Abstract
Background:
The mechanism of adverse limb events associated with peripheral artery disease remains incompletely understood. We investigated whether microvascular disease is associated with amputation in a large cohort of veterans. To determine whether microvascular disease diagnosed in any location increases the risk of amputation alone and in concert with peripheral artery disease.
Methods:
Participants in the Veterans Aging Cohort Study from April 1, 2003 through December 31, 2014. We excluded participants with known prior lower limb amputation. Using time-updated Cox proportional hazards regression, we analyzed the effect of prevalent microvascular disease (retinopathy, neuropathy, and nephropathy) and peripheral artery disease status on the risk of incident amputation events after adjusting for demographics and cardiovascular risk factors.
Results:
Among 125,674 veterans without evidence of prior amputation at baseline, the rate of incident amputation over a median of 9.3 years of follow up was 1.16 per 1000 person-years yielding a total of 1185 amputations. In time-updated multivariable-adjusted analyses, compared to those without peripheral artery disease or microvascular disease: microvascular disease alone was associated with a 3.7-fold [95% CI=3.0, 4.6] increased risk of amputation; peripheral artery disease alone conferred a 13.9-fold [11.3, 17.1] elevated risk of amputation; and the combination of peripheral artery disease and microvascular disease was associated with a 22.7-fold [18.3, 28.1] increased risk of amputation.
Conclusion:
Independent of traditional risk factors, the presence of microvascular disease increases the risk of amputation alone and synergistically increases risk in patients with peripheral artery disease. Further research is needed to understand the mechanisms by which this occurs.
Keywords: Peripheral Artery Disease, Microvascular Disease, Amputation, Retinopathy, Nephropathy, Neuropathy
Introduction
Peripheral artery disease (PAD) is a cardinal manifestation of atherosclerosis and is associated with an increased risk of cardiovascular events, such as myocardial infarction and stroke, and with adverse limb events, including the need for revascularization and/or amputation. Although the severity of lower extremity artery occlusive disease in PAD, as measured by an ankle brachial index (ABI), strongly associates with cardiovascular events like myocardial infarction1, it does not relate as strongly to the development of limb complications1, 2. These observations suggest the existence of other important contributors to the development of limb symptoms beyond large artery atherosclerosis.
Abnormalities in the microvasculature (vessels with a diameter of ~100 um) represent a putative candidate mechanism for the development of adverse limb events in individuals with peripheral artery disease. Although the term microcirculation has been applied to both small (pedal) artery disease and microvascular disease3, the microvasculature stands apart from the small pedal arteries (1–2 mm in diameter) of the foot. Pedal vessels are, in fact, ten-fold larger than the vessels that are compromised in microvascular disease.
MVD often occurs alongside atherosclerosis but is not a direct manifestation of atherosclerotic disease. MVD is commonly ascribed to diabetes because of the significantly increased risk of microvascular dysfunction compared to patients without diabetes4. However, the aggregate population burden of retinopathy5, neuropathy6, and nephropathy7 is much greater in the general population, creating a larger group at potential risk for MVD than just in persons with diabetes. To better characterize the impact of microvascular disease on adverse limb events, we examined the association between PAD, MVD, and amputation in an older, predominantly male population of veterans. We chose amputation as our endpoint because of its definitive nature and clarity in the electronic health record. Although there is likely impact of MVD on the development of critical limb ischemia (CLI), ulceration, and infection, amputation is a well-defined objective end point that neither varies in definition between clinicians nor requires nuanced adjudication.
Methods
The VA does not allow identifiable datasets to be used by non-VA investigators. However, after meeting all requirements per VA regulations, a de-identified dataset can be provided to qualified investigators that would allow for replication of our results.
Study Sample
The Veterans Aging Cohort Study (VACS) is a prospective longitudinal cohort of HIV-infected and uninfected Veterans matched 1:2 for sex, age, clinical site, race/ethnicity, and calendar year of enrollment. VACS participants are identified using a validated algorithm deployed in the US Department of Veterans Affairs (VA) national electronic medical record system8. Information regarding demographic and clinical data are extracted from the VA electronic medical record, Health Factor data set and the VA Corporate Data Warehouse. Vital status is determined using the VA vital status file, the Veterans Health Administration Medical Statistical Analysis Systems inpatient data sets, the Social Security Administration death master file, and the Beneficiary Identification and Records Locator Subsystem. Institutional review boards from Vanderbilt University Medical Center, West Haven VA Medical Center, and Yale University approved this study.
We included all VACS participants in this analysis who were alive as of April 1, 2003 with the baseline as a participant’s first clinic visit on or after this date. Participants were followed from baseline to the minimum of: date of lower extremity amputation, death, or December 31, 2014. Our analyses were truncated at the end of 2014 to coincide with end of our Medicare data files. We excluded participants with previous amputation based upon administrative International Classification of Diseases (ICD)-9 or Current Procedural Terminology (CPT) codes before their baseline date or within 180 days post-baseline to account for delayed reporting. Because VACS was initially designed to look at the impact of HIV, but was not a focus of this manuscript, we additionally excluded participants who seroconverted to HIV+ during follow-up. Following these exclusions (n=7,931), our final sample included 125,674 veterans.
Independent Variable
The presence of MVD was determined on the basis of at least 1 inpatient and/or 2 or more outpatient VA, VA fee for service, or Medicare ICD-9 or CPT codes for peripheral neuropathy or retinopathy related to MVD (Table S1) prior to baseline or within 180 days post-baseline, or a urine protein test result of “+1” or greater indicative of proteinuria within 180 days on either side of baseline. PAD was defined using the description from Bali et al.9 which we have previously used in VACS10, less codes for lower extremity amputation based on presence of 1 or more inpatient or at least 2 outpatient VA, VA fee for service, or Medicare ICD-9 or CPT codes prior to baseline or within 180 days post-baseline (Table S2). We then defined a 4-level categorical variable to serve as our exposure of interest, with the 4 levels being: neither MVD nor PAD (referent), MVD alone, PAD alone, and both MVD and PAD.
Dependent Variable
Our primary outcome, incident lower extremity amputation, was based on the presence of 1 or more inpatient or at least 2 outpatient VA, VA fee for service, or Medicare ICD-9 or CPT codes in the absence of any exclusion codes (Table S2). Lower extremity amputation and exclusion codes described by Bali et al.9 were used in this analysis.
Covariates
In the present analysis, age, sex, race/ethnicity, HIV infection, hypertension, diabetes mellitus, lipid levels, smoking status, hepatitis C infection, renal disease, body mass index, anemia, total bilirubin, chronic obstructive pulmonary disease, alcohol abuse or dependence, cocaine abuse or dependence, and prevalent cardiovascular disease served as our covariates. Ascertainment sources and definitions of these variables in VACS have been previously described10.
Statistical Analysis
We analyzed descriptive statistics for all variables by baseline MVD and PAD status using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. Next, we plotted the cumulative incidence of amputation over the follow-up period by baseline vascular disease status and performed log-rank tests to compare the various groups. We determined that there were no serious violations of the proportional hazards assumption through inspection of log(-log) plots where we observed approximately parallel lines, and then utilized unadjusted and multivariable-adjusted Cox proportional hazards regression models to compare the risk of incident amputation in veterans with at least one subtype of vascular disease compared to veterans free of MVD and PAD. Because participants could change vascular disease status during follow-up, the primary analyses included vascular disease status as a time-varying exposure. Our multivariable-adjusted Cox proportional hazards regression model included age, sex, race/ethnicity, established atherosclerotic risk factors and other comorbidities described above. In supplemental analyses, baseline vascular disease status was assumed constant throughout the follow-up period.
Additional analyses were further limited to participants free of diabetes mellitus, participants free of renal disease, never smokers, and those free of HIV infection since these factors are associated with MVD and/or PAD10. In further supplemental analyses, we investigated the association between vascular disease groups and incident major-adverse cardiac events (MACE) in order to support our MVD definition in comparison to prior findings regarding the impact of MVD and PAD on MACE. Here, as described by Hawn et al, MACE was defined as acute myocardial infarction or coronary revascularization with and without all-cause mortality11. For these analyses, individuals with prevalent cardiovascular disease were excluded (n=19,954) for an analytic sample of 105,720 individuals.
In the present investigation, we employed multiple imputation by chained equation techniques that generated 5 complete data sets to handle missing covariate data through predictive mean-matching. A two-sided p-value of <0.05 was used to determine statistical significance. Multiple imputation was performed in R version 3.2.5 and all other analyses were performed using SAS version 9.4 (Cary, NC).
Results
There were 125,674 veterans without evidence of amputation prior to baseline. We analyzed veterans according to the presence or absence of prevalent PAD, MVD, or the combination of PAD and MVD (Table 1). Compared to participants with neither manifestation of vascular disease, participants with PAD, MVD, or the combination were more likely to have hypertension, diabetes, anemia, and chronic obstructive pulmonary disease.
Table 1.
Baseline Characteristics of Veterans stratified by PAD and Microvascular Disease
| Baseline Characteristic* | No Microvascular Disease or PAD (n = 109,447) |
Microvascular Disease Only (n = 9125) |
PAD Only (n = 5313) |
Microvascular Disease and PAD (n = 1789) |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 49.1 (10.1) | 52.7 (9.6) | 57.4 (10.4) | 58.6 (10.2) |
| Median | 49.0 | 53.0 | 56.0 | 58.0 |
| Male sex, % | 97.1 | 97.8 | 98.5 | 99.2 |
| Race/ethnicity, % | ||||
| African American | 47.2 | 56.0 | 46.0 | 53.9 |
| White | 39.5 | 34.0 | 44.6 | 36.2 |
| Hispanic | 7.7 | 6.6 | 6.1 | 6.9 |
| Other | 5.5 | 3.4 | 3.3 | 3.0 |
| Prevalent CVD, % | 12.3 | 26.4 | 54.2 | 65.5 |
| HIV Infection, % | 29.1 | 44.7 | 27.2 | 32.4 |
| Framingham Risk Factors, % | ||||
| Hypertension† | ||||
| None | 36.8 | 18.5 | 11.8 | 6.8 |
| Controlled | 34.3 | 43.7 | 52.7 | 50.9 |
| Uncontrolled | 29.0 | 37.9 | 35.5 | 42.3 |
| Diabetes mellitus, % | 12.9 | 40.7 | 32.3 | 61.3 |
| Lipids, mg/dL† | ||||
| LDL cholesterol <100 | 37.2 | 47.3 | 47.5 | 57.5 |
| LDL cholesterol 100–129 | 32.3 | 28.4 | 29.0 | 23.8 |
| LDL cholesterol 130–159 | 20.2 | 15.6 | 16.0 | 12.0 |
| LDL cholesterol ≥160 | 10.4 | 8.7 | 7.5 | 6.8 |
| HDL cholesterol ≥60 | 13.3 | 12.7 | 11.1 | 11.6 |
| HDL cholesterol 40–59 | 44.6 | 39.0 | 41.8 | 36.8 |
| HDL cholesterol <40 | 42.1 | 48.4 | 47.1 | 51.7 |
| Triglycerides ≥150 | 40.5 | 46.1 | 43.3 | 44.9 |
| Smoking, %† | ||||
| Current | 51.8 | 49.2 | 56.8 | 48.9 |
| Former | 17.3 | 20.4 | 21.2 | 25.1 |
| Never | 30.9 | 30.4 | 22.0 | 26.0 |
| Other risk factors, % | ||||
| HCV infection, % | 17.0 | 24.9 | 20.8 | 24.5 |
| Renal disease, mL/min/1.73m2 † | ||||
| eGFR ≥60 | 95.1 | 80.6 | 83.6 | 60.6 |
| eGFR 30–59 | 4.4 | 13.9 | 11.5 | 23.1 |
| eGFR <30 | 0.5 | 5.5 | 5.0 | 16.3 |
| Body Mass Index, kg/m2 † | ||||
| Mean (SD) | 28.3 (5.8) | 28.3 (6.6) | 28.4 (6.9) | 28.6 (6.8) |
| Median | 27.6 | 27.3 | 27.6 | 27.6 |
| Anemia, g/dL† | ||||
| Hemoglobin ≥14 | 68.4 | 48.5 | 51.5 | 35.4 |
| Hemoglobin 12–13.9 | 26.0 | 34.4 | 34.2 | 37.6 |
| Hemoglobin 10–11.9 | 4.7 | 13.3 | 11.0 | 20.5 |
| Hemoglobin <10 | 1.0 | 3.8 | 3.3 | 6.6 |
| Bilirubin, mg/dL† | ||||
| Mean (SD) | 0.7 (0.6) | 0.7 (0.6) | 0.7 (0.6) | 0.6 (0.7) |
| Median | 0.6 | 0.6 | 0.6 | 0.5 |
| History of Alcohol Abuse, % | 26.1 | 28.9 | 30.9 | 28.1 |
| History of Cocaine Abuse, % | 16.2 | 18.2 | 16.8 | 17.2 |
| COPD, % | 9.9 | 15.7 | 26.7 | 27.1 |
Abbreviations; ART, antiretroviral therapy; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; PAD, peripheral artery disease;
SI conversion factors: To convert HDL and LDL to millimoles per liter, multiply by 0.0259; hemoglobin to grams per liter, multiply by 10; and triglycerides to millimoles per liter, multiply by 0.0113.
All characteristics were statistically different among Veterans in various groups (P<.05) using χ2 test or Kruskal-Wallis.
All variables had complete data except the following: Hypertension data were available on 105,436 (no micro or PAD), 5240 (PAD only), 9041 (microvascular disease only), and 1771 (micro and macrovascular disease); LDL cholesterol data were available on 85,544 (no micro or macrovascular disease), 4529 (PAD only), 8022 (microvascular disease only), and 1589 (micro and macrovascular disease); HDL cholesterol data were available on 86,627 (no micro or PAD), 4571 (PAD), 8089 (microvascular disease only), and 1600 (micro and macrovascular disease); triglyceride data were available on 86,977 (no micro or PAD), 4603 (PAD only), 8170 (microvascular disease only), and 1623 (micro and macrovascular disease); smoking data were available on 74,253 (no micro or PAD), 4000 (PAD only), 6787 (microvascular disease only), and 1373 (micro and macrovascular disease); eGFR data were available on 98,789 (no micro or macro vascular disease), 5044 (PAD only), 8883 (microvascular disease only), and 1735 (micro and macrovascular disease); BMI data were available on 103,830 (No Micro or Macro Vascular Disease), 5186 (PAD only), 9004 (microvascular disease only), and 1753 (micro and PAD); anemia data were available on 98,288 (no micro or PAD), 5056 (PAD only), 8838 (microvascular disease only), and 1719 (micro and macrovascular disease); bilirubin data were available on 95,243 (no micro or PAD), 4925 (PAD only), 8720 (microvascular disease only), and 1709 (micro and macro vascular disease).
Amputation
The rate of incident amputation over a median of 9.3 years of follow up was 1.16 / 1000 person-years. At the time of amputation, retinopathy was present in 69%, nephropathy in 67%, and neuropathy in 78% of participants. In unadjusted analyses that were time-updated for changes in PAD and MVD status to reflect this, the presence of MVD alone was associated with a 6.8-fold [95% CI=5.6, 8.4] increased risk of amputation; PAD alone conferred a 19.7-fold [16.3, 23.8] elevated risk of amputation; and the combination of PAD and MVD was associated with a 56.9-fold [47.8, 67.8] increased risk of amputation (Table 2). Even after multivariable adjustment for demographic characteristics, cardiovascular disease risk factors, and other potential confounders, our results were highly significant. Compared to those without either vascular disease: presence of MVD alone was associated with a 3.7-fold [3.0, 4.6] increased risk of amputation; PAD alone conferred a 13.9-fold [11.3, 17.1] elevated risk of amputation; and, notably, the combination of PAD and MVD was associated with a 22.7-fold [18.3, 28.1] increased risk of amputation (Table 2).
Table 2.
Time-Updated Risk of Amputation by combination of Microvascular disease and PAD
| Group | Person-Years | Events | Incidence Rate per 1000 PY | Unadjusted Model | Adjusted Model | ||
|---|---|---|---|---|---|---|---|
| Hazard Ratio [95% CI] |
p-value | Hazard Ratio [95% CI] |
p-value | ||||
| No Microvascular disease or PAD | 795,802 | 182 | 0.23 | 1.00 | --- | 1.00 | --- |
| Microvascular disease | 130,584 | 207 | 1.59 | 6.84 [5.60, 8.35] |
<0.0001 | 3.74 [3.03, 4.62] |
<0.0001 |
| PAD | 58,395 | 265 | 4.54 | 19.68 [16.25, 23.83] |
<0.0001 | 13.86 [11.25, 17.07] |
<0.0001 |
| Microvascular disease and PAD | 40,182 | 531 | 13.22 | 56.92 [47.80, 67.78] |
<0.0001 | 22.71 [18.34, 28.12] |
<0.0001 |
Adjusted Model includes age, sex, race, HIV, prevalent CVD, hypertension, diabetes mellitus, LDL cholesterol, HDL cholesterol, triglycerides, smoking status, HCV Infection, renal failure, BMI, anemia, total bilirubin, alcohol abuse or dependence, cocaine abuse or dependence, and COPD.
The location of amputation also varied by vascular bed involvement (Table 3). Using the type of vascular disease at the time of amputation, participants with microvascular disease alone suffered 18% of all amputations, 21% of below ankle amputations, 15% of below knee amputations, and 6% of all above knee amputations. Participants with PAD alone at baseline suffered 22% of all amputations, 17% of below ankle, 25% of below knee, and 39% of above knee amputations. The combination of MVD and PAD accounted for 45% of all amputation and caused the most amputation at all limb levels. There was a statistically significant variation in vascular involvement and level of amputation, with MVD more likely to cause a below-ankle amputation and PAD more likely to cause below and above knee amputations (p < 0.001). Further, we assessed the location of MVD at the time of amputation. Amputation at all levels was associated with all manifestations of MVD, alone and in combination (Table S4).
Table 3:
Level of Amputation by Type of Vascular Disease Manifestation at time of Amputation
| Vascular Disease Manifestation | Level of Amputation | |||
|---|---|---|---|---|
| Below Ankle Amputation | Below Knee Amputation | Above Knee Amputation | Unknown Location | |
| No Microvascular Disease or PAD | 110 (15%) | 34 (14%) | 31 (16%) | 7 (26%) |
| Microvascular Disease Only | 152 (21%) | 37 (15%) | 11 (6%) | 7 (26%) |
| PAD Only | 124 (17%) | 62 (25%) | 73 (39%) | 6 (22%) |
| Microvascular Disease and PAD | 333 (46%) | 117 (47%) | 74 (39%) | 7 (26%) |
| Number of Amputations | 719 | 250 | 189 | 27 |
Cells contain N (column percent).
There is an association between level of amputation and vascular disease manifestation (χ2=60.5, df=9, p<0.0001).
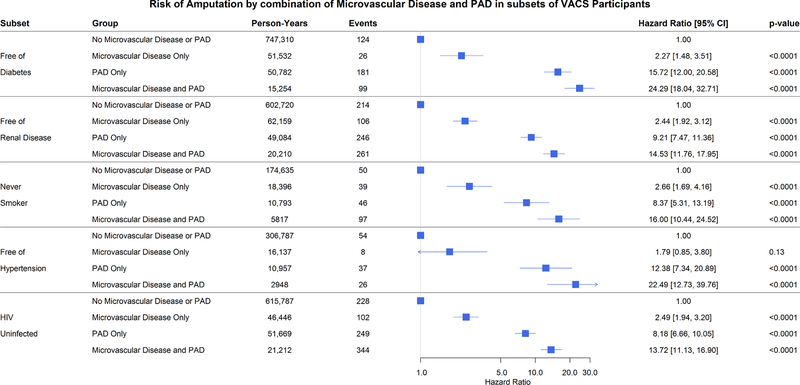
The graded risk of amputation from neither vascular disease to MVD alone, PAD alone, and the combination was maintained after adjustment, using time-updated exposures, in participants without diabetes mellitus, free of renal disease, never smokers, and those free of hypertension or HIV infection (Figure 1).
Figure 1: Risk of Amputation by Time-Updated Combination of Microvascular Disease and PAD in Subsets of VACS Participants.
Specific subsets include those who were free of diabetes mellitus, free of renal disease (eGFR>60), never smokers, free of hypertension, and HIV uninfected.
Analyses assuming baseline vascular disease status remained constant throughout follow-up also demonstrated increased risk of amputation among those with at least one vascular disease subtype compared to those free of vascular disease, but effects were greatly attenuated (Figure 2, Table S3) as at study baseline only 31% of the participants who later one required amputation had been diagnosed with microvascular disease.
Figure 2: Cumulative Incidence of Amputation by baseline MVD and PAD status.
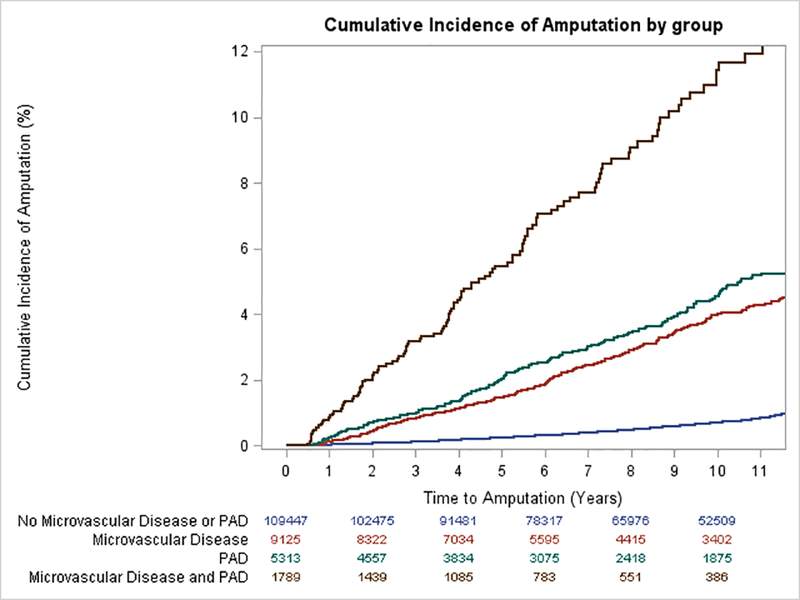
Kaplan-Meier survival curves illustrating the time to first amputation incident over 9.3 median years of follow up for veterans with neither MVD nor PAD, MVD alone, PAD alone, and MVD and PAD at baseline.
Diabetes
Because of the strong association of diabetes with microvascular disease, we assessed the graded risk of amputation in subjects with diabetes as well. Participants with diabetes comprise 41% of those with microvascular disease alone, 32% of those with PAD alone and 61% of those with microvascular disease and PAD compared with 13% of those with neither manifestation. Time-updating vascular disease status reinforced the role of both MVD and PAD in lower extremity amputation incidence in participants with diabetes. After multivariable adjustment, compared to those diabetic participants without either vascular disease: presence of MVD alone was associated with a 3.1-fold [2.4, 4.0] increased risk of amputation; PAD alone conferred a 7.9-fold [5.9, 10.5] elevated risk of amputation; and, even more impressively, the combination of PAD and MVD was associated with a 15.9-fold [12.4, 20.6] increased risk of amputation (Table S5).
Major Adverse Cardiovascular Events
The rate of incident MACE over a median 9.2 years of follow up was 24 events / 1000 person-years. In unadjusted analyses, compared to those without vascular disease: presence of MVD alone was associated with a 2.0-fold [95% CI=1.9, 2.1] increased risk of MACE; PAD alone conferred a 2.7-fold [2.5, 2.9] elevated risk of MACE; and the combination of PAD and MVD was associated with a 3.9-fold [3.4, 4.3] increased risk of MACE (Table S6). After adjustment for demographic characteristics, cardiovascular disease risk factors, and other potential confounders, the association was attenuated for all vascular disease subsets, but remained statistically significant. These results were similar when death was removed from the MACE endpoint (Table S6).
Discussion
In this cohort of veterans, the presence of MVD was associated with a significantly increased risk of lower extremity amputation. The increase in amputation risk among those with MVD was independent of the presence of PAD, augmented the risk when PAD was present, and remained robust after adjusting for demographics, cardiovascular risk factors, and other factors associated with vascular disease. In contrast, adjusting for similar covariates attenuated the association between MVD and MACE and MVD did not augment the MACE risk when added to PAD.
In this cohort, we show that MVD is common and strongly associated with adverse limb events in a manner that is potentiated in participants with concomitant PAD. This suggests that MVD plays an important and independent role in creating conditions that place patients at risk for amputation. In a large series of patients with CLI undergoing revascularization, 21% did not have evidence of reduced perfusion pressure12; numbers similar to our population. MVD may play an important role in determining the PAD population at particularly high-risk for CLI and amputation in addition to suggesting the need for active foot surveillance in patients with MVD alone. In our cohort, more than 40% of all amputations occurred in the group with both PAD and MVD despite representing about 4% of the population. The novelty of these findings becomes clear when put into the current framework of critical limb ischemia. In a recent state of the art review of CLI, MVD as a whole or its components did not receive a single mention13. Our work shows that MVD helps identify a population not previously considered at particularly high risk for amputation and, when added to PAD, identify a group of patients at very high risk for amputation. One take-home message from this work is that MVD alone is associated with 18% of all amputations and 15% of all below-knee amputations implicating MVD as an important risk for amputation. The results of this work provide additional avenues of inquiry to reduce rates of amputation.
In contrast to the strong association between MVD and amputation, we describe a more modest relationship for myocardial infarction, coronary revascularization, and death. Our findings are similar to those previously reported and provides a positive control for our definition, links our definition for MVD to those previously reported, and helps validate our definition for MVD7, 14.
MVD may modify symptomatic status importantly in patients with atherosclerosis more generally15. For example, coronary microvascular dysfunction is a stronger predictor of symptom severity than measured stenosis in patients with CAD16–19 and well associates with functional capacity even in patients without CAD20. When measured in more than 900 patients, coronary flow reserve predicted cardiovascular death and heart failure, but not myocardial infarction21. MVD may represent an important mediator of symptoms in both CAD and PAD and a target for new therapies.
We examined the impact of MVD in subpopulations free of conditions known to cause MVD. Notably, the risk of amputation was similar to the cohort as a whole in participants who never smoked, were free of diabetes, free of renal disease, and free of hypertension. These results suggest the risk of amputation associates with MVD, per se. Indeed, the organizing principle may be that symptoms are dependent on MVD rather than the specific disease that may cause it22.
One important question in these findings is the role of diabetes. Our data demonstrate a similar increase in associated risk of amputation in the presence or absence of diabetes. Diabetes is an important contributor to the microvascular disease and PAD population burden and provides multiple mechanisms by which amputation may develop. Although we cannot exclude the possibility of patients with undiagnosed diabetes, the likelihood that undiagnosed diabetes contributes significantly to the findings in our nondiabetic population is modest. The validated definition of diabetes we use23 considers glucose measurements, antidiabetic agent use, and/or at least 1 inpatient and/or at least 2 outpatient ICD-9 codes for this diagnosis.
Interestingly, participants with diabetes represent the minority of patients who develop MVD in our cohort. This finding mirrors other work in MVD prevalence. In the Diabetes Prevention Program, approximately 8% of all participants with prediabetes had retinopathy, similar to the number of prediabetic participants with retinopathy in the Gutenberg Health Study in Germany24, 25. In the Prevention of Renal and Vascular Endstage Disease (PREVEND) study, 7% of the participants had evidence of microalbuminuria7. Of 3200 participants in PREVEND with urinary albumin excretion of >20 mg/L, 7% had diabetes, 21% had hypertension, and the 82% had neither. Diabetes remains an important contributor to MVD development but focusing on it alone may miss opportunities for amputation prevention present in a larger population at risk.
This work advances the notion that microvascular dysfunction may be a systemic phenomenon that leads to adverse clinical events akin to that described for conduit arteries26,27, 28. Dysfunction of the microvasculature in beds remote from clinical presentation has been similarly demonstrated. Both retinal arteriolar and skin arteriolar dysfunction directly correlate with albuminuria, in the presence or absence of diabetes29. The presence of retinopathy30 and nephropathy31, 32 have been shown to associate inversely with coronary flow reserve. With particular relevance to amputation, both retinopathy and nephropathy are associated with impaired skin microvessel function and lower extremity amputation33–35. We surmise that clinical evidence of microvascular disease diagnosed in any vascular bed increases the risk for dermal microvascular dysfunction, poor wound healing, and amputation. We believe that MVD is a systemic phenomenon. Viewed as such, our work extends the multiple previous observations of individual bed MVD and amputation36. We aggregated the three major MVD manifestations into a single phenotype, demonstrating the independent association of this combined MVD phenotype and amputation, and showed its additive effect to PAD in fostering adverse limb outcomes. Indeed, similar to atherosclerosis, the associated risk of amputation increases with the presence of symptoms in more than one microvascular bed raising the possibility of a similar polyvascular disease phenomenon.
There are some limitations of this work to be noted. First, the diagnostic codes used to identify both PAD and MVD do not find wide agreement in the literature. For PAD, we have used the criteria defined by Bali and colleagues9, 10. The criteria we selected for MVD are listed in the supplement. We would submit that missing MVD cases would likely be distributed evenly through the groups, bias the results toward the null, and should not significantly affect the findings of this study. A single, unifying MVD phenotype has not been studied before; however, our MVD definition associated with MACE events similar to other cohorts of MVD components supporting our contention that it is a valid marker. As our study population is a cohort of veterans, our results are most generalizable to a cohort of men with a relatively high burden of disease. Finally, as this is an observational cohort, we cannot exclude the possibility of residual or unmeasured confounding.
We conclude that, in the VACS cohort, the diagnosis of MVD alone increased the risk of amputation, further increased the risk in patients with PAD, and identified a greater population of patients than previously recognized at risk for amputation. MVD had a modest effect on MACE and did not increase the risk beyond the diagnosis of PAD. MVD likely participates importantly in the development of adverse limb events in PAD and suggests additional patient populations who may benefit from greater foot surveillance to minimize amputation.
Supplementary Material
Clinical Perspective.
What is New?
The presence of microvascular disease (MVD) increases the risk of amputation significantly in the absence of peripheral artery disease (PAD).
As many as one in six below-knee amputations may result from MVD without PAD.
MVD potentiates the amputation risk in persons with PAD to more than 20-fold compared to persons with neither PAD nor MVD.
What Are the Clinical Implications?
Patients with MVD, with or without diabetes mellitus, are at increased risk of amputation.
The combination of MVD and PAD identifies a population at particularly high risk for amputation.
Clinicians should routinely solicit clinical complaints and physical signs of lower extremity injury or lesion to ensure the addition of therapies to minimize amputation in patients with MVD and PAD, and, particularly, the combination of both.
Funding Sources:
This work was supported by grants AHA Strategically Focused Research Network in Vascular Disease 18SFRN33960373 (Drs Beckman, Freiberg, Wasserman, Wells, and Barnett), HL131977 (Dr. Beckman), and HL125032 and U01 AA020790 (Dr. Freiberg). SMD is supported by the U.S. Department of Veterans Affairs (IK2-CX001780). This publication does not represent the views of the Department of Veterans Affairs or the United States government.
Footnotes
Disclosure Statement:
Dr. Beckman reports consulting with Astra Zeneca, Bristol Myers Squibb, Amgen, Merck, Sanofi, Antidote Pharmaceutical, and Boehringer Ingelheim. He serves on the DSMC for Bayer and Novartis. No other authors have relationships to report.
References
- 1.Ankle Brachial Index C, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d’Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J and McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquino R, Johnnides C, Makaroun M, Whittle JC, Muluk VS, Kelley ME and Muluk SC. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962–970. [DOI] [PubMed] [Google Scholar]
- 3.Shishehbor MH and Reed GW. Personalized approach to revascularization of critical limb ischemia. Circulation Cardiovascular interventions. 2014;7:642–644. [DOI] [PubMed] [Google Scholar]
- 4.Beckman JA and Creager MA. Vascular Complications of Diabetes. Circ Res. 2016;118:1771–1785. [DOI] [PubMed] [Google Scholar]
- 5.Keenan JD, Fan AZ and Klein R. Retinopathy in nondiabetic persons with the metabolic syndrome: findings from the Third National Health and Nutrition Examination Survey. Am J Ophthalmol. 2009;147:934–44, 944 e1–2. [DOI] [PubMed] [Google Scholar]
- 6.Katon JG, Reiber GE and Nelson KM. Peripheral neuropathy defined by monofilament insensitivity and diabetes status: NHANES 1999–2004. Diabetes Care. 2013;36:1604–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE and Prevend Study G. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. [DOI] [PubMed] [Google Scholar]
- 8.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S and Justice AC. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44:S25–30. [DOI] [PubMed] [Google Scholar]
- 9.Bali V, Yermilov I, Coutts K and Legorreta AP. Novel screening metric for the identification of at-risk peripheral artery disease patients using administrative claims data. Vasc Med. 2016;21:33–40. [DOI] [PubMed] [Google Scholar]
- 10.Beckman JA, Duncan MS, Alcorn CW, So-Armah K, Butt AA, Goetz MB, Tindle HA, Sico JJ, Tracy RP, Justice AC and Freiberg MS. Association of Human Immunodeficiency Virus Infection and Risk of Peripheral Artery Disease. Circulation. 2018;138:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG and Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. [DOI] [PubMed] [Google Scholar]
- 12.Sukul D, Grey SF, Henke PK, Gurm HS and Grossman PM. Heterogeneity of Ankle-Brachial Indices in Patients Undergoing Revascularization for Critical Limb Ischemia. JACC Cardiovascular interventions. 2017;10:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farber A Chronic Limb-Threatening Ischemia. N Engl J Med. 2018;379:171–180. [DOI] [PubMed] [Google Scholar]
- 14.de Jong PE, Gansevoort RT and Bakker SJ. Macroalbuminuria and microalbuminuria: do both predict renal and cardiovascular events with similar strength? J Nephrol. 2007;20:375–380. [PubMed] [Google Scholar]
- 15.Kim DH, Grodstein F, Newman AB, Chaves PH, Odden MC, Klein R, Sarnak MJ, Patel KV and Lipsitz LA. Prognostic implications of microvascular and macrovascular abnormalities in older adults: cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2014;69:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulman DS, Lasorda D, Farah T, Soukas P, Reichek N and Joye JD. Correlations between coronary flow reserve measured with a Doppler guide wire and treadmill exercise testing. Am Heart J. 1997;134:99–104. [DOI] [PubMed] [Google Scholar]
- 17.Youn HJ, Park CS, Moon KW, Oh YS, Chung WS, Kim JH, Choi KB and Hong SJ. Relation between Duke treadmill score and coronary flow reserve using transesophageal Doppler echocardiography in patients with microvascular angina. Int J Cardiol. 2005;98:403–408. [DOI] [PubMed] [Google Scholar]
- 18.Kalayci S, Kalayci B, Sahan E and Boyaci AAA. Association between fractional flow reserve and Duke treadmill score in patients with single-vessel disease. Kardiol Pol. 2017;75:877–883. [DOI] [PubMed] [Google Scholar]
- 19.Blomster JI, Svedlund S, H UW and Gan LM. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int J Cardiol. 2016;217:161–166. [DOI] [PubMed] [Google Scholar]
- 20.Eroglu S, Sade LE, Polat E, Bozbas H and Muderrisoglu H. Association between coronary flow reserve and exercise capacity. Hellenic J Cardiol. 2015;56:201–207. [PubMed] [Google Scholar]
- 21.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R and Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B, Harrap S, Marre M, Chalmers J and Group AC. Microvascular and Macrovascular Disease and Risk for Major Peripheral Arterial Disease in Patients With Type 2 Diabetes. Diabetes Care. 2016;39:1796–803. [DOI] [PubMed] [Google Scholar]
- 23.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M and Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–119. [DOI] [PubMed] [Google Scholar]
- 24.Lamparter J, Raum P, Pfeiffer N, Peto T, Hohn R, Elflein H, Wild P, Schulz A, Schneider A and Mirshahi A. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: the Gutenberg Health Study. J Diabetes Complications. 2014;28:482–487. [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program Research G. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson TJ, Gerhard MD, Meredith IT, Charbonneau F, Delagrange D, Creager MA, Selwyn AP and Ganz P. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71B–74B. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC and Creager MA. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78:1210–1214. [DOI] [PubMed] [Google Scholar]
- 28.Yataco AR, Corretti MC, Gardner AW, Womack CJ and Katzel LI. Endothelial reactivity and cardiac risk factors in older patients with peripheral arterial disease. Am J Cardiol. 1999;83:754–758. [DOI] [PubMed] [Google Scholar]
- 29.Martens RJH, Houben A, Kooman JP, Berendschot T, Dagnelie PC, van der Kallen CJH, Kroon AA, Leunissen KML, van der Sande FM, Schaper NC, Schouten J, Schram MT, Sep SJS, Sorensen BM, Henry RMA and Stehouwer CDA. Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. J Hypertens. 2018;36:1178–1187. [DOI] [PubMed] [Google Scholar]
- 30.Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S and Yoshikawa J. Retinopathy identifies marked restriction of coronary flow reserve in patients with diabetes mellitus. J Am Coll Cardiol. 1997;30:935–941. [DOI] [PubMed] [Google Scholar]
- 31.Lin C, Zhang P, Xue Y, Huang Y and Ji K. Link of renal microcirculatory dysfunction to increased coronary microcirculatory resistance in hypertensive patients. Cardiology journal. 2017;24:623–632. [DOI] [PubMed] [Google Scholar]
- 32.Potier L, Chequer R, Roussel R, Mohammedi K, Sismail S, Hartemann A, Amouyal C, Marre M, Le Guludec D and Hyafil F. Relationship between cardiac microvascular dysfunction measured with 82Rubidium-PET and albuminuria in patients with diabetes mellitus. Cardiovascular diabetology. 2018;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen TT, Shaw JE, Robinson C, Kawasaki R, Wang JJ, Kreis AJ and Wong TY. Diabetic retinopathy is related to both endothelium-dependent and -independent responses of skin microvascular flow. Diabetes Care. 2011;34:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss SE, Klein R and Klein BE. The 14-year incidence of lower-extremity amputations in a diabetic population. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1999;22:951–959. [DOI] [PubMed] [Google Scholar]
- 35.Schmiedel O, Schroeter ML and Harvey JN. Microalbuminuria in Type 2 diabetes indicates impaired microvascular vasomotion and perfusion. Am J Physiol Heart Circ Physiol. 2007;293:H3424–H3431. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BE, Moss SE and Wong TY. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 2007;114:1884–1892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.