ABSTRACT
Intravesical Bacille Calmette-Guérin (BCG) remains the most effective treatment for high-risk non-muscle-invasive bladder cancer (NMIBC), unfortunately there is no validated biomarker to predict clinical outcome. Here we tried to explore the possibility that a combination of the density of peritumoral infiltrating cells (Th1, Th2 and PD-L1) and the composition of peripheral immune cells (neutrophil and lymphocyte counts) could generate a more reliable prognostic biomarker. Twenty-two patients with high-risk NMIBC treated with BCG (10 BCG nonresponders and 12 BCG responders) were selected. BCG responders had significantly lower level of peritumoral T-bet+ cells with an associated higher GATA-3+/T-bet+ ratio (p = 0.04, p = 0.02, respectively). Furthermore, the immune polarization in tissue (GATA-3+/T-bet+ ratio) adjusted for the systemic inflammation (neutrophil-to-lymphocyte ratio) showed a significantly higher association with the BCG response (p = 0.004). A survival analysis demonstrated prolonged recurrence-free survival (RFS) in patients with a lower T-bet+/Lymphocyte ratio and higher GTR/NLR (p = 0.01). No association was observed between peritumoral PD-L1+ expression and the BCG response. In conclusion, alterations in overall immune function, both local and systemic, may influence the therapeutic response to BCG, therefore a combined analysis of tumoral (Th2/Th1 ratio) and peripheral (NLR) immune composition prior to treatment may be a promising approach to predict the BCG response in high-risk NMIBC patients.
KEYWORDS: NMIBC, BCG immunotherapy, GATA-3, T-bet, NLR, biomarker
Introduction
In patients with high-risk non-muscle-invasive bladder cancer (NMIBC), immunotherapy with Bacillus Calmette-Guérin (BCG) is the most successful adjuvant treatment;1 nevertheless, the reported response rate is only 60% with 5-year recurrence rates of 30% to 40% and progression rates from 9% to 13%.2 Despite many efforts, no biomarkers are currently able to predict patient prognosis to discriminate individuals who will respond to BCG treatment from individuals who would be best served by more aggressive therapies, such as cystectomy.3
Tumor-infiltrating lymphocytes (TILs) have a strong influence on cancer-specific survival.4 In patients with metastatic urothelial carcinoma (UC) receiving platinum-based chemotherapy, a recent study has shown that TILs perform as a significant prognostic factor,5 which is in line with other studies demonstrating the positive prognostic value of tumor-infiltrating CD3+ and CD8+ T cells in survival.6,7 Nevertheless, the impact will depend on the phenotype, localization and density of the immune cells present in the tumor,8 with peritumoral cells exhibiting a more critical role than intratumoral cells in the clinical outcome of UC,5 melanoma9 and breast cancer.10
Although the exact mechanism of BCG-induced antitumor activity is not fully understood, it is known that BCG acts as a localized Th1-polarizing immunomodulator;11,12 therefore, one emerging concept in this respect relates to the prognostic and/or predictive value that the pretreatment Th1/Th2 polarization of infiltrating cells may have on the BCG response. Interestingly, in a mixed NMIBC population (Ta, T1 and CIS)13 and a group of patients with carcinoma in situ,14 an increased density of intratumoral Th2 cells and a higher Th2/Th1 ratio in BCG responders prior treatment have been described, which suggests that BCG is effective only when the tumor microenvironment converts from Th2 to Th1.
Programmed Death Ligand 1 (PD-L1) is abundantly present in the tumor microenvironment, where it is expressed by many malignant cells, as well as immune cells. The expression of this inhibitor receptor suppresses anti-tumor immunity and promotes tumor progression.15 In contrast, PD-L1 expression in TILs has been correlated with improved overall survival in patients with UC who developed metastatic disease.16 In NMIBC, the role of PD-L1 expression in the tumor prior to BCG treatment has not previously been characterized; however, a high expression of PD-L1 within BCG-induced granulomas has been associated with resistance to the therapy.17 These data suggest that the accumulation of PD-L1-expressing cells in bladder tissues could abrogate the effectiveness of BCG immunotherapy, a hypothesis reinforced by the recent finding that BCG and anti-PD-L1 combination therapy enhance the antitumor immunity in an animal model.18
In addition to TILs, systemic immunological parameters have also been correlated with cancer outcome. The peripheral absolute lymphocyte count has been suggested to not only be a prognostic factor of survival in several tumor types19 but also a predictor of treatment efficacy.20 Furthermore, the neutrophil-to-lymphocyte ratio (NLR), a measure of systemic inflammation and a strong predictor for prognosis in patients with different diseases,21,22 has been suggested to be a prognostic predictor for overall survival and disease free survival and a predictive marker of the response to treatment in many cancers.23 In bladder cancer, high NLR has been associated with poor clinical outcome in individuals with muscle-invasive bladder cancer (MIBC),24,25 it has been postulated as a prognostic factor for individuals with MIBC undergoing neoadjuvant chemotherapy26-28 and it correlates with disease progression and recurrence in individuals with NMIBC.29,30
Overall, immune biomarkers based on TILs or blood cells have been independently described in cancer patients; however, the possibility that a combination of both factors could generate a more reliable prognostic biomarker has not previously been explored. In recent years, the hypothesis that the ratios between different immune subsets, such as CD4/CD8 or NLR, are more predictive of prognosis has gained substantial attention as these ratios may provide a more comprehensive view of the complexity of the immune system. Therefore, the aim of our study was to determine the densities of Th1 (T-bet+), Th2 (GATA-3+) and PD-L1+ peritumoral cells in bladder tissue samples, as well as the systemic immunity (absolute neutrophil and lymphocyte counts) prior to BCG treatment in a homogeneous population of high-risk NMIBC patients to elucidate whether a combination of these immune parameters could generate a new biomarker useful for predicting the response to BCG.
Results
Participant characteristics
Twenty-two individuals with primary T1HG NMIBC were included in the study. The median age was 70.5 years (IQR: 62–78), and all patients, except for one patient, were men (96%). Ten individuals were classified as BCG nonresponders with a median (range) time to recurrence of 3.5 (2–6) months, and 12 individuals were characterized as BCG responders (median to recurrence not reached) after at least 30 months of follow-up. Both groups were similar in age, gender and BMI and did not exhibit significant differences in the peripheral neutrophil and lymphocyte counts (Table 1).
Table 1.
Participant Characteristics.
| BCG Responders (n = 12) |
BCG Nonresponders (n = 10) |
p-valuea | |
|---|---|---|---|
| Age (years); median (IQR) | 67 (61–78) | 72 (66–80) | 0.41 |
| Gender (male); n (%) | 12 (100%) | 9 (90%) | 0.45b |
| BMI (kg/m2); median (IQR) | 28.2 (26.1–30.4) | 28.7 (26.7–34.6) | 0.41 |
| Time to tumor recurrence; months (range) | Not reached | 3.5 (2–6) | |
| Absolute neutrophils count (x109/L); median (IQR) | 4.5 (3.3–4.8) | 4.6 (4–6) | 0.37 |
| Absolute lymphocyte count (x109/L); median (IQR) | 2.1 (1.6–2.6) | 1.8 (1.3–2.4) | 0.32 |
| NLR; median (IQR) | 1.9 (1.6–2.7) | 2.3 (1.8–4.2) | 0.12 |
BMI: Body Mass Index. NLR: neutrophil-to-lymphocyte ratio. aMann-Withney U-test. bFisher exact test
Tumor-infiltrating Th1 and Th2 polarized lymphocytes
The expression of transcription factor T-bet (Th1) and GATA-3 (Th2) was found in the peritumoral tissues prior to BCG treatment in all individuals tested. A significantly higher infiltration of Th2 cells than Th1 cells was identified, with a median (IQR) number of total GATA-3+ and T-bet+ cells of 644 (369–1064) and 170 (64–277), respectively (p < 0.0001, Figure 1(a)), and a GATA-3+/T-bet+ ratio (GTR) of 4.9 (2.4–8.4). There was no significant correlation between the total density of peritumoral GATA-3+ cells and T-bet+ cells in all patients (Spearman r = 0.3; p = 0.15. Figure 1(b)). When BCG response was taken into account, we observed that the response was significantly associated with a lower level of peritumoral T-bet+ cells, with no changes in the total density of peritumoral GATA-3+ cells (median 134 and 246 cells; p = 0.04 and 708 and 604 cells; p = 0.4 in BCG responders and BCG nonresponders, respectively, Figure 2(a,b)). Furthermore, a higher GTR was identified in the BCG responders than in the BCG nonresponders (7.2 and 2.6, respectively; p = 0.02, Figure 2(c)). Representative immunohistochemical stainings of GATA-3, T-bet and hematoxylin-eosin in bladder tissue obtained in the RTU prior to BCG treatment of a BCG responder (left) and a BCG nonresponder (right) are shown in Figure 3. Lower level of T-bet+ cells in the BCG responder than in the nonresponder (111 vs 325 T-bet+ cells, respectively), similar values of GATA-3+ cells (1090 vs 908 cells, respectively) and higher GTR (9.8 vs 2.7, respectively) can be observed.
Figure 1.
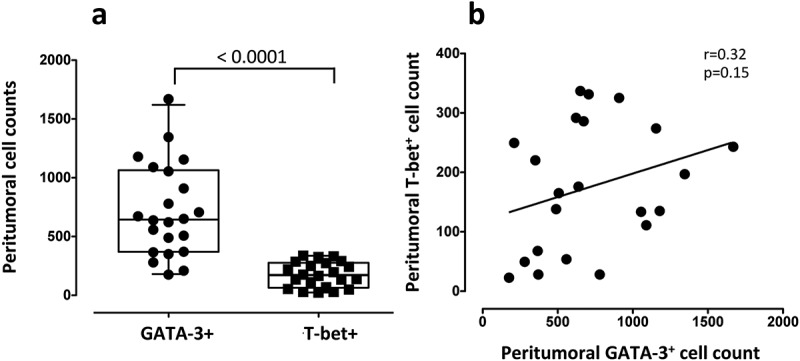
Tumor-infiltrating Th1 (T-bet) and Th2 (GATA-3) lymphocytes in bladder tissues prior to BCG treatment in high-risk NMIBC patients. (a) Total density of peritumoral T-bet+ and GATA-3+ cells in all patients. The boxes represent the median and interquartile range of the values. Individual data of all subjects is displayed. The median values were compared using a non-parametric Mann-Whitney U-test. (b) Correlation analysis of peritumoral GATA-3+ and T-bet+ cell. Linear correlation (Spearman) r and p-values are shown.
Figure 2.
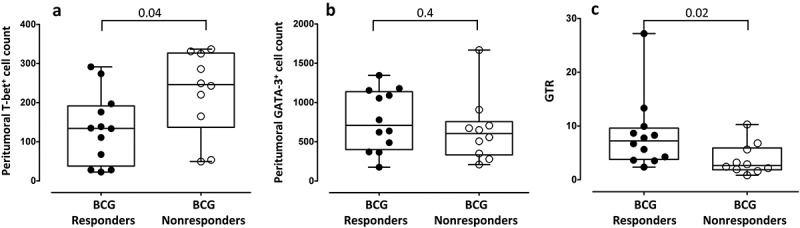
Peritumoral lymphocytes in bladder tumors prior to BCG treatment in BCG responders and BCG nonresponders. (a) Total density of T-bet+ cells and (b) GATA-3+ cells in the tumor before BCG treatment in responders and nonresponder to BCG. (c) GTR in BCG responders and nonresponders. Median and IQR values are shown. Nonparametric Mann-Whitney U test was used to analyze differences between both groups. GTR (GATA-3+/T-bet+ ratio).
Figure 3.

Immunohistochemistry analysis of peritumoral TILs and hematoxylin/eosin (HE) staining. Representative images of bladder tissue stained with anti-GATA-3, anti-T-bet and HE in a BCG responder and a BCG nonresponder patient. 10X and 40X magnification (left and right part or each patient, respectively) are shown.
Relationship between systemic immunity and bladder immune infiltration and their association with the response to subsequent BCG treatment
The levels of peripheral absolute lymphocytes, neutrophils, and the systemic inflammatory marker NLR prior to BCG treatment were obtained for all individuals (Table 1). The levels of neutrophils, lymphocytes and the NLR were 4.5 × 109 cells/L, 1.9 × 109 cells/L and 2.3, respectively, in the total population with no significant differences in any of these parameters between the two treatment groups; however, a slightly higher NLR in the BCG nonresponder population was observed (median 1.9 vs 2.3 for responders and nonresponders, respectively; p = 0.12, Table 1). Spearman correlation analysis between the peripheral and local immunity prior to BCG treatment showed that the peritumoral density of T-bet+ and GATA-3+ cells was not correlated with the peripheral absolute neutrophil or lymphocyte counts in the total population, the BCG responders or the nonresponders (Figure S1). However, when the T-bet+ and GATA-3+ cell density was normalized with the absolute lymphocyte count, a significantly lower T-bet+/Lymphocyte ratio was identified in the individuals with a BCG response (median (IQR): 53 (23–95) and 141 (78–211) in BCG responders and nonresponders, respectively; p = 0.04, Figure 4(a)), with no differences between the normalized GATA-3+ cell levels between both groups (median (IQR): 321 (214–490) and 403 (197–597) in BCG responders and nonresponders, respectively; p = 0.77, Figure 4(b)). In addition, when a new immune score GTR/NLR was calculated by combining the local immune polarization (GATA-3+/T-bet+ ratio) with the systemic inflammation using the NLR value, BCG responders had a significantly higher ratio than BCG nonresponders (median (IQR): 3.6 (1.5–5.6) and 0.78 (0.63–1.8), respectively; p = 0.004, Figure 4(c)).
Figure 4.
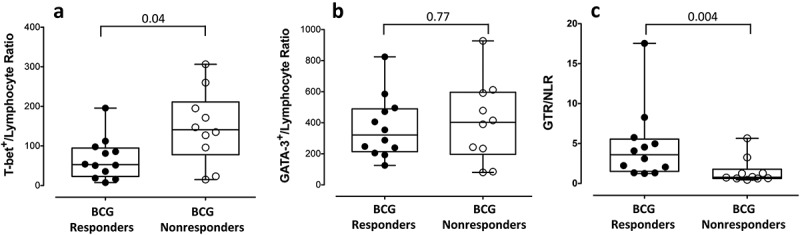
Combination of local and systemic immunity biomarkers in BCG responders and BCG nonresponders. (a) Total cell density of T-bet+ cells and (b) GATA-3+ cell normalized to the absolute lymphocyte counts. (c) The GTR/NLR immune score was obtained by normalizing the immune polarization in the tissue to the level of systemic inflammation (NLR). Differences were tested using Mann-Whitney U nonparametric test. GTR (GATA-3+/T-bet+ ratio). NLR (neutrophil-to-lymphocyte ratio).
Influence of pretreatment immunity on recurrence-free survival (RFS) after BCG therapy
To perform survival analyses, all individuals were divided into two groups (low vs high counts), based on the median value of the different variables analyzed (median cell density: 170 for T-bet+ cell count; 644 for GATA-3+ cell count; 4.95 for GTR; 4.45 × 109 cells/L for neutrophils; 1.85 × 109 cells/L for lymphocytes; 90.81 for T-bet+/Lymphocyte ratio, 2.26 for NLR, 1.69 for GTR/NLR ratio, and 70.5 years for age). The individuals with a lower level of peritumoral T-bet+ cells showed a trend towards better RFS, although significance was not reached (p = 0.11, Figure 5(a)). The inclusion of systemic immunity showed that patients with a low T-bet+/Lymphocyte ratio prior to BCG treatment had a significantly prolonged RFS compared with the individuals with a high ratio (HR = 5.3; 95% CI:1.4–19.9; p = 0.01, Figure 5(b)). The same significant difference in RFS was identified when the GTR/NLR index was evaluated, with a prolonged RFS in the patients with a higher index (HR = 0.18; 95% CI:0.05–0.7; p = 0.01, Figure 5(c)). The difference in RFS between individuals with low and high T-bet+/Lymphocyte ratios was maintained even when data was separated by age. However, the difference was not significant, likely because of the low number of individuals in each group (Figure S2). The sensitivity ranged from 66.7% (95% CI: 34.9–90.1%) for peritumoral T-bet+ cell count to 75% (95% CI: 42.8–94.5%) for T-bet+/Lymphocyte ratio and GTR/NLR index and the specificity ranged from 70% (95% CI: 34.8–93.3%) for peritumoral T-bet+ cell count to 80% (95% CI: 44.4–97.5%) for T-bet+/Lymphocyte ratio and GTR/NLR index. No association was identified for other parameters, such as age, GATA-3+ cells, neutrophils, lymphocytes or NLR.
Figure 5.
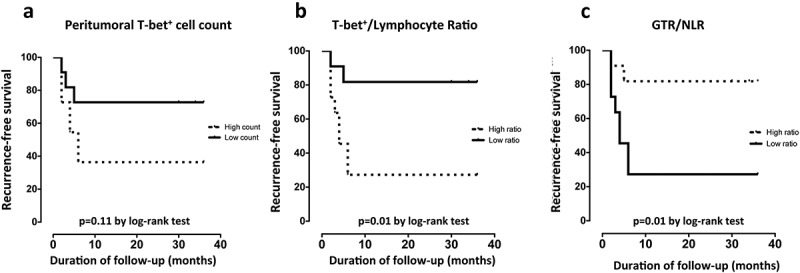
Kaplan Meyer survival curves for recurrence-free survival (RFS) in months. RFS according to (a) total peritumoral T-bet+ cells, (b) T-bet+/lymphocyte ratio and (c) the immune score GTR/NLR stratified by high or low count based on the median (≤median = low count; >median = high count). P values by log-rank test.
PD-L1 expression in tumoral cells and peritumoral infiltrating immune cells
The PD-L1 expression in tumor cells prior to BCG treatment was mostly negative with only 2 individuals (9%) being positives in the population. In contrast, 16 of the 22 samples (73%) had positive PD-L1 expression (≥ 1%) in peritumoral infiltrating immune cells. Thirteen (59%), 9 (41%), 8 (36%), 5 (23%) and 2 (9%) samples had expressions of ≥5%, ≥10%, ≥20%, ≥25% and ≥35%, respectively. Representative IHC staining is shown in Figure S3. No differences in the expression pattern of PD-L1 were identified between the BCG responder and nonresponder individuals. Expression was observed in 70% (7 of 10) of the BCG responders and 75% (9 of 12) of the BCG nonresponders with the same distribution among the different levels of positivity (data not shown). To evaluate the relationship between PD-L1 in tissue, systemic and other tissue immune cells, samples were classified according to their PD-L1 level of expression as (≤10% or >10%) and according to BCG response. The individuals with the highest percentages of peritumoral PD-L1+ cells had a significantly lower number of peripheral blood lymphocytes (p = 0.04, Table 2), however no association with age, other systemic or local immune parameters or BCG response was found (Table 2).
Table 2.
Relationship between PD-L1 in tissue and other immune parameters.
| PD-L1 expression in Peritumoral immune cells |
p-valuea |
||||||
|---|---|---|---|---|---|---|---|
| ≤10% (n = 14) |
> 10% (n = 8) |
||||||
| BCG Responders (n = 8) |
BCG Nonresponders (n = 6) |
p- valueb |
BCG Responders (n = 4) |
BCG Nonresponders (n = 4) |
p-valueb | ||
| Age (years); median (IQR) | 63 (60–77) | 72 (61–78) | 0.6 | 74 (64–85) | 75 (68–84) | 1 | 0.12 |
| Absolute neutrophils count (x109/L); median (IQR) | 4.5 (3.3–4.8) | 4.6 (4–5.6) | 0.6 | 4 (2.8–5.1) | 5.4 (3–8.7) | 0.6 | 0.9 |
| Absolute lymphocyte count (x109/L); median (IQR) | 2.1 (1.7–2.5) | 2.1 (1.7–2.8) | 1 | 2 (1.4–2.6) | 1.2 (1.1–1.7) | 0.1 | 0.04 |
| NLR; median (IQR) | 1.8 (1.6–2.7) | 2 (1.8–2.6) | 0.5 | 2.3 (1.3–3) | 4.5 (2.7–5.2) | 0.1 | 0.07 |
| Total Peritumoral GATA-3+ cells; median (IQR) | 630 (400–1012) | 454(262–757) | 0.2 | 1105 (395–1173) | 662 (543–1420) | 0.9 | 0.18 |
| Total Peritumoral T-bet+ cells; median (IQR) | 125(38–192) | 235 (53–327) | 0.2 | 134 (50–239) | 265 (185–324) | 0.1 | 0.6 |
| GTR ratio; median (IQR) | 6.2 (3.6–12.5) | 2.5(1.5–6.8) | 0.08 | 8 (5.1–8.6) | 2.8 (2–6) | 0.06 | 0.9 |
aComparison of low PD-L1 expression (≤10%) and high PD-L1 expression (> 10%); bComparison of BCG responders and BCG nonresponders.
Discussion
The search for predictive markers of the clinical response to intravesical BCG in NMIBC has been a constant feature in urological research in recent years. Given that up to 30–50% of patients will experience treatment failure, and up to 15% of these cases can progress, the early identification of patients who will not respond to BCG instillations is essential, so that other treatments can be used and/or radical treatments (early cystectomy) are not delayed.
Several studies have indicated that TILs have a major impact on the clinical course of several cancers (for a review, see ref. 4). In our study, the total cell density of peritumoral cells in bladder tissues prior to BCG treatment was evaluated, as it has been reported that cells surrounding the tumor (peritumoral TILs) are more crucial than intratumoral TILs for survival.5,9 Regarding phenotype, we observed a predominant GATA-3+ (Th2) over T-bet+ (Th1) peritumoral infiltrate. This Th1/Th2 imbalance is in accordance with other reports that evaluated tissues from NMIBC patients13,14,31 or other cancer types in which the imbalance was described not only in tissues but also in peripheral blood mononuclear cells and cytokine production.32,33 We found no correlation between the density of tumor-infiltrating GATA-3+ and the density of T-bet+, which suggests that although they are mutually antagonistic,34 other cytokines/chemokines produced within the tumor microenvironment are tipping the observed immune imbalance.35
The influence of the presence of different TIL subpopulations in the tissue prior to treatment on the BCG response in homogeneous populations of high-risk NMIBC patients has not been fully investigated. Increased intratumoral CD4+, GATA-3+ T-cells and GTR have been associated with the BCG response and a longer RFS in a mixed population of low and high-risk NMIBC (Ta, T1 and CIS).13 A similar tendency towards increased numbers of GATA-3+ cells in BCG responders has also been reported by the same authors, although only 4 BCG nonresponders were evaluated.31 Consistent with these data, the GTR has been described as a predictor of the BCG response in one study restricted to CIS patients.14 These observations led authors to propose that an intratumoral Th2 predisposition prior to treatment determined the clinical response to BCG. In our population of high-risk NMIBC patients (T1HG), we observed the same significant association of GTR with the BCG response. However, a significantly lower level of T-bet+ cells was identified in BCG responders without significant changes in the total density of peritumoral GATA-3+ cells; these findings indicate that the total density of T-bet+ cells, rather than the density of peritumoral GATA-3+ cells, is the determinant factor for treatment response. These data provide novel arguments to reinforce the hypothesis that in high-risk NMIBC patients, tumor-infiltrating Th1 cells present prior to BCG treatment could be a population of inactive cells, due to immune escape or tolerance, and therefore, the expansion of these cells after the addition of BCG is unlikely to be effective.14,31 We can speculate that the detection of those cells is indicating the presence of a paradoxically immunosuppressive environment. In line with this hypothesis is the fact that, although T-bet is a transcription factor required for differentiation of and IFN-γ secretion by CD4+ Th1 T cells, some activated Treg cells express T-bet, which have been suggested to provide Treg cells with enhanced suppressive capacity.36,37 Although this T-bet+Treg are currently a subject of research, it has been described that despite the T-bet expression, this T-bet+Treg subtype is characterized by regulatory, rather than pro-inflammatory properties, producing less IFN-γ and TNF-α as compared with regular T-bet+ Th1 cells38 and therefore, in patients with high-risk NMIBC the presence of those cells in the tissue could be contributing to the BCG failure. Thus, the development of new therapeutics targeting these cells prior to BCG treatment could be a new strategy to improve BCG’s response.
In addition to local immunity, the peripheral absolute lymphocyte count has been associated with an inferior outcome in various cancers.19 Furthermore, peripheral NLR has been postulated to be a prognostic factor for individuals with MIBC26-28 and higher values of NLR have been associated with an increased risk of disease recurrence and overall survival in NMIBC.29,30 In the present study and consistent with a recently published report,39 a higher NLR prior to BCG treatment value was identified in BCG nonresponders, although the difference did not reach significance.
As indicated, immune alterations at both the tumor site and in peripheral circulation may be present in individuals with cancer. Nevertheless, the relationship between peripheral and tumor-infiltrating immune cells prior treatment and the development of an immune score that takes into account systemic and local immune markers has not been previously evaluated. In our population of high-risk NMIBC patients, no correlation was identified between TILs (GATA-3+ or T-bet+) and peripheral cells (neutrophils or lymphocytes) prior to BCG treatment. These data are in agreement with previous work in ovarian cancer, in which no association was found between the lymphocyte count and infiltrating CD8+ or CD20+ cells;40 thus, the tumor infiltration is not directly associated with the level of peripheral cells. Interestingly, however, the value of infiltrating T-bet+ cells, normalized to the peripheral blood lymphocyte levels, was significantly associated with recurrence-free survival. Furthermore, the immune polarization in tissue (GTR) adjusted for the systemic inflammation (NLR) showed a significantly higher difference between BCG responders and nonresponders, with a positive association between an increased GTR/NLR immune score and a higher RFS. These results show that the tumor-infiltrating immune cell profile and systemic inflammation play important roles in the response to BCG; therefore, an immune biomarker that includes both parameters could increase the accuracy of the prediction of clinical outcome after BCG treatment.
Finally, the levels of PD-L1 expression in tumor cells have been shown to predict localized UC stage progression.17 Moreover, PD-L1 expression has been associated with increased resistance to BCG therapy, with high expression of this marker within BCG-induced bladder granulomata in patients failing the therapy.17 In contrast, PD-L1 expression in tissue prior BCG treatment was not significantly associated with BCG response in our study cohort. However, a higher local PD-L1 expression was observed in individuals with lower peripheral lymphocyte counts and higher NLR, although this last association was not statistically significant. These data could suggest a relationship between systemic inflammation and local immunosuppression and, therefore, an unfavorable clinical outcome in NMIBC patients. In individuals with cholangiocarcinoma, Sangkhamanon et al. found that an increased PD-L1 expression was also associated with a higher NLR,41 although this association, to our knowledge, has not been previously described in patients with NMIBC.
The limitations of this study are primarily associated with its retrospective design and small patient number, which decrease the statistical power. However, the high-risk population investigated is highly homogeneous and includes a relatively high number of nonresponders compared to similar studies.13,31 Further prospective studies with larger populations are required to validate these preliminary findings. In addition, the expression of PD-L1 could be slightly lower than the expression reported in other studies, as differences in the percentage of positive cells have been described for different anti PD-L1 antibody clones.42
In conclusion, the BCG response in high-risk NMIBC patients is associated with local and systemic immune alterations in the bladder tissue prior to BCG treatment. Our results suggest the potential predictive value of the combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity composition for the identification of individuals who will obtain more benefit from BCG treatment.
Patients and methods
Study subjects
This retrospective study included 22 patients treated by complete transurethral resection (TUR) and with high-grade (T1HG) NMIBC, without associated in situ carcinoma and who received treatment with BCG. The BCG treatment consisted of once-weekly instillation of BCG for 6 weeks with a maintenance course with 3 weekly instillations at 3, 6 and 12 months after the initial induction course. Patients were stratified based on their response to BCG treatment. BCG responders were defined as patients without a recurrence or progression based on follow-up cystoscopy and urinary cytology for at least 30 months after BCG treatment initiation. Neutrophil and lymphocyte counts were obtained 3–4 weeks before surgery. Demographic, biometric characteristics and immunological data, including age, gender, Body Mass Index, and numbers of neutrophils and lymphocytes, were retrieved from medical records. The neutrophil and lymphocyte counts have been obtained from the routine blood tests realized before the TUR using an automated haematology analyser that measures the volume and the size of the cells using impedance, conductivity and light scatter. The values are shown as the numbers of neutrophil and lymphocyte counts x109/L of blood.
The institutional review board and the ethical committee of the Hospital Germans Trias i Pujol approved the study (code: PI-18–137). The methods were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Evaluation of GATA-3, T-bet and PD-L1 protein expression by immunohistochemistry (IHC) in peritumoral cells
Immunohistochemical analysis was conducted on 4 μm serial sections from archived formalin-fixed, paraffin-embedded (FFPE) bladder tumor tissues. Monoclonal antibodies specific for the Th2-associated transcription factor GATA-3 (clone L50-823, Ventana Medical Systems) or the Th1-associated transcription factor T-bet (clone H-210, Santa Cruz Biotechnology) were used. IHC staining was performed using an automated immunostainer (Ventana Medical Systems Inc.) according to the manufacturer’s protocol. The total numbers of peritumoral GATA-3 and T-bet-positive cells were manually counted in 5 high-power fields by three independent observers unaware of the evolution of the patients, and the mean values were obtained. PD-L1 immunohistochemistry was performed using the Ventana SP142 assay (Ventana Medical Systems Inc.). The PD-L1 expression was evaluated on both tumor cells (TC) and peritumoral TILs. Percentages of >1% were considered positive for PD-L1. Peritumoral lymphocytes were defined as lymphocytes surrounding the tumor mass.
Statistical analysis
Continuous variables were expressed as the medians with interquartile ranges and were compared using nonparametric tests (two-tailed Mann-Whitney U-test). Discrete variables were described as percentages and were analyzed using the chi-square or Fisher’s exact tests. Spearman’s correlation coefficient was calculated to identify associations between variables. All analyses and graphical representations were performed in Graph-Pad Prism v5.0a (GraphPad Software, Inc.). The median values of each parameter were used as a cut-off point for the Kaplan-Meier survival analysis and comparison by the log-rank test. The prognostic value was calculated using the HR and 95% CIs. Sensitivity and specificity were determined for different cut-offs to predict recurrence. 95% CI were also calculated. All statistical analyses used 2-sided p-values of ≤ 0.05 to define statistically significant differences.
Funding Statement
This study was funded by funds generated at the Institut de Recerca de la SIDA-IrsiCaixa. PD-L1 immunohistochemistry was funded by Pangea Biotech. JB and CC are researchers at the Fundació Institut de Recerca en Ciències de la Salut Germans Trias i Pujol supported by the Health Department of the Catalan Government/Generalitat de Catalunya and ISCIII grant numbers PI14/01307 (to JB) and PI15/01053 (to CC).
Acknowledgments
We are grateful to Pangea Biotech for the PD-L1 stainings and the technical staff of the Pathology Department for their help with all Immunohistochemical analyses. The authors would like to thank all individuals who were participants in the study and the clinical staff of the Urology Department. IrsiCaixa and IGTP are part of the CERCA Program/Generalitat de Catalunya.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BWG, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FCD, Oosterlinck W, Prescott S, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in Non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 3.Kamat AM, Li R, O‘Donnell MA, Black PC, Rouprêt M, Catto JW, Compérat E, Ingersoll MA, Witjes WP, McConkey DJ, et al. Predicting response to intravesical bacillus calmette-guérin immunotherapy: are we there yet? A systematic review. Eur Urol. 2018;73(5):738–748. doi: 10.1016/j.eururo.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW.. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H-S, Su HY-L, Li P-H, Chiang P-H, Huang C-H, Chen C-H, Hsieh M-C.. Prognostic impact of tumor infiltrating lymphocytes on patients with metastatic urothelial carcinoma receiving platinum based chemotherapy. Sci Rep. 2018;8:7485. doi: 10.1038/s41598-018-25944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, Bajorin DF, Reuter VE, Herr H, Old LJ, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci USA. 2007;104:3967–3972. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraj SF, Munari E, Guner G, Taube J, Anders R, Hicks J, Meeker A, Schoenberg M, Bivalacqua T, Drake C, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology. 2015;85:703.e1–6. doi: 10.1016/j.urology.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Park CK, Kim SK. Clinicopathological significance of intratumoral and peritumoral lymphocytes and lymphocyte score based on the histologic subtypes of cutaneous melanoma. Oncotarget. 2017;8:14759–14769. doi: 10.18632/oncotarget.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saint F, Patard JJ, Groux Muscatelli B, Lefrere Belda MA, Gil Diez de Medina S, Abbou CC, Chopin DK. Evaluation of cellular tumour rejection mechanisms in the peritumoral bladder wall after bacillus Calmette-Guérin treatment. BJU Int. 2001;88:602–610. PMID:11678759. [DOI] [PubMed] [Google Scholar]
- 12.Kamat AM, Briggman J, Urbauer DL, Svatek R, Nogueras González GM, Anderson R, Grossman HB, Prat F, Dinney CP. Cytokine panel for response to intravesical therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guérin. Eur Urol. 2016;69:197–200. doi: 10.1016/j.eururo.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pichler R, Fritz J, Zavadil C, Schäfer G, Culig Z, Brunner A. Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical bacillus calmette-guérin therapy in bladder cancer. Oncotarget. 2016;7(26):39916–39930. doi: 10.18632/oncotarget.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunez-Nateras R, Castle EP, Protheroe CA, Stanton ML, Ocal TI, Ferrigni EN, Ochkur SI, Jacobsen EA, Hou Y-X, Andrews PE, et al. Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urol Oncol. 2014;32(1):45.e23–30. doi: 10.1016/j.urolonc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrand-Rosenberg S, Horn LA, Haile ST. The programmed death-1 immune-suppressive pathway: barrier to antitumor immunity. J Immunol. 2014;193:3835–3841. doi: 10.4049/jimmunol.1401572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellmunt J, Mullane SA, Werner L, Fay AP, Callea M, Leow JJ, Taplin ME, Choueiri TK, Hodi FS, Freeman GJ, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 17.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Liu J, Yang X, Liu Y, Liu Y, Li Y, Sun L, Yang X, Niu H. Bacillus Calmette-Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets Ther. 2018;11:2891–2899. doi: 10.2147/OTT.S165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, Tredan O, Verweij J, Biron P, Labidi I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lissoni P, Brivio F, Fumagalli L, Messina G, Ghezzi V, Frontini L, Giani L, Vaghi M, Ardizzoia A, Gardani GS. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–140. PMID:15255546. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 21.Bhat TM, Afari ME, Garcia LA. Neutrophil lymphocyte ratio in peripheral vascular disease: a review. Expert Rev Cardiovasc Ther. 2016;14:871–875. doi: 10.1586/14779072.2016.1165091. [DOI] [PubMed] [Google Scholar]
- 22.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S, Stankovic N, Abazovic D, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically Ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediators Inflamm. 2018;3758068. doi: 10.1155/2018/3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 24.Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 25.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, Thompson RH, Tollefson MK. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 26.Buisan O, Orsola A, Oliveira M, Martinez R, Etxaniz O, Areal J, Ibarz L. Role of inflammation in the perioperative management of urothelial bladder cancer with squamous-cell features: impact of neutrophil-to-lymphocyte ratio on outcomes and response to neoadjuvant chemotherapy. Clin Genitourin Cancer. 2017;15:e697–e706. doi: 10.1016/j.clgc.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Buisan O, Orsola A, Areal J, Font A, Oliveira M, Martinez R, Ibarz L. Low pretreatment neutrophil-to-lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer. 2017;15:145–151.e2. doi: 10.1016/j.clgc.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser J, Li H, North SA, Leibowitz-Amit R, Seah J-A, Morshed N, Chau C, Lee-Ying R, Heng DYC, Sridhar S, et al. The prognostic role of the change in neutrophil-to-lymphocyte ratio during neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: a retrospective, multi-institutional study. Bladder Cancer. 2018;4:185–194. doi: 10.3233/BLC-170133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vartolomei MD, Porav-Hodade D, Ferro M, Mathieu R, Abufaraj M, Foerster B, Kimura S, Shariat SF. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol Oncol. 2018:S1078–1439(18)30166-2. doi: 10.1016/j.urolonc.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget. 2017;8:12891–12901. doi: 10.18632/oncotarget.14179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichler R, Gruenbacher G, Culig Z, Brunner A, Fuchs D, Fritz J, Gander H, Rahm A, Thurnher M. Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunol Immunother. 2017;66:427–440. doi: 10.1007/s00262-016-1945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori T, Takada R, Watanabe R, Okamoto S, Ikeda Y. T-helper (Th)1/Th2 imbalance in patients with previously untreated B-cell diffuse large cell lymphoma. Cancer Immunol Immunother. 2001;50:566–568. PMID:11776379. doi: 10.1007/s00262-001-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210. doi: 10.3389/fphys.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. PMID:9846495. doi: 10.1016/S1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 35.Burkholder B, Huang R-Y, Burgess R, Luo S, Jones VS, Zhang W, Lv Z-Q, Gao C-Y, Wang B-L, Zhang Y-M, et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim Biophys Acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Koch M, Tucker-Heard G, Perdue N, Killebrew J, Urdahl K, Campbell D. T-bet controls regulatory T cell homeostasis and function during type-1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine A, Medoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, Hoyos BE, Putintseva EV, Chaudhry A, Dikiy S et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017;546(7658):421–425. doi: 10.1038/nature22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kachler K, Holzinger C, Trufa D, Sirbu H, Finotto S. The role of Foxp3 and Tbet co-expressing Treg cells in lung carcinoma. OncoImmunology. 2018;7:8. doi: 10.1080/2162402X.2018.1456612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Gianfrancesco L, Racioppi M, Ragonese M, Palermo G, Sacco E, Pf B. Neutrophil-to-lymphocyte ratio: A new prognostic factor in high risk non muscle invasive bladder cancer treated with BCG? Eur Urol Suppl. 2018;17(4):e2078. Modena, Italy. [Google Scholar]
- 40.Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, Gilks CB, Clarke B, Köbel M, Nelson BH. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangkhamanon S, Jongpairat P, Sookprasert A, Wirasorn K, Titapun A, Pugkhem A, Ungareevittaya P, Chindaprasirt J. Programmed Death-Ligand CJ 1 (PD-L1) expression associated with a high neutrophil/lymphocyte ratio in cholangiocarcinoma. Asian Pac J Cancer Prev. 2017;18:1671–1674. doi: 10.22034/APJCP.2017.18.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgson A, Slodkowska E, Jungbluth A, Liu SK, Vesprini D, Enepekides D, Higgins K, Katabi N, Xu B, Downes MR. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol. 2018;42(8):1059–1066. doi: 10.1097/PAS.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


