0347; Figure S1 in Supplementary Material). We used MutSigCV algorithm to identified significantly mutated genes (SMGs) of melanoma followed by additional filtering procedures (Methods). In total, we identified 60 SMGs (Figure 1 and Table S2 in Supplementary Material). Apart from well-known driver oncogenes in melanoma (e.g. BRAF, NRAS, NF1, PREX2, ARID2, PPP6C, PTEN), we identified eight new SMGs (i.e., COL5A1, COL28A1, SEMA3E, DGKG, RAPGEF5, GLDN, NCF2 and RCAN2), whose mutations were associated with improved overall survival (log-rank test, P < .05; Figure S2A in Supplementary Material and Figure 1). The mutation types of these eight new SMGs are shown in Figure S3 in Supplementary Material and Table S3 in Supplementary Material. COL5A1 and COL28A1 were the members of fibrillar forming collagen; rs13301426 C > T in COL5A1 was reported as potentially functional SNPs in melanoma.20 DGKG was mutated in 8.6% of melanoma samples; 24 of the 29 non-silent mutations in DGKG were missense mutations. SEMA3E plays an important role in signaling via the cell surface receptor, and modulates immunological synapse and migration of thymocytes.21 The Rap guanine nucleotide exchange factor 5 encoding gene RAPGEF5 regulated nuclear translocation of β-Catenin in Wnt signaling.22 NCF2, mutated in 3.5% of melanoma cases, was reported to be involved in autoimmune disease.23 RCAN2, also known as MCIP1, inhibits calcineurin-dependent transcriptional responses by binding to the catalytic domain of calcineurin.24 GLDN, mutated in 5.3% samples, plays a role in the formation and maintenance of the nodes of Ranvier on myelinated axons.25
Figure 1.
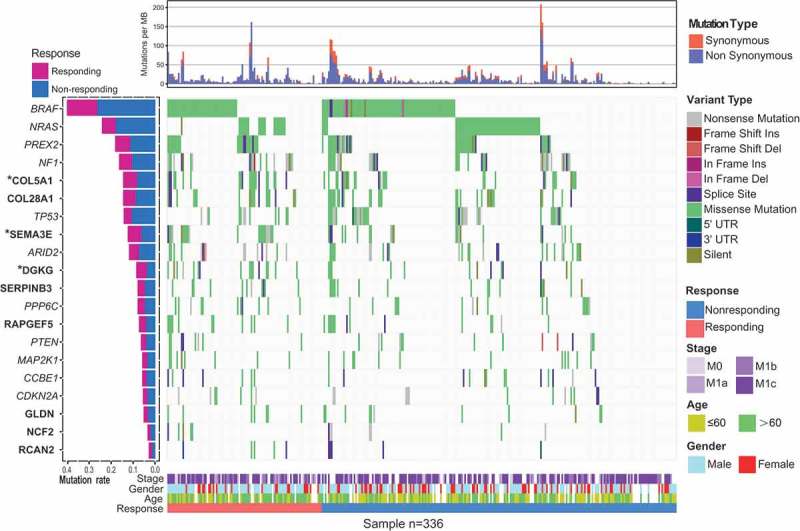
Mutational landscape of significantly mutated genes (SMGs) of melanoma patients treated with ICB. The left panel is mutation frequency. Upper panel shows mutation load stratified by synonymous and non-synonymous mutations. Middle panel depicts mutation relation of SMGs across analyzed cases with mutation types color coded differently. Bottom panel displays clinical features such as immune response status, age, gender and stage. Prognosis-related SMGs were highlighted in bold and immune response-related genes are highlighted in upper left asterisk.
COL5A1 mutation predictive of survival and immune cell infiltration
Three out of eight new SMGs COL5A1, SEMA3E and DGKG were enriched in patients benefited from ICB therapy (Fisher’s exact test, P < .05; Figure S2B in Supplementary Material). Mutations in COL5A1 were associated with better survival (log-rank test, P = .031; Figure 2(a)). The association between COL5A1 mutations with immunotherapy survival remained statistically significant after taking into account age, gender and stage (Figure 2(b)). The TMLs in samples of COL5A1 mutations are significantly higher than those without mutations (Figure 2(d)). In the subgroup analysis, we found that patients with COL5A1 mutation in anti-PD-1 cohort (n = 111) and anti-CTLA-4 cohort (n = 179) exhibited a trend with better survival outcome (Figure S4 in Supplementary Material). Furthermore, we found that CD8+ T cells and activated NK cells were enriched in COL5A1 mutant group; nevertheless, resting memory T cells and neutrophils were enriched in COL5A1 wild-type group (Figure 2(c)). These findings support previous observations that CD8+ T cells and NK cells promote immune response,26,27 whereas neutrophils impede immune response.28 We also observed that COL5A1 mutant samples had higher abundance of CD4+T cells in melanoma patients that estimated by TIMER algorithm (P < .05; Figure S5 in Supplementary Material).
Figure 2.
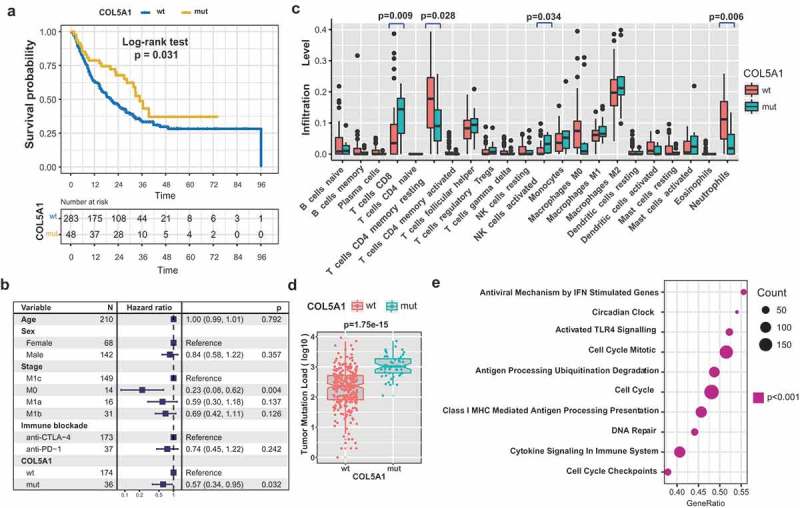
COL5A1 mutation were associated with ICB treatment response. (a) Kaplan–Meier survival analysis of COL5A1 mutations. (b) Multivariate Cox regression analysis of COL5A1 mutations with age, gender, tumor stage and ICB types were taken into account. (c) Relative abundance of tumor infiltrating leukocytes in COL5A1 mutant-type versus COL5A1 wild-type samples. (d) Tumor mutation load in melanoma samples was compared by COL5A1 mutation status. The notch in the box shows the 95% confidence interval of the median. (e) GSEA pathway enrichment of the top differentially expressed gene sets in COL5A1 mutant versus COL5A1 wild-type group.
Gene set enrichment analysis revealed that signaling pathways involved in cytokine-mediated immune system, antigen processing presentation, cell cycle and IFN stimulation were significantly upregulated in samples with COL5A1 mutations compared with those without COL5A1 mutation (P < .001; Figure 2(e)). Collectively, these evidences suggested that COL5A1 may be associated with regulation of immune response.
Mutational signatures correlated with ICB response
The overall mutational pattern was dominated by C > T mutations and mutation patterns are similar between the responding versus non-responding group (Figure 3(a)). The profiles of mutational signatures extracted from ICB treated samples are comparable with mutational signatures extracted from TCGA melanoma cohort (Figure S6 in Supplementary Material). We extracted six mutational signatures (i.e., signatures 1, 3, 7, 11, 18 and 26; Figure 3(b)) from mutation data of ICB-treated melanoma with varying mutational activities. The six signatures were annotated against the COSMIC signature nomenclature29,30 (Figure S7 in Supplementary Material). Signature 1 was dominated by C > T mutation that is associated with age-related accumulation of spontaneous deamination of 5-methylcytosine. Signatures 7 and 11 were associated with large numbers of C > T substitutions and found predominantly in melanoma, which were likely due to ultraviolet light exposure. Signatures 3 and 26, characterized by T > C mutations, are associated with deficiency in genomic integrity maintenance and have been found in breast, ovarian, and pancreatic cancers.31 Signatures 3 and 7 were also extracted and identified in TCGA melanoma cohort (cosine similarity, signature 3, 0.78; signature 7, 0.99) (Figure S8A in Supplementary Material and Table S4 in Supplementary Material).
Figure 3.

Mutational signatures extracted from the aggregated melanoma samples. (a) Lego plot representation of mutation patterns in responding patients (a, up) and non-responding patients (a, down). Single-nucleotide substitutions are divided into six categories with 16 surrounding flanking bases. The pie chart in upper left shows the proportion of six categories of mutation patterns. (b) The mutational activities of corresponding extracted mutational signatures. (c) Forest plot representation of the association between mutational activities of signature 3 with ICB treatment response status. The non-responder group was taken as the baseline, and the odds ratio of the responder group was evaluated with respect to the non-responder group. Age, sex, stage, and ICB types were likewise taken into account.
To identify mutagenic factors responsible for immune response, multivariate regression analyses were performed to analyze the relationship between mutation signature activities with the immune response/resistance. We observed that signature 3 was significantly associated with immune resistance in multivariable regression analysis (logistic regression model: OR, 2.59 [95% CI, 1.07 to 7.00], P = .043, Figure 3(c)). Signaling pathways involved in deficiency of genomic integrity maintenance were significantly altered in patients with mutational signature 3 compared with those without mutational signature 3 (Figure S8B in Supplementary Material). We also compared the signature 3 with TML and found a significantly lower TML in patients with signature 3 (Wilcoxon rank sum test, median TML, 52 vs. 380, P < .001, Figure S8C in Supplementary Material). Besides, patients with signature 3 in anti-PD-1 cohort were significantly associated with immune resistance, and a similar tendency was also observed in anti-CTLA-4 cohort although not statistically significant (Figure S9 in Supplementary Material).
Neoantigen qualities model identified the immunotherapy survivor
Kaplan–Meier analysis was performed to compare the neoantigen load (median neoantigen load as cutoff) with the immunotherapy survival and response in melanoma patients. However, we have not found significant difference in prognosis and response rate between high and low neoantigen load (log-rank test, P = .78; Wilcoxon rank sum test, P = .202; Figure S10A-B in Supplementary Material).
We then calculated the weight mean scores of each patient and divided into neoantigen quality high and quality low groups based on median quality scores. High neoantigen quality was significantly associated with favorable overall survival in the neoantigen quality model (log-rank test, P = .009; Figure 4(a)). Association of high neoantigen quality remained statistically significant in multivariate Cox model with age, gender, stage and hypermutation status (Cox proportional hazards model, HR, 0.56 [95%CI, 0.38 to 0.82], P = .003; Figure 4(b)). We further compared the tumor mutation load and immune response in different neoantigen quality group, and noticed a higher TML and preferable immune response in high neoantigen quality group (Wilcoxon rank sum test, P < .001 for TML, P = .043 for immune response; Figure S10C-D in Supplementary Material).
Figure 4.
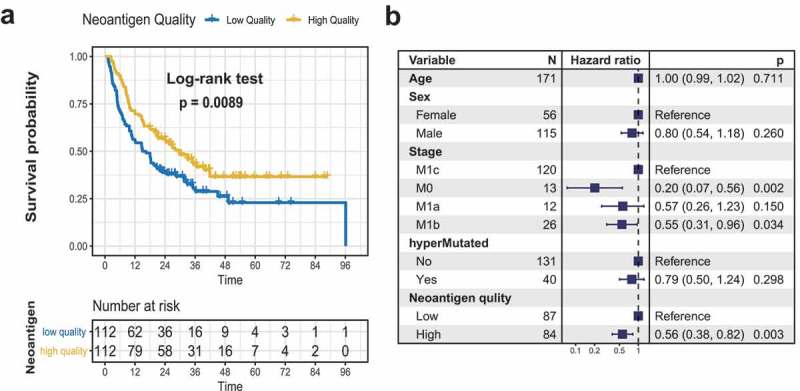
Neoantigen quality is associated with overall survival of melanoma patients treated with ICB. (a) Kaplan–Meier survival analysis of neoantigen quality. (b) Multivariate Cox regression analysis of neoantigen quality model by taking into account confounding factors such as age, gender, tumor stage and hypermutation status.
We found that patients with COL5A1 mutation and high neoantigen quality were associated with better survival, whereas presence of mutational signature 3 was associated with worse survival. Additionally, we combined COL5A1 mutation, high neoantigen quality and lack of mutational signature 3 as a single marker. Samples with the combined marker exhibited better association with ICB response than any of each individual marker (hazard ratio: combined marker,0.25 [95%CI, 0.09 to 0.70], Figure S11 in Supplementary Material; COL5A1 mutation, 0.57[95%CI, 0.34 to 0.95]; mutational signature 3, 0.66 [95%CI, 0.43 to 1.02], neoantigen quality, 0.56 [95%CI, 0.38 to 0.82]).
Discussion
The development of anti-PD-1 and anti-CTLA-4 agents has revolutionized therapeutic strategies for melanoma, non-small cell lung cancer and clear cell renal cell carcinoma.5,32,33 In this study, we performed systematic analysis of genomic data from 336 melanoma patients treated with ICB. We observed that mutational signature 3 and neoantigen quality were associated with naïve immune response. Besides, we also identified COL5A1 gene mutation as improved prognosticators for ICB therapy.
The ability to redefine mutational significance with expanding sample sizes may identify new biologically and clinically relevant genes and pathways not previously appreciated. In this study, we identified COL5A1 as a new SMG. Our analysis found that mutations of COL5A1 were associated with antigen process and presentation, immune system and interferon pathways (Figure 2(e)), and enrichment of CD8+ T cell and activated NK cells (Figure 2(c)). This implicated that COL5A1 was involved in the pathogenesis of immune response in melanoma. COL5A1 (Collagen type V α1 chain) encoded an alpha chain for one of the low abundance fibrillar collagens, whose high expression could contribute to tumor progression.34-36 COL5A1 is highly immunogenic,37 and decreased and abnormal synthesis of collagen type V α1 chain was found associated with immune response and endothelial death in cancer microenvironment.38 Besides, collagens were expressed in stromal cell fibroblasts and high levels of some collagens (e.g. collagens type I, III, V) suppressed immune activation.39
In addition to examining association between gene mutation and survival, we further extracted and characterized the mutation signatures. Patients with signature 3 harbored weaker immune response when merely treated by ICB agents. Recent advances have reported that PARP inhibitor (i.e., olaparib), treatment of DNA homologous recombination (HR) deficiency tumors (e.g. with signature 3),40,41 enhanced the immunogenicity and activation of host anti-tumor immune cells in breast and ovarian cancer.42,43 Collectively, we hypothesize that melanoma patients with significant presence of signature 3 may benefit from combination treatment of ICB and PARPi. These findings bridge the gap between PARPi and immune therapy with somatic mutations in relation to immune response.
Besides, it has been reported that higher neoantigen load was associated with better antitumor activity to ICB treatment.5,44 We did not observe significant association of the neoantigen load with response to immunotherapy in this collected data set. However, we found melanoma patients with high neoantigen quality significantly outlived patients with low neoantigen quality. We posited TILs could cross-reactively recognize both tumor neoantigens and homologous non-tumor microbial antigens, so that the neoantigen quality could identify bona fide neoantigens targeted by T cells.45,46 Our study used by far the largest number of samples to identify molecular markers for the patients who may benefit from ICB treatment.
Calculation of neoantigen quality required HLA type and MHC binding affinity data; however, such information was only available for anti-CTLA-4 cohort but not anti-PD-1 cohort; therefore, association between neoantigen quality and immune response in anti-PD-1 cohort is still unknown. In the aforementioned subgroup analysis, we observed that such identified markers in both anti-PD-1 and anti-CTLA-4 cohort were associated with better immune response status. Anti-PD-1 regimen exhibited stronger association with overall survival than the anti-CTLA-4 regimen even though the later had a large number of patients. Recent studies reported that anti-PD-1 regimen (such as nivolumab and pembrolizumab) resulted in longer overall survival than anti-CTLA-4 (such as ipilimumab) in metastatic melanoma patients.47,48 Presumably, the association of COL5A1 mutation and signature 3 with clinical benefit from ICB treatment were better in anti-PD-1 cohort, but less significant in anti-CTLA-4 cohort.
In this study, we assembled and characterized genomic data and clinical information from 336 patients treated by ICB to determine whether the tumor genetic landscape affects the clinical benefit. These studies have identified putative genomic mutation signature and molecular biomarkers of response or resistance to ICB treatment, demonstrating the complex interplay of host and tumor in immune response. More broadly, we identify neoantigen quality as a biomarker for prognostic estimation that may guide the application of immunotherapies.
Materials and methods
Genomic data and clinical information
A total of 225,691 somatic mutations were acquired from six previous studies totaling 336 pre-treatment melanoma cases.5-10 Mutations were re-annotated by oncotator.49 Clinicopathological information including age, gender, stage, immune blockades type, immune response status, and survival were curated from supplemental materials of these six studies (Tables S5 and S6 in Supplementary Material). The predicted MHC binding affinity scores, HLA types, and clinical features were collected from the previous studies5,6,9 (n = 224). Patients with complete or partial responses were considered to be efficacious to ICB treatment and the rest were regarded as the non-responding. Gene expression data were available in Riaz. N and Hugo. W cohorts.7,8 Overall survival for the Zaretsky. J.M cohort were not available,10 and progression-free survival were only found in Van Allen. E.M and Zaretsky. J.M cohorts.5,10 Somatic mutations for 467 melanoma samples in the TCGA were downloaded from Genome Data Commons (https://portal.gdc.cancer.gov).
Identification of significantly mutated genes
We identified SMGs by using MutSigCV algorithm.50 MutSigCV measures the significant enrichment of non-silent somatic mutations in a gene by addressing mutational context specific background mutation rates. Candidate SMGs were required to meet these criteria: statistically significant (q < 0.1), expressed in human cancer cell lines51 and in the TCGA pan-cancer dataset.30
Deciphering mutational signature operative in the genome
The framework proposed by Kim et al.52 was used to extract mutational signatures. This framework is based on Bayesian variant nonnegative matrix factorization and it can automatically determine the optimal number of mutational signatures. The mutation portrait matrix A was factorized into two nonnegative matrices W and H, where W representing mutational signatures and H representing the corresponding mutational activities. The number of columns of matrix W indicates the number of extracted signatures and the rows indicated the 96 mutational contexts, which derived from combinations of 6 mutational types (i.e., C > A, C > G, C > T, T > A, T > C, and T > G) and their 5ʹ and 3ʹ adjacent bases. The rows of matrix H represented the signatures the columns represented the mutational activities of each samples. Mutational signatures were annotated by calculating cosine similarity against 30 validated mutational signatures in the Catalogue of Somatic Mutations in Cancer (COSMIC, v85, released 08-May-18d).
Tumor infiltrating lymphocyte cell analysis
We curated gene expression data of 72 pre-treatment samples from Riaz and Hugo et al.7,8 The CIBERSORT algorithm was used to estimate the relative abundance of 21 tumor infiltrating lymphocyte cells (TILs)53 with ICB response status. Besides, the abundance of six infiltrating immune cells (B cells, CD4+T cells, CD8+T cells, neutrophils, macrophages, and dendritic cells) were also estimated with the Tumor Immune Estimation Resource (TIMER) algorithm.54
Gene set enrichment analysis
We partitioned 72 cases whose gene expression profiles were available into two groups according to mutation status of COL5A1. The limma R package was used to calculate the differential expressed statistics. We used these statistics as input to R -function in ClusterProfile package to do gene set enrichment analysis (GSEA). The gene sets examined in GSEA of REACTOME pathways and GO Biological Process Ontology were obtained from MSigDB database (v6.2).55,56
Neoantigens quality scoring
We applied and followed the neoantigen fitness modeling proposed by Balachandran et al.17 to quantify the neoantigen quality. Consistent with filtering criterion used in Balachandran et al., somatic mutations with predicted MHC binding affinity below 500nM were kept for neoantigen quality analysis. We obtained sequence alignment scores by aligning in silico translated amino acid sequences centered on mutated nucleotides to human infectious disease-derived, class I-restricted peptide sequences with positive immune assays from the Immune Epitope Database (IEDB).57 We then inferred its probability of TCR recognition using a nonlinear logistic dependence on alignment score, and calculated quality scores by amplifying these binding probabilities by inferred relative wild-type and mutant peptide-MHC class I affinities (Table S7 in Supplementary Material). The neoantigen quality of each sample was calculated as weighted mean neoantigen qualities of all mutations in that sample. The predicted affinity binding scores of the mutant peptides in that sample were used weights. These samples were split into two groups by using the median value of neoantigen quality score of all samples.
Statistical analyses
The statistical analyses in this study were performed with R software (version 3.2.3). The continuous variables between groups were compared by the Wilcoxon rank sum test. The association between proportion of mutated genes and immune therapy response was evaluated by Fisher’s exact test. Kaplan–Meier survival analysis and Cox proportional hazards model were used to analyze the association between mutated genes and prognosis with the R survival package. Association of mutational signatures with immune response was examined by the logistic regression by including confounding factors such age, sex, stage and ICB regimens. All comparisons were two-sided with a significance level of 0.05, and the Benjamini-Hochberg method was applied to control false discovery rate (FDR) for multiple hypothesis testing.58
Funding Statement
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University in China [grant number IRT_14R40 to CK), Natural Science Foundation of Tianjin [grant number 16JCYBJC24700 to CK and LB) and the National Foundation for Cancer Research, the National Natural Science Foundation of China [grant number 31801117 to LX).
Abbreviations
| ICB | Immune check-point blockade |
| CTLA-4 | Cytotoxic T-lymphocyte protein 4 |
| PD-1 | Programmed cell death protein 1 |
| TML | Tumor mutation load |
| HR | Hazards ratio |
| OR | Odds ratio |
| TCGA | The Cancer Genome Atlas |
| PARPi | Poly ADP-ribose polymerase inhibitor |
| COSMIC | Catalog of Somatic Mutations in Cancer |
| GSEA | Gene set enrichment analysis |
| TIMER | Tumor Immune Estimation Resource |
| SKCM | Skin Cutaneous Melanoma |
Acknowledgments
We thank Yichen Yang and Wei Chong from Tianjin Cancer Institute for protocols and helpful discussions.
Disclosure statement
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
All studies have been approved by the Institutional Research Board.
Availability of data and materials
All relevant data and materials within this work are made available in this manuscript and TCGA databases.
Authors contributions
KXC and XCL contributed to the conception and design. KXC, XCL, and HC contributed to the development of methodology. KXC, XCL, and HC contributed to the acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.). XCL, HC, MY, QHW, and FJS contributed to the analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis). XCL, KXC, and HC contributed to the writing, review, and/or revision of the manuscript. XCL and HC contributed to the administrative, technical, or material support (i.e., reporting or organizing data, constructing databases). KXC and XCL contributed to the study supervision.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with Nivolumab in CHECKMATE 025. Eur Urol. 2017;72:368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall survival with combined Nivolumab and Ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, Gopalakrishnan V, Wang F, Cooper ZA, Reddy SM, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9. doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riaz N, Havel JJ, Makarov V, Desrichard A, Urba WJ, Sims JS, Hodi FS, Martin-Algarra S, Mandal R, Sharfman WH, et al. Tumor and microenvironment evolution during immunotherapy with Nivolumab. Cell. 2017;171:934–949 e15. doi: 10.1016/j.cell.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DSM, Pauken KE, Huang AC, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167:1540–1554 e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz-Adalia A, Ramirez-Santiago G, Osuna-Perez J, Torres-Torresano M, Zorita V, Martinez-Riano A, Boccasavia V, Borroto A, Martinez Del Hoyo G, Gonzalez-Granado JM, et al. Conventional CD4(+) T cells present bacterial antigens to induce cytotoxic and memory CD8(+) T cell responses. Nat Commun. 2017;8:1591. doi: 10.1038/s41467-017-01661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DB, Frampton GM, Rioth MJ, Yusko E, Xu Y, Guo X, Ennis RC, Fabrizio D, Chalmers ZR, Greenbowe J, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. 2016;4:959–967. doi: 10.1158/2326-6066.CIR-16-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, Komoto S, Katsube R, Ninomiya T, Tazawa H, et al. Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3(+) T Cells Via IL6 in the tumor microenvironment. Clin Cancer Res. 2018;24:4820–4833. doi: 10.1158/1078-0432.CCR-18-0205. [DOI] [PubMed] [Google Scholar]
- 15.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD.. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topalian SL, Taube JM, Anders RA, Pardoll DM.. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, Herbst B, Askan G, Bhanot U, Senbabaoglu Y, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512–516. doi: 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Kim HS, Kim E, Lee MG, Shin EC, Paik S, Kim S. Neopepsee: accurate genome-level prediction of neoantigens by harnessing sequence and amino acid immunogenicity information. Ann Oncol. 2018;29:1030–1036. doi: 10.1093/annonc/mdy022. [DOI] [PubMed] [Google Scholar]
- 19.Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Wang Y, Liu H, Shi Q, Li H, Wu W, Zhu D, Amos CI, Fang S, Lee JE, et al. Genetic variants of PDGF signaling pathway genes predict cutaneous melanoma survival. Oncotarget. 2017;8:74595–74606. doi: 10.18632/oncotarget.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda Y, Kondo N, Ozawa M, Yasuda K, Tomiyama T, Kinashi T. Sema3e/Plexin D1 modulates immunological synapse and migration of thymocytes by Rap1 inhibition. J Immunol. 2016;196:3019–3031. doi: 10.4049/jimmunol.1502121. [DOI] [PubMed] [Google Scholar]
- 22.Griffin JN, Del Viso F, Duncan AR, Robson A, Hwang W, Kulkarni S, Liu KJ, Khokha MK. RAPGEF5 regulates nuclear translocation of beta-catenin. Dev Cell. 2018;44:248–260 e4. doi: 10.1016/j.devcel.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim-Howard X, Sun C, Molineros JE, Maiti AK, Chandru H, Adler A, Wiley GB, Kaufman KM, Kottyan L, Guthridge JM, et al. Allelic heterogeneity in NCF2 associated with systemic lupus erythematosus (SLE) susceptibility across four ethnic populations. Hum Mol Genet. 2014;23:1656–1668. doi: 10.1093/hmg/ddt532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. [DOI] [PubMed] [Google Scholar]
- 25.Wambach JA, Stettner GM, Haack TB, Writzl K, Skofljanec A, Maver A, Munell F, Ossowski S, Bosio M, Wegner DJ, et al. Survival among children with “Lethal” congenital contracture syndrome 11 caused by novel mutations in the gliomedin gene (GLDN). Hum Mutat. 2017;38:1477–1484. doi: 10.1002/humu.23297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daud AI, Loo K, Pauli ML, Sanchez-Rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Invest. 2016;126:3447–3452. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 28.Ferrucci PF, Ascierto PA, Pigozzo J, Del Vecchio M, Maio M, Antonini Cappellini GC, Guidoboni M, Queirolo P, Savoia P, Mandala M, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27:732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 29.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014;15:585–598. doi: 10.1038/nrg3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, Norton C, Bosse D, Wankowicz SM, Cullen D, et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science. 2018;359:801–806. doi: 10.1126/science.aan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB, Abu-Akeel M, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–852 e4. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Wei H, Gao Z, Chen G, Liu Y, Gao X, Bai G, He S, Liu T, Xu W, et al. COL5A1 may contribute the metastasis of lung adenocarcinoma. Gene. 2018;665:57–66. doi: 10.1016/j.gene.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 35.Ren W, Zhang Y, Zhang L, Lin Q, Zhang J, Xu G. Overexpression of collagen type V alpha1 chain in human breast invasive ductal carcinoma is mediated by TGF-beta1. Int J Oncol. 2018. doi: 10.3892/ijo.2018.4317. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Li X, Yang M, Xu L, Deng S, Ran L. Prediction of biomarkers of oral squamous cell carcinoma using microarray technology. Sci Rep. 2017;7:42105. doi: 10.1038/srep42105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B, Wilkes DS. Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol. 2000;23:62–70. doi: 10.1165/ajrcmb.23.1.3924. [DOI] [PubMed] [Google Scholar]
- 38.Souza P, Rizzardi F, Noleto G, Atanazio M, Bianchi O, Parra ER, Teodoro WR, Carrasco S, Velosa AP, Fernezlian S, et al. Refractory remodeling of the microenvironment by abnormal type V collagen, apoptosis, and immune response in non-small cell lung cancer. Hum Pathol. 2010;41:239–248. doi: 10.1016/j.humpath.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 39.Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwe H, Pircher A, Van Den Eynde K, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 40.Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Aspects Med. 2013;34:1217–1256. doi: 10.1016/j.mam.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoonen PM, Talens F, Stok C, Gogola E, Heijink AM, Bouwman P, Foijer F, Tarsounas M, Blatter S, Jonkers J, et al. Progression through mitosis promotes PARP inhibitor-induced cytotoxicity in homologous recombination-deficient cancer cells. Nat Commun. 2017;8:15981. doi: 10.1038/ncomms15981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, Gimotty PA, Adams SF. CTLA-4 blockade synergizes therapeutically with parp inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res. 2015;3:1257–1268. doi: 10.1158/2326-6066.CIR-15-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23:3711–3720. doi: 10.1158/1078-0432.CCR-16-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and anticancer immunosurveillance. Cell. 2016;165:276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Routy B, Gopalakrishnan V, Daillere R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 47.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 49.Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, Meyerson M, Getz G. Oncotator: cancer variant annotation tool. Hum Mutat. 2015;36:E2423–E2429. doi: 10.1002/humu.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klijn C, Durinck S, Stawiski EW, Haverty PM, Jiang Z, Liu H, Degenhardt J, Mayba O, Gnad F, Liu J, et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat Biotechnol. 2015;33:306–312. doi: 10.1038/nbt.3080. [DOI] [PubMed] [Google Scholar]
- 52.Kim J, Mouw KW, Polak P, Braunstein LZ, Kamburov A, Kwiatkowski DJ, Rosenberg JE, Van Allen EM, D‘Andrea A, Getz G. Somatic ERCC2 mutations are associated with a distinct genomic signature in urothelial tumors. Nat Genet. 2016;48:600–606. doi: 10.1038/ng.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li B, Severson E, Pignon JC, Zhao H, Li T, Novak J, Jiang P, Shen H, Aster JC, Rodig S, et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17:174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR, Wheeler DK, Gabbard JL, Hix D, Sette A, et al. The immune epitope database (IEDB) 3.0. Nucleic Acids Res. 2015;43:D405–D412. doi: 10.1093/nar/gku938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data and materials within this work are made available in this manuscript and TCGA databases.


