Abstract
Alcoholic liver disease (ALD) is one of the most common liver diseases worldwide. However, the exact mechanisms underlying ALD remain unclear. Previous studies reported that sphingosine kinase 2 (SphK2) plays an essential role in regulating hepatic lipid metabolism. In the current study, we demonstrate that compared to wild-type (WT) mice, SphK2 deficient (SphK2−/−) mice exhibited a greater degree of liver injury and hepatic lipid accumulation after feeding with an alcohol diet for 60 days. This is accompanied by a down-regulation of steroid 7-alpha-hydroxylase (Cyp7b1) and an up-regulation of pro-inflammatory mediators (Tnfα, F4/80, Il-1β). In vitro experiments showed that alcohol induced SphK2 expression in mouse primary hepatocytes and cultured mouse macrophages. Furthermore, alcohol feeding induced a more severe intestinal barrier dysfunction in SphK2−/− mice than WT mice. Deficiency of SphK2 impaired the growth of intestinal organoids. Finally, SphK2 expression levels were down-regulated in the livers of human patients with alcoholic cirrhosis and hepatocellular carcinoma compared to healthy controls. In summary, these findings suggest that SphK2 is a crucial regulator of hepatic lipid metabolism and that modulating the SphK2-mediated signaling pathway may represent a novel therapeutic strategy for the treatment of ALD and other metabolic liver diseases.
Keywords: sphingosine 1-phosphate, inflammation, hepatic steatosis, gut-liver axis
Introduction
Alcoholic liver disease (ALD) is one of the most common liver diseases worldwide and exists as a spectrum ranging from hepatic steatosis, alcoholic hepatitis (AH), cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. Interestingly, 90% of patients who consume more than 60 g/day of alcohol develop fatty liver but only 10–30% progress to AH and cirrhosis [3, 4]. Several risk factors have been shown to be associated with ALD progression including the female gender, obesity, nutrition, drugs, smoking, genetic factors and drinking pattern [5–12]. Among these risk factors, an individual’s drinking pattern has the most profound effect on alcohol-induced liver injury [13–15]. The mechanisms by which alcohol causes liver injury is complex and is not fully understood but appears to be a combination of hepatic lipid metabolism dysregulation, induction of endoplasmic reticulum (ER) stress, and activation of inflammation [16–18]. There is no effective treatment for ALD and liver transplantation remains the only definitive treatment option for advanced stage alcoholic cirrhosis [3].
Sphingosine 1-phosphate (S1P) is a potent signaling molecule that mediates various physiologic responses including cellular differentiation, migration, survival, and metabolism [19, 20]. S1P is synthesized by two kinases, sphingosine kinase 1 (SphK1) and sphingosine kinase 2 (SphK2) [21]. SphK1 generates cytosolic S1P levels while SphK2 contains a nuclear localization signal that allows the synthesis of nucleus S1P. SphK2-generated nuclear S1P is a potent natural inhibitor of histone deacetylases (HDAC1/2). Increased histone acetylation is often associated with an increase in the transcriptional activity of genes involved in cell proliferation, migration, and metabolism [22]. Intracellular S1P can directly mediate various cellular functions or be transported out of the cell by ATP-binding cassette (ABC) transporter proteins such as Spinster homolog 2 (SPNS2) and activate five different G-protein coupled receptors termed sphingosine 1-phosphate receptor 1–5 (S1PR1–5) [23–27].
We have previously reported that conjugated bile acids can activate S1PR2 to regulate SphK2 expression and hepatic lipid metabolism [28]. Moreover, we demonstrated that S1PR2 deficient (S1PR2−/−) and SphK2 deficient (SphK2−/−) mice developed overt fatty liver on a two-week high fat diet feeding [28]. Indeed, SphK2 was also reported to ameliorate hepatic steatosis and improve insulin sensitivity through the activation of ER stress in non-alcoholic fatty liver disease (NAFLD) mouse model [29]. Since ALD and NAFLD share similarities in disease progression, our previous observations in NAFLD prompted us to examine the role of SphK2 in alcohol-induced liver injury.
In this study, we examine the effects of SphK2 deficiency on alcohol-induced liver injury using the most widely used ad-libitium Lieber-DeCarli alcohol diet chronic feeding mouse model [30–32]. The results indicated that alcohol exacerbates liver steatosis and injury in SphK2−/− mice on a 60-day alcohol diet.
Materials and Methods
Reagents
Oil Red O, maltodextrin and common laboratory chemicals were purchased from Sigma Aldrich (St. Louis, MO). Lieber-Decarli Diet was purchased from Bioserv (Flemington, NJ). The SphK2 antibody was purchased from Proteintech (Chicago, IL). F4/80 conjugated with Alexa Fluor® 488 and CD11b conjugated with FITC antibodies were purchased from BioLegend (San Diego, CA). BD Matrigel Basement Membrane Matrix was purchased from BD Biosciences (Bedford, MA). Dulbecco’s PBS (DPBS), 100 × Penicillin/Streptomycin, 100 × GlutaMAX, 1M HEPES, 50× B-27 Supplement and 100× N-2 Supplement were obtained from Life Technologies, Gibco (Waltham, MA). Heat Inactivated Fetal Bovine Serum (FBS) was from Atlanta Biologicals (Flowery Branch, GA). Recombinant murine epithelial growth factor (EGF), recombinant murine Noggin and recombinant human R-Spondin-1 were purchased from Pepro Tech (Rocky Hill, NJ).
Animal Studies
WT and SphK2−/− mice with C57BL/6J background (both male and female, 8–10 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed under a 12-hour light/12-hour dark cycle with free access to water and regular chow. The 60-day chronic alcohol feeding was performed by feeding mice ad libitum Lieber-DeCarli control diet or 5% alcohol diet for 60 days. At the end of the experiment, mice were sacrificed. The blood, liver and small intestines were collected for subsequent biochemical analysis. All animal study protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University (Richmond, VA).
Histological and immunohistochemical staining
Tissues (liver, small intestine) were processed for Hematoxylin and Eosin (H&E) and Masson’s Trichrome staining at the clinical pathology laboratory at the Medical College of Virginia Hospital (Richmond, VA). All histological samples are blinded to a board-certified pathologist for analysis. Frozen tissue sections cut to 10 μm in thickness and preserved in 3.7% formaldehyde for 20 min were used for Oil Red O (ORO) staining and immunofluorescence (IF) staining. For ORO staining, frozen tissue sections were washed with H2O twice followed by a wash with 60% isopropanol for 30 sec. The sections were incubated in 0.3% ORO solution for 20 min at room temperature followed by two H2O washes and a subsequent wash with 60% isopropanol for 30 sec. The sections were counter-stained with hematoxylin. For IF staining of frozen liver tissue, sections were blocked in 2% bovine serum albumin (BSA) with 0.1% Triton-X for 30 min followed by overnight incubation of primary antibodies F4/80 conjugated with Alexa Fluor® 488 and CD11B conjugated with FITC. DAPI was used as a counter-stain for nuclei. Staining images were all taken using a Zeiss Axio Scope A1 microscope (Carl Zeiss, Germany).
Isolation of intestinal crypts and intestinal organoid culture
The small intestine was cut open longitudinally and washed with ice-cold PBS to remove intestinal contents. After removal of intestinal villi with a sterile cell scraper, remains were cut into small pieces, washed with 10% FBS-Dulbecco’s PBS (DPBS) several times, transferred into 2 mM EDTA-DPBS and incubated at 4°C on a shaker for 30 min. After sedimentation, EDTA was removed, followed by vigorously pipetting up and down in 10% FBS-DPBS to dissociate intestinal crypts. After passing through a 100 μm cell strainer, the cell suspension containing the intestinal crypts was centrifuged and then washed with Basic Medium (Advanced DMEM/F12 medium with the supplement of 1× B27, 1× N2, 500 mM N-Acetyl-L-cysteine, 10 mM HEPES, 1× GlutaMAX and 1× Penicillin/Streptomycin). The isolated crypts were resuspended with BD Matrigel Basement Membrane Matrix for intestinal organoid culture. The crypts were cultured in Intestinal Organoid Medium (Basic Medium supplemented with 30 ng/mL recombinant murine EGF, 100 ng/mL recombinant murine Noggin and 500 ng/mL recombinant human R-Spondin-1). Three dimensional-cultured intestinal organoids were photographed every 2 days for 10 days using a 10× or 20× objective lens of an Olympus 1×71 microscope (Olympus Corp., PA) [33].
Isolation of primary mouse hepatocytes
Primary mouse hepatocytes were prepared from mice by the collagenase-perfusion technique of Bissell and Guzelian has been previously described [34]. Cells were plated at 2 × 106 cells per collagen-coated 60-mm dish in serum-free Williams E medium containing penicillin, dexamethasone (0.1 μM), and thyroxine (1 μM).
Biochemical analyses of serum
Blood was obtained from the inferior vena cava and allowed to coagulate for 30 min. Serum was collected through centrifugation. Biochemical analyses of the serum were performed using VetScan and mammalian liver profile rotors purchased from Abaxis (Union City, CA).
RNA Isolation and RT-PCR
Total RNA was extracted using Trizol reagent (ThermoFisher) following the manufacturer’s protocol. cDNA synthesis and Quantitative RT-PCR analysis of relative mRNA expression levels of target genes were previously described [35]. Primer sequences will be provided upon request.
Western blot analysis
Cells were lysed in cold RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1× Protease Inhibitor) and centrifuged for 15 min at 4°C. Tissue samples were homogenized in cold RIPA buffer using a tissue grinder. Protein concentrations were determined using the Bio-Rad Protein Assay reagent and Bradford protein assay. Samples were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% skim milk in TBS-T (150 mM NaCl, 50 mM Tris-HCl, pH 8.0, and 0.05% Tween 20), the membranes were probed with the indicated primary antibodies overnight at 4°C followed by incubation with a horseradish peroxidase-conjugated secondary antibody. The antibody-antigen complexes were detected using the ECL system.
FITC-Dextran permeability assay
FITC-Dextran solution (100mg/ml) was prepared in PBS. 60 mg/100g of FITC-Dextran was administered to mice by oral gavage, and blood samples were taken after 3 hr. Serum concentration of FITC-dextran was measured using Victor Multilabel Plate Counter (PerkinElmer, Waltham, MA) with an excitation wavelength of 490 nm and an emission wavelength of 530 nm [36].
Human Liver Samples
Frozen patient liver tissues were obtained through the Liver Tissue Cell Distribution System (Minneapolis, MN) funded by NIH Contract# HSN276201200017C.
Statistical Analysis
Results are presented as the mean ± SE and are from at least three independent experiments. One-way analysis of variance and posttest was performed to analyze the differences between multiple groups by GraphPad Prism (version 5; GraphPad Software Inc., San Diego, CA). P-values of ≤ 0.05 were considered statistically significant.
Results
SphK2 Deficiency Potentiates Alcohol-induced Hepatic Steatosis and Liver Injury
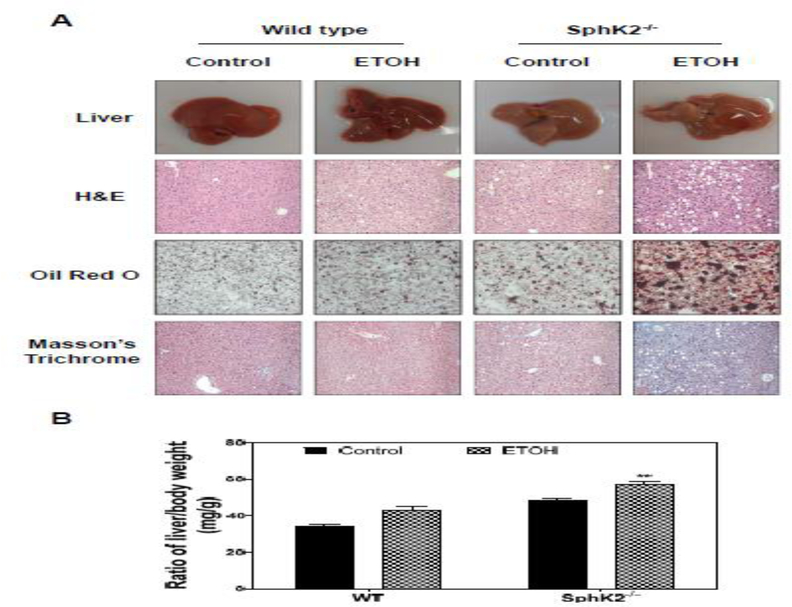
To investigate the role of SphK2 in alcohol-induced liver injury, the mouse chronic feeding model with Lieber-DeCarli alcohol diet was used. Both age- and sex-matched WT and SphK2−/− mice were fed an alcohol diet (Lieber-DeCarli) containing 5% alcohol by volume or Lieber-DeCarli control diet for 60 days. As shown in Fig. 1A, compared to WT mice, SphK2−/− on an alcohol diet had an overall increase in liver size and hepatic lipid accumulation suggestive of fatty liver. Histological analysis revealed that SphK2−/− mice on an alcohol diet exhibited a greater extent of liver injury characterized by an increase in the number and size of lipid droplets and inflammatory cell infiltration (Fig. 1A). Consistent with the dramatic increase in the accumulation of hepatic lipids shown in H&E staining, Oil Red O staining revealed an overt amount of lipid droplets contained in the livers of SphK2−/− mice on an alcohol diet (Fig. 1A). The histopathologic analysis also revealed evidence of necroinflammation and hepatocyte ballooning in the livers of SphK2−/− mice on an alcohol diet (data not shown). However, Masson’s trichrome staining showed no evidence of significant fibrosis (Fig. 1A). SphK2−/− mouse livers weighed 32% more than WT mouse livers on an alcohol diet (Fig. 1B).
Figure 1.
Effect of alcohol on hepatic lipid accumulation in SphK2−/− mice. Wild type (WT) and SphK2−/− mice (male and female, 20 weeks old) were fed Lieber-DeCarli control diet or 5% alcohol diet for 60 days. The liver sections were subjected to H&E staining, Oil Red O, and Masson’s Trichrome staining. The images were taken with an Olympus microscope equipped with an image recorder using a 400× lens. (A) Representative images of livers, H&E staining, Oil Red O staining, Masson are shown. (B) The ratio of liver weight and body weight (mg/g). Results are represented as mean ± SE from each group (n = 7). Statistical significance relative to the control, *P < 0.05; **P < 0.01.
To determine whether hepatic lipid accumulation was associated with liver injury, the serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, cholesterol, and total bile acid levels (TBA) were measured. As shown in Fig. S1A, S1B and S1F, the ALT, AST, and TBA levels were elevated in SphK2−/− mice-fed alcohol. Interestingly, there were no significant changes in total bilirubin, albumin, and cholesterol levels (Fig. S1C, S1D, and S1E).
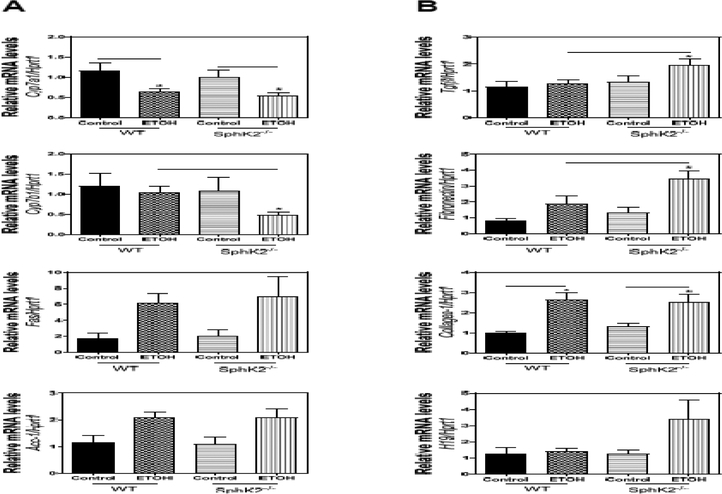
To determine the potential underlying mechanisms by which SphK2−/− mice developed overt fatty livers, we examined the mRNA expression levels of several genes that play a crucial role in hepatic lipid metabolism. Real-time PCR results showed that the mRNA level of Cyp7a1 was reduced in both WT and SphK2−/− mice on an alcohol diet compared to the control diet group, but the mRNA level of Cyp7b1 was only down-regulated in SphK2−/− mice-fed alcohol (Fig. 2A). In addition, mRNA levels of fatty acid synthase (Fas) and acetyl-CoA carboxylase-1 (Acc-1) were up-regulated in both WT and SphK2−/− mice on an alcohol diet (Fig. 2A). Although Masson’s trichrome staining did not reveal extensive liver fibrosis (Fig. 1A), there was a marked increase in the mRNA levels of fibrogenic genes (Tgfβ, fibronectin, collagen-1, H19) in SphK2−/− mice on an alcohol diet (Fig. 2B).
Figure 2.
Effect of alcohol on the mRNA expression of the hepatic lipid and fibrogenic genes in SphK2−/− mice. WT) and SphK2−/− mice (male and female, 20 weeks old) were fed Lieber-DeCarli control diet or 5% alcohol diet for 60 days. Total liver RNA was isolated, and the mRNA levels of target genes were determined using quantitative RT-PCR and normalized to Hprt1 as an internal control. (A) The mRNA levels of key genes in bile acid synthesis and lipid metabolism. (B) The mRNA levels of fibrogenic genes. Results are represented as mean ± SE from each group (n = 7). Statistical significance relative to the WT group, *P < 0.05.
SphK2 Deficiency Promotes Inflammation in Alcohol-induced Liver Injury
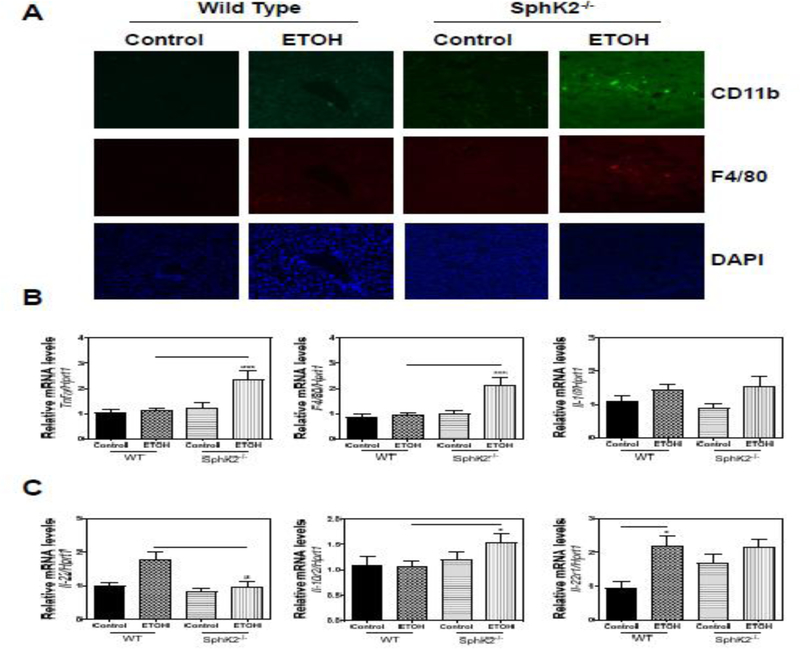
Inflammation is the characteristic hallmark of alcohol-induced liver injury and has been implicated in the progression of ALD. Consistent with H&E staining showing inflammatory cell infiltration (Fig. 1A), immunofluorescence (IF) staining of CD11b and F4/80 revealed increased recruitment of immune cells expressing CD11b+ and F4/80+ inflammatory cells in the SphK2−/− mouse livers on an alcohol diet (Fig. 3A). As expected, real-time PCR results showed that the pro-inflammatory markers including tumor necrosis factor α (Tnfα) and EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80) were increased in SphK2−/− mice-fed alcohol (Fig. 3B). Although alcohol feeding upregulated interleukin-1β (Il-1β) expression, there was no significant difference between WT and SphK2−/− mice.
Figure 3.
SphK2 deficiency promotes a hepatic inflammatory response. (A) Representative images of immunofluorescence staining against CD11b and F4/80 with DAPI as a counterstain are shown. (B and C) Total liver RNA was isolated. Relative mRNA levels of target genes in WT and SphK2−/− mice on a 60-day control diet or 5% alcohol Lieber-DeCarli diet were determined using quantitative RT-PCR and normalized to Hprt1 as an internal control. Results are represented as mean ± SE from each group (n = 7). Statistical significance relative to the WT group, *P < 0.05; ***P < 0.001.
Interleukin-22 (IL-22) has been shown to protect the liver from damage and promote regeneration after injury [37]. To access whether SphK2−/− mice on an alcohol diet had reduced levels of IL-22, we used real-time PCR to analyze the relative mRNA levels of Il-22 and its associated receptors Il-10r2 and Il-22r1. Il-22 was up-regulated in the livers of WT mice-fed alcohol but stayed at relatively baseline levels in SphK2−/− mice-fed alcohol (Fig. 3C). Interestingly, Il-10r2 was modestly up-regulated in SphK2−/− mice-fed alcohol and Il-22r1 was increased in WT mice-fed alcohol compared to WT mice-fed control group (Fig. 3C). These results suggest that SphK2−/− promotes an inflammatory process when exposed to alcohol.
Effect of Alcohol on SphK2 Expression in Hepatocytes and Macrophages
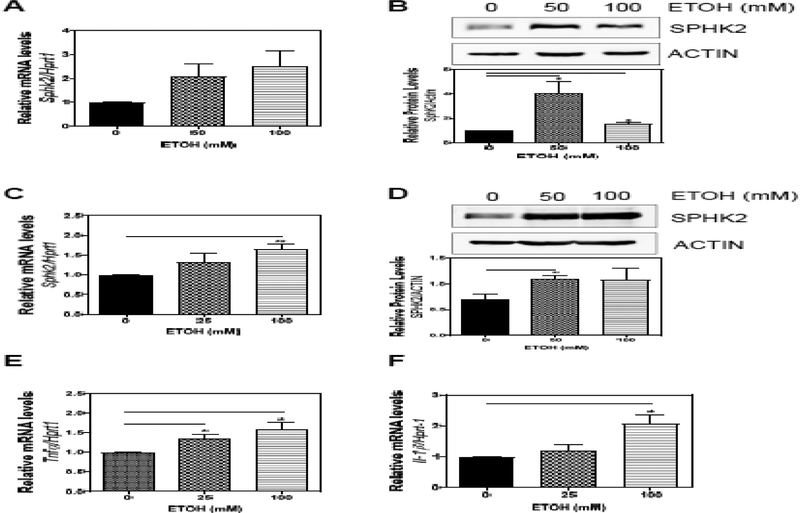
Previous studies have shown that the up-regulation of SphK2 can attenuate hepatic steatosis in the NAFLD mouse model [29]. To investigate whether alcohol could induce the expression of SphK2, we isolated primary mouse hepatocytes and treated the cells with alcohol. Real-time PCR and Western blot analysis revealed that both mRNA and protein levels of SphK2 were up-regulated in hepatocytes treated with alcohol for 6 h (Fig. 4A–B). Macrophages are important players in alcohol-induced liver inflammation, and SphK2 is also highly expressed in macrophages. Interestingly, alcohol also induced both the mRNA and protein expression of SphK2 in mouse macrophages (Fig. 4C–D), but did not affect S1PR2 mRNA expression (data not shown). Furthermore, alcohol upregulated the expression of inflammatory cytokines,Tnfα and Il-1β (Fig. 4E–F).
Figure 4.
Effect of alcohol on SphK2 expression in hepatocytes and macrophages. Mouse primary hepatocytes were treated with alcohol (0, 50, or 100 mM) for 6 hours. (A) Total RNA was isolated, and the mRNA level of SphK2 was determined using quantitative RT-PCR and normalized to Hprt1 as an internal control. (B) Total protein lysates were prepared, and the protein level of SPHK2 was determined by Western Blot analysis. Relative protein levels were determined by normalizing to loading control ACTIN. (C) RAW 264.7 cells were treated with alcohol (0, 25 or 100 mM) for 48 hours. Total cellular RNA was isolated. Relative mRNA levels of SphK2, S1pr2, Tnfα, Il-1β were determined using quantitative RT-PCR and normalized to Hprt1 as an internal control. (D) RAW 264.7 cells were treated with alcohol (0, 50 or 100 mM) for 6 hours and total cellular protein was isolated. Relative protein levels of SPHK2 were determined and normalized to ACTIN. Results are represented as mean ± SE from each group (n = 3). Statistical significance relative to the no treatment group, *P < 0.05; **P < 0.01.
SphK2 Deficiency Suppresses Intestinal Stem Cell Growth and Increases Alcohol-induced Intestinal Barrier Dysfunction
Alcohol has been shown to disrupt intestinal barrier integrity which allows the translocation of bacteria and antigens to sensitize the liver to inflammation. To investigate whether alcohol-induced liver inflammation is associated with intestinal barrier dysfunction, we examined the intestinal permeability in WT and SphK2−/− mice after a 60-day alcohol feeding. As shown in Fig. S2A, the serum levels of FITC-Dextran were significantly higher in SphK2−/− mice than WT mice fed the control diet. Alcohol-feeding did not impact intestinal permeability in WT mice. Consistently, the H&E staining revealed that the intestinal barrier was disrupted in WT mice-fed alcohol diet compared to WT mice-fed control diet. The intestinal disruption was substantial in SphK2−/− mice-fed control diet and most profound in SphK2−/− mice-fed alcohol diet (Fig. S2B). These results suggest that SphK2 deficiency impair intestinal epithelial cell growth.
Our recent study reported that the activation of ER stress induced the loss of stemness in intestinal stem cells [33]. To further determine whether SphK2 plays a critical role in maintaining normal function of intestinal stem cells, we isolated intestinal stem cells from WT and SphK2−/− mice and cultured them in a Matrigel. As shown in Fig. S2C, the SphK2−/− intestinal organoids have a slower growth rate than those compared to WT.
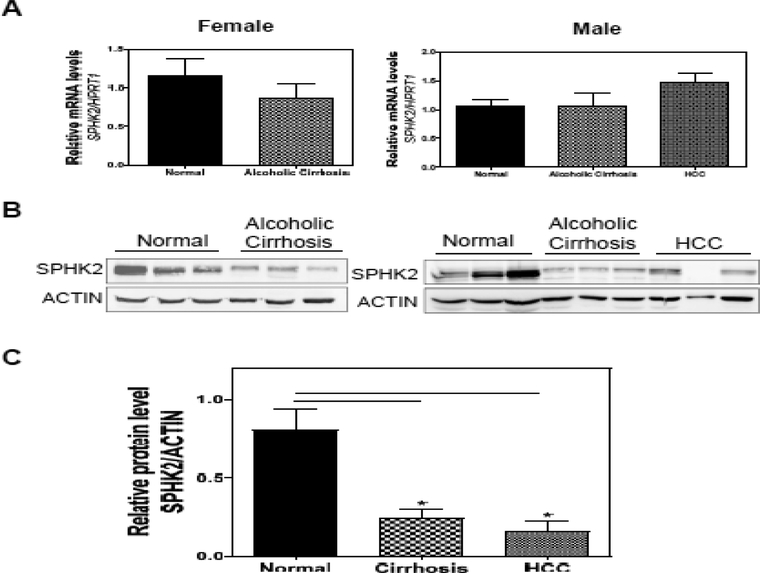
SphK2 is Downregulated in Human Alcoholic Cirrhosis and Hepatocellular Carcinoma
We have determined that SphK2 deficiency potentiates alcohol-induced liver injury in mice. To further determine whether these findings are translatable to human alcoholic patients, we examined the expression levels of hepatic SphK2 in patients with alcoholic cirrhosis and HCC. Real-time PCR results showed that there were no significant changes in the mRNA expression levels of SphK2 (Fig. 5A). However, Western blot analysis revealed that there was a significant down-regulation of SphK2 protein expression in the livers with alcoholic cirrhosis and HCC (Fig. 5B). These results underscored the importance of SphK2 in ALD.
Figure 5.
The hepatic SphK2 expression is downregulated in the patients of alcoholic cirrhosis and hepatocellular carcinoma (HCC). (A) Total RNA was isolated from human liver samples from normal control, alcoholic cirrhosis or HCC patients (both male and female). The mRNA levels of SphK2 were determined using quantitative RT-PCR and normalized to HPRT1 as an internal control. Results are represented as mean ± SE from each group (n = 8). (B) The protein levels of SphK2 were determined by Western Blot analysis. Representative immunoblot images of SphK2 and actin are shown. (C) The relative protein level of SPHK2 was determined by normalizing to ACTIN as a loading control. Results are represented as mean ± SE from each group (n = 3–6). Statistical significance relative to the normal control group, *P < 0.05.
We have also examined the mRNA expression levels of genes that play a key role in bile acid metabolism and inflammation. Although we didn’t find the downregulation of CYP7B1 and other major transporters (Data not shown), the inflammatory mediators, such as IL-1β, IL-6, MCP-1, TNF-α, IL-22, and IL-22R1, were upregulated in alcoholic cirrhosis and HCC (Fig. S3).
Discussion
We have previously reported that S1PR2−/− and SphK2−/− mice rapidly developed overt fatty liver after two-week feeding with a high-fat diet [28]. Here, this current study identified a protective role for SphK2 in alcohol-induced liver injury. Our results indicate that SphK2−/− mice potentiated alcohol-induced liver injury, hepatic steatosis, and inflammation.
Hepatic steatosis is the hallmark feature of early-stage ALD and is present in up to 90% of patients who consume large quantities of alcohol [3]. It has been previously reported that alcohol causes lipid accumulation in the liver through the dysregulation of genes that regulate lipid metabolisms such as Fas, Acc-1, sterol regulatory element binding protein-1 (Srebp-1), and peroxisome proliferator-activated receptor α (Pparα) [38]. In the context of our study, we have previously demonstrated that SphK2−/− mice exhibited a down-regulation of key genes involved in hepatic lipid metabolism, which suggests the importance of SphK2 as a regulator of hepatic lipid metabolism [28]. Moreover, another study reported that SphK2 attenuated hepatic steatosis by inducing the expression of fatty acid oxidation genes such as Pparα, carnitine palmitoyltransferase 1 (Cpt1), and peroxisomal acyl-coenzyme A oxidase 1 (Acox1) [29]. Our results showed that SphK2−/− mice on a 60-day alcohol diet exhibited enlarged livers and a dramatic increase in lipid accumulation in the liver. We observed an increase in the genes that regulate fatty acid metabolism such as Fas and Acc-1 in both WT and SphK2−/− mice on a 60-day alcohol diet. 3
Interestingly, there was a down-regulation of Cyp7b1 in SphK2−/− mice-fed alcohol compared to WT mice-fed alcohol. Cyp7b1 encodes for the enzyme oxysterol 7-alpha-hydroxylase, which synthesizes the bile acid chenodeoxycholic acid in the alternative pathway [39]. Studies have shown that bile acids regulate lipid metabolism through pathways involving FXR and small heterodimer partner (SHP) [40]. In this regard, we speculate that a decrease in the synthesis of certain bile acids could alter the function of the liver to transport and metabolize lipids effectively. This is supported by a recent study demonstrating that FXR agonists attenuate alcohol-induced liver injury and steatosis [41]. In addition, a down-regulation of Cyp7b1 has been shown to cause an accumulation of oxysterols which promote liver inflammation and fibrosis [42]. Inflammation has been implicated in ALD progression from hepatic steatosis to AH, fibrosis, and cirrhosis [43]. SphK2−/− mice on an alcohol diet produced a more profound inflammatory response as shown by an increase in inflammatory markers Tnfα, F4/80, and Il-1β. It is interesting to note that Il-22 has been shown to protect mice from alcohol-induced liver injury through the activation of signal transducer and activator of transcription 3 (STAT3) [44]. We observed that the mRNA levels of Il-22 were up-regulated in the livers of WT mice-fed alcohol but stayed at relatively baseline levels in SphK2−/− mice-fed alcohol. This would suggest that SphK2 may play a role in IL-22 activation. Further studies are warranted to determine whether this is a direct effect of SphK2.
One of the functions of the intestine is to provide a physical barrier against bacteria and associated antigens from reaching the liver [45]. Given that 70% of the blood drains from the intestines to the liver, compromised intestinal integrity would result in transient endotoxemia that could promote an inflammatory process in the liver known as leaky gut syndrome [46]. Previous studies have highlighted the importance of S1P in promoting intestinal stem cell growth and the maintenance of the intestinal barrier [47, 48]. Our results showed that SphK2−/− mice exhibited compromised intestinal integrity and attenuation of intestinal crypt growth, which is consistent with the notion that S1P plays an essential role in the maintenance of intestinal epithelial cell growth. Interestingly, it has been reported that alcohol alters the composition of the gut microbiome which may shift the levels of bacteria favoring the production of potent inflammatory antigens such as lipopolysaccharide (LPS) [49]. Moreover, it has been shown that patients who are active drinkers with alcoholic cirrhosis have elevated levels of secondary bile acids compared to those who are abstinent with alcoholic cirrhosis [50].
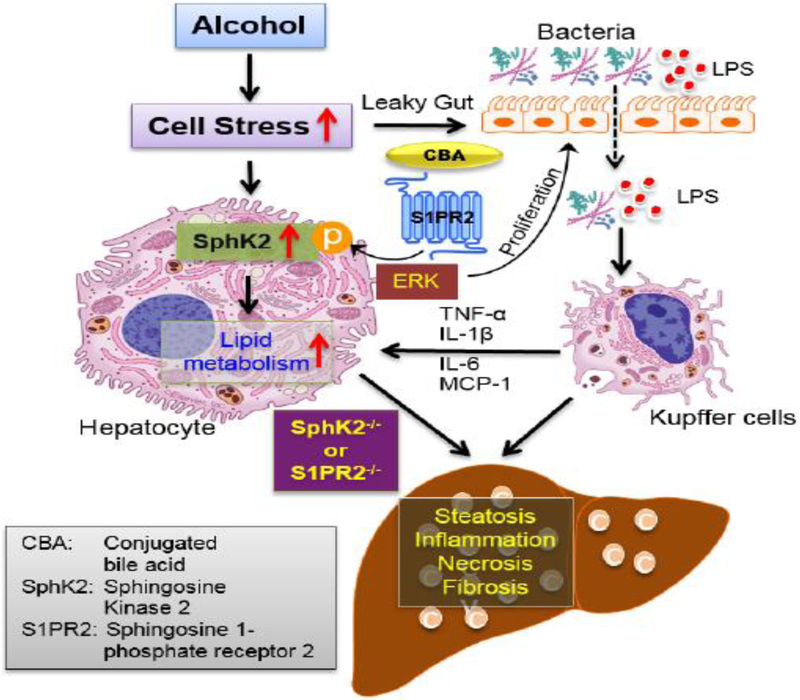
In summary, the current study demonstrated that SphK2−/− mice on an alcohol diet potentiates alcohol-induced liver injury through the increased accumulation of hepatic lipids and inflammatory response. The data also highlight the importance of the gut-liver axis as SphK2−/− mice have compromised intestinal integrity that is worsened by alcohol (Fig. 6). Moreover, SphK2 is induced in hepatocytes when exposed to alcohol and the striking observation that SphK2 is down-regulated in advanced stages of human ALD demonstrates the importance of SphK2 in maintaining liver homeostasis. Here, we have provided evidence to suggest that targeting the SphK2-mediated signaling pathway represents a therapeutic strategy against ALD as well as other various liver diseases.
Figure 6.
Schematic diagram of the proposed mechanisms underlying SphK2-mediated regulation of lipid metabolism in alcohol-induced liver injury.
Supplementary Material
Financial Support
This study was supported by National Institutes of Health Grant R01 DK104893 and R01DK-057543 (HZ and PBH); VA Merit Award I01BX004033 and 1I01BX001390 (HZ); Research Career Scientist Award (HZ, IK6BX004477). Massey Cancer Center research fund.
Abbreviations:
- ABC
ATP-binding cassette
- Acc-1
acetyl-CoA carboxylase-1
- AH
alcoholic hepatitis
- ALD
alcoholic liver disease
- Cyp7b1
steroid 7-alpha-hydroxylase
- ER
endoplasmic reticulum
- Fas
fatty acid synthase
- HCC
hepatocellular carcinoma
- LPS
lipopolysaccharide
- NAFLD
non-alcoholic fatty liver disease
- S1P
sphingosine 1-phosphate
- S1PR2
sphingosine 1-phosphate receptor 2
- SHP
small heterodimer partner
- SphK1
sphingosine kinase 1
- SphK2
sphingosine kinase 2
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial interest: The authors declare no competing financial interest
References
- (1).Cholankeril G, Ahmed A. Alcoholic Liver Disease Replaces Hepatitis C Virus Infection as the Leading Indication for Liver Transplantation in the United States. Clin Gastroenterol H. 2018;16:1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Chacko KR, Reinus J. Spectrum of Alcoholic Liver Disease. Clin Liver Dis. 2016;20:419–+. [DOI] [PubMed] [Google Scholar]
- (3).Frazier TH, Stocker AM, Kershner NA, et al. Treatment of alcoholic liver disease. Therapeutic advances in gastroenterology. 2011;4:63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. Journal of hepatology. 2015;62:S38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Frezza M, Dipadova C, Pozzato G, et al. High Blood-Alcohol Levels in Women - the Role of Decreased Gastric Alcohol-Dehydrogenase Activity and 1st-Pass Metabolism. New Engl J Med. 1990;322:95–9. [DOI] [PubMed] [Google Scholar]
- (6).Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterology & Hepatology. 2013;9:633–9. [PMC free article] [PubMed] [Google Scholar]
- (7).Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Mahli A, Hellerbrand C. Alcohol and Obesity: A Dangerous Association for Fatty Liver Disease. Digestive diseases. 2016;34 Suppl 1:32–9. [DOI] [PubMed] [Google Scholar]
- (9).Nahon P, Sutton A, Rufat P, et al. Liver iron, HFE gene mutations, and hepatocellular carcinoma occurrence in patients with cirrhosis. Gastroenterology. 2008;134:102–10. [DOI] [PubMed] [Google Scholar]
- (10).Yuan JM, Ross RK, Wang XL, et al. Morbidity and mortality in relation to cigarette smoking in Shanghai, China. A prospective male cohort study. Jama. 1996;275:1646–50. [PubMed] [Google Scholar]
- (11).Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological endpoints by zygosity among male veterans. Alcoholism, clinical and experimental research. 1981;5:207–15. [DOI] [PubMed] [Google Scholar]
- (12).Whitfield JB. Meta-analysis of the effects of alcohol dehydrogenase genotype on alcohol dependence and alcoholic liver disease. Alcohol and alcoholism. 1997;32:613–9. [DOI] [PubMed] [Google Scholar]
- (13).Askgaard G, Gronbaek M, Kjaer MS, et al. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. Journal of hepatology. 2015;62:1061–7. [DOI] [PubMed] [Google Scholar]
- (14).Llerena S, Arias-Loste MT, Puente A, et al. Binge drinking: Burden of liver disease and beyond. World journal of hepatology. 2015;7:2703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ventura-Cots M, Watts AE, Bataller R. Binge drinking as a risk factor for advanced alcoholic liver disease. Liver Int. 2017;37:1281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Seminars in liver disease. 2009;29:141–54. [DOI] [PubMed] [Google Scholar]
- (17).Galicia-Moreno M, Gutierrez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Revista de gastroenterologia de Mexico. 2014;79:135–44. [DOI] [PubMed] [Google Scholar]
- (18).Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. International journal of hepatology. 2012;2012:853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hannun YA, Obeid LM. Principles of bioactive lipid signaling: lessons from sphingolipids. Nat Rev Mol Cell Bio. 2008;9:139–50. [DOI] [PubMed] [Google Scholar]
- (20).Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Olivera A, Kohama T, Edsall L, et al. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hait NC, Allegood J, Maceyka M, et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science. 2009;325:1254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mitra P, Oskeritzian CA, Payne SG, et al. Role of ABCC1 in the export of sphingosine-1-phosphate from mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kawahara A, Nishi T, Hisano Y, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–7. [DOI] [PubMed] [Google Scholar]
- (25).Pyne NJ, McNaughton M, Boomkamp S, et al. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Advances in biological regulation. 2016;60:151–9. [DOI] [PubMed] [Google Scholar]
- (26).Karimian G, Buist-Homan M, Schmidt M, et al. Sphingosine kinase-1 inhibition protects primary rat hepatocytes against bile salt-induced apoptosis. Biochimica et Biophysica Acta. 2013;1832:1922–9. [DOI] [PubMed] [Google Scholar]
- (27).Kihara Y, Maceyka M, Spiegel S, et al. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. British journal of pharmacology. 2014;171:3575–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Nagahashi M, Takabe K, Liu RP, et al. Conjugated Bile Acid-Activated S1P Receptor 2 Is a Key Regulator of Sphingosine Kinase 2 and Hepatic Gene Expression. Hepatology 2015;61:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Lee SY, Hong IK, Kim BR, et al. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology. 2015;62:135–46. [DOI] [PubMed] [Google Scholar]
- (30).Cohen JI, Roychowdhury S, McMullen MR, et al. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–74, 74e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mandrekar P, Ambade A, Lim A, et al. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nath B, Levin I, Csak T, et al. Hepatocyte-Specific Hypoxia-Inducible Factor-1 alpha Is a Determinant of Lipid Accumulation and Liver Injury in Alcohol-Induced Steatosis in Mice. Hepatology. 2011;53:1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Liu R, Li X, Huang Z, et al. C/EBP homologous protein-induced loss of intestinal epithelial stemness contributes to bile duct ligation-induced cholestatic liver injury in mice. Hepatology. 2018;67:1441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Heuman DM, Hernandez CR, Hylemon PB, et al. Regulation of bile acid synthesis. I. Effects of conjugated ursodeoxycholate and cholate on bile acid synthesis in chronic bile fistula rat. Hepatology. 1988;8:358–65. [DOI] [PubMed] [Google Scholar]
- (35).Wang YQ, Aoki H, Yang J, et al. The Role of Sphingosine 1-Phosphate Receptor 2 in Bile-Acid-Induced Cholangiocyte Proliferation and Cholestasis-Induced Liver Injury in Mice. Hepatology. 2017;65:2005–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Wu X, Sun L, Zha W, et al. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Carmo RF, Cavalcanti MSM, Moura P. Role of Interleukin-22 in chronic liver injury. Cytokine. 2017;98:107–14. [DOI] [PubMed] [Google Scholar]
- (38).Donohue TM Jr,. Alcohol-induced steatosis in liver cells. World journal of gastroenterology. 2007;13:4974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annual review of biochemistry. 2003;72:137–74. [DOI] [PubMed] [Google Scholar]
- (40).Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. The Journal of clinical investigation. 2004;113:1408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Iracheta-Vellve A, Calenda CD, Petrasek J, et al. FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After Binge or Prolonged Alcohol Feeding in Mice. Hepatology communications. 2018;2:1379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kakiyama G, Marques D, Takei H, et al. Inflammasome Activation by Chronic down Regulation of Cyp7b1 and Its Causative Increased Oxysterol Accumulation, Represents the Key Initial Step in Fatty Liver’s Progression toward Inflammation. Gastroenterology. 2017;152:S1069–S. [Google Scholar]
- (43).Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016;150:1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Ki SH, Park O, Zheng MQ, et al. Interleukin-22 Treatment Ameliorates Alcoholic Liver Injury in a Murine Model of Chronic-Binge Ethanol Feeding: Role off Signal Transducer and Activator of Transcription 3. Hepatology. 2010;52:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nature Reviews Gastroenterology & Hepatology. 2017;14:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Compare D, Coccoli P, Rocco A, et al. Gut-liver axis: the impact of gut microbiota on nonalcoholic fatty liver disease. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2012;22:471–6. [DOI] [PubMed] [Google Scholar]
- (47).Greenspon J, Li RY, Xiao L, et al. Sphingosine-1-Phosphate Protects Intestinal Epithelial Cells from Apoptosis Through the Akt Signaling Pathway. Digestive diseases and sciences. 2009;54:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Chen TZ, Lin RY, Jin SS, et al. The Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis in Intestinal Epithelial Cells Regulates Intestinal Barrier Function During Intestinal Epithelial Cells-CD4(+)T-Cell Interactions. Cell Physiol Biochem. 2018;48:1188–200. [DOI] [PubMed] [Google Scholar]
- (49).Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of hepatology. 2014;60:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kakiyama G, Hylemon PB, Zhou HP, et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol-Gastr L. 2014;306: G929–G37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.