Abstract
Mobility deficits, including gait disturbance, balance impairments and falls, are common features of Parkinson’s disease (PD) that negatively impact quality of life. Mobility deficits respond poorly to dopaminergic medications, indicating a role for additional neurotransmitters. Due to the critical role of cortical input to gait and balance, acetylcholine—an essential neurotransmitter system for attention—has become an area of interest for mobility. This review aimed to identify the role of cholinergic function on gait, balance, and falls in PD using three techniques; pharmacological, imaging, and electrophysiological. Studies supported the role of the cholinergic system for mobility in PD, with the most promising evidence indicating a role in falls. Imaging studies demonstrated involvement of anterior cholinergic (basal forebrain) systems in gait, and posterior (brainstem) systems in balance. However, this review identified a small number of studies which used varying protocols, making comparisons difficult. Further studies are warranted, measuring comprehensive gait and balance characteristics as well as gold standard falls detection to further quantify the relationship between ACh and mobility in PD.
Keywords: acetylcholine, gait, balance, falls, Parkinson’s disease
1. Introduction
Gait disturbances, difficulty with balance, and falls are prominent features of Parkinson’s disease (PD) that lead to reduced quality of life, increased healthcare costs and caregiver burden [1, 2]. Mobility deficits in people with PD are particularly problematic because they show limited improvement, and in some instances worsen, with treatments such as levodopa [3] and deep brain stimulation [4]. This clinical observation and mounting research make it increasingly clear that neurotransmitter systems besides the dopaminergic system play a role in regulation of gait and balance in PD.
One neurotransmitter system that may be particularly important for control of mobility is acetylcholine (ACh), which is reduced in people with PD compared to age-matched, healthy controls [5, 6]. Beyond subcortical cholinergic systems in the striatum, thalamus and cerebellum critical to mobility, ACh is primarily associated with alertness and cognition, specifically executive function and sustained attention [7]. The ACh system is of particular interest in PD because of the prominence of cognitive impairments from early in the disease course, which mainly impacts frontal lobe executive function and attention mechanisms [8]. Executive function and attention are important not only for cognitive functioning but also for mobility, as cognitive control of gait and balance is essential for safe mobilization [9, 10]. Gait and balance are complex sensorimotor tasks from which a number of comprehensive characteristics are measured. Due to the high number of characteristics, previous literature has sought to comprise characteristics onto domains of gait (e.g. pace, variability, asymmetry and trunk movement), and domains of balance (e.g. sway and jerkiness, anteroposterior and mediolateral sway) [11, 12] in order to simplify interpretation. Specific gait and balance domains, such as pace, variability and dynamic balance, may be more dependent on cortical control than others, such as gait asymmetry or sway [9]. Thus, it is hypothesized that ACh deficiencies contribute to mobility deficits via executive function and attentional mechanisms.
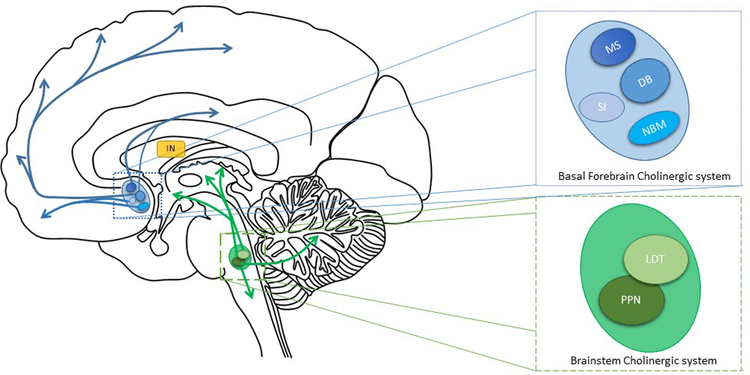
There are a number of sources of ACh in the brain. First, the basal forebrain nuclei, which contains the medial septal nucleus, diagonal band of Broca, nucleus basalis of Meynert (nbM) and substantia innominata, see Figure 1. The basal forebrain cholinergic nuclei project to the cerebral cortex (including prefrontal cortex), thalamus and striatum. Second, located in the brainstem and composed of two nuclei; the pedunculopontine nucleus (PPN) and the laterodorsal tegmental nucleus (LDT), which project to the thalamus, brainstem, basal forebrain and cerebellum (Figure 1) [13, 14]. Third, cholinergic interneurons make up 1–2% of striatal cells that modulate dopaminergic and GABAergic pathways [15] (Figure 1). The cholinergic systems degenerate in PD, with basal forebrain degeneration producing cortical cholinergic denervation and brainstem degeneration producing thalamic cholinergic denervation [16–20].
Figure 1:
Cholinergic projections of the central nervous system. Blue: basal forebrain cholinergic system which contains the medial septal nucleus (MS), the diagonal band of Broca (DB), nucleus basalis of meynert (NBM) and the substantia innominate (SI). The basal forebrain nuclei project to the prefrontal cortex, thalamus and striatum. Green: the brainstem cholinergic system contains the pedunculopontine nucleus (PPN) and the laterodorsal tegmental nucleus (LDT) which project to the thalamus, brainstem and cerebellum. IN= cholinergic striatal interneurons.
Three main techniques assess the central cholinergic system: pharmacological, imaging, and electrophysiological. First, pharmacological studies use acetylcholinesterase (AChE) inhibitors to increase the available amount of central ACh. AChE inhibitors such as donepezil or rivastigmine increase ACh concentration within cholinergic synapses by inhibiting the cholinesterase enzyme [21, 22]. Second, molecular imaging, such as single-photon emission computerized tomography (SPECT) and positron emission tomography (PET) targeting cholinergic markers, has enabled study of the cholinergic system in PD. Imaging targets include markers of cholinergic activity, which can be both presynaptic (VAChT and AChE) and postsynaptic (α4β2 nicotinic acetylcholine receptors [nAChR] and muscarinic acetylcholine receptors [mAChRs]) [18]. Third, transcranial magnetic stimulation (TMS) measures short latency afferent inhibition (SAI), an electrophysiological technique used to investigate cortical cholinergic activity [23, 24]. Evidence for the cholinergic contribution to SAI is demonstrated by muscarinic antagonists, which significantly decrease SAI in healthy adults (Di Lazzaro et al., 2000). Additionally, patients with Alzheimer’s disease, which is known to impair cholinergic function, have significantly worse SAI than otherwise healthy controls [25].
Due to recent interest in the relationship between the cholinergic system and mobility, we sought to review the role of cholinergic function in gait, balance, and falls in people with PD. This review will focus on the three different measurement techniques – pharmacological, imaging and electrophysiological – to collate current evidence for the role of the cholinergic system in gait, balance, and falls in PD. We hypothesize that these three techniques will support the role of the cholinergic system for mobility in PD.
2. Methods
2.1. Search Strategy
Three databases were used for the search: Medline, Scopus, and PsychInfo. For each of the databases used, separate searches were performed for the three focuses of the review: pharmacological, imaging, and electrophysiology techniques. The search terms used are shown in Table 1.
Table 1.
Structured review search terms.
| Medline | PsychInfo | Scopus | ||
|---|---|---|---|---|
| Mobility and Cohort (for all three searches) | Mobility |
MESH: Gait, locomotion, walking, postural balance, fall |
MESH: Gait, locomotion, walking, postural balance, fall |
Keyword Search: Gait OR locomotion OR walking OR symmetry OR asymmetry OR frequency OR variability OR speed OR velocity OR stance OR step OR swing OR stride OR double limb, OR balance OR sway OR postural control, OR fall |
| Keyword Search: Gait OR locomotion OR walking OR symmetry OR asymmetry OR frequency OR variability OR speed OR velocity OR stance OR step OR swing OR stride OR double limb, OR balance OR sway OR postural control, OR fall | Keyword Search: Gait OR locomotion OR walking OR symmetry OR asymmetry OR frequency OR variability OR speed OR velocity OR stance OR step OR swing OR stride OR double limb, OR balance OR sway OR postural control, OR fall | |||
| Cohort | MESH: Parkinson’s disease | MESH: Parkinson’s disease | Keyword Search: Parkinson* OR Parkinson disease OR Parkinson’s disease OR Idiopathic Parkinson’s disease | |
| Keyword Search: Parkinson* OR Parkinson disease OR Parkinson’s disease OR Idiopathic Parkinson’s disease | Keyword Search: Parkinson* OR Parkinson disease OR Parkinson’s disease OR Idiopathic Parkinson’s disease | |||
| Measurement | Pharmacological | MESH: Cholinesterase inhibitors | MESH: Cholinesterase inhibitors | Keyword Search: Cholinesterase inhibitors OR Anticholinesterase drugs OR Anticholinesterases OR Anticholinesterase Agents OR Anti-cholinesterasers OR Acetylcholinesterase inhibitors OR Rivastigmine OR Exelon OR Donepezil OR Aricept OR Galantamine OR Galanthamine OR Reminyl OR Nivalin OR Razadyne |
| Keyword Search: Cholinesterase inhibitors OR Anticholinesterase drugs OR Anticholinesterases OR Anticholinesterase Agents OR Anti- cholinesterasers OR Acetylcholinesterase inhibitors OR Rivastigmine OR Exelon OR Donepezil OR Aricept OR Galantamine OR Galanthamine OR Reminyl OR Nivalin OR Razadyne | Keyword Search: Cholinesterase inhibitors OR Anticholinesterase drugs OR Anticholinesterases OR Anticholinesterase Agents OR Anti-cholinesterasers OR Acetylcholinesterase inhibitors OR Rivastigmine OR Exelon OR Donepezil OR Aricept OR Galantamine OR Galanthamine OR Reminyl OR Nivalin OR Razadyne | |||
| Imaging | MESH: positron-emission tomography, ‘Tomography, Emission-Computed, Single-Photon’, magnetic resonance imaging, functional magnetic resonance imaging | MESH: positron-emission tomography, ‘Tomography, Emission-Computed, SinglePhoton’, magnetic resonance imaging, functional magnetic resonance imaging |
Keyword search: PET OR SPECT OR vesicular acetylcholine transporter PET ligand OR pedunculopontine nucleus (PPN) OR nucleus basalis of meynart (NbM) OR [123I]IBVM OR [18F]FEOBV OR 18F]Fluoroethoxy-Benzovesamicol OR [18F]Fluoroethoxybenzovesamic ol OR [11 C]MP4A OR [11C]PMP OR [123I]5IA OR [18F]2FA 2- [18F]fluoro-3- (2(S)azetidinylmethoxy)pyridine (2FA) OR [11 C]nicotine, [11 C]N- methyl-4-piperidyl benzilate OR [11C]NMPB, 3-quinuclidinyl-4- iodobenzilate (QNB) OR [123I]QNB OR [123I]-(R,R)-I- QNB |
|
|
Keyword search: PET OR SPECT OR vesicular acetylcholine transporter PET ligand OR pedunculopontine nucleus (PPN) OR nucleus basalis of meynart (NbM) OR [123I]IBVM OR [18F]FEOBV OR [18F]Fluoroethoxy-Benzovesamicol OR [18F]Fluoroethoxybenzovesamicol OR [11C]MP4A OR [11C]PMP OR [123I]5IA OR [18F]2FA 2- [18F]fluoro-3- (2(S)azetidinylmethoxy)pyridine (2FA) OR [11 C]nicotine, [11 C]N- methyl-4-piperidyl benzilate OR [11C]NMPB, 3-quinuclidinyl-4- iodobenzilate (QNB) OR [123I]QNB OR [123I]-(R,R)-I-QNB |
Keyword search: PET OR SPECT OR vesicular acetylcholine transporter PET ligand OR pedunculopontine nucleus (PPN) OR nucleus basalis of meynart (NbM) OR [123I]IBVM OR [18F]FEOBV OR [18F]Fluoroethoxy- Benzovesamicol OR [18F]Fluoroethoxybenzovesamicol OR [11 C]MP4A OR [11 C]PMP OR [123I]5IA OR [18F]2FA 2- [18F]fluoro-3-(2(S)azetidinylmethoxy)pyridine (2FA) OR [11 C]nicotine, [11 C]N- methyl-4-piperidyl benzilate OR [11C]NMPB, 3-quinuclidinyl-4- iodobenzilate (QNB) OR [123I]QNB OR [123I]-(R,R)-I-QNB |
|||
| SAI | Keyword Search: Short-Latency Afferent Inhibition OR Short Latency Afferent Inhibition OR SAI OR Cholinergic | Keyword Search: Short-Latency Afferent Inhibition OR Short Latency Afferent Inhibition OR SAI OR Cholinergic | Keyword Search: Short-Latency Afferent Inhibition OR Short Latency Afferent Inhibition OR SAI OR Cholinergic |
The search was limited to full journal articles written in the English language published from February 1990 to March 2018. Three master databases were produced for each focus of the review, duplicates were deleted and an initial title screen was performed (RM, DM and TM). After the initial title screen, abstracts were reviewed by the three reviewers (RM, DM and TM). Full review of the text was completed when it remained unclear from the abstract whether the paper was suitable for inclusion.
2.2. Inclusion and Exclusion criteria
Articles were included if (1) they assessed cholinergic function via pharmacological, imaging or electrophysiological techniques in people with PD, and (2) they associated cholinergic function with mobility, inclusive of comprehensive objective measures of balance and gait as well as falls (including falls status), in PD.
2.3. Data Extraction
Three separate extraction forms were used for each focus of the review. Information from the forms were then transferred to the review table. Information extracted included participant groups, participant characteristics, mobility assessed (including individual variables of balance and gait), analysis tools, medication status and key findings.
3. Results
3.1. Search yield
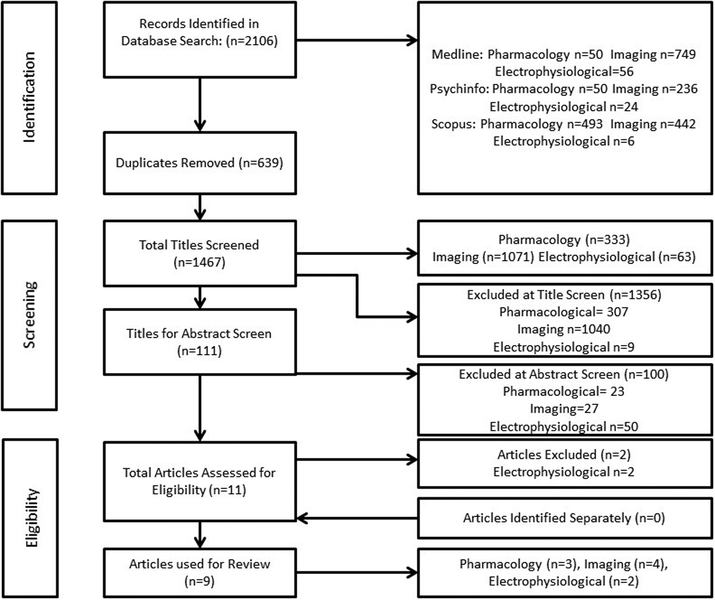
The search strategy generated a total of 2106 papers. After duplicates, a total of 1467 papers were yielded from the search. The total number of papers were split into three arms of the search: pharmacology studies (333), imaging studies (1071) and electrophysiological techniques (63). After an abstract screen, 11 papers were eligible for data extraction (n=3 for pharmacology studies, n=4 for imaging studies and n=4 for electrophysiological techniques). Two papers did not meet inclusion criteria and were excluded; thus, a total of nine papers were included in this review. The search yield and all parameters are demonstrated in Figure 2. All articles were published in the English language, and publication dates ranged from 2009 to 2016.
Figure 2:
PRISMA diagram of search yield used for structured review.
3.2. Pharmacological Studies
The results of the three phase 2 clinical trials [26–28] assessing pharmacological intervention in relation to gait, balance and falls are presented in Table 2. All three studies used AChE inhibitors, either donepezil or rivastigmine. All were randomized, double-blind studies, with two randomized placebo-controlled parallel group trials [27, 28], and one crossover trial [26]. One study assessed gait [27], measuring characteristics of gait speed (m/s) and step time variability (s). This study identified that those receiving AChE significantly increased gait speed and reduced step time variability under both single and dual-task conditions (gait assessment whilst completing a concurrent cognitive or motor task) compared to the control group. Two studies assessed balance, one using the Berg Balance Scale [26] and the other study using the controlled leaning balance score [27]. The Berg Balance Score did not change (Chung, et al, 2010) but the controlled leaning balance score improved [27] with ACh supplementation. All three of pharmacological studies assessed number of falls [26–28] before and after intervention, and all identified a reduced number of falls when taking either donepezil or rivastigmine. Thus, three RCT’s show a benefit of ACh enhancement via AChE inhibitors on gait, balance, or falls. However, interpretation is limited due to the differences in outcome measures used.
Table 2.
Main characteristics of the studies observing gait, balance, and falls in relation to pharmacological studies.
| Study | Participant Characteristics | Study Type | Medication | Mobility Measured | Analysis Tool | Main Study Findings |
|---|---|---|---|---|---|---|
| Chung et al., 2010 | Number: 19 Gender: 15M & 4F Age: 68.3 ± 10.8 Years since diagnosis: 10 ± 5.6 Med: NR |
Randomised, crossover, double-blind study |
Treatment phase: Donepezil, 5mg for 3 weeks, increased to 10mg for 3 weeks. 3- week washout period before placebo phase. Placebo Phase: aesthetically identical medication |
Balance: Balance scale score Falls: Number of falls and near falls |
Balance: Berg Balance Scale Falls: Falls postcards |
Balance: No significant change on Berg Balance Scale score. Falls: During treatment phase, subjects fell significantly less than during placebo phase. Those who fell most frequently at baseline showed greatest reduction in falls. |
| Henderson et al., 2016 | Treatment group: Number: 65 Gender: 35M & 30F Age: 71 (54–90) Years since diagnosis: 8 (5–13) Med: NR Placebo group: Number: 65 Gender: 46M & 19F Age: 69 (46–88) Years since diagnosis: 9 (5–13) Med: NR |
Randomised, double-blind, placebo- controlled, phase 2 trial |
Treatment group: 3mg Rivastigmine (1.5mg 2 × per day), titrated up in 3mg increments every 4 weeks to maximum of 12mg per day from week 13 onwards. Placebo: placebo tablet titrated as with treatment group. |
Gait: gait speed (m/s), step time variability (s) Balance: Controlled leaning balance score Falls: rate of falls per month |
Gait: Triaxial accelerometer (DynaPort Hybrid, McRoberts, Netherlands), 22m flat covered walkway under three conditions: 1) single task, 2) simple dual task and 3) complex dual task Balance: Controlled leaning balance test Falls: monthly falls diaries. Posted monthly, telephoned once per month for additional information. |
Gait: Gait speed increased in treatment group. Step time variability was 28% and 21% lower in single and dual task conditions respectively in the treatment group. Balance: Treatment group had significantly better scores on controlled leaning balance test. Falls: Treatment group had 45% falls reduction. |
| Li et al., 2015 | Treatment group: Number: 41 Gender: 30M & 11F Age: 67.5 (52.7–71.1) Years since diagnosis: 5.3 (2.4–7.1) Med: NR Placebo group: Number: 40 Gender: 21M & 19F Age: 66.9 (53.8–70.3) Years since diagnosis: 5.5 (2.6–8.0) |
Randomised, double-blind, placebo controlled trial | Treatment group: 3mg Rivastigmine for 12 months Placebo group: NR |
Falls: Number of falls per person in years, Incidence of falls (number) | Falls: Weekly Telephone Follow-up calls | Falls: Number and incidence of falls were significantly reduced in the treatment arm compared to placebo arm. |
NR=not reported
3.3. Imaging Studies
Four PET studies assessing the cholinergic system were all from the same laboratory, as shown in Table 3 [5, 16, 17, 29]. All used1C-methylpiperidin-4-yl propionate (11C-PMP PET) as a radioligand for pre-synaptic AChE. Two of the four studies assessed the relationship between AChE levels and gait speed [5, 29]. One study of a total of 101 participants assessed both neocortical and thalamic AChE and found neither were significantly associated with gait speed [5] but in the 31 subjects with low neocortical AChE, the relationship between cholinergic function and gait speed approached significance. In contrast, the second study in 125 subjects showed that gait speed was slower in those with nigrostriatal dopaminergic and basal forebrain cholinergic denervation [29]. One study of the relationship between cholinergic activity and balance ([17] identified that decreased thalamic cholinergic innervation, not cortical cholinergic or striatal dopaminergic deficits, was associated with increased center of pressure sway speed (indicating impaired balance) when controlling for cognitive and motor impairments. Two of the four studies assessed falls using the Unified Parkinson’s disease Rating Scale (UPDRS) part II [16] and self-reported falls history [5]. PD fallers had significantly reduced cortical and thalamic AChE, but PD non-fallers only had reduced cortical AChE compared to controls [16]. The second study split PD subjects into groups by AChE activity (low combined neocortical and thalamic, low isolated neocortical, or low isolated thalamic) [5] and identified that PD fallers had lower thalamic AChE compared to non-fallers.
Table 3.
Main characteristics of the studies observing gait, balance, and falls and imaging techniques.
| Study | Participant Characteristics | Imaging Technique | Mobility Measured | Analysis Tool | Main Study Findings |
|---|---|---|---|---|---|
| Bohnen et al., 2009 |
PD fallers: Number: 17 Gender: NR Age: 72.5 ± 9.3 Years since diagnosis: 8.8 ± 4.3 Med: NR PD non-fallers: Number: 27 Gender: NR Age: 66.6±9.1 Years since diagnosis: 6.0 ± 3.9 Med: NR Control: Number: 15 Gender: 7M & 8F Age: 64.4 ± 9.6 (50–81) |
MRI and PET (PMP & DTBZ) | Falls: Fall status | Falls: UPDRS, Part II, Question 13 (score of 0 is non-faller, score of >1 classified as faller) | Falls: Significantly reduced cortical AChE hydrolysis rates seen in PD fallers followed by PD non-fallers compared to control subjects. |
| Bohnen et al., 2012 |
PD: Number: 101 Gender: 76M & 25F Age: 65.3 ± 7.2 (50–84) Years since diagnosis: 5.9 ± 3.9 (0.5–19) Med: Off Control: Number: 29 Gender: 16M & 13F Age: 66.8 ± 10.9 (50–84) |
MRI and PET (PMP & DTBZ). Patients classified as low (n=31) or normal neocortical (n=70) AChE and low (n=18) or normal (n=83) thalamic AChE. | Gait: Speed Falls: Fall History |
Gait: Time to walk 8.5m Falls: Fall history selfreported clinically |
Gait: Gait speed difference near significance between low and normal neocortical AChE groups. No gait speed difference between low and normal thalamic AChE groups. Falls: Increased history of falls in the low thalamic AChE group (18% of subjects). |
| Bohnen et al., 2013 |
PD DA+: Number: 87 Gender: 61M & 26F Age: 64.2 ± 7.1 Med: OFF PD DA+/Ach+: Number: 38 Gender: 34M & 4F Age: 68.7 ± 7.0 Med: OFF Controls” Number: 32 Gender: 18M & 14F Age: 66.0 ± 10.6 |
MRI and PET (PMP & DTBZ) | Gait: Speed | Gait: Time to walk 8.5m | Gait: The group with DA+/Ach+ had significantly slower gait speed. No association with gait speed and low PPN ACh levels. |
| Muller et al, 2013 |
PD: Number: 124 Gender: 32F & 92M Age: 65.5 ± 7.4 Motor disease duration: 6.0 ± 4.2 Med: OFF Control: Number: 25 Gender: 10F & 15M Age: 66.8 ± 10.1 |
PET,11C-methylpiperidin- 4-yl propionate AChE positron emission tomography (11C-PMP) | Balance: Centre of Pressure (COP) speed and COP RMS | Balance: EquiTest balance platform (NeuroCom, a division of Natus) sensory organisation test | Balance: Increased postural sway associated with lower thalamic AChE activity No association of neocortical cholinergic innervation with postural sensory integration |
ACh= acetylcholine, AChE= Acetylcholinesterase, DTBZ= [(11)C] dihydrotetrabenazine, MRI= Magnetic Resonance Imaging, NR= not reported, PET= Positron Emission Tomography, PMP= 1C-methylpiperidin-4-yl propionate, PPN= pedunculopontine nucleus, RMS= root mean square.
In summary, four PET studies consistently show that balance problems and falls history are linked to reduced thalamic, but not cortical, ACh levels whereas gait abnormalities relate to cortical ACh in people with PD. Thus, ACh may play a role in both balance and gait, but in different brain networks.
3.4. Short-latency Afferent Inhibition (SAI)
Table 4 presents the results of the two studies using SAI to assess cortical cholinergic activity and relate this to gait and falls [30, 31]. Both studies were cross-sectional, included subjects who were ON levodopa medication and used the GAITRite electronic walkway to assess spatiotemporal gait characteristics. Rochester et al., used the interstimulus intervals of N20+0 to N20+4, in increments of one millisecond, whereas Pelosin et al., used the interstimulus intervals of N20–2 to N20+8, in increments of two milliseconds. SAI was calculated as the grand mean of the percentage of the unconditioned trial for each interstimulus interval. Rochester et al. included a PD group regardless of fall status, whereas Pelosin et al., only assessed a PD faller group. Both groups found SAI to be significantly worse in the PD group compared to controls. Gait speed (m/s) was reported in both investigations, though Rocehster et al., also reported stride length (m), stride time (s), step width (cm), as well as the variability (SD) of these variables. Rochester et al., found that both slower single-task gait speed and shorter step length were significantly correlated with worse SAI in PD only. In contrast, Pelosin et al., reported the dual-task cost, defined as dual-task gait speed minus single-task gait speed, with increased dual-task through to reflect a greater need for cortical control of walking. They identified a significant correlation between increased dual-task cost of gait speed and worse SAI. One study assessed the number of falls in six months prior to participation [31] and demonstrated worse SAI in both the PD faller and older adult faller groups than the older adult non-faller group, indicating that cortical cholinergic activity may play a role in falls, regardless of PD status.
Table 4.
Main characteristics of the studies observing gait, balance, and falls and electrophysiological techniques
| Study | Study Type | Participant Characteristics | Method | Mobility Measured | Analysis Tool | Main Study Findings |
|---|---|---|---|---|---|---|
| Rochester et al., 2012 | Cross Sectional |
PD: Number: 22 Gender: 16M & 6F Age: 70.2(9.7) Months since diagnosis: 19.8(8.6) Med: ON Control: Number: 22 Gender: 9M & 13F Age: 67.4(8.4) |
TMS: SAI from N20 to N20+4 | Gait: gait speed (m/s), stride length (m), stride time (s) and step width (cm), variability of gait speed, stride length, stride time and step width described as the standard deviation | Gait: GAITRite electronic walkway | Gait: SAI was significantly correlated with gait speed and stride length in PD only. SAI was a significant independent predictor of gait speed in PD only. SAI remained a significant predictor when adjusting for age, cognition, depression, and disease severity. |
| Pelosin et al., 2016 | Cross Sectional |
PD Fallers (PD-F): Number: 33 Gender: 17M & F16 Age: 72.6(4.4) Years since diagnosis: NR Med: ON Older Adult Fallers (OA-F): Number: 17 Gender: 7M & 10F Age: 73.4(4.2) Older Adult Non-Fallers (OA-NF): Number: 14 Gender: 9M & 5F Age: 72.1(4.9) |
TMS: SAI from N20–2 to N20+8, by 2’s. | Gait: gait speed (m/s) and DTC of gait speed. Falls: number of falls in 6 months prior to study. |
Gait: GAITRite electronic walkway Clinical Assessments: MOCA, and UPDRS-III |
Gait: During single task alking, gait speed was significantly lower in both PD-F and OA-F compared to OA-NF with no difference between PD-F and OAF. During dual task walking, gait speed was significantly lower in PD-F than in both OA-F and OA- NF and in OA-F than in OA- NF. SAI was significantly worse in PDF compared to both OA-F and OA-NF. SAI was significantly worse in OA-F compared to OA- NF. SAI significantly correlated with dual task change in gait speed. SAI was a significant independent predictor of dual task change in gait speed. SAI remaind a significant predictor when adjusting for age and cognition. |
DTC= dual task cost, NR= not reported, SAI= short-latency afferent inhibition
Thus, two small SAI studies show that lower ACh levels are associated with reduced gait speed, stride length and increased dual-task cost, as well as falls in people with PD. However, no studies to date have evaluated the relationship between SAI and balance.
4. Discussion
This structured review aimed to provide an overview of the role of the cholinergic system in gait, balance, and falls in people with PD. We reviewed pharmacological, imaging, and electrophysiological techniques to improve understanding of this central neural transmitter system on mobility in PD. Evidence from all three measurement techniques appear to support the hypothesis that ACh plays a role in gait, balance, and falls in people with PD. Although evidence to date is promising, it is limited due to a small number of studies with varying assessments of balance, gait and falls.
The cholinergic system and gait
This review showed significant associations between cholinergic activity and gait. Gait speed was the most commonly assessed gait characteristic and was strongly associated with ACh levels (both PET and SAI), supporting the hypothesis for a cholinergic role in gait. However, comprehensive gait characteristics, beyond gait speed, improve utility due to the sensitive and specific nature of discrete gait characteristics [9]. For example, step length and step time variability were associated with cholinergic function. Step length is thought to be a characteristic of gait highly dependent on cognition [9] as demonstrated by improvement in step length when using attentional cues [32] and its sensitivity to cognitive decline in PD [33]. Although this result is unsurprising, step length was only assessed in one study so further work is needed to validate these findings.
In contrast to gait speed, step time variability, assessed in two studies, was not related to cholinergic function as measured by SAI, although it was improved with rivastigmine [27, 30]. The response to rivastigmine suggests a cholinergic role in gait variability, which was previously demonstrated to be cortically controlled in PD but not in older adults [9]. The reason for these contradictory findings may be twofold. First, different gait measurement techniques were used with body worn sensors having increased sensitivity to characteristics of variability compared to the electronic walkway [34]. Second, the SAI study was in an incident cohort of PD [30] whereas the pharmacological study by Henderson et al., was in a more advanced PD cohort. The differences in cohorts suggest dopaminergic and cholinergic loss may mirror findings in older adults in which gait variability, in mild disease, does not appear to be mediated by cognition [9]. Thus, in an early PD cohort, dopaminergic and cholinergic loss may not have reached a threshold at which attention is needed to compensate for deficits in gait variability.
The findings for gait speed, step length and gait variability support the notion that gait requires higher cognitive function, namely executive function and attention, for safe and purposeful mobility [35]. The gait-cognition relationship can be assessed via dual-task paradigms in which concurrent cognitive tasks are performed whilst walking [36]. Decline in gait performance under dual-task compared to single-task conditions are thought to reflect impaired automaticity of gait control and/or an increase in the need to use cognitive processes, such as attention, to control gait [35]. In light of this, one reviewed article identified an association between cholinergic activity and dual-task gait, further indicating the role of attention under challenging gait conditions.
Poorer performance on gait was only associated with neocortical ACh, but not thalamic ACh levels, suggesting that the basal forebrain, but not brainstem, cholinergic system contributes to cholinergic control of gait. This finding complements evidence of higher cognitive control for gait characteristics and the underpinning role of attention for gait in PD [9, 11]. A small number of studies in this review identified better performance on executive function and attention assessments correlated with poorer gait with lower ACh levels, thus, suggesting an indirect role for ACh on gait via cognition [5, 27, 30]. However, more comprehensive assessments of gait, balance and cognition in association with ACh are required to further understand the extent to which ACh affects mobility both indirectly and directly via attentional contributions and sensorimotor control of gait and balance.
The cholinergic system and balance
Evidence from this review supported the role of ACh in balance deficits in PD, with two of three studies identifying an association. However, there were some contradictory results, which are most likely due to the different balance outcome measures used across studies. Balance improved when measured by the controlled leaning balance scale in those taking rivastigmine [27] and poorer balance was noted in those with lower thalamic AChE when using an objective measure of postural sway [17]. In contrast, there was no improvement in the Berg Balance Scale when taking donepezil [26, 37]. This was the only study that did not use an objective measure of postural sway and it is likely that the Berg Balance scale is not sensitive enough to detect subtle changes in balance in the PD cohorts for these studies. Although other subjective paper-based outcome measures are more sensitive to milder deficits in PD [38], discrete changes in balance are difficult to measure with such scales [39].
As measured by PET studies, poorer balance was associated only with thalamic ACh but not neocortical ACh, which implies the brainstem cholinergic system has greater influence for static balance than the basal forebrain system. In support of this, stereology techniques identified those with PD and impaired balance have fewer cholinergic neurons in the PPN compared to those without balance deficits [40]. Furthermore, the PPN has a critical role in regulation of Rapid Eye Movement (REM) in humans with REM sleep disorder, a common feature of PD, and may be associated with direct targeting of the PPN to treat balance deficits; however, deep brain stimulation of the PPN has so far shown mixed clinical improvements in balance [43]; therefore alternative therapies to improve activity of the brainstem cholinergic system are indicated.
The cholinergic system and falls
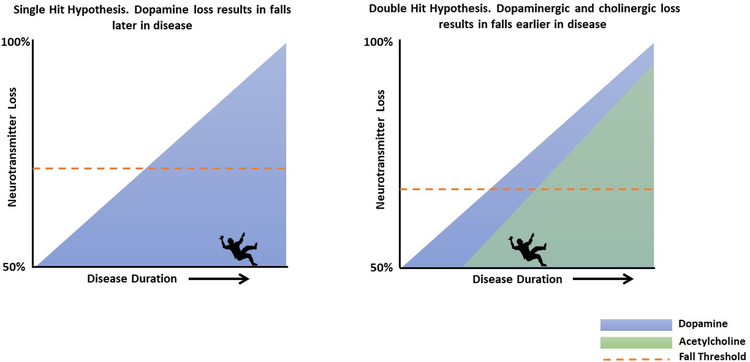
The most promising evidence that emerged from this review supported the association between reduced ACh activity and increased incidence of falls. All three pharmacological studies found a reduction in fall rates when patients took either donepezil or rivastigmine. All three studies used the gold standard outcome measure of self-report falls diaries [44], further validating these findings. The imaging work from this review revealed reduced thalamic AChE was associated with PD fallers, and although those with low neocortical AChE had a higher incidence of falls, this was not significant [5]. Previous work has identified the ‘dual-hit hypothesis’ which describes dual dopaminergic and cholinergic loss [45] in PD. Studies in rodents demonstrate that dual dopaminergic and cholinergic loss results in a significant increase in falls, when using an agility course that reflects balance capabilities in humans. Furthermore, an increase in falls was correlated with poorer performance on an attention task [45]. The authors hypothesize that attentional control can no longer compensate for striatal impairments in rodents. In PD, cognitive reserve may compensate for gait and balance deficits, but when cognitive reserve is diminished, gait and dynamic balance may no longer be compensated for by attentional control, leading to an increased falls risk (see Figure 3). Interestingly, SAI was reduced in older adult fallers compared to older adult non-fallers [31], suggesting reduced ACh, likely from the brainstem, increases falls risk unrelated to PD. However, SAI was significantly worse in PD fallers compared to older adult fallers in the same article [31]. This further demonstrates the dual-hit hypothesis in PD, in which dual dopaminergic and cholinergic loss further increases falls risk.
Figure 3:
Single and double hit hypothesis of dopaminergic and cholinergic loss for falls risk in Parkinson’s disease. Single hit hypothesis; mainly loss of dopamine with minimal, age-related loss of acetylcholine. Double hit hypothesis; loss of both dopamine and acetylcholine. Dotted red line indicates the decreased fall threshold with both dopaminergic and cholinergic loss causing falls to occur earlier in disease
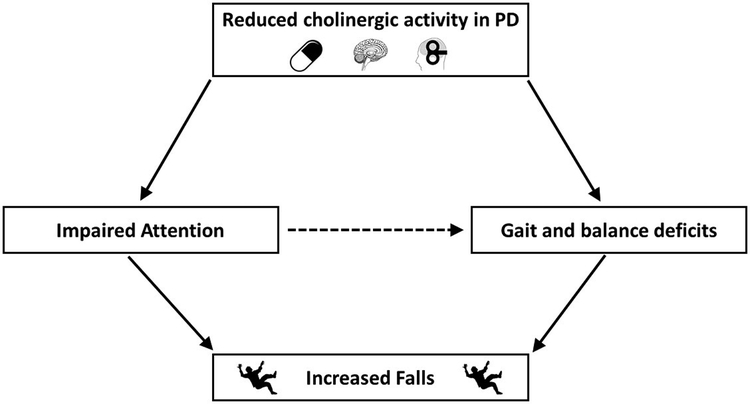
The above results suggest a critical role of the brainstem cholinergic system as a neural correlate for falls in PD, but it is likely that underlying cognitive deficits associated with neocortical ACh from the basal forebrain play a role that further exacerbates falls in PD. Studies in humans suggest that the role of attention in falls may be important later in disease. In an incident cohort of PD, discrete gait characteristics predicted future falls risk but not cognition [46, 47]. However, in more advanced disease, an intervention that incorporated cognitive challenges, a virtual reality paradigm combined with treadmill training, was shown to reduce falls compared to treadmill training alone [48]. Thus, in later disease, when ACh levels are further reduced, training to increase attentional resources may override dopaminergic loss. This indicates that attention could mediate the impact of gait and balance deficits on falls risk (see Figure 4). In accordance with figure 4, reduced cholinergic activity may be a consequence of anticholinergic medications in addition to PD pathology. Anticholinergic drugs are commonly used in PD to treat both motor symptoms and non-motor symptoms. In turn, such medications have demonstrated increased falls risk and reduced physical function [49, 50].
Figure 4:
Reduced cholinergic activity in PD causes deficit in attention (direct relationship) and gait and balance (direct relationship). Impaired attention, gait and balance then lead to an increased falls risk (direct relationship). It is proposed that reduced cholinergic activity leads to poorer attention which indirectly affects gait and balance leading to increased falls risk (dashed line).
One caveat to our findings is that the imaging studies from this review utilized clinical self- reported falls measures, which limits the scope of interpretation around falls. The use of the gold-standard falls diaries to classify recurrent fallers and faller phenotype [51–53] would help to determine the association of neocortical and thalamic ACh with detailed fall characteristics. In addition, there were no electrophysiological studies assessing SAI between PD fallers and PD non-fallers, and therefore future work using this technique would further validate the role of ACh in falls in this population.
Future Implications
The recent evidence for the role of ACh in gait, balance, and falls has future therapeutic implications for PD. One of the main challenges is the variety of protocols used to assess gait, balance and falls; however- this is unsurprising due to the small number of current publications. To further collate evidence of ACh for mobility deficits in PD, future research should use standardized, validated protocols assessing comprehensive measures of gait and balance. The use of current gait and balance models would allow for comprehensive variables and reduce heterogeneity allowing for replication and validation of research [11, 12, 54]. Pharmacological studies show promising results for improving mobility in PD; however, side effects of AChE inhibitors are burdensome and especially problematic in the older adult population [27]. Furthermore, current AChE inhibitors have limited brain uptake and therefore limited efficacy. Thus, other treatment options to increase cholinergic function need to be sought. Exercise may provide a non-pharmacological alternative therapy based upon evidence from animal studies in which exercise interventions increased ACh production [55, 56]. In humans, exercise interventions that include cognitive components have demonstrated beneficial effects on mobility and falls [48], but to the best of our knowledge such interventions have not been related specifically to ACh. Other techniques to improve cholinergic output such as deep brain stimulation of the NbM [57, 58] and non-invasive vagus nerve stimulation [59] are in the early stages of investigation, and so far, demonstrate mixed results.
Limitations
Limitations to this review include the lack of use of a quality assessment tool to assess quality of individual articles; however, due to the small number and the variability of study designs included in this review, we did not see this as appropriate. In addition, we chose to focus on gait, balance, and falls in PD and not other motor symptoms such as Freezing of Gait; this would be of interest for future studies. Finally, we have to acknowledge that imaging and electrophysiological techniques bias measurement for cortical over subcortical neural networks and therefore the role of subcortical circuits in mobility may be underrepresented in our findings. Although 11C-PMP PET provides a precise index of AChE activity in brain regions with a low AChE concentration (i.e., cortex), estimates of AChE activity in areas of high activity (i.e, cerebellum, basal ganglia) are poorer. This is due to the combination of a relatively high hydrolysis rate of 11C-PMP by AChE and high concentration of AChE resulting in the uptake of the tracer being limited by delivery to the tissue, resulting in low sensitivity to change [60]. Thus, papers were implicitly limited to examining group differences in cholinergic innervation of areas with relatively low AChE concentration. For SAI, as TMS is a cortical assessment, the outcome measure is cortical in nature. This does not rule out subcortical input on MEP response, including the striatal cholinergic interneurons. For example, Nardone et al. (2008) identified worse SAI in subcortical ischemic vascular disease (SIVD) but findings are difficult to interpret as the authors were not able to rule out concurrent AD pathology from their SIVD group, confounding TMS results [61]. Ultimately, the direct role of subcortical neural input on motor output following cortical stimulation with TMS remains unknown [62–64].
Conclusions
This review aimed to synthesize current evidence for the role of cholinergic function in gait, balance, and falls in people with PD. Current evidence from pharmacological, imaging, and electrophysiological studies support the role of ACh for mobility in PD. Given the prevalence of mobility problems in PD and their poor response to current treatment, an improved understanding of how ACh influences mobility in PD has the potential to inform future interventions with the aim to improve patient quality of life and reduce caregiver burden. To better inform future work, comprehensive measurements of balance, gait, falls and cognition are required in order to extract both the direct and indirect effects of ACh on mobility in PD.
Acknowledgements
This research was supported by the Pacific Udall Center (P50 NS062684–07) and the Department of Veterans Affairs Northwest Parkinson’s Disease Research, Education and Clinical Care Center.
Footnotes
Conflicts of Interest
FH has a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology- this potential institutional and individual conflict has been reviewed and managed by OHSU
References
- [1].Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ, Determinants of disability and quality of life in mild to moderate Parkinson disease, Neurology 70(23) (2008) 2241–7. [DOI] [PubMed] [Google Scholar]
- [2].Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M, Caregiver-burden in parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability, Parkinsonism Relat Disord 12(1) (2006) 35–41. [DOI] [PubMed] [Google Scholar]
- [3].Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB, Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease, Mov Disord 30(10) (2015) 1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fasano A, Appel-Cresswell S, Jog M, Zurowkski M, Duff-Canning S, Cohn M, Picillo M, Honey CR, Panisset M, Munhoz RP, Medical Management of Parkinson’s Disease after Initiation of Deep Brain Stimulation, Canadian Journal of Neurological Sciences 43(5) (2016) 626–34. [DOI] [PubMed] [Google Scholar]
- [5].Bohnen NI, Müller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, Albin RL, Frey KA, Heterogeneity of Cholinergic Denervation in Parkinson’s Disease without Dementia, Journal of Cerebral Blood Flow & Metabolism 32(8) (2012) 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calabresi P, Picconi B, Parnetti L, Di Filippo M, A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine–acetylcholine synaptic balance, The Lancet Neurology 5(11) (2006) 974–983. [DOI] [PubMed] [Google Scholar]
- [7].Taylor P, Heller Brown J, Acetylcholine, in: Sigel G (Ed.), Basic Neurochemistry: Molecular, Cellular and Medical Aspects, Elsevier Academic Press, Philadelphia: 2006, pp. 185–210. [Google Scholar]
- [8].Yarnall AJ, Rochester L, Burn DJ, Mild cognitive impairment in Parkinson’s disease, Age and Ageing 42(5) (2013) 567–576. [DOI] [PubMed] [Google Scholar]
- [9].Morris R, Lord S, Bunce J, Burn D, Rochester L, Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease, Neurosci Biobehav Rev 64 (2016) 326–45. [DOI] [PubMed] [Google Scholar]
- [10].Mc Ardle R, Morris R, Wilson J, Galna B, Thomas AJ, Rochester L, What Can Quantitative Gait Analysis Tell Us about Dementia and Its Subtypes? A Structured Review, J Alzheimers Dis 60(4) (2017) 1295–1312. [DOI] [PubMed] [Google Scholar]
- [11].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L, Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach, J Gerontol A Biol Sci Med Sci 68(7) (2013) 820–7. [DOI] [PubMed] [Google Scholar]
- [12].Horak FB, Mancini M, Carlson-Kuhta P, Nutt JG, Salarian A, Balance and Gait Represent Independent Domains of Mobility in Parkinson Disease, Physical Therapy 96(9) (2016) 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yarnall A, Rochester L, Burn DJ, The interplay of cholinergic function, attention, and falls in Parkinson’s disease, Mov Disord 26(14) (2011) 2496–503. [DOI] [PubMed] [Google Scholar]
- [14].Mesulam M-M, Mufson EJ, Levey AI, Wainer BH, Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey, Journal of Comparative Neurology 214(2) (1983) 170–197. [DOI] [PubMed] [Google Scholar]
- [15].Lim SA, Kang UJ, McGehee DS, Striatal cholinergic interneuron regulation and circuit effects, Front Synaptic Neurosci 6 (2014) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bohnen NI, Muller MLTM, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL, History of falls in Parkinson’s disease is associated with reduced cholinergic activity, Neurology (73) (2009) 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Müller MLTM, Albin RL, Kotagal V, Koeppe RA, Scott PJH, Frey KA, Bohnen NI, Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease, Brain 136(11) (2013) 3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bohnen NI, Albin RL, The cholinergic system and Parkinson disease, Behavioural Brain Research 221(2) (2011) 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bohnen NI, Albin RL, Cholinergic denervation occurs early in Parkinson disease, Neurology 73(4) (2009) 256–7. [DOI] [PubMed] [Google Scholar]
- [20].Kotagal V, Muller ML, Kaufer DI, Koeppe RA, Bohnen NI, Thalamic cholinergic innervation is spared in Alzheimer disease compared to parkinsonian disorders, Neuroscience letters 514(2) (2012) 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krall WJ, Sramek JJ, Cutler NR, Cholinesterase Inhibitors: A Therapeutic Strategy for Alzheimer Disease, Annals of Pharmacotherapy 33(4) (1999) 441–450. [DOI] [PubMed] [Google Scholar]
- [22].Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM, Acetylcholinesterase inhibitors: pharmacology and toxicology, Current neuropharmacology 11(3) (2013) 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC, Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex, Exp Brain Res 135(4) (2000) 455–61. [DOI] [PubMed] [Google Scholar]
- [24].Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC, Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation, Neurology 59(3) (2002) 392–397. [DOI] [PubMed] [Google Scholar]
- [25].Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali P, Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease, Journal of Neurology, Neurosurgery & Psychiatry 75(4) (2004) 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chung KA, Lobb BM, Nutt JG, Horak FB, Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease, Neurology (75) (2010) 1263–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Henderson EJ, Lord SR, Brodie MA, Gaunt DM, Lawrence AD, Close JCT, Whone AL, Ben-Shlomo Y, Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial, The Lancet Neurology 15(3) (2016) 249–258. [DOI] [PubMed] [Google Scholar]
- [28].Li Z, Yu Z, Zhang J, Wang J, Sun C, Wang P, Zhang J, Impact of Rivastigmine on Cognitive Dysfunction and Falling in Parkinson’s Disease Patients, European Neurology 74(1–2) (2015) 86–91. [DOI] [PubMed] [Google Scholar]
- [29].Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Scott PJH, Albin RL, Müller MLTM, Gait speed in Parkinson disease correlates with cholinergic degeneration, Neurology 81(18) (2013) 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rochester L, Yarnall AJ, Baker MR, David RV, Lord S, Galna B, Burn DJ, Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease, Brain 135(Pt 9) (2012) 2779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pelosin E, Ogliastro C, Lagravinese G, Bonassi G, Mirelman A, Hausdorff JM, Abbruzzese G, Avanzino L, Attentional Control of Gait and Falls: Is Cholinergic Dysfunction a Common Substrate in the Elderly and Parkinson’s Disease?, Front Aging Neurosci 8 (2016) 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baker K, Rochester L, Nieuwboer A, The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson’s disease, Arch Phys Med Rehabil 88(12) (2007) 1593–600. [DOI] [PubMed] [Google Scholar]
- [33].Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Rochester L, Gait Rather Than Cognition Predicts Decline in Specific Cognitive Domains in Early Parkinson’s Disease, The Journals of Gerontology: Series A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Din SD, Godfrey A, Rochester L, Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson's Disease: Toward Clinical and at Home Use, IEEE Journal of Biomedical and Health Informatics 20(3) (2016) 838–847. [DOI] [PubMed] [Google Scholar]
- [35].Yogev-Seligmann G, Hausdorff JM, Giladi N, The role of executive function and attention in gait, Mov Disord 23(3) (2008) 329–42; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kelly VE, Eusterbrock AJ, Shumway-Cook A, A review of dual-task walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications, Parkinsons Dis 2012 (2012) 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Berg KO, Wood-Dauphinee SL, Williams JI, Maki B, Measuring balance in the elderly: validation of an instrument, Canadian journal of public health = Revue canadienne de sante publique 83 Suppl 2 (1992) S7–11. [PubMed] [Google Scholar]
- [38].King LA, Priest KC, Salarian A, Pierce D, Horak FB, Comparing the Mini-BESTest with the Berg Balance Scale to Evaluate Balance Disorders in Parkinson’s Disease, Parkinson’s Disease 2012 (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Horak F, King L, Mancini M, Role of Body-Worn Movement Monitor Technology for Balance and Gait Rehabilitation, Physical Therapy 95(3) (2015) 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Karachi C, Grabli D, Bernard FA, Tande D, Wattiez N, Belaid H, Bardinet E, Prigent A, Nothacker HP, Hunot S, Hartmann A, Lehericy S, Hirsch EC, Francois C, Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease, The Journal of clinical investigation 120(8) (2010) 2745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Dowd S, Galna B, Morris R, Lawson RA, McDonald C, Yarnall AJ, Burn DJ, Rochester L, Anderson KN, Poor Sleep Quality and Progression of Gait Impairment in an Incident Parkinson’s Disease Cohort, J Parkinsons Dis 7(3) (2017) 465–470. [DOI] [PubMed] [Google Scholar]
- [42].Gallea C, Ewenczyk C, Degos B, Welter ML, Grabli D, Leu-Semenescu S, Valabregue R, Berroir P, Yahia-Cherif L, Bertasi E, Fernandez-Vidal S, Bardinet E, Roze E, Benali H, Poupon C, Francois C, Arnulf I, Lehericy S, Vidailhet M, Pedunculopontine network dysfunction in Parkinson’s disease with postural control and sleep disorders, Mov Disord 32(5) (2017) 693–704. [DOI] [PubMed] [Google Scholar]
- [43].Wang J-W, Zhang Y-Q, Zhang X-H, Wang Y-P, Li J-P, Li Y-J, Deep Brain Stimulation of Pedunculopontine Nucleus for Postural Instability and Gait Disorder After Parkinson Disease: A Meta-Analysis of Individual Patient Data, World Neurosurgery 102 (2017) 72–78. [DOI] [PubMed] [Google Scholar]
- [44].Hunter H, Rochester L, Morris R, Lord S, Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition, Disability and Rehabilitation (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [45].Kucinski A, Paolone G, Bradshaw M, Albin RL, Sarter M, Modeling fall propensity in Parkinson’s disease: deficits in the attentional control of complex movements in rats with cortical-cholinergic and striatal-dopaminergic deafferentation, J Neurosci 33(42) (2013) 16522–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lord S, Galna B, Yarnall AJ, Morris R, Coleman S, Burn D, Rochester L, Natural history of falls in an incident cohort of Parkinson’s disease: early evolution, risk and protective features, J Neurol 264(11) (2017) 2268–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L, Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naive cohort, Mov Disord 31(12) (2016) 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, Rikkert MO, Bloem BR, Pelosin E, Avanzino L, Abbruzzese G, Dockx K, Bekkers E, Giladi N, Nieuwboer A, Hausdorff JM, Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial, The Lancet 388(10050) (2016) 1170–1182. [DOI] [PubMed] [Google Scholar]
- [49].Aizenberg D, Sigler M, Weizman A, Barak Y, Anticholinergic Burden and the Risk of Falls Among Elderly Psychiatric Inpatients: A 4-Year Case-Control Study, International Psychogeriatrics 14(3) (2005) 307–310. [DOI] [PubMed] [Google Scholar]
- [50].Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, Bernabei R, Onder G, Anticholinergic drugs and physical function among frail elderly population, Clinical pharmacology and therapeutics 81(2) (2007) 235–41. [DOI] [PubMed] [Google Scholar]
- [51].Hunter H, Rochester L, Morris R, Lord S, Longitudinal falls data in Parkinson’s disease: feasibility of fall diaries and effect of attrition, Disabil Rehabil (2017) 1–6. [DOI] [PubMed] [Google Scholar]
- [52].Mactier K, Lord S, Godfrey A, Burn D, Rochester L, The relationship between real world ambulatory activity and falls in incident Parkinson’s disease: influence of classification scheme, Parkinsonism Relat Disord 21(3) (2015) 236–42. [DOI] [PubMed] [Google Scholar]
- [53].Ross A, Yarnall AJ, Rochester L, Lord S, A novel approach to falls classification in Parkinson’s disease: development of the Fall-Related Activity Classification (FRAC), Physiotherapy 103(4) (2017) 459–464. [DOI] [PubMed] [Google Scholar]
- [54].Verghese J, Wang C, Lipton RB, Holtzer R, Xue X, Quantitative gait dysfunction and risk of cognitive decline and dementia, J Neurol Neurosurg Psychiatry 78(9) (2007) 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fordyce DE, Farrar RP, Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning, Behavioural Brain Research 46(2) (1991) 123–133. [DOI] [PubMed] [Google Scholar]
- [56].Brown BM, Peiffer JJ, Martins RN, Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease?, Molecular psychiatry 18(8) (2013) 864–74. [DOI] [PubMed] [Google Scholar]
- [57].Barnikol TT, Pawelczyk NB, Barnikol UB, Kuhn J, Lenartz D, Sturm V, Tass PA, Freund HJ, Changes in apraxia after deep brain stimulation of the nucleus basalis Meynert in a patient with Parkinson dementia syndrome, Mov Disord 25(10) (2010) 1519–20. [DOI] [PubMed] [Google Scholar]
- [58].Gratwicke J, Zrinzo L, Kahan J, Peters A, Beigi M, Akram H, Hyam J, Oswal A, Day B, Mancini L, Thornton J, Yousry T, Limousin P, Hariz M, Jahanshahi M, Foltynie T, Bilateral Deep Brain Stimulation of the Nucleus Basalis of Meynert for Parkinson Disease Dementia: A Randomized Clinical Trial, JAMA Neurol 75(2) (2018) 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Engineer ND, Moller AR, Kilgard MP, Directing neural plasticity to understand and treat tinnitus, Hearing research 295 (2013) 58–66. [DOI] [PubMed] [Google Scholar]
- [60].Koeppe RA, Frey KA, Snyder SE, Meyer P, Kilbourn MR, Kuhl DE, Kinetic Modeling of N-[11C]Methylpiperidin-4-yl Propionate: Alternatives for Analysis of an Irreversible Positron Emission Tomography Tracer for Measurement of Acetylcholinesterase Activity in Human Brain, Journal of Cerebral Blood Flow & Metabolism 19(10) (1999) 1150–1163. [DOI] [PubMed] [Google Scholar]
- [61].Nardone R, Bergmann J, Tezzon F, Ladurner G, Golaszewski S, Cholinergic dysfunction in subcortical ischaemic vascular dementia: a transcranial magnetic stimulation study, J Neural Transm (Vienna) 115(5) (2008) 737–43. [DOI] [PubMed] [Google Scholar]
- [62].Hallett M, Transcranial Magnetic Stimulation: A Primer, Neuron 55(2) (2007) 187–199. [DOI] [PubMed] [Google Scholar]
- [63].Rossini PM, Rossi S, Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential, Neurology 68(7) (2007) 484–8. [DOI] [PubMed] [Google Scholar]
- [64].Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits, Eur J Neurosci 19(7) (2004) 1950–62. [DOI] [PubMed] [Google Scholar]