Abstract
Background and Aims:
The United States Food and Drug Administration has proposed regulation to require cigarettes contain very low nicotine content (VLNC). In contrast, reducing the number of cigarettes per day (CPD) is the most common current method to reduce nicotine. This trial aims to explore whether gradually transitioning to VLNC cigarettes plus nicotine patch or reducing CPD plus nicotine patch is more effective at decreasing nicotine dependence.
Design:
A two-arm, individually randomized open label trial.
Setting:
Community setting, Vermont, USA
Participants:
68 adult daily smokers (40% female) of ≥ 10 cigarettes/day who were not planning to quit in the next 30 days.
Interventions:
All participants smoked study cigarettes with a nicotine yield similar to most commercial cigarettes ad lib for 1 week (baseline). Participants then gradually reduced to 70%, 35%, 15% and 3% of baseline nicotine over 4 weeks by either a) transitioning to lower nicotine content cigarettes (N=36) or b) reducing the number of full nicotine cigarettes (N=32). All participants received nicotine patches.
Measurements:
The primary outcome was change in nicotine dependence assessed at baseline and weekly during the intervention with the Nicotine Dependence Syndrome Scale.
Findings:
Dependence declined over time for both VLNC and CPD participants but declined more for VLNC (mean decrease in z-score of 1.0) than CPD (mean decrease in z-score of 0.5) participants over time (interaction p=.018).
Conclusions:
Transitioning to very low nicotine content cigarettes reduced nicotine dependence over a 4-week period to a greater extent than reducing cigarettes per day when both conditions were aided by nicotine patch.
Keywords: Cigarette smoking, Nicotine dependence, Reduction, Very low nicotine content cigarettes, Tobacco regulatory policy, Harm reduction
Introduction
Currently, more than 20% of the world’s population smokes cigarettes and approximately 6 million people die from smoking related illness each year (1). In the United States, the prevalence of smoking has declined dramatically since 1964 but the decline has appeared to slow to less than 1% per year (2–4). Most smokers do not plan to quit in the near future (4, 5). This may be because actual or perceived dependence is a barrier to making a quit attempt (2). One way to reduce dependence is to decrease smokers’ nicotine intake (6–8). Presently, reducing cigarettes per day (CPD) is a common strategy to reduce nicotine intake and dependence (5, 9, 10). A second method is to transition to very low nicotine content (VLNC) cigarettes. The US Food and Drug Administration (FDA) recently proposed regulation to require that all US cigarettes have minimally addictive levels of nicotine in cigarettes; i.e., could force smokers to transition to VLNC cigarettes (11). In contrast to traditional “light” cigarettes (12), VLNC cigarettes contain tobacco with reduced nicotine content and result in reduced nicotine intake and minimal compensatory smoking (13).
It is unclear whether switching to VLNC cigarettes or reducing CPD is a) more effective at decreasing dependence and b) more acceptable. One prior trial found a transition to “lighter” cigarettes (12) appeared more acceptable than reducing CPD (14), but none have tested transitioning to cigarettes containing tobacco with reduced nicotine content (i.e., VLNC cigarettes) vs reducing CPD. Though both methods aim to reduce nicotine intake, switching to VLNC cigarettes and reducing CPD may work in different ways. Switching to VLNC cigarettes reduces the magnitude of cigarettes’ pharmacological reinforcement, which could decrease dependence by disrupting operant and Pavlovian conditioning. In contrast, reducing CPD restricts the pattern and frequency of smoking behavior. This could decrease dependence by providing increased opportunity to practice not smoking in the presence of stimuli that would have otherwise prompted smoking.
Policy to regulate cigarettes could a) mandate an abrupt switch to the lowest nicotine content VLNC cigarettes, b) mandate progressively lower nicotine content over time to gradually transition to the lowest nicotine VLNC cigarettes, or c) create a market where cigarettes with various levels of nicotine are available simultaneously to allow smokers to choose whether or how to transition to the lowest nicotine VLNC cigarettes. Some have proposed the latter scenario with taxation according to nicotine content to incentivize transitions to cigarettes with less nicotine (15). A recent multi-site trial found that an abrupt switch to VLNC cigarettes was more effective at reducing biomarkers and dependence than a gradual transition but increased withdrawal symptoms more and was less acceptable (16). Despite its efficacy, a mandated abrupt switch to a market with only the lowest nicotine cigarettes available may be less politically feasible than a market that allows smokers the option to gradually transition to VLNC cigarettes. Acceptable implementation of VLNC cigarettes is especially important given that the overall impact will be determined by both efficacy and feasibility. Therefore, further research is needed to examine feasibility of transitioning to VLNC cigarettes and how that strategy compares to current common nicotine reduction strategies.
In this exploratory randomized trial, we compared gradual reduction via transitioning to VLNC cigarettes versus reducing CPD. Our VLNC condition explores a reduction strategy that could be used if cigarettes were regulated to have reduced nicotine. In contrast our CPD condition explores a common reduction strategy. We instructed participants to make large reductions in nicotine content or number of CPD in order to detect differences between reduction strategies and dose-response relationships in dependence and acceptability. We provided both groups with nicotine replacement therapy (NRT) to aid reduction via VLNC or CPD for the reasons described below. This trial aimed to determine: a) which reduction strategy decreases dependence more and b) which reduction strategy is more acceptable. We will examine intention to quit, quit attempts, cessation, self-efficacy, carbon monoxide, and participants’ evaluation of cigarettes in subsequent papers.
Methods
Participants
Research personnel recruited 74 participants via Internet advertising, flyers, and word of mouth in the Burlington, VT area. Major inclusion criteria were a) ≥ 18 years old, b) smoke ≥10 cigarettes/day seven days per week c) meet DSM-5 criteria for Tobacco Use Disorder, and d) have no plans to stop smoking in the next 30 days (see Supplemental Methods). All participants smoked ad lib during the baseline week of the study. Only the 68 participants who attended the initial visit and the visit at the end of the baseline week were retained for analysis (Figure 1). Table S1 provides a description of the 6 excluded participants. The study was approved by the Committee on the Use of Human Subjects in Research at the University of Vermont and registered on ClinicalTrials.gov (http://www.webcitation.org/73yePQUWH), record: . All participants provided written informed consent.
Figure 1: Participant flow diagram.
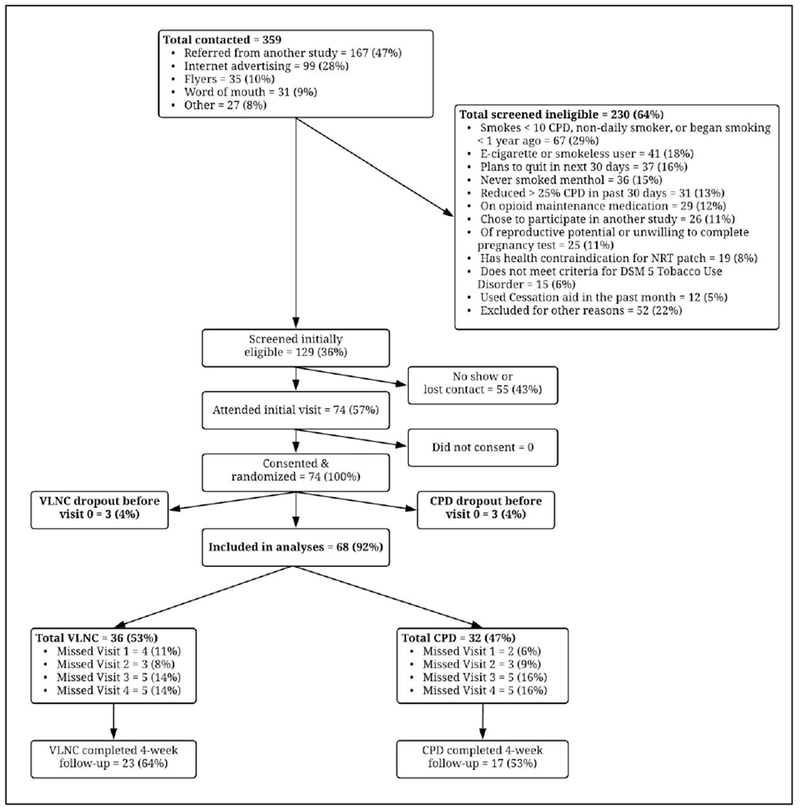
CPD=Condition that reduced cigarettes/day; DSM-5=Diagnostic and Statistical Manual of Mental Disorders Fifth Edition; NRT=Nicotine replacement therapy; TCORS=Tobacco Center on Regulatory Science; VLNC=Condition that switched to very low nicotine content cigarettes.
Design
Data were collected between February 2017 and January 2018 for this unblended parallel group, randomized trial. All participants attended an initial visit and five subsequent weekly visits (Table 1) to answer questionnaires, provide urine samples, and receive study cigarettes and NRT. Participants also answered nightly questionnaires during weeks 0 through 4 via telephone using an Interactive Voice Response system or online survey using smartphone or computer. Participants completed an online follow-up survey 1 month after the final study visit (i.e., 9 weeks after their initial study visit).
Table 1:
Schedule of reduction and study visits.
| Study Visit: | Initial | b0 | b1 | b2 | b3 | c4 |
|---|---|---|---|---|---|---|
| aWeek 0 | Week 1 | Week 2 | Week 3 | Week 4 | ||
| Transitioned to VLNC cigarettes | Cigarettes/day | Ad lib | 100% of Week 0 | 100% of Week 0 | 100% of Week 0 | 100% of Week 0 |
| Nicotine content | 100% (17.6 mg) | 70% (12.3 mg) | 35% (6.3 mg) | 15% (2.3 mg) | 3% (0.6 mg) | |
| Reduced number of full nicotine CPD | Cigarettes/day | Ad lib | 70% of Week 0 | 35% of Week 0 | 15% of Week 0 | d3% of Week 0 |
| Nicotine content | 100% (17.6 mg) | 100% (17.6 mg) | 100% (17.6 mg) | 100% (17.6 mg) | 100% (17.6 mg) |
Baseline cigarettes/day established during this time;
Laboratory visits when participants received 21-mg nicotine replacement therapy patches and instructions to use one patch per day;
Laboratory visit when participants were advised to quit;
Participants received a minimum of 1 cigarette/day;
CPD=Cigarettes per day; mg/g=Milligram nicotine per gram tobacco; VLNC=Very low nicotine content. The presented nicotine contents reflect the ratings by RTI International for the batches of Spectrum cigarettes used in this trial (see Table S3). In the VLNC condition, study cigarettes at week 0 = NRC601, week 1 = NRC501, week 2 = NRC401, week 3 = NRC301, and week 4 = NRC103. In the CPD condition study cigarettes for all weeks = NRC601. Participants completed an online follow-up survey 1 month after the end of week 4
Study cigarettes were packaged with labels displaying their varying nicotine content. We used the same study cigarettes (Spectrum cigarettes, 22nd Century Group, Inc.) as prior studies of VLNC cigarettes (8, 17) and report the nicotine contents estimated by RTI International (http://www.webcitation.org/744KC5wXz) for the cigarettes used in this trial (Table S3). During the baseline week (week 0), we provided participants in both conditions with 17.6 mg/g nicotine content study cigarettes. Because access to free cigarettes could increase smoking (18), we provide smokers with 150% of their self-reported number of cigarettes/day. This cigarette has a nicotine yield (0.97 mg/cigarette) similar to most commercial cigarettes. Participants were instructed to smoke only study cigarettes, but to smoke as usual during the baseline week. After the baseline week, we scheduled a similar magnitude of reduction in nicotine provided to participants via either VLNC cigarettes or reduced number of CPD (Table 1).
We provided participants who were randomized to use VLNC cigarettes with 100% of their mean number of cigarettes/day during the baseline week to use throughout weeks 1 to 4 and instructed them to only smoke cigarettes provided by the study. We limited VLNC participants to 100% of their baseline (i.e., week 0) cigarettes/day so that reducing cigarettes’ nicotine content over the study period would match the magnitude of reduction in total nicotine provided to CPD participants (Table 1). Cigarettes in the VLNC condition had progressively lower nicotine content beginning with 12.3 mg/g during week 1; 6.3 mg/g during week 2; 2.3 mg/g during week 3; and 0.6 mg/g during week 4 to provide approximately 70%, 35%, 15% and 3% of baseline nicotine (Table 1).
We provided participants randomized to reduce CPD with full nicotine study cigarettes (17.6 mg/g) during weeks 1 to 4 and instructed them to only smoke cigarettes provided by the study. They received progressively fewer number of cigarettes beginning with 70% of the mean number of week 0 cigarettes/day for week 1; 35% for week 2; 15% for week 3; and 3% for week 4 (Table 1).
At the time of this trial, the only available Spectrum study cigarettes that had consistent qualities (e.g., filter type) across nicotine contents were mentholated. Therefore we recruited only participants who had past (n= 52) or current (n=16) experience smoking menthol cigarettes. We used a computer generated stratified blocked randomization schedule so that the proportion of current menthol smokers was similar between groups. Participants were randomized at their initial visit in a 1:1 allocation ratio.
We provided all participants with 21-mg NRT patches and instructed them to use patches daily during weeks 1 through 4 (see Supplemental Methods) for two reasons. First, in order to increase internal validity, our CPD condition required large magnitudes of reduction to be comparable to nicotine reduction from transitioning to VLNC cigarettes. In prior studies, large reductions in cigarettes/day without NRT were very rare (19). For example, in our prior study, reduction without NRT produced only a 19% reduction in cigarettes/day (20). In contrast multiple studies have found NRT aided reduction produces large reductions in cigarettes/day (21–23). Second, many researchers believe that the provision of alternate nicotine sources is essential for a tobacco regulatory policy to be successful (24) and that many who transition to VLNC cigarettes will supplement with alternative nicotine products such as nicotine patch (25).
Participants reported compliance with our instruction to use NRT patches on nightly questionnaires. At the final study visit, we a) stopped providing study cigarettes, b) advised smokers to quit, and c) offered an additional 1-month supply of NRT patches to increase the likelihood of cessation. Participants completed a 1-month online follow-up survey.
Measures
Dependence:
Our primary measure of dependence was the Nicotine Dependence Syndrome Scale, which includes an overall score (NDSS-OS) and five subscales: 1) Drive (NDSS-D) to assess craving, withdrawal, and compulsion to smoke, 2) Priority (NDSS-P) to assess preference for smoking over other reinforcers, 3) Tolerance (NDSS-T) to assess sensitivity to the effects of smoking, 4) Continuity (NDSS-C) to assess the regularity of smoking rate, and 5) Stereotypy (NDSS-S) to assess changes in invariance of smoking (26). The NDSS scales produce z-scores using weighted parameters and have good reliability and predictive validity (26, 27). We also administered the Fagerstrom Test for Cigarette Dependence (FTCD) (28) questionnaire modified to exclude the cigarettes/day item, the Glover-Nilsson Smoking Behavioral Questionnaire (GN-SBQ) (29), and a single-item Addiction Ladder to assess cigarette addiction on a 10-point Likert scale (30). The NDSS, FTCD, and GN-SBQ were measured weekly and the Addiction Ladder was measured nightly. All were assessed at the 1-month follow-up.
Acceptability:
Although both groups were instructed to only smoke study cigarettes, they were also told that honest reporting of use of non-study cigarettes was important because we were interested in the acceptability of each strategy. The primary measure of acceptability was the percent of participants’ daily cigarettes that were non-study cigarettes. Participants reported their number of study and non-study cigarettes/day on nightly questionnaires and via timeline follow-back at weekly study visits. In order to increase the validity of participants’ self-reports, we a) informed participants that self-reported noncompliance would not influence their payment or participation and b) employed a bogus pipeline technique (31) by falsely telling participants we could detect non-study cigarettes via breath and urine tests. In fact, biochemical estimation of compliance using cotinine was not possible because participants used NRT and using cotinine to estimate use of study vs non-study cigarettes containing full or moderate levels of nicotine does not appear accurate (32). Participants were debriefed about this deception at the end of the study period. Each week we also asked participants to rate on five-point Likert scales how willing and able they would be to smoke the type and number of cigarettes provided to them over the past week for the next year if only those study cigarettes were available.
Nicotine exposure:
Weekly urine samples were analyzed for cotinine using semi-quantitative enzyme immunoassay (Microgenics, Fremont, CA, USA). Weekly breath samples were analyzed for carbon monoxide (CO) using a handheld Covita Micro Smokelyzer.
Baseline:
We administered the modified cigarette evaluation questionnaire (mCEQ) (33) after the baseline week of ad-lib smoking study cigarettes.
Adverse events (AE):
We assessed AEs at each study visit by asking if participants experienced any new problems over the past week. We defined AEs based on FDA guidelines (http://www.webcitation.org/74ZSRWgTl, http://www.webcitation.org/74ZSSwysz).
Analyses
We used nQuery Advisor module MTT3-1 (http://www.webcitation.org/74ilQcdg4) to calculate that a sample size of 32 per group would provide power of 0.8 with a two-sided alpha of 0.05 to detect a 10% within-group difference (0.5 units) in dependence using the NDSS-OS trend across time for the two conditions. We assumed a between-participant standard deviation of 1.0 and within-participant correlation in NDSS-OS of 0.8 (20, 26). We stopped recruitment after consenting 74 participants to account for attrition. Sixty-eight participants attended the visit at the end of baseline and were retained for analysis. We used multi-level modeling with restricted maximum likelihood (REML) and participant as a random effect to examine the condition by time interaction for dependence, acceptability, and cotinine outcomes. Covariance structure was chosen to minimize Akaike and Bayesian information criteria (Table S4). For measures assessed nightly, we used weekly means as the time variable because parameter estimates are less accurate in multi-level models when there are small sample sizes and a large number of time-points (34). Participants were instructed to smoke only study cigarettes throughout the entire study (weeks 0-4) and use NRT patches daily during weeks 1-4. Thus, we examined percent of days using NRT and percent non-study cigarettes/day as time-varying covariates for all models testing an interaction to determine if dependence or nicotine intake were influenced by compliance factors. Finally, we tested sex and pre-study menthol vs non-menthol status as moderators of all outcomes. Neither variable moderated any outcome (Table S11). We used t-tests to compare participants’ evaluation of study cigarettes after the baseline week using the mCEQ. We did not include p-value corrections in this exploratory trial because, like others, we are concerned that these adjustments are based on arbitrary cutoffs and increase the probability of type II errors (35).
At the 1-month follow-up, we used linear regression to make between- and within-participant comparisons as well as condition by time interactions for dependence measures. In addition, we tested all condition by time interactions after controlling for patch use during follow-up.
Missing data did not significantly differ between conditions (Table S5). Of the 68 participants included in our analyses, 58 (85%) attended the final study visit. Across the 2,380 total nightly surveys (68 participants × 35 day study period), 326 (14%) were missing. We imputed missing daily values using weekly retrospective surveys when possible. This decreased the number of missing values to 209 (9%). In our primary analyses, missing data were handled as prescribed in multi-level modeling (36). Most participants (59%) completed the 1-month follow-up survey. Missing data at follow-up were treated as missing.
Results
Participant Characteristics
Compared with US current daily smokers (37, 38), participants in this study appear younger, more likely to be white, non-Hispanic males, more educated, more dependent, and smoked more cigarettes/day (Table 2). Characteristics did not significantly differ between conditions (Table S2).
Table 2:
Participant demographics and smoking history.
| VLNC (n=36) | CPD (n=32) | Total (N=68) | NHISa and other surveysb,c | |
|---|---|---|---|---|
| Demographics | ||||
| Mean age | 38.4 (SD=13.4) | 39.3 (SD=13.6) | 38.8 (SD=13.4) | 42a |
| No. (%) women | 14 (39) | 13 (41) | 27 (40) | (46)a |
| No. (%) white, non-Hispanic | 28 (78) | 28 (88) | 56 (82) | (78)a |
| No. (%) with > 12 years of education | 26 (72) | 23 (72) | 49 (72) | (39)a |
| No. (%) employed full time | 17 (47) | 12 (38) | 29 (43) | - |
| No. (%) married | 10 (31) | 8 (25) | 18 (28) | - |
| dSmoking history | ||||
| Mean cigarettes/day | 19.0 (SD=6.1) | 19.8 (SD=10.4) | 19.4 (SD=8.4) | 16a |
| Mean total Fagerstrom Test for Cigarette Dependence (0=lowest, 10=highest) | 5.3 (SD=1.9) | 5.0 (SD=2.2) | 5.1 (SD=2.0) | 4.3-4.6b |
| Mean intention to quit in the next month (0=very definitely no, 10=very definitely yes) | 3.4 (SD=3.0) | 3.5 (SD=3.0) | 3.5 (SD=3.0) | - |
| Mean cotinine ng/ml | 1,364.1 (SD=739.4) | 1,464.5 (SD=703.2) | 1,412.1 (SD=718.7) | - |
| No. (%) menthol smokers | 8 (22) | 8 (25) | 16 (24) | (39)c |
| Mean age at onset of smoking | 16.7 (SD=4.7) | 15.6 (SD=3.3) | 16.2 (SD=4.1) | 17a |
| Mean number of times attempted to reduce but not quit in past 12 months | 1.0 (SD=1.5) | 2.4 (SD=6.3) | 1.7 (SD=4.5) | - |
| Median number of QAs in life | 2 | 3 | 2 | - |
| No. (%) used NRT during a past QA | 19 (53) | 13 (40) | 32 (47) | - |
Baseline Smoking, NRT Patch Compliance, and Adverse Events
During the baseline week, there were no significant between- or within-participant differences across the two groups for items assessed daily (Table S6). Prior research indicates that participants smoke more cigarettes when they are free (18). We used a t-test to compare pre-study (mean=19.4, between-subjects standard deviation [SD]=8.4) vs baseline cigarettes/day (mean=20.6, SD=10.5), and found differences were marginally significant (t= −1.8, p=.084). During baseline, participants in both conditions reported smoking few non-study cigarettes (median=1.2%). With regard to evaluation of study cigarettes on the mCEQ, there were no differences between conditions or between participants who identified as menthol vs non-menthol smokers (Table S7). Compliance with NRT patch was high (median=91% of days) and a t-test found no difference between conditions (t=0.7, p=.468). There were no serious adverse events related to participation in either condition.
Dependence
In the whole sample, dependence decreased from baseline to the end of the study period on the NDSS-OS, NDSS-D, FTCD without cigarettes/day, GN-SBQ, and Addiction Ladder (Table 3). Among VLNC participants, there were decreases in NDSS-OS, NDSS-D, FTCD without cigarettes/day, GN-SBQ, and Addiction Ladder scores over time. Among CPD participants there were decreases in NDSS-OS, NDSS-D, GN-SBQ, and Addiction Ladder scores over time (Table S8). Main effects for differences between conditions were not significant on any dependence measure (Table 3).
Table 3:
F (p) values for dependence, acceptability, and cotinine outcomes.
| VLNC vs CPD | Week | Condition × Week | Condition × Week (controlling for NRT patch use) | Condition × Week (controlling for % non-study cigarette/day) | |
|---|---|---|---|---|---|
| Dependence | |||||
| NDSS Overall Score | 0 (.78) | 13.9 (<.001) | 3.2 (.018) | 3.0 (.023) | 1.0 (.41) |
| NDSS Drive | 0.5 (.47) | 14.7(<.001) | 3.3 (.013) | 3.5 (.011) | 5.1 (.004) |
| NDSS Stereotypy | 1.0 (.33) | 1.4 (.24) | 0.8 (.56) | 0.9 (.45) | 0.2 (.92) |
| NDSS Priority | 0.6 (.43) | 0.6 (.68) | 0.6 (.68) | 0.3 (.88) | 0.7 (.63) |
| NDSS Tolerance | 1.4 (.23) | 0.9 (.46) | 0.1 (.97) | 0.2 (.92) | 0.7 (.63) |
| NDSS Continuity | 3.4 (.071) | 0.8 (.50) | 0.7 (.61) | 0.5 (.76) | 1.2 (.34) |
| FTCD without cigarettes/day | 0.1 (.70) | 4.4 (.003) | 0.5 (.70) | 0.4 (.79) | 1.2 (.33) |
| GN-SBQ | 0 (.94) | 12.8 (<.001) | 0.1 (.99) | 0.2 (.93) | 1.2 (.33) |
| Addiction Ladder | 0 (.85) | 24.1 (<.001) | 2.4 (.049) | 1.8 (.14) | 0.5 (.75) |
| Acceptability | |||||
| % Non-Study Cigarettes/Day | 6.6 (.013) | 26.3 (<.001) | 8.0 (<.001) | 29.5 (<.001) | - |
| Willingness to smoke number of cigarettes | 10.4 (.002) | 5.7 (<.001) | 4.0 (.005) | 6.0 (<.001) | - |
| Ability to smoke number of cigarettes | 16.0 (<.001) | 4.8 (.002) | 3.5 (.009) | 4.1 (.005) | - |
| Willingness to smoke type of cigarette | 0.01 (.94) | 0.2 (.92) | 0.7 (.60) | 0.5 (.75) | - |
| Ability to smoke type of cigarette | 0.5 (.50) | 1.0 (.44) | 0.8 (.56) | 0.4 (.80) | - |
| Nicotine Exposure | |||||
| Cotinine | 8.3 (.005) | 5.3 (<.001) | 7.7 (<.001) | 9.3 (<.001) | 2.3 (.094) |
CPD=Condition that reduced cigarettes per day; FTCD=Fagerstrom Test for Cigarette Dependence; GN-SBQ=Glover-Nilsson Smoking Behavioral Questionnaire; NDSS=Nicotine Dependence Syndrome Scale; NRT=Nicotine replacement therapy; VLNC=Condition that switched to very low nicotine content cigarettes; Bold findings represents primary outcomes. See Table S4 for covariance structures and Table S9 for mean dependence, acceptability, and cotinine outcomes.
There were condition by time interactions where VLNC participants’ dependence decreased more over time than CPD participants on the NDSS-OS, NDSS-D, and the Addiction Ladder (Table 3, Figure 2). The difference in change in NDSS-OS remained significant after controlling for NRT use but was no longer significant when controlling for percent non-study cigarettes/day. The difference in change in NDSS-D remained significant after controlling for both NRT and non-study cigarette use. Change in the Addiction Ladder outcome did not differ between conditions after controlling for either covariate (Table 3). Post-hoc t-tests comparing conditions at each week found that, at week 4 (3% nicotine), the VLNC condition had numerically less dependence on the NDSS-OS and significantly less dependence on the NDSS-D. Differences between groups were not significant for the Addiction Ladder at any single week (Figure 2).
Figure 2: Dependence outcomes.
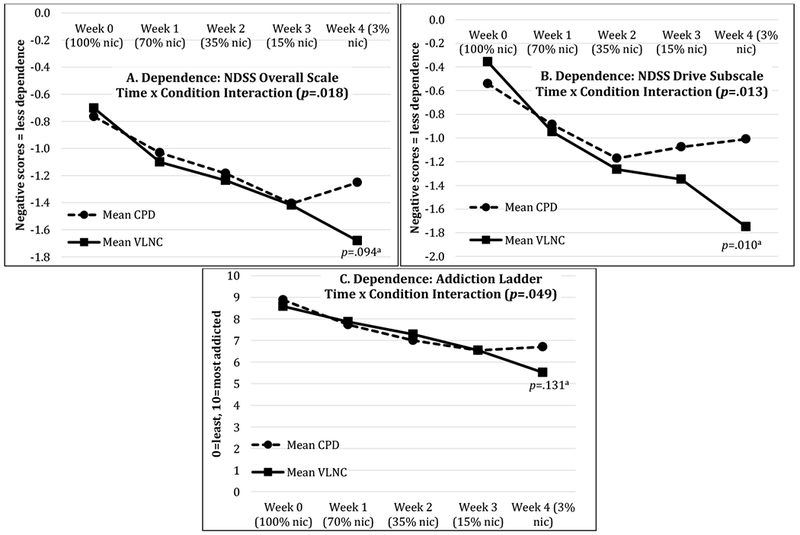
aPost-hoc between-condition t-tests at week 4; CPD=Condition that reduced cigarettes/day; NDSS=Nicotine Dependence Syndrome Scale; nic=Nicotine; VLNC=Condition that smoked low nicotine cigarettes. The presented values are estimated marginal means from the mixed model.
At the 1-month follow-up, there were no significant between-group, within-group, or time by condition interactions for any dependence measure (Table S10).
Acceptability
The percent of cigarettes/day that were non-study cigarettes was greater in CPD participants than VLNC participants, indicating that reducing CPD was less acceptable than transitioning to VLNC cigarettes (Table 3). Percent non-study cigarettes/day is influenced by number of total cigarettes/day (study + non-study), which differed between conditions. For example, though CPD participants reduced from a mean total cigarettes/day (study + non study) of 21.1 (SD=12.2) to 8.5 (SD=6.9), their non-study cigarettes/day increased by a mean 64.7% (Figure S1). Further, CPD participants reported a decrease in willingness and ability to smoke the number of cigarettes provided (Table S8). VLNC participants also reduced from a mean total of 20.1 (SD=8.8) to 16.2 (SD=9.8) cigarettes/day. However, there were no changes over time in their percent non-study cigarettes/day, willingness, or ability to smoke the progressively reduced nicotine content study cigarettes provided (Table S8).
There were condition by time interactions where the percent non-study cigarettes/day increased and self-reported willingness and ability to smoke the number of cigarettes provided decreased among CPD but not VLNC participants over time (Table 3, Figure 3). These findings remained significant after controlling for NRT use (Table 3). Post-hoc t-tests found that, in comparison to VLNC participants, CPD participants smoked more non-study cigarettes/day and reported that they were less willing and able to smoke the number of study cigarettes provided at week 2 (35% nicotine), week 3 (15% nicotine), and week 4 (3% nicotine; Figure 3).
Figure 3: Acceptability and Nicotine Exposure Outcomes.
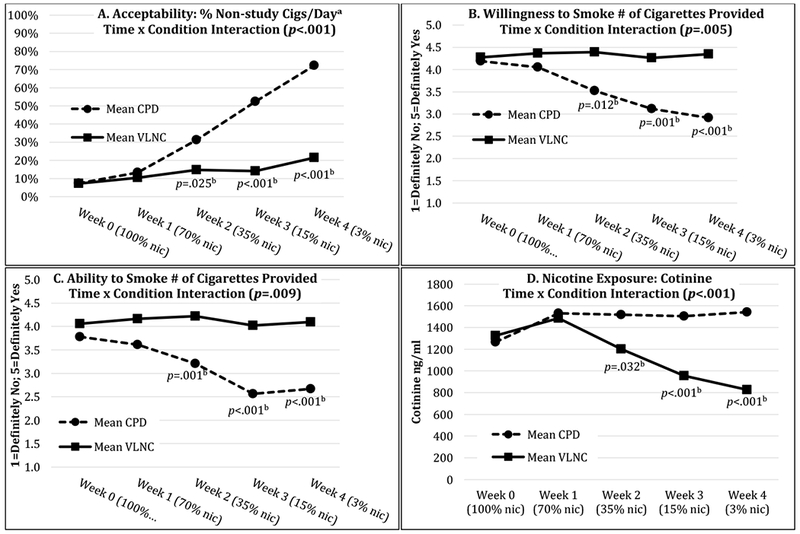
aSee Figure S2 for daily change; bSignificant post-hoc between-condition t-tests at each week; CPD=Condition that reduced cigarettes/day; nic=Nicotine; VLNC=Condition that smoked low nicotine cigarettes. The presented values are estimated marginal means from the mixed model.
Nicotine Exposure
Cotinine values were significantly less for VLNC than CPD participants (Table 3). Over time, VLNC participants’ cotinine decreased. In contrast, there was an unexpected small but significant increase in cotinine for CPD participants (Table S8) despite a 59.7% reduction in mean total cigarettes/day (study + non study; Figure S1).
There was an interaction where cotinine decreased for VLNC but increased for CPD participants over time (Table 3, Figure 3). This remained after controlling for NRT use but was no longer significant after controlling for percent non-study cigarettes/day (Table 3). Post-hoc t-tests found that, in comparison to CPD participants, VLNC participants had less cotinine at week 2 (35% nicotine), week 3 (15% nicotine), and week 4 (3% nicotine; Figure 3).
To further examine the CPD condition’s unexpected increase in nicotine exposure, we controlled for participants’ weekly CO levels and found the condition by time interaction described above remained significant (F=6.2, p=.001). In addition we plotted changes in CO (Figure S3) and tested NRT patch use, CO, and time as predictors of cotinine in a multivariable model. Time remained significant but neither patch use nor CO predicted cotinine among the CPD or VLNC group (Table S12). This suggests the increase in nicotine exposure was not directly due to compensatory smoking per se.
Discussion
This exploratory randomized trial directly compared transitioning to VLNC cigarettes versus the commonly used strategy of reducing CPD as methods to decrease dependence and provide adequate acceptability. We found that gradually reducing nicotine by transitioning to VLNC cigarettes is more effective at decreasing dependence and cotinine and more acceptable than reducing number of CPD. The only prior similar trial compared a 2-week taper to abstinence without NRT via transitioning to “light” cigarettes (i.e., cigarettes low in nicotine due to dilution of smoke) vs reducing CPD (14). In contrast, we used a 4-week taper with NRT in both groups and cigarettes containing tobacco with less nicotine content. Despite these methodological differences, the prior trial also found that switching to reduced nicotine cigarettes was more acceptable than reducing CPD. The prior trial did not measure change in dependence or cotinine (14).
Transitioning to VLNC cigarettes with approximately 3% nicotine appeared to decrease dependence more than instruction to make equivalent reductions in CPD. One possible mechanism is that responding to environmental or internal cues by smoking cigarettes with less pharmacological reward (i.e., VLNC cigarettes) disrupts conditioning and decreases the drive to smoke cigarettes. In contrast, the mechanism for reducing full nicotine CPD could be that it helps smokers temporarily resist the drive to smoke with less actual change in dependence per se. Differences between conditions appeared to emerge during week 4, suggesting that the influence on dependence may differ only at the greatest magnitudes of reduction.
There was a large decline in acceptability as CPD participants were instructed to reduce 97% of their cigarettes. In contrast, a similar magnitude of reduction via transitioning to VLNC cigarettes remained relatively acceptable. Thus, reducing the frequency of smoking behavior appears less acceptable than reducing the magnitude of pharmacological reward associated with each cigarette. This finding is particularly notable because it suggests that regulation to reduce cigarettes’ nicotine content could result in an option for harm reduction that is more, not less, acceptable than the current common practice of reducing CPD among smokers not ready to quit.
Nicotine exposure (cotinine) decreased for VLNC but increased for CPD participants over time. The decrease in cotinine in the VLNC condition supports prior research that suggests minimal compensatory smoking with VLNC cigarettes (13). The reason for an increase in cotinine in the CPD condition is less clear. The CPD condition received full nicotine research cigarettes and had a 59.7% reduction in total cigarettes/day (study + non-study). One potential explanation could be that CPD participants inhaled their remaining cigarettes more vigorously (i.e., compensatory smoking), which caused a net increase in cotinine. However, higher CO levels, a measure of smoke inhalation, were not associated with higher cotinine levels or the time by condition interaction. Further, we could not find any prior research on whether reducing number of full nicotine Spectrum research cigarettes causes compensatory smoking. Another potential explanation is that supplemental nicotine from NRT caused the increase in cotinine for CPD participants. However, NRT use did not differ between conditions or influence outcomes in our analyses. Thus it remains unclear why CPD participants had an increase in nicotine exposure. Further research is needed to parse out the influences on change in cotinine from reducing CPD. Nonetheless, transitioning to VLNC cigarettes appears to be a more effective strategy to reduce nicotine exposure than reducing CPD.
Limitations and Considerations
We provided NRT to aid reduction and increase internal validity of our comparisons. Though this may have decreased the generalizability of our findings, if mandated to use VLNC cigarettes, many smokers will likely seek alternative nicotine sources such as NRT (25). On the other hand, provision of nicotine via NRT may have blunted the impact of reduction, thereby decreasing the sensitivity of our test. However, we found reliable differences in our primary outcomes suggesting the study had sufficient sensitivity.
All study cigarettes were mentholated because of limitations at the time of recruitment, which limits the generalizability of our findings. However, participants’ current versus past menthol status did not a) differ between groups, b) influence subjective evaluation of study cigarettes, or c) moderate any outcome. We were unable to biochemically verify compliance with study cigarettes. We attempted to increase the validity of self-report by informing participants that payment and participation would be unaffected by noncompliance and employing a bogus pipeline technique (31). We instructed participants to make large reductions over a short period of time: it is possible our results might differ if reduction goals were smaller or the duration of reduction was longer. We tested a single reduction schedule. It is unclear how the FDA will regulate cigarettes’ nicotine content or smokers will transition to VLNC cigarettes. In addition, our findings for two dependence measures and cotinine were no longer significant after controlling for non-study cigarettes/day which suggests that the effectiveness of the VLNC reduction strategy is likely influenced by its acceptability. Time-by-condition interactions were not significant on multiple secondary measures of dependence and thus future research is needed to replicate our primary findings. This trial was unblinded because reducing CPD is an observable behavior. Thus expectancy may have influenced outcomes. The study entered only smokers not planning to quit in the near future. Our findings could differ among smokers who intend to quit now. Finally, findings at follow-up are limited because VLNC participants could not, but CPD participants could continue their assigned reduction strategy.
Conclusions
We compared a proposed (VLNC) vs a common (CPD) reduction strategy and found that a gradual transition to VLNC cigarettes appears to be more effective at decreasing dependence and nicotine exposure and more acceptable than gradually reducing CPD. Future research is needed to a) replicate findings in a larger sample size with a control condition of no attempt to reduce, b) test different reduction goals and schedules, c) test reduction with the aid of e-cigarettes or no nicotine replacement, and d) examine potential biological and behavioral moderators and mediators of reduction’s effects on dependence. Our major conclusion is that a policy to reduce the nicotine content of cigarettes could provide smokers not ready to quit with a reduction strategy that is more acceptable and effective at decreasing dependence and nicotine exposure than the common strategy of reducing CPD.
Supplementary Material
Acknowledgements
We thank Stephen Higgins, Matthew Carpenter, Mark Bouton, and Matthew Price for their consultation and Taylor Ochalek and Shae Rowlandson for their assistance.
Funding: This work was supported by research grant DA-025089 and training grant T32 DA 7242 from the National Institute on Drug Abuse.
Footnotes
Declaration of Interests: EMK, PWC, JAB, and NEM have nothing to disclose. JRH has received consulting and speaking fees from several companies that develop or market pharmacological and behavioral treatments for smoking cessation or harm reduction and from several non-profit organizations that promote tobacco control. He also consults for Altria, Philip Morris and Swedish Match on their harm reduction products.
Clinical Trial Registration: ClinicalTrials.gov (http://www.webcitation.org/73yePQUWH), trial record:
REFERENCES
- 1.World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2015 Geneva, Switzerland: World Health Organization; 2015. Report No.: 924156492X. [Google Scholar]
- 2.US Department of Health and Human Services. The health consequences of smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014;17. [Google Scholar]
- 3.Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, et al. Current cigarette smoking among adults–United States, 2005-2014. Morbidity and Mortality Weekly Report (MMWR). 2015;64(44):1233–40. [DOI] [PubMed] [Google Scholar]
- 4.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–64. [DOI] [PubMed] [Google Scholar]
- 5.PROPEL Centre for Population Health Impact. Tobacco Use in Canada: Patterns and Trends 2017 Edition Waterloo, Ontario: 2017. [Available from: www.tobaccoreport.ca. [Google Scholar]
- 6.Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, et al. Progressive commercial cigarette yield reduction: biochemical exposure and behavioral assessment. Cancer Epidemiol Biomarkers Prev. 2009;18(3):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P 3rd. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2479–85. [DOI] [PubMed] [Google Scholar]
- 8.Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med. 2015;373(14):1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West R, Brown J. Smoking and smoking cessation in England 2011: Findings from the smoking toolkit study. Age (years). 2012;1:1.01–1.2. [Google Scholar]
- 10.Beard E, Fidler J, West R. Is use of nicotine replacement therapy while continuing to smoke associated with increased nicotine intake? Evidence from a population sample. Psychopharmacology. 2011;218:609–10. [DOI] [PubMed] [Google Scholar]
- 11.Administration FaD. Advanced Notice of Proposed Rulemaking: Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. In: HHS, editor. 2018. p. 11818–43. [Google Scholar]
- 12.Benowitz NL, Jacob P 3rd, Bernert JT, Wilson M, Wang L, Allen F, et al. Carcinogen exposure during short-term switching from regular to “light” cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1376–83. [DOI] [PubMed] [Google Scholar]
- 13.Hatsukami DK, Donny EC, Koopmeiners JS, Benowitz NL. Compensatory smoking from gradual and immediate reduction in cigarette nicotine content. Cancer Epidemiol Biomarkers Prev. 2015;24(2):472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berecz JM. Superiority of a low-contrast smoking cessation method. Addict Behav. 1984;9(3):273–8. [DOI] [PubMed] [Google Scholar]
- 15.Laugesen M Modelling a two-tier tobacco excise tax policy to reduce smoking by focusing on the addictive component (nicotine) more than the tobacco weight. NZ Med J. 2012;125(1367):35–48. [PubMed] [Google Scholar]
- 16.Hatsukami DK, Luo X, Jensen JA, al’Absi M, Allen SS, Carmella SG, et al. Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA. 2018;320(9):880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter P, Steven PR, Bravo R, Lisko JG, Damian M, Gonzalez-Jimenez N, et al. Characterization of SPECTRUM Variable Nicotine Research Cigarettes. Tob Regul Sci. 2016;2(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiffman S, Scholl S. Increases in Cigarette Consumption and Decreases in Smoking Intensity when Non-Daily Smokers are Provided with Free Cigarettes. Nicotine Tob Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes JR, Carpenter MJ. The feasibility of reduced smoking: An update. Addiction. 2005;100:1074–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction, and usual care interventions for smokers who are not ready to quit: A randomized controlled trial. Addiction. 2016:n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindson-Hawley N, Banting M, West R, Michie S, Shinkins B, Aveyard P. Gradual Versus Abrupt Smoking Cessation: A Randomized, Controlled Noninferiority Trial. Annals of Internal Medicine. 2016;164(9):585–92. [DOI] [PubMed] [Google Scholar]
- 22.Stein JH, Bushara M, Bushara K, McBride PE, Jorenby DE, Fiore MC. Smoking cessation, but not smoking reduction, reduces plasma homocysteine levels. Clin Cardiol. 2002;25(1):23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagerstrom KO, Hughes JR, Callas PW. Long-term effects of the Eclipse cigarette substitute and the nicotine inhaler in smokers not interested in quitting. Nicotine Tob Res. 2002;4 Suppl 2:S141–5. [DOI] [PubMed] [Google Scholar]
- 24.Hatsukami DK, Donny EC. The Debate About Nicotine Addiction and the Role of Medicinal Products: Commentary on Zeller. Nicotine Tob Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatsukami DK, Luo X, Dick L, Kangkum M, Allen SS, Murphy SE, et al. Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial. Addiction. 2017;112(1):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2004;6(2):327–48. [DOI] [PubMed] [Google Scholar]
- 27.Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: A guide to measure evaluation and selection. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2006;8:339–51. [DOI] [PubMed] [Google Scholar]
- 28.Fagerstrom KO. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14(1):75–8. [DOI] [PubMed] [Google Scholar]
- 29.Glover E, Nilsson F, Westin A, Glover P, Laflin M, Persson B. Developmental history of the Glover-Nilsson Smoking Behavior Questionnaire. American Journal of Health Behavior. 2005;29:443–55. [DOI] [PubMed] [Google Scholar]
- 30.Hughes JR, Oliveto AH, Riggs R, Kenny M, Liguori A, Pillitteri JL, et al. Concordance of different measures of nicotine dependence: Two pilot studies. Addictive Behaviors. 2004;29:1527–39. [DOI] [PubMed] [Google Scholar]
- 31.Aguinis H, Pierce CA, Quigley BM. Conditions Under Which a Bogus Pipeline Procedure Enhances the Validity of Self‐Reported Cigarette Smoking: A Meta‐Analytic Review1. J Appl Soc Psychol. 1993;23(5):352–73. [Google Scholar]
- 32.Benowitz N Biochemical verification for compliance with very low nicotine content cigarettes. Personal communication. 2015.
- 33.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the Modified Cigarette Evaluation Questionnaire. Addict Behav. 2007;32:912–23. [DOI] [PubMed] [Google Scholar]
- 34.Bijleveld C, van der Kamp L, Mooijaart A, van der Kloot W, van der Leeden R, van der Burg E. Longitudinal Data Analysis: Designs, Models and Methods. London: Sage Publications; 1998 1998. [Google Scholar]
- 35.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Field A Discovering statistics using IBM SPSS statistics: Sage; 2013. [Google Scholar]
- 37.Hughes JR, Callas PW. Data to assess the generalizability of samples from studies of adult smokers. Nicotine & Tobacco Research; 2010;12:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagerstrom K, Furgerg H. A comparison of the Fagerstrom Test for Nicotine Dependence and smoking prevalence across countries. Addiction. 2008;103:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004-2014. Tob Control. 2016:tobaccocontrol-2016-053329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


