ABSTRACT
The formation of new lymphatic vessels, or lymphangiogenesis, is a critical step of the tissue repair program. In pathological conditions involving chronic inflammation or tumorigenesis, this process is often dysregulated and can contribute to disease progression. Yet, lymphangiogenesis is still incompletely understood. In this study, we identified A2a adenosinergic signaling as an important regulator of inflammatory and tumor-associated lymphangiogenesis. Using Adora2a (A2a)-deficient mice, we demonstrated that A2a signaling was involved in the formation of new lymphatic vessels in the context of peritoneal inflammation. We also demonstrated that tumor-associated and sentinel lymph node lymphangiogenesis were impaired in A2a-deficient mice, protecting them from lymph node metastasis. Notably, A2a signaling in both hematopoietic and non-hematopoietic cells contributed to sentinel lymph node metastasis. In A2a-deficient tumor-draining lymph nodes, impaired lymphangiogenesis was associated with a reduced accumulation of B cells and decreased VEGF-C levels. Supporting a role for non-hematopoietic A2a signaling, we observed that primary murine lymphatic endothelial cells (LEC) predominantly expressed A2a receptor and that A2a signaling blockade altered LEC capillary tube formation in vitro. Finally, we observed that Adora2a, Nt5e and Entpd1 gene expression positively correlated with Lyve1, Pdpn and Vegfc in several human cancers, thereby supporting the notion that adenosine production and A2a receptor activation might promote lymphangiogenesis in human tumors.
In conclusion, our study highlights a novel pathway regulating lymphangiogenesis and further supports the use of A2a or adenosine blocking agents to inhibit pathological lymphangiogenesis in cancers and block the dissemination of tumor cells through the lymphatic system.
KEYWORDS: Adenosine, A2a receptors, lymphangiogenesis, lymphatic vessels, inflammation, cancer, metastasis, sentinel lymph node, tumor-associated lymphangiogenesis
Introduction
The formation of new lymphatic vessels, or lymphangiogenesis, is induced during acute and chronic inflammatory episodes to enhance extracellular fluid drainage and reduce edema.1,2 Inflammation-induced lymphangiogenesis also enhances the drainage of immune cells and macromolecules (including pathogens, cellular debris, immune complexes) to the draining lymph nodes, favoring the initiation of adaptive immunity and preventing tissue damage by limiting excessive immune reaction at the inflammatory site.1,2 While lymphangiogenesis is a dynamic physiological process during embryogenesis and childhood, it is most often associated to pathological conditions in adults.3 Indeed, in adults, lymphangiogenesis is mainly restricted to areas subjected to acute or chronic inflammation, wound healing, tissue remodeling and tumor growth. Accordingly, lymphangiogenesis has been associated to the development of various disorders including obesity, auto-immune disease like arthritis, graft rejection, tissue fibrosis and tumor metastasis.4
Akin to neoangiogenesis, de novo lymphangiogenesis is a multistep process involving sprouting, migration, proliferation and tube formation of pre-existing lymphatic endothelial cells (LEC).3,5 In addition, myeloid-like lymphatic endothelial cells (M-LEC), recruited to inflammatory sites, further contribute to lymphatic vessel formation.6–9 M-LEC and progenitors co-express lymphatics markers, such as Lyve1 and podoplanin, together with myeloid cell markers, including CD11b and F4/80.7,10 Differentiation of lymphatic-promoting cells from myeloid precursors is reminiscent of myeloid-derived blood vascular endothelial progenitors that contribute to neoangiogenesis.11
From a molecular point of view, the main pathway involved in the regulation of lymphangiogenesis is the vascular endothelial growth factor receptor-3 (VEGFR-3) pathway.5 VEGFR-3 is a tyrosine kinase receptor predominantly expressed by LEC that is activated upon binding of VEGF-C or VEGF-D, the two main pro-lymphangiogenenic factors. VEGF-C and VEGF-D are upregulated in inflammatory microenvironments and produced by a variety of cells including macrophages, neutrophils, B cells and some tumor cells.5,12 Other factors such as VEGF-A, angiopoietins, b-FGF, HGF, sphingosine-1-phosphate and TNFα, also display pro-lymphangiogenic properties and participate to embryonic and inflammatory lymphangiogenesis.1,5,12
The A2a receptor belongs to the adenosine receptor family which comprises 3 additional members namely the A1, A3 and A2b receptors.13 Adenosine receptors are G-protein coupled receptors (GPCRs) with a wide tissue distribution that participate in numerous physiopathological processes.14 All adenosine receptors are activated locally upon binding with adenosine, a purine nucleoside with a short half-life. Adenosine can be released from intracellular pools in the extracellular medium by specific membrane transporters or directly produced extracellularly following ATP catabolism by the concerted action of the ecto-enzymes CD39 and CD73.15 Physiological adenosine concentrations usually range between 10 nM to a few hundred nanomolar but can rapidly increase to several hundred micromolar following tissue damage or stress due to hypoxia and inflammation.16 In these situations, adenosine receptors, and in particular high affinity A2a receptor, have a pivotal tissue-protective function limiting tissue damage due to excessive immune activation and inflammation.17,18 Activation of A2a receptors on immune cells such as neutrophils, T cells, monocytes/macrophages and dendritic cells strongly inhibits the release of cytotoxic and pro-inflammatory mediators thereby reducing tissue injury.19 Moreover, the A2a receptors have been reported to be involved in tissue repair after injury by promoting critical steps of the wound healing process including extracellular matrix remodeling and neoangiogenesis.20,21 Whether A2a signaling is involved in the regulation of lymphangiogenesis is currently unknown.
In the current study, we investigated the role of A2a signaling during inflammation-induced and tumor-associated lymphangiogenesis. Using two different models of peritonitis, we compared inflammatory lymphangiogenesis on the diaphragms of WT and A2a-deficient mice. We also investigated the role of A2a signaling during tumor-associated lymphangiogenesis and sentinel lymph node metastasis. Our study demonstrated that inflammatory lymphangiogenesis is significantly suppressed in A2a-deficient mice. In the context of tumors, deficiency in A2a signaling altered both tumor-associated and sentinel lymph node lymphangiogenesis leading to protection against lymphatic metastasis. Finally, supporting the transposition of our findings to humans, we observed that Adora2a and ectonucleotidases gene expression correlated with lymphatic/lymphangiogenesis markers in multiple human tumors.
Results
A2a-deficient mice display impaired LPS-induced lymphangiogenesis in the diaphragm
To assess the role of A2a receptor signaling in the formation of new lymphatic vessels during inflammatory responses, we used two well-described models of peritonitis to study lymphangiogenesis in vivo. In the first model, mice received intraperitoneal injections of LPS, which induces lymphangiogenesis in the diaphragm.22,23 Seven days after treatment initiation we observed a significant reduction of lymphatic endothelial cells (LEC; defined as CD45neg/Lyve1+/CD31low) in the diaphragm of A2a-deficient mice compared to WT mice (Figure 1(a,b)). We also detected a significant decrease in blood endothelial cells (BEC; defined as CD45neg/Lyve1neg/CD31hi), consistent with the described role of A2a in angiogenesis.24,25 The identity of BEC and LEC populations was further confirmed using additional markers (Figure S1). Notably, we found no significant difference in the expression levels of CD31, CD34, podoplanin, Lyve1 and prox-1 on BEC and LEC (Figure S1). To support our flow cytometry-based analysis of LEC, we performed quantitative immunofluorescence microscopy analysis of the lymphatic marker Lyve1 on the diaphragms of A2a-deficient or WT mice. As shown in Figure 1(c,d), we observed a significant decrease in lymphatic vessel density (LVD) and complexity (branching) in LPS-challenged A2a-deficient mice compared to WT ones.
Figure 1.
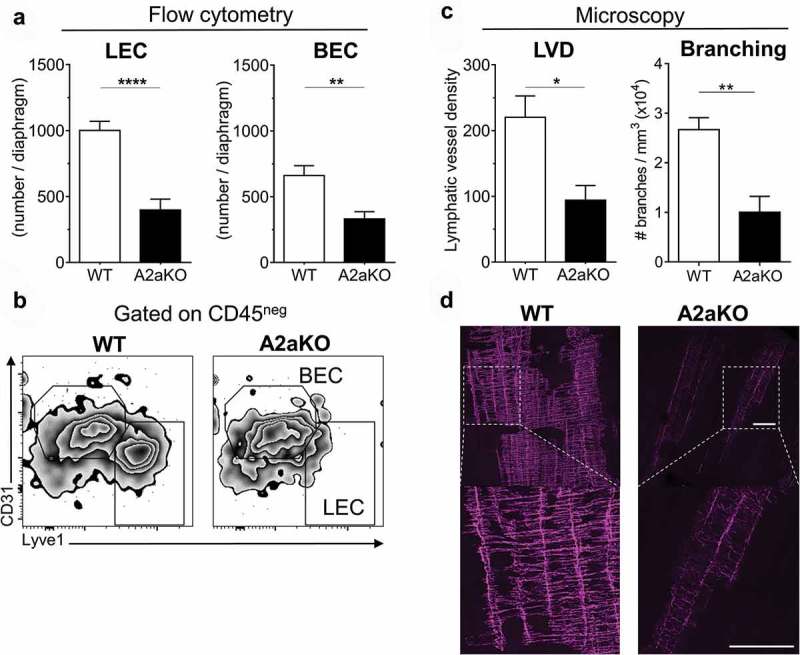
LPS-induced lymphangiogenesis is impaired in A2a-deficient mice. WT and A2a-deficient mice (6–10 mice per group) were injected with LPS (10 μg, i.p) for 7 days and diaphragms were collected, disaggregated and analyzed by flow cytometry on day 7 to evaluate lymphatic endothelial cells (LEC) infiltration. (A) Absolute number of CD45neg/Lyve1neg/CD31hi blood endothelial cells (BEC) and CD45neg/Lyve1hi/CD31lo LEC per diaphragm. (B) Representative FACS plots of data are presented in panel A, gated on CD45-negative cells, showing BEC and LEC populations. (C) Quantitation of Lyve1 whole-mount immunofluorescent staining on diaphragms of WT and A2aKO mice treated with LPS. The lymphatic vessel density (LVD; normalized to tissue thickness) and the total number of lymphatic vessel branches are displayed. (D) Representative confocal microscopy of Lyve1 whole mount staining of WT or A2aKO diaphragms (white bar = 200 microns). Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results are representative of 2 independent experiments.
A2a deficiency blocks IFA-induced lymphangiogenesis and the development of lymphangioma in the diaphragm
We next tested the role of A2a on lymphangiogenesis using a second model of peritonitis consisting of intraperitoneal injections of incomplete Freund’s adjuvant (IFA). In this model, mice develop begnin lymphangioma on the diaphragm.26 We observed that A2a-deficient mice developed lymphangiomas that were significantly reduced in size compared to WT mice (Figure 2(a,b)). When we analyzed the frequency of lyve1+ cells in the diaphragms by flow cytometry, we observed that two populations of could be identified based on the myeloid marker CD11b: lyve1+/CD11bneg (LEC) and lyve1+/CD11b+ cells (M-LEC) (Figure 2(f)). The frequency of both LECs and M-LEC were strongly reduced in A2a-deficient mice compared to WT mice (Figure 2(c,d)). In contrast, the frequency of lyve1neg/CD11b+ myeloid cells was unchanged (Figure 2(e)).
Figure 2.

IFA-induced lymphangiogenesis is altered in A2a-deficient mice. WT and A2a-deficient mice (10 mice per group) were challenge with two i.p injections of IFA (day 0 and day 10) and sacrificed 10 days after the second injection to evaluate LEC infiltration in diaphragms. (A) Begnin lymphangioma (circled in yellow) on the diaphragm of WT and A2aKO animals (day 20). (B) Quantitation of lymphangioma weights after dissection. Absolute number of Lyve1+/CD11bneg conventional LEC (C), CD11b+/Lyve1+ myeloid-like LEC (M-LEC) (D) and lyve1neg/CD11b+ myeloid cells (E) per diaphragm were determined by flow cytometry. (F) Representative FACS plots illustrating the data obtained in the panels C, D and E are shown (FMO: Fluorescence Minus One). Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results are representative of 2 independent experiments.
Together, our results in two distinct models of peritonitis demonstrated that A2a receptor signaling is involved in inflammation-driven lymphangiogenesis and that A2a signaling deficiency impairs the accumulation of both conventional LEC and myeloid-derived LEC.
Inhibition of inflammatory lymphangiogeneis in the diaphragm of A2a-deficient mice is associated with a reduction in tissue-infiltrating myeloid cells
Inflammatory lymphangiogenesis is highly dependent on the recruitment of myeloid cells, in particular macrophages, that secrete large amounts of pro-lymphangiogeneic factors.22,23,27–29 We thus investigated the infiltration of myeloid cell subsets in the diaphragm of WT and A2a-deficient mice challenged with intraperitoneal injections of LPS or IFA. As shown in supplemental Figure S2A and S2F, we observed a global decrease in the number of CD11b+ myeloid cells in the diaphragm of A2a-deficient mice compared to WT mice. We next phenotyped the myeloid cells infiltrating the diaphragms to identify (i) CD11b+/F4/80neg/Ly6Gneg/Ly6C+ inflammatory monocytes, (ii) CD11b+/F4/80+/Ly6Gneg macrophages, (iii) CD11b+/F4/80neg/Ly6G+/Ly6C+ neutrophils and (iv) CD11b+/F4/80low/Ly6Gneg/Ly6C+/SCChi eosinophils (see supplemental Figure S3 for the gating strategy and the article of Rose et al.30). In both LPS and IFA peritonitis models, we found that A2a-deficient diaphragms were significantly less infiltrated by inflammatory monocytes, macrophages, neutrophils and eosinophils than WT diaphragms (Figure S2). The reduction was particularly pronounced for neutrophils, macrophages and inflammatory monocytes indicating a role of A2a signaling for the accumulation of various myeloid cells upon LPS and IFA challenge.
Tumor-associated lymphangiogenesis is reduced in A2a-deficient mice
Lymphangiogenesis occurs in the inflamed tumor microenvironment (TME) and favors metastasis of several types of cancers, including melanoma.31–33 In view of our above-described results, we hypothesized that A2a signaling could be involved in tumor-associated lymphangiogenesis. To test this hypothesis, we used B16F10 melanoma cells injected in the foot pad in order to investigate tumor-associated lymphangiogenesis and regional lymph node metastasis.34–36 B16F10 melanoma cells expressing the adenosine-generating enzyme CD73 (B16-CD73) were injected in the foot pad of WT and A2a-deficient mice and, two weeks later, mice were evaluated for tumor lymphangiogenesis. Sections of B16-CD73 tumors immunostained with anti-Lyve1 antibody revealed that tumor-associated lymphatic vessel density (LVD) was significantly reduced in A2aKO mice compared to WT (Figure 3(a,b)).
Figure 3.

Tumor-associated lymphangiogenesis is impaired in A2a-deficient mice. (A-B). B16-CD73+ foot pad tumors were collected two weeks after inoculation and stained for Lyve1 in order to determine the surface of lyve1+ area (or lymphatic vessel density; LVD). (C-F) In another set of experiments, foot pad tumors were collected two weeks after inoculation, disaggregated to obtain a cell suspension and analyzed by flow cytometry to evaluate the number of lymphatic endothelial cells and myeloid cells. Absolute numbers of (C) CD45neg/CD31+/Podoplanin+ lymphatic endothelial cells (LEC), (D) CD45neg/CD31+/Podoplaninneg blood endothelial cells (BEC), (E) CD45+/CD11b+/Lyve1+ myeloid-like lymphatic endothelial cells (M-LEC), (F) total CD11b positive myeloid cells, are presented. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results are representative of 2 independent experiments with 5 to 10 mice per group.
In accordance with this observation, we observed a significant decrease in the frequency of both tumor-associated LEC (CD45neg/CD31+/Podoplanin+) and M-LEC (CD11b+/Lyve1+) in A2a-deficient mice compared to WT mice (Figure 3(c,e)). Most M-LEC present in the B16-CD73 TME co-expressed Lyve1 and F4/80, suggesting a macrophage origin (Figure S4). A significant reduction in tumor-associated BEC (CD45neg/CD31+/Podoplaninneg) was also observed in A2a-deficient mice (Figure 3(d)). These results thus reveal that tumor-associated lymphangiogenesis is defective in A2a-deficient mice. In a similar fashion to what we had observed in the peritonitis models, melanoma tumors implanted in A2a-deficient animals were also significantly less infiltrated by CD11b+ myeloid cells (Figure 3(f)), including macrophages and inflammatory monocytes (Figure S5).
Tumor-draining lymph node lymphangiogenesis is impaired in A2a-deficient mice
Tumor-induced sentinel lymph node lymphangiogenesis is a crucial event for lymph node metastasis and occurs prior to the establishment of lymphatic metastases.34,37–39 Given that lymphangiogenesis is altered in the TME of A2a-deficient mice, we investigated whether A2a-deficient mice had impaired lymphangiogenesis in the tumor-draining lymph node. Lyve1 immunostaining of tumor-draining lymph node sections revealed that A2a-deficient sentinel lymph nodes had decreased LVD compared to WT counterparts (Figure 4(a,b)). Moreover, while WT tumor-draining lymph nodes displayed a 2-fold increase in LVD relative to their contralateral pairs, LVD remained unchanged in A2a-deficient lymph nodes indicating an impaired lymphangiogenesis (Figure 4(a)).
Figure 4.
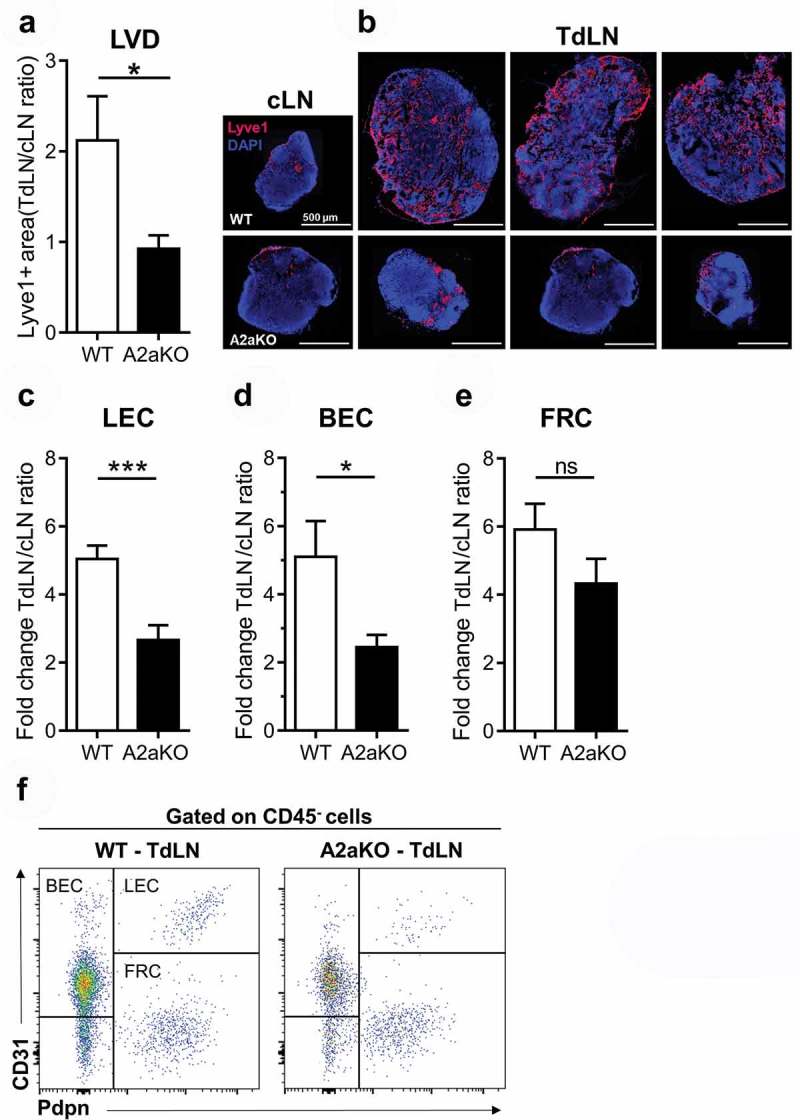
Tumor-draining lymph node vascular and immune cell remodeling is altered in A2a-deficient mice. Two weeks after tumor cell inoculation in the foot pad tumors, tumor-draining (TdLN) and contralateral (cLN) lymph nodes were collected and stained for lyve1 to to determine lymphatic vessel density (LVD). (A) LVD ratios between TdLN and matched cLN were calculated for each WT and A2a-deficient group (10 mice/group; means ± standard errors are shown). (B) Representative lyve1 staining is shown. (C-F) In another set of experiments, TdLN and cLN were collected two weeks after tumor inoculation, disaggregated to obtain a cell suspension and analyzed by flow cytometry to evaluate the cellular composition. Fold-increase in cell population is shown for (C) CD45neg/CD31+/Podoplanin+ lymphatic endothelial cells (LEC), (D) CD45neg/CD31+/Podoplaninneg blood endothelial cells (BEC), (E) CD45neg/CD31neg/Podoplanin+ follicular reticular cells (FRC). (F) Representative FACS plot showing the gating strategy to identify LEC, BEC and FRC in the CD45-negative population of lymph node cells. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results of 2 independent experiments with 10 mice per group were pooled.
Using flow cytometry, we compared the immune and vascular composition of both sentinel and contralateral lymph nodes of WT and A2a-deficient mice bearing B16F10-CD73 foot pad tumors. Two weeks after tumor inoculation, we observed that the cellular composition of WT sentinel lymph nodes was drastically modified compared to their contralateral counterparts. We observed an enrichment in T and B cells (Figure S6A and S6B) and an accumulation of vascular cells including LEC (Figure 4(c,d)), reflecting increased lymphangiogenesis in tumor-draining versus non-draining lymph nodes in WT mice. In sharp contrast, tumor-draining lymph nodes from A2a-deficient mice displayed limited vascular and immune remodeling when compared to WT sentinel lymph nodes. The accumulation of LEC, BEC and B cells in sentinel lymph nodes (relative to contralateral lymph nodes) was significantly lower (i.e. reduced by 50%) in A2a-deficient mice compared to WT mice (Figure 4(c,d), S6A and S6B). In contrast, the accumulation of myeloid cells and follicular reticular cells (FRC) was not different (Figure 4(e), S6C and S6D). Together, these observations indicate that A2a-deficiency is associated with a general decreased responsiveness to tumor-derived signals that normally promote sentinel lymph node lymphangiogenesis and immune remodeling.
Tumor-draining lymph node metastasis is reduced in A2a-deficient mice
We next investigated the role of A2a in lymph node metastasis. For this purpose, we inoculated WT and A2a-deficient mice with B16-CD73 tumors cells in the foot pad, and assessed the development of tumor lesions in the draining lymph node. One month after tumor cell inoculation, primary tumor masses (Figure 5(a)) and animal survival (Figure 5(b)) were similar in WT and A2a-deficient mice. Yet, when tumor-draining popliteal lymph nodes were analyzed, we observed a significant reduction in lymph node weight (Figure 5(c)) and metastatic tumor burden (Figure 5(d,e)) in A2a-deficient mice compared to WT. Tumor-draining popliteal lymph nodes of WT mice were more frequently invaded by macroscopic metastatic lesions compared to A2a-deficient mice (Figure 5(f)). In fact, 85.7% (18/21) of WT mice developed macroscopic metastases in popliteal tumor-draining lymph nodes while only 40% (6/15) of A2a-deficient mice did (Figure 5(d)).
Figure 5.

Locoregional metastasis to tumor draining lymph node is reduced in A2a-deficient mice bearing B16-CD73 foot pad tumors. WT and A2a-deficient mice were injected with 2.5 × 105 B16-CD73+ melanoma cells in the foot pad and euthanized when tumors reach a size of 120 mm2. (A-B) Tumor growth was similar in WT and A2aKO mice as illustrated by tumor weights at end point and survival. (C-F) Tumor metastasis to the draining popliteal lymph node in WT and A2aKO mice was compared at end point by measuring (C) lymph node weight, (D) metastatic incidence, (E) GFP fluorescence in homogenized popliteal draining lymph nodes and (F) by visual inspection. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test (except for D where a Fisher’s test was used). Results of 3 independent experiments were pooled.
A2a signaling on both hematopoietic and non-hematopoietic cells favors locoregional metastasis to tumor-draining lymph node
To better understand the contribution of hematopoietic versus non-hematopoietic A2a signaling, we performed bone marrow (BM) transplantations and evaluated tumor-draining lymph node metastasis of B16F10 melanoma cells (Figure 6). As expected, A2a-deficient mice transplanted with A2a-deficient BM (A2a into A2a) displayed a significant reduction in sentinel lymph node size and metastasis incidence compared to WT mice transplanted with WT BM (WT into WT; Figure 6(a,b)). Most notably, transplantation of WT BM into A2a-deficient mice (WT into A2a) or A2a BM into WT mice (A2a into WT) failed to phenocopy the reduction in lymph node metastasis observed in A2a-deficient mice (Figure 6). Therefore, our results indicate that both hematopoietic and non-hematopoietic A2a signaling promote locoregional sentinel lymph node metastasis.
Figure 6.
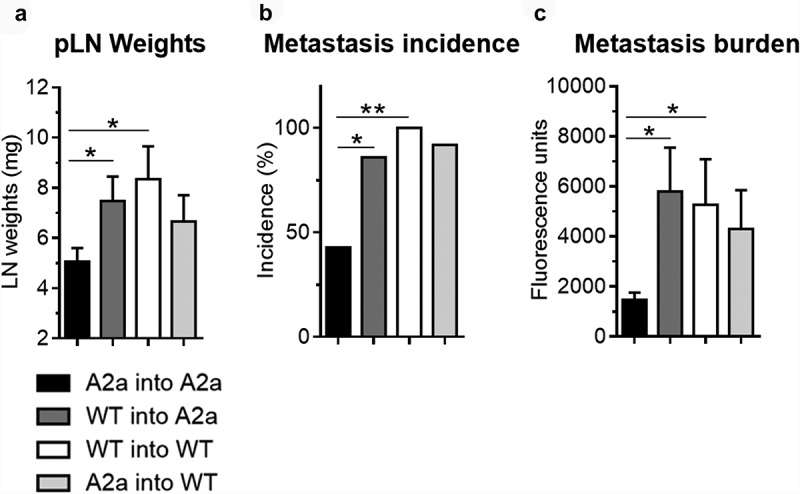
A2a signaling on both hematopoietic and non-hematopoietic cells promotes sentinel lymph node metastasis. WT and A2a-deficient mice were lethally irradiated, adoptively transferred with WT or A2a-deficient bone marrow, and injected 8 weeks later with 2.5 × 105 B16-CD73+ melanoma cells in the foot pad. Tumor metastasis to the draining popliteal was evaluated 30 days later by measuring (A) popliteal lymph node weights, (B) metastatic incidence and (C) GFP fluorescence on homogenized tumor-draining lymph nodes. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results are representative of one experiment with 15-20 mice per group.
A2a-deficient sentinel lymph nodes have reduced VEGF-C concentrations
The VEGF-C/VEGFR3 pathway is considered as the main pathway promoting pathological lymphangiogenesis in tumors and draining lymph nodes.12,33 To investigate whether A2a signaling modulate tumor-associated lymphangiogenesis through the VEGF-C/VEGFR3 pathway, we analyzed the concentration of VEGF-C in primary tumors, sentinel lymph nodes and contralateral lymph nodes collected from WT and A2a-deficent mice. As shown in Figure 7(a), we observed a significant reduction in VEGF-C levels in tumor-draining lymph nodes from A2a-deficient mice (p < 0.001). In contrast, similar lymph node levels of VEGF-A and VEGF-D were observed, and similar tumoral levels of VEGF-A, VEGF-C and VEGF-D were observed between WT and A2a-deficient mice. Therefore, our data suggest that reduced VEGF-C levels is associated with A2a-deficiency and could, at least in part, contribute to the impairment of sentinel lymph node lymphangiogenenis observed.
Figure 7.

VEGF-C levels are reduced in A2a-deficient sentinel lymph nodes.
WT and A2a-deficient mice were injected with 2.5 × 105 B16-CD73+ melanoma cells in the foot pad and two weeks later tumor-draining lymph nodes and contralateral lymph nodes were collected. Primary tumors and lymph node were homogenized, centrifuged and supernatants were assayed for VEGF levels by ELISA. (a) Fold-increase in VEGF-A, C and D in tumor-draining lymph nodes (TdLN) over matched contraleral lymph nodes (cLN). (b) Levels of VEGF-A, C and D in tumors. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results of 2 independent experiments with 10 mice per group were pooled.
Decreased VEGF-C levels in A2a-deficient tumor-draining lymph nodes are associated with a reduction of B cell number
In lymph nodes, B cells are critical for the induction of inflammation-driven lymphangiogenesis, notably through the secretion of VEGF isoforms.40–42 We observed a concomitant reduction of VEGF-C levels and B cell frequency in A2a-deficient sentinel lymph nodes (Figure 8(a,b)). In contrast, macrophages, another source of VEGF-C, were equally present in both WT and A2a-deficient lymph nodes (Figure 8(c)). Supporting the notion that tumor-draining B cells produce VEGF-C in our model, we observed co-staining of the B cell marker B220 and VEGF-C in WT mice (Figure 8(d)). Moreover, BCR stimulation effectively triggered production of VEGF-C by purified lymph node B cells (Figure 8(e)). However, A2a-deficiency had no direct impact on VEGF-C production by activated B cells in vitro (Figure 8(e)).
Figure 8.
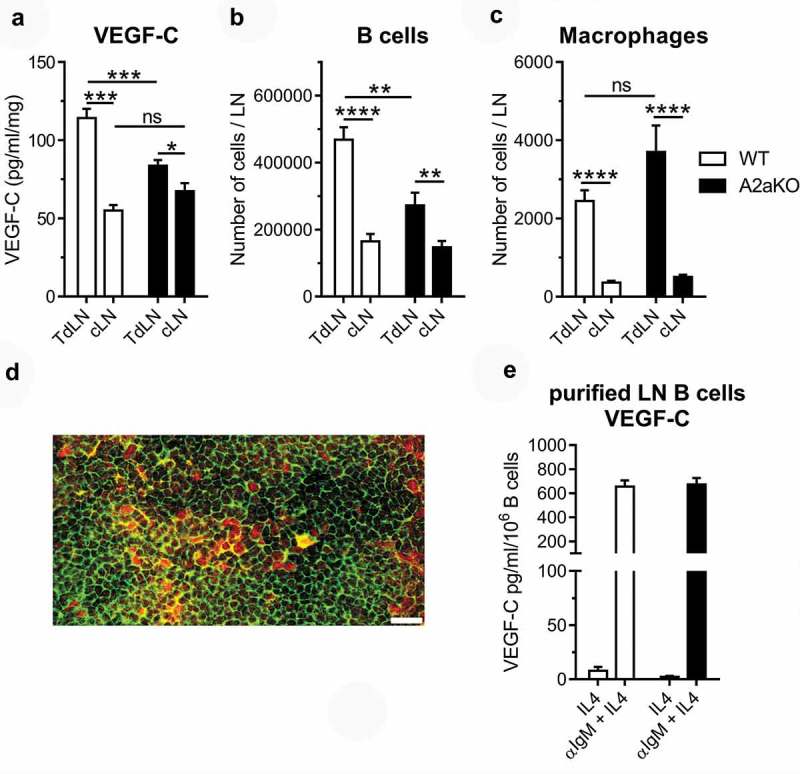
The number of VEGF-C secreting B cells is reduced in A2a-deficient sentinel lymph nodes.
WT and A2a-deficient mice were injected with 2.5 × 105 B16-CD73+ melanoma cells in the foot pad. (a) Two weeks later, tumor-draining (TdLN) and contralateral (cLN) lymph nodes were collected and assayed for VEGF-C levels by ELISA and for flow cytometry for (b) CD45.2+/CD19+ B cells, and (c) CD45.2+/CD11b+/F4/80+ macrophages. (d) Representative section of a WT sentinel lymph node showing B220 and VEGF-C co-staining (scale bar = 20 microns). (e) VEGF-C production by WT and A2aKO purified lymph node B cells. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results of 2 independent experiments with 10 mice per group were pooled.
Blockade of A2a signaling impairs capillary-like tube formation of primary murine LEC
To better understand the contribution non-hematopoietic A2a-signaling on lymphangiogenesis, we next investigated the effect of A2a blockade on LEC function in vitro. Firstly, we observed that A2a was the predominant adenosine receptor subtype expressed by murine LEC, which also expressed CD39 and CD73 (Figure S7). To understand how A2a signaling might regulates LEC function, we evaluated the formation of capillary-like structures by primary murine LEC treated with a selective and competitive A2a antagonist (SCH58261). As shown in Figure 9(a,b), specific blockade of A2a significantly impaired LEC tube formation by reducing capillary thickness and branching, an effect that was partly reversed by adding exogenous adenosine (which competes with SCH58635 for A2a binding).
Figure 9.
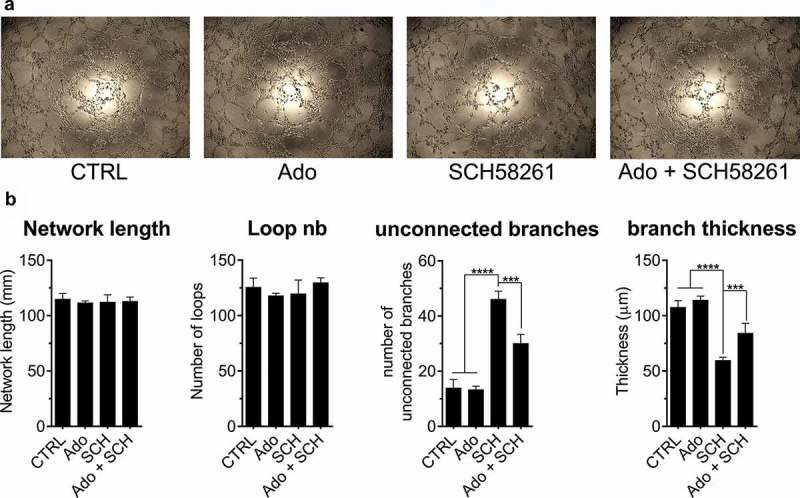
A2a blockade impairs the formation of capillary-like structure by primary murine LEC. Primary murine dermal lymphatic endothelial cells were seeded on Matrigel to evaluate tube formation. In some conditions, cells were treated with 50 µM adenosine (Ado), 10 µM A2a antagonist SCH58261 (SCH) or a combination of Ado + SCH. In every condition, including control wells (CTRL), 10 µM EHNA was added to block adenosine deaminase activity. (A) After 8 hours, pictures of the capillaries were taken and analyzed using Zen Blue software. (B) Total network length, number of loops, number of unconnected branches and thickness of branches were determined. Means ± standard errors are shown. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 using a Student t test. Results are representative of 2 independent experiments.
Expression levels of Adora2a, Nt5e and Entpd1 positively correlate with lymphatic markers in multiple human tumors.
We finally evaluated whether Adora2a gene expression correlated with markers of lymphatic vessels (Lyve1, Pdpn) or lymphangiogenesis (Vegfc) in various types of human cancer. As shown in Figure 10, we indeed observed a significant positive correlated between Adora2a and Lyve1, Pdpn or Vegfc. Correlations were particularly pronounced and significant in melanoma, colorectal cancers and breast cancers. Interestingly, genes encoding for CD73 (Nt5e) and CD39 (Entpd1) also positively correlated with Lyve1, Pdpn and Vegfc in multiple tumor types. Together, these data support the notion that adenosine and A2a-signaling may favor the formation of lymphatic vessels in human cancers.
Figure 10.
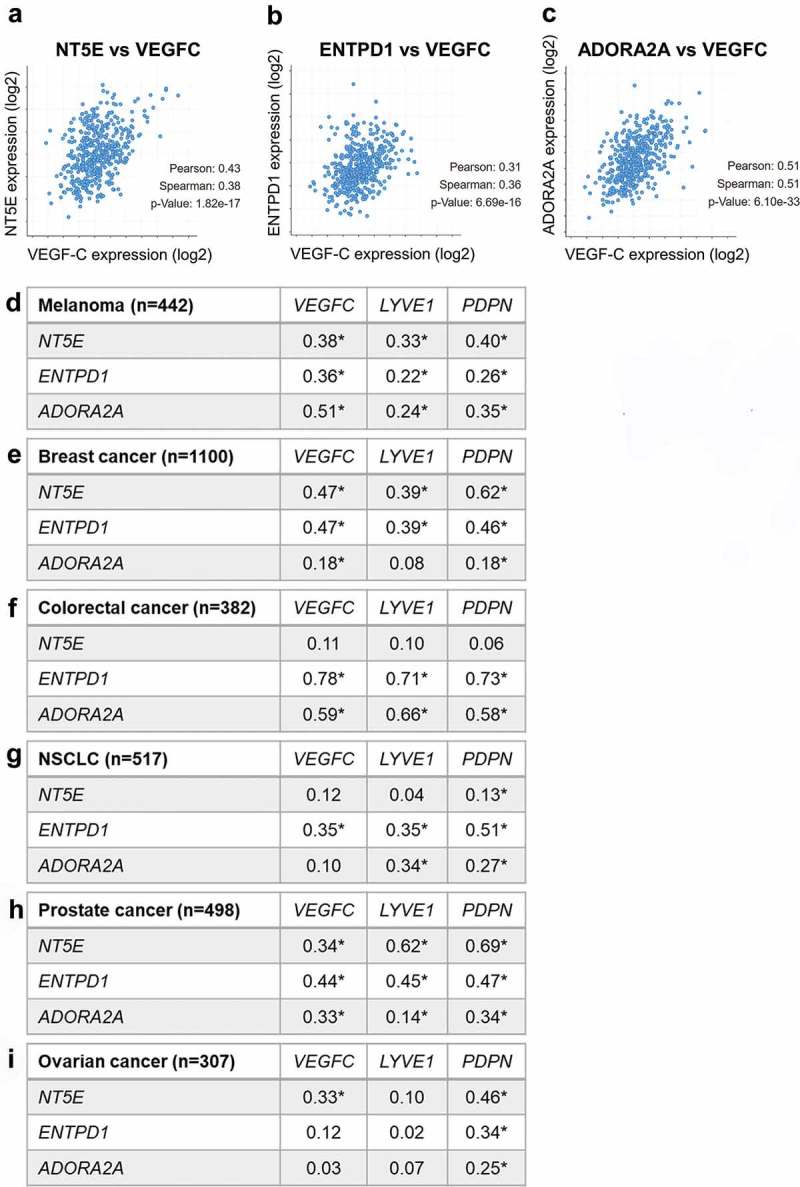
Expression levels of Adora2a, Nt5e and Entpd1 correlates with markers of lymphatics and lymphangiogenesis in human tumors.
(A-C) Correlation analysis of the indicated genes in the TCGA melanoma dataset. (D-I) Tables summarizing the correlations between the indicated genes for each type of cancer. The number of patients analyzed in indicated (n). For each gene combination, Spearman correlation coefficients are displayed (*p < 0.01).
Discussion
In this study, we provide conclusive evidence that signaling through the A2a adenosine receptor promotes lymphangiogenesis in response to inflammation and cancer, thereby favoring tumor cell metastasis to lymph nodes. Our findings support prior work from Lenoir et al.,43 which demonstrated pro-lymphangiogenenic properties of 2-chloro-adenosine in Matrigel plug assays.
Using well-established in vivo models of peritonitis, we observed a significant reduction of lymphatic vessel density in A2a-deficent mice compared to WT mice. Interestingly, in the IFA model of peritonitis, we were able to evaluate the effect of A2a-deficiency on the recruitment of conventional LEC (CD11bneg/Lyve1+) and myeloid-like LEC (CD11b+/Lyve1+). The recruitment of myeloid-like LEC was selectively reduced in A2a-deficient mice, suggesting a role for A2a in the recruitment, survival or differentiation of these cells. In support of this model, A2a activation has been shown to promote monocyte/macrophage chemotaxis and differentiation into an anti-inflammatory M2 phenotype characterized by the secretion of VEGF.44–46 Whether A2a can promote the acquisition of LEC markers by macrophage and/or favor their contribution to lymphatic vessel formation warrants further investigations. In both peritonitis models, A2a-deficient mice presented a strong decrease in myeloid cell subsets known to be important sources of lymphangiogenic factors including VEGF-C and VEGF-D.28,29 We postulate that activation of A2a signaling on myeloid cells in the context of inflammation favor their accumulation, thereby promoting lymphangiogenesis.
In the second part of our study we investigated the role A2a signaling in the context of tumor-associated lymphangiogenesis. We took advantage of a well-established tumor model, the B16F10 footpad model, that is commonly used to study tumor lymphangiogenesis and sentinel lymph node metastasis.34–36 We used B16F10 cells overexpressing CD73 to favor the accumulation of adenosine in the TME and engage surrounding A2a receptors.47,48 Our results demonstrated that B16-CD73+ tumors implanted in A2a-deficient animals had diminished tumor-associated LVD, reduced numbers of LEC and M-LEC, and showed a decrease in tumor-infiltrating myeloid cells. Together, our results using B16-CD73+ tumors are in agreement with those obtained in the two peritonitis models and further support an important role for A2a signaling in the accumulation of lymphatic cells and myeloid cells in the TME.
Recent work indicated that tumor-driven lymphangiogenesis not only occurs in the tumor bed but also in tumor draining lymph nodes and that this event is crucial for lymphatic metastasis.34,37–39 We thus compared lymphangiogenesis occurring in tumor-draining and contralateral lymph node of WT and A2a-deficient mice bearing B16-CD73+ footpad tumors. We noted that LEC accumulation and LVD was significantly reduced in A2a-deficient sentinel lymph nodes compared to WT ones. Notably, our data further highlighted that A2a-signaling is not only important for local lymphangiogenesis, but also for orchestrating a more global lymph node remodeling in response to a primary tumor.
Notably, deficiency in A2a-signaling conferred a significant protection against sentinel lymph node metastasis. While the effect of A2a on immune surveillance against lung metastasis has been documented,47,48 our study demonstrates for the first time that A2a signaling is also involved in locoregional metastasis to tumor-draining lymph nodes. Interestingly, we found that silencing A2a on hematopoietic cells was required but not sufficient to induce protection against lymphatic metastasis, indicating that A2a signaling in both hematopoietic and non-hematopoietic cells promotes lymph node metastasis.
In the tumor-microenvironment, we observed no difference in VEGF-C or VEGF-D levels between WT and A2a-deficient mice. However, our results indicate that the regulation of sentinel lymph node lymphangiogenesis by A2a signaling is associated with modulation of the VEGF-C/VEGFR3 pathway. Interestingly, our data ruled-out a major contribution for myeloid A2a signaling in the regulation of sentinel lymph node lymphangiogenesis as no significant difference in number of myeloid cells, including macrophages, was detected between WT and A2a-deficient mice. Instead, we found that the number of B cells was significantly reduced in A2a-deficient tumor-draining lymph nodes. Immunofluorescence analysis of draining lymph nodes support the notion that B cells can be an important source of VEGF-C. In support of this, B cells were previously shown to contribute to lymph node lymphangiogenesis.34,40–42 In B16F10 models of foot pad tumors, B cell depletion indeed suppresses sentinel lymph node lymphangiogenesis.34 In models of infection or immunization, lymph node B cells were also shown to secrete VEGF-C and VEGF-A, promoting lymphangiogenesis to enhance dendritic cell mobilization.40,41 Interestingly, lack of A2a-signaling on B cells had no direct impact on their ability to produce VEGF-C, proliferate or survive in vitro following stimulation. In light of our data, we propose that A2a signaling indirectly promotes the production of VEGF-C by tumor-draining lymph node B cells, which in turns enhances local lymphangiogenesis and favors the development of lymphatic metastases.
A possible indirect mechanism could be through regulation of lymphotoxin beta receptor (LTβR)-mediated crosstalk between FRC and B cells, which has been shown to promote lymph node B cell production of VEGF-A and VEGF-C.41 Another possibility by which A2a signaling may indirectly regulate VEGF-C production by B cells may be through macrophages. Hence, the exact mechanisms by which A2a signaling on hematopoietic cells promote tumor-associated lymphangiogenesis and lymph node metastasis remains to be determined.
With regards to non-hematopoietic cells, we explored the possibility that A2a signaling on LEC regulates their capacity to form new lymphatic vessels. We observed that A2a receptor was predominantly expressed by primary dermal LEC and that exposure to a selective A2a antagonist impaired their capacity to form capillary-like structures in vitro. These observations support for a contribution of LEC-derived A2a signaling to lymphangiogenesis.
Lymphangiogenesis is increasingly recognized as an important process favoring the development and the progression of multiple inflammatory disorders including cancer.33 In various human tumors, the accumulation of intra or peri-tumoral lymphatic vessels has been consistently documented and very often associated with lymph node metastasis and worse prognosis for patients.32,49-54Very recently, the contribution of tumor-associated lymphangiogenesis and lymph node metastasis for the actual establishment of distant metastasis was distinctly evidenced in two landmark studies.55,56 Lymphangiogenesis was also shown to occur in distant organs with established metastases where it promoted further spread of the tumor cells to other organs.57 Supporting the clinical relevance of our findings, we found that expression of Adora2a and adenosine-generating ectonucleotidases were positively correlated with the levels of lymphatic or pro-lymphangiogenic markers such as Lyve1, Pdpn or Vegfc, in several types of human tumors. In this context, our findings are highly relevant as they identify a novel pathway that could be targeted to block tumor-associated lymphangiogenesis and sentinel lymph node metastasis. With various A2a receptors antagonists currently under clinical evaluation for cancer immunotherapy, our results highlight a new tumor-promoting function of A2a signaling in the TME and thus further support the development of A2a inhibitors and adenosine-targeting agents for cancer treatment.
Materials and methods
Mice
A2a-deficient mice on a C57Bl/6N background were obtained from Dr. JF Chen (Boston, MA) and bred at the CHUM research center. WT C57Bl/6N mice were purchased from Charles Rivers. All mice were maintained 5 per cages in a specific pathogen–free facility with free access to food and water. Mice used for the experiments were females between 8 to 12 weeks of age. All experiments were conducted with at least 5 mice per group and were approved by the CHUM research center Animal Ethics Committee.
LPS and IFA-induced lymphangiogenesis in the diaphragm
For LPS-induced peritonitis, 10 µg of LPS (from E coli 0111:B4; Sigma-Aldrich) in 50 µl of phosphate buffer saline (PBS) was daily injected in the peritoneal cavity as previously described.22,23 After 7 days, mice were sacrificed to collect diaphragms and evaluate lymphatic vessel formation. For IFA-induced peritonitis, mice were injected with 200 µl of a 50:50 v/v emulsion containing PBS and incomplete Freund’s adjuvant (Sigma Aldrich). A second injection was performed after 10 days and mice were euthanized on day 20. IFA injections induced the formation of lymphangiomas on the diaphragm and liver as described by Mancardi et al.26
Detection of lymphatic endothelial cells and myeloid cells by flow cytometry
Diaphragms of mice treated with LPS or IFA were collected, finely minced and enzymatically disaggregated in RPMI containing 1 mg/ml of collagenase IV and 20 µg/ml of DNAse I (enzymes from Worthington Biochemical, Lakewood, NJ). After 1 hour of incubation at 37°C under agitation, disaggregated diaphragms were passed through a 40 µm cell strainer, rinsed twice with PBS- 2% FBS and resuspended in 100 µl of PBS- 2% FBS containing Fc block (BD bioscience). Then, samples were stained with a cocktail of fluorochrome-labelled antibodies to detect both lymphatic endothelial cells and myeloid cells. The following antibodies were used: anti-Lyve1-efluor660 (clone ALY7) and anti-podoplanin-Alexa488 (clone eBio8.1.1) from Ebioscience, anti-CD31-PE (clone 390), anti-CD45.2-BUV747 (clone 104), anti-CD34-BV421 (clone RAM34), anti-CD11b-BUV395 (clone M1/70), anti-Ly6C-BV711 (clone RB68C5), anti-Ly6G-APC-H7 (clone 1A8) from BD Bioscience, anti-F4/80-BV605 (clone BM8) and anti-podoplanin-PE-Cy7 (clone 8.1.1) from Biolegend, anti-prox1 (# ab101851) from Abcam. The same procedure as well as the same antibody cocktail was used to disaggregate and stain immune and endothelial cells extracted from lymph nodes and from B16-CD73 foot pad tumors
Whole mount staining of diaphragms
Diaphragms of WT and A2a-deficient mice challenged with LPS for 7 days were collected, briefly rinsed in PBS to remove excess of blood and fixed overnight in 2% PFA at 4°C. The following day, diaphragms were blocked 1h at room temperature in a solution of PBS containing 3% BSA and 5% FBS. Diaphragms were then stained overnight at 4°C with efluor 660-coupled anti-Lyve1 antibody (Ebioscience). The following day, diaphragms were rinsed twice in PBS and mounted on microscope slides with Prolong gold (Thermofisher). Diaphragms were imaged using a confocal microscope (Leica Microsystems TCS SP5). 5 pictures of each diaphragm were used for the analysis. Each picture corresponds to the maximal projection of a 4 × 4 tiling scanned across a 50 to 100 µm thickness. Determination of lyve1 positive area was performed on Image J using a script elaborated by Dr. Nicolas Stifani (University of Montreal). Results were normalized according to the thickness of each picture. Branching analysis was also performed on Image J using the “skeleton” plugin.
Foot pad B16-CD73 melanoma model
WT and A2a-deficient mice were injected in the rear foot pad with 2.5 × 105 B16F10 cells expressing CD73 and Green fluorescent protein (previously described47,48 and obtained from Mark J. Smyth, Australia) diluted in 20 µl of PBS. Tumor size was evaluated using a digital caliper. Mice were sacrificed when the tumor size reached 120 mm2. Tumors and popliteal draining lymph nodes were collected and weighted. Tumor-draining popliteal lymph node were crushed in 50 µl of a solution of PBS-tween 80 0.1% containing Halt™ protease inhibitors (Thermofisher). Then, GFP fluorescence of the supernatant was measured using a Wallac fluorometer (Perkin Elmer). In some experiments, mice were euthanized 14 days after tumor cell inoculation to collect tumors and lymph nodes. In that case, samples were disaggregated and analyzed by flow cytometry.
Immunofluorescence on fresh-frozen tumors and lymph nodes
B16-CD73 footpad tumors, tumor-draining popliteal lymph nodes and contralateral lymph nodes were collected two weeks after tumor cell inoculation, embedded in OCT (Optimal Cutting Temperature, Sakura) and flash frozen in liquid nitrogen. Using a cryostat, 5-micron sections were cut, fixed 10 min in ice-cold acetone, blocked 1h with Dako protein block solution and stained overnight with efluor 660-coupled anti-Lyve1 antibody (Ebioscience), Alexa fluor-488-coupled anti-B220 antibody (Biolegend, clone RA3-6B2) or Alexa fluor-647-coupled anti-VEGF-C antibody (Santa Cruz, clone E-6). The following day, slides were rinced, counstained with DAPI and mounted using Prolong gold (Thermofisher). Slides were then imaged using an Olympus BX61VS slide scanner. Lyve1 positive areas were determined using Zen blue software (Zeiss).
Bone marrow transplantation
Eight- to 10-wk-old A2aKO (CD45.1+) or WT B6N (CD45.2+) or B6N.CD45.1 (CD45.1+) hosts were lethally irradiated (1100 rad whole body, first day 2 times 350 rad in 4-h interval, next day 400 rad) as described.58 Hosts were reconstituted within 24 h of last irradiation by i.v. injection of 5 million bone marrow donor cells coming from femurs and tibias of either A2aKO (CD45.2+) or B6.CD45.1 (CD45.1+) mice. Donor cell engraftment was confirmed by flow cytometry 8 weeks after transplantation.
Lymph node B cell purification
B cells from WT and A2aKO lymph nodes were purified using a negative selection magnetic mouse B cell purification kit (Stem Cell) according to the manufacturer’s recommendations. B cell purity was over 99%. Purified B cells were then plated in 96-well plates (100 000 cells per well) and stimulated with 10 μg/ml of anti-mouse IgM Fab’2 (Jackson Immunoresearch) with 10 ng/ml recombinant mouse IL-4. Unstimulated wells contained IL-4 only. Supernantants were collected after 48h and the number of viable cells per well was determined by flow cytometry.
VEGF ELISA
Supernatant of stimulated B cells were collected, centrifuged and assayed for VEGF-A, C and D concentrations using manufacturer’s recommendations of the ELISA kits. VEGF-A and VEGF-D ELISA kits were purchased for R&D systems. VEGF-C ELISA kit was purchased from Cusabio. To determine VEGF isoforms concentration in lymph nodes, the latter were crushed in 50 µl of a solution of PBS-tween 80 0.1% containing Halt™ protease inhibitors (Thermofisher) and centrifuge to collect the supernatant.
Capillary-like tube formation in vitro
C57BL/6 mouse primary dermal lymphatic endothelial cells (#C57-6064L, Cell Biologics, Chicago, IL) were plated in 96 well plates on a matrigel (BD bioscience) layer at a density of 20 000 cells per well and placed in an incubator. In some wells, cells were treated with 50 µM adenosine and/or 10 µM of SCH58261 (Tocris Bioscience), a specific A2a inhibitor. In every condition, 10 µM of the adenosine deaminase inhibitor EHNA (Tocris Bioscience) was added to increase adenosine stability. After 8 hours, pictures of the capillary network were taken and analyzed manually using the Zen Blue software (Zeiss). Total network length, number of loops, number of unconnected branches and thickness of branches were determined.
Gene expression analysis
The TCGA database was interrogated through the cbioportal website and correlations of expression (mRNA) between the targeted genes were evaluated for several cohorts of cancer patients. All cohorts presented in this analysis are TCGA provisional cohorts dating from March 2019.
Statistical analysis
Data are shown as means ± SEM. Statistics are calculated using unpaired Student’s T test or one-way ANOVA with Bonferroni correction when multiple comparisons were made. Data were analyzed using GraphPad prism software.
Funding Statement
JS received a Project Grant from the Canadian Institutes for Health Research (CIHR). JS received a salary award from the CIHR and support from the Jean-Guy Sabourin Research Chair in Pharmacology. BA received postdoctoral scholarships from MITACS, the Montreal Cancer Institute and from the Fonds de Recherche du Québec en Santé (FRQS)Institute of Cancer Research;
Disclosure of Potential Conflicts of Interest
J. Stagg is a paid consultant, SAB member and owns stocks of Surface Oncology and received sponsored research grants from Surface Oncology.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Kim H, Kataru RP, Koh GY.. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124(3):936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Card CM, Yu SS, Swartz MA.. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124(3):943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Investigation. 2014;124(3):888–897. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. 2014;124(3):874–877. doi: 10.1172/JCI74854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. J Clin Invest. 2014;124(3):878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Kröber SM, Greinix H, Rosenmaier A, Karlhofer F, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12(2):230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Rooijen NV, Takenaka H, D’Amore PA, Stein-Streilein J, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow–derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106(13):4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 9.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012;7(3):e31794. doi: 10.1371/journal.pone.0031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska‐Hansen B, Kurzen H, Ugurel S, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209(1):67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 11.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, Heikamp E, McDevitt MR, Scheinberg DA, Benezra R, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124(3):922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessi S, Merighi S, Varani K. Adenosine receptors: the status of the art In: The adenosine receptors. Cham: Humana Press; 2018. p. 1–11. (The Receptors). [Google Scholar]
- 14.Merighi S, Gessi S, Borea PA. Adenosine receptors: structure, distribution, and signal transduction In: The adenosine receptors. Cham: Humana Press; 2018. p. 33–57. (The Receptors). [Google Scholar]
- 15.Boison D. Regulation of extracellular adenosine In: The adenosine receptors. Cham: Humana Press; 2018. p. 13–32. (The Receptors). [Google Scholar]
- 16.Antonioli L, Fornai M, Blandizzi C, Haskó G. Adenosine regulation of the immune system In: The adenosine receptors. Cham: Humana Press; 2018. p. 499–514. (The Receptors). [Google Scholar]
- 17.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22(1):657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 18.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414(6866):916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 19.Milne GR, Palmer TM. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. Sci World J. 2011;11:320–339. doi: 10.1100/tsw.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montesinos MC, Desai A, Chen J-F, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A2A receptors. Am J Pathol. 2002;160(6):2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang C-K, Hirschhorn R, et al. Wound healing is accelerated by agonists of adenosine A2 (Gαs-linked) Receptors. J Exp Med. 1997;186(9):1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang S, Lee S-P, Kim KE, Kim H-Z, Mémet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009;113(11):2605–2613. doi: 10.1182/blood-2008-07-166934. [DOI] [PubMed] [Google Scholar]
- 23.Kim KE, Koh Y-J, Jeon B-H, Jang C, Han J, Kataru RP, Schwendener RA, Kim J-M, Koh GY. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide-induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175(4):1733–1745. doi: 10.2353/ajpath.2009.090133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A2A receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol. 2004;164(6):1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai A. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67(5):1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- 26.Mancardi S, Stanta G, Dusetti N, Bestagno M, Jussila L, Zweyer M, Lunazzi G, Dumont D, Alitalo K, Burrone OR. Lymphatic endothelial tumors induced by intraperitoneal injection of incomplete Freund’s adjuvant. Exp Cell Res. 1999;246(2):368–375. doi: 10.1006/excr.1998.4270. [DOI] [PubMed] [Google Scholar]
- 27.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 29.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81(4):343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dadras SS, Lange‐Asschenfeldt B, Muzikansky A, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. J Cutan Pathol. 2005;32(1):84. doi: 10.1111/j.0303-6987.2005.320bd.x. [DOI] [PubMed] [Google Scholar]
- 32.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162(6):1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 34.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170(2):774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hood JL, Roman SS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011. April 8. doi: 10.1158/0008-5472.CAN-10-4455 [DOI] [PubMed] [Google Scholar]
- 36.Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13(8):748–757. doi: 10.1593/neo.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C–induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109(3):1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian C-N, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et al. Preparing the “Soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66(21):10365–10376. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 40.Angeli V, Ginhoux F, Llodrà J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Dubey LK, Karempudi P, Luther SA, Ludewig B, Harris NL. Interactions between fibroblastic reticular cells and B cells promote mesenteric lymph node lymphangiogenesis. Nat Commun. 2017;8(1):367. doi: 10.1038/s41467-017-00504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrestha B, Hashiguchi T, Ito T, Miura N, Takenouchi K, Oyama Y, Kawahara K-I, Tancharoen S, Ki-I Y, Arimura N, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. 2010;184(9):4819–4826. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 43.Lenoir B, Wagner DR, Blacher S, Sala-Newby GB, Newby AC, Noel A, Devaux Y. Effects of Adenosine on Lymphangiogenesis. PLoS One. 2014;9(3):e92715. doi: 10.1371/journal.pone.0092715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons G-J, Leibovich SJ. An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am J Pathol. 2003;163(2):711–721. doi: 10.1016/S0002-9440(10)63698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM, Gause WC, Leibovich SJ, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26(1):376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36(4):921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci USA. 2013;110(36):14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittal D, Young A, Stannard K, Yong M, Teng MWL, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74(14):3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- 49.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95(1):32–39. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renyi-Vamos F, Tovari J, Fillinger J, Timar J, Paku S, Kenessey I, Ostoros G, Agocs L, Soltesz I, Dome B. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non–small cell lung cancer. Clin Cancer Res. 2005;11(20):7344–7353. doi: 10.1158/1078-0432.CCR-05-1077. [DOI] [PubMed] [Google Scholar]
- 51.Mohammed RAA, Green A, El-Shikh S, Paish EC, Ellis IO, Martin SG. Prognostic significance of vascular endothelial cell growth factors -A, -C and -D in breast cancer and their relationship with angio- and lymphangiogenesis. Br J Cancer. 2007;96(7):1092–1100. doi: 10.1038/sj.bjc.6603678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–256. [PubMed] [Google Scholar]
- 53.Miyata Y, Kanda S, Ohba K, Nomata K, Hayashida Y, Eguchi J, Hayashi T, Kanetake H. Lymphangiogenesis and angiogenesis in bladder cancer: prognostic implications and regulation by vascular endothelial growth factors-A, -C, and -D. Clin Cancer Res. 2006;12(3):800–806. doi: 10.1158/1078-0432.CCR-05-1284. [DOI] [PubMed] [Google Scholar]
- 54.Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, Kubista E, Hausmaninger H, Samonigg H, Gnant M, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004;240(2):306–312. doi: 10.1097/01.sla.0000133355.48672.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brown M, Assen FP, Leithner A, Abe J, Schachner H, Asfour G, Bago-Horvath Z, Stein JV, Uhrin P, Sixt M, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359(6382):1408–1411. doi: 10.1126/science.aal3662. [DOI] [PubMed] [Google Scholar]
- 56.Pereira ER, Kedrin D, Seano G, Gautier O, Meijer EFJ, Jones D, Chin S-M, Kitahara S, Bouta EM, Chang J, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359(6382):1403–1407. doi: 10.1126/science.aal3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma Q, Dieterich LC, Ikenberg K, Bachmann SB, Mangana J, Proulx ST, Amann VC, Levesque MP, Dummer R, Baluk P, et al. Unexpected contribution of lymphatic vessels to promotion of distant metastatic tumor spread. Sci Adv. 2018;4(8):eaat4758. doi: 10.1126/sciadv.aat4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chrobak P, Charlebois R, Rejtar P, El Bikai R, Allard B, Stagg J. CD73 plays a protective role in collagen-induced arthritis. J Immunol. 2015;194(6):2487–2492. doi: 10.4049/jimmunol.1401416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


