Abstract
Objective:
Individuals with active major depressive disorder (MDD) have shown affective biases in cognitive flexibility and memory, particularly for negatively valenced stimuli. We evaluated whether impairments in affective flexibility would remain even during remission (rMDD), potentially representing trait- or scar-like effects of illness. Method: Participants completed the Emotion Card Sort Test (ECST), a measure of cognitive flexibility containing emotionally valenced stimuli, and the Emotion Word Stimulus Test (EWT), a measure of affective biases in delayed recall and recognition memory, and several self-report measures.
Results:
Healthy controls (HCs; n=35) and individuals with rMDD (n=93) did not differ on performance for any of the 3 word types on the ECST or EWT. However, individuals with rMDD demonstrated greater negative bias on EWT recognition trials relative to HCs (d=.36). On self-report measures, individuals with rMDD exhibited greater levels of neuroticism, problems with attentional control, pessimistic attributional style, and negative automatic thoughts compared to HCs.
Conclusions:
These results provide initial evidence that some performance, but not self-reported, indices of affective bias may improve during remission from MDD. Results of this study could suggest that some components of affective bias may represent state feature of illness and others trait-like risk or scar features.
Keywords: Depression, Affective Bias, Affective Flexibility, Cognitive Bias, Remitted Depression, Cognitive Style
Depression is a common, potentially debilitating, episodic mental health disorder. Beck’s (1967) cognitive theory of depression states that depression is associated with depressed individuals having negative cognitive structures (schemas) about the self, the world, and the future. According to Gotlib & Joormann (2010) “depression is characterized by increased elaboration of negative information, by difficulties disengaging from negative material, and by deficits in cognitive control when processing negative information.” Because these cognitive deficits are specific to negatively valenced stimuli, Gotlib and Joormann’s (2010) characterization fits with Beck’s cognitive theory of depressive schemas and suggests that individuals with MDD have a cognitive bias for negatively valenced information. Numerous studies have supported the presence of cognitive biases for negatively valenced information, as well as negative appraisals of neutrally valenced information (for a review see Mathews & MacLeod, 2005).
Beck suggests that following remission, negative schemas and downstream cognitive biases may remain as latent vulnerability factors (Beck, 1987; Beck & Bredemeier, 2016). Results have been less clear as to which negative cognitive biases persist in remission (Gotlib & Joormann, 2010; Mathews & MacLeod, 2005). NIMH’s Research Domain Criteria (RDoC; Insel et al., 2010) emphasizes the potential utility in examining interactions between cognitive domains (e.g., cognitive control and attention) and negative valence systems such as loss (Woody & Gibb, 2015) for understanding mechanisms that might underlie psychopathology. Depression in particular often is characterized by cognitive and attentional biases (Beck & Bredemeier, 2016), particularly those that are relevant to loss and attenuated responsiveness to reward (Langenecker, Jacobs, & Passarotti, 2014; Woody & Gibb, 2015). Although these biases often appear present during the active illness state, it is vital to explore which biases persist in remission of MDD (rMDD), as they may also predate the illness as vulnerability factors and could confer vulnerability to recurrence. Moreover, it would be ideal to have psychometrically sensitive measures of affective biases that are independent of self-report measures, which have significant, but still limited, predictive value.
In active MDD, negative memory bias is sometimes referred to as mood-congruent memory (MCM; Bower, 1981, 1987), and occurs when individuals selectively attend to stimuli that match their emotional state. There is also evidence that it is easier to access memories that are emotionally congruent. Actively depressed individuals tend to recall more negatively valenced information when compared to healthy controls (HCs) (Hamilton & Gotlib, 2008). Furthermore, people with active MDD (Hsu et al., 2010; Ridout et al., 2009) and rMDD (Alloy et al., 1997; Romero et al., 2014) have demonstrated stronger recall and recognition performance for negatively valenced information compared to positively valenced information.
Individuals with active MDD also display impairments on measures of executive functioning (EF) (Langenecker et al., 2005; Xu et al., 2012; Trivedi & Greer, 2014; Cotrena et al., 2016), which interfere with cognitive and behavioral flexibility (Stange, Alloy, & Fresco, 2017). People with MDD may have particular difficulty disengaging from negative aspects of stimuli (Gotlib & Joorman, 2010), perhaps due to negative attentional biases or difficulty with filtering out negative stimuli (Joorman & Gotlib, 2008; Beblo et al., 2010). For example, individuals with MDD experience significantly greater negative interference on an emotional Stroop test relative to HCs (Dai & Feng, 2011). Numerous studies have found the Wisconsin Card Sorting Test (WCST), a measure of executive control and cognitive flexibility, to be sensitive to active depression (Davis & Nolen-Hoeksema, 2000; Cotrena et al., 2016), and others have demonstrated that impairments on the WCST tend to improve when depression remits (Biringer et al., 2005; Nakano et al., 2008).
Other work has suggested that impairments in cognitive flexibility in MDD may be particularly pronounced in the contexts of negative affective information (e.g., Murphy et al., 2012). For example, in a modified version of the WCST called the Emotion Card Sort Test (ECST), people with MDD made more perseverative errors (PEs) when sorting negatively valenced words compared to positively or neutrally valenced words (Deveney & Deldin, 2006). Thus, individuals with active MDD show deficits in set shifting compared to healthy individuals, particularly for negatively valenced stimuli, perhaps due to executive functioning deficits in conjunction with a negative affective bias, or interfering affective stimuli. However, little work has examined the state-independence of these deficits in affective set shifting, such as whether they would be apparent during the remitted state of depression (Hasselbalch et al., 2011, 2012; Stange et al., 2017). Specifically, the ECST has never been administered to an rMDD sample. If negative affective bias on the ECST persists in remission, this might suggest its value as a trait-like vulnerability marker for depression (e.g., Stange et al., 2016), or a scar of experiencing depression in the past (Just, Abramson, & Alloy, 2001). This study included the ECST to determine whether affective set shifting difficulties found by Deveney & Deldin (2006) persist in rMDD.
This study aims to examine which negative affective biases persist outside of active depressive episodes by examining performance on the ECST and EWT among a sample of individuals with remitted major depressive disorder (rMDD) and healthy control (HC) participants. Studying individuals in the remitted state of illness may allow for the detection of stable, trait-like effects of illness, which should persist outside of the context of an active episode of depression; in contrast, state-like effects, such as mood and affect, may not persist once individuals remit. In addition to examining behavioral measures of negative affective bias, we also examined whether rMDD and HC groups would differ in various self-reported indices of negative cognitive and affective functioning that are also associated with active illness, such as neuroticism, rumination, negative automatic thoughts, and attentional control (Costa & McCrae, 1992; Treynor, et al., 2003; Nolen-Hoeksema, 1991; Derryberry & Reed, 2002). Examining multiple behavioral and self-report measures in rMDD is important given that prior studies often have examined tasks independent of one another. Thus, it is unclear whether mixed findings in the literature are due to differences in measurement or sample characteristics between studies, or to affective biases being stronger in certain domains of cognitive functioning. The present study aims to address these limitations by comparing individuals with rMDD and HCs across multiple behavioral and self-reported domains of cognitive-affective functioning. We hypothesize that individuals with rMDD will display a bias for negatively valenced stimuli on the ECST and EWT relative to neutrally or positively valenced stimuli.
Method
Participants and Procedures
Participants were recruited using flyers, transportation and social media ads in a major Midwestern city near a state university. The rMDD group comprised 93 individuals with a history of MDD who were in remission at the time of the study, as defined by DSM-IV-TR criteria. We recruited an rMDD sample that was primarily euthymic; however, consistent with RDoC (Insel et al., 2010), we allowed individuals into the study even if they were not fully asymptomatic. The HC group comprised 35 individuals who did not meet current or past criteria for MDD or any other Axis I psychiatric disorder. HCs who had a history of psychiatric disorder in a first degree relative were also excluded. Participants were between 18 and 30 years of age (69% Female). Participants were required to either be taking no psychiatric medications or to have had no medication changes for 30 days prior to the assessment. Twelve rMDD participants reported taking antidepressant medication. Individuals with substance abuse or dependence within the past six months were excluded. Those with a history of bipolar disorder or psychosis were excluded. Participant demographics and clinical characteristics are presented in Table 1. Written informed consent was obtained according to the guidelines of the Institutional Review Board of UIC and consistent with the Declaration of Helsinki. Participants completed a series of interviews and neuropsychological tasks in the laboratory.
Table 1.
Demographic comparisons between groups.
| HC (n = 35) | rMDD (n = 93) | |
|---|---|---|
| M (SD) / N (%) | M (SD) / N (%) | |
| Female | 57% | 73% |
| Age | 22.40 (3.08) | 23.17 (3.40) |
| Race | ||
| African American/Black | 5 (14%) | 21 (23%) |
| Asian or Indian | 10 (29%) | 7 (8%) |
| Caucasian/White | 12 (34%) | 46 (49%) |
| Latino(a) | 5 (14%) | 13 (14%) |
| More than One Race | 2 (6%) | 5 (5%) |
| Not Reported | 1 (3%) | 1 (1%) |
| Hispanic | 5 (9%) | 13 (14%) |
| HDRS* | 0.32 (0.59) | 3.63 (4.14) |
| Age at onset | n/a | 15.26 (4.09) |
| Number of depressive episodes | n/a | 4.93 (6.73) |
| Education | 14.91 (1.99) | 14.90 (1.96) |
| Estimated Verbal IQ | 105.06 (7.64) | 106.12 (10.32) |
| Brooding* | 7.07 (2.73) | 12.76 (4.25) |
| Neuroticism* | 68.02 (21.69) | 102.88 (28.20) |
| ACS Focusing* | 16.41 (3.95) | 22.05 (6.16) |
| ACS Shifting* | 20.06 (4.44) | 23.21 (5.40) |
| ATQ Negative* | 32.59 (10.45) | 58.15 (30.88) |
p < .05.
Note. HC, Healthy control; rMDD, remitted major depressive disorder; HDRS, Hamilton Rating Scale for Depression; ACS, Attentional Control Scale; ATQ, Automatic Thoughts Questionnaire.
Measures
Diagnostic interview.
Semistructured diagnostic interviews were conducted using either a dimensional version of the SCID-I/P (First et al., 2002), or the Diagnostic Interview for Genetic Studies (DIGS; Nurnberger et al., 1994), which assess symptoms related to mood, anxiety, eating, psychotic, and substance use disorders over the lifetime. Interviews to assess diagnoses were completed by intensively trained doctoral students in clinical psychology and postbaccalaureate research assistants, all of whom were supervised by a clinical psychologist with extensive experience in diagnosing mood disorders. Diagnosis of past MDD or HC was confirmed using a modified Family Interview for Genetic Studies (FIGS; Maxwell, 1992) completed with a parent, guardian, or close sibling. The SCID, DIGS, and FIGS have demonstrated good reliability and validity (First et al., 2002; Maxwell, 1992).
Emotion Card Sort Test.
The ECST (Deveney & Deldin, 2006) is a computerized measure of cognitive flexibility that was modeled after the Heaton version of the Wisconsin Card Sort Test (WCST; Heaton, 1981). Whereas the WCST cards display different shapes, the ECST displays emotionally valenced words on each card. Like the WCST, the ECST requires participants to match a target card to one of four key cards based on category (color, font, and number of times a word appears on a card). Sorting principle order was counterbalanced across participants. Participants receive feedback after each response. When ten correct consecutive matches are made, the target category shifts without warning and the participant receives feedback based on the new category rule. A maximum of 88 cards or six correctly completed categories are administered per round. The ECST is split into three rounds based on emotional valence (positive, negative, neutral). Presentation of each valence is counterbalanced to account for practice effects. Like the WCST, the ECST variables included: total categories completed, total errors, perseverative errors, and failures to maintain set. The primary variable of interest in this study was perseverative errors (PEs), which occurred when participants made an error that matched the first unambiguous error or a previously completed set. PEs were computed separately for each word valence (negative, neutral, and positive). Given that prior work has suggested the presence of negative affective bias in MDD (Koster et al., 2005; Peckham et al., 2010), we examine the degree to which negative affective biases were present, by computing a difference score between the numbers of PEs on negative versus neutral trials. The ECST was chosen because performance on the ECST has been shown to be sensitive to current mood state. A previous study suggests that participants with MDD tend to make more PEs when shown cards containing negatively valenced words and HCs tend to make more PEs when shown cards with positively valenced words (Deveney & Deldin, 2006). ECST data were available for 107 participants.
Emotion Word Stimulus Test.
The Emotion Word Stimulus Test (EWT; Hsu et al., 2010) is a test in which a total of 162 emotionally valenced (positive, negative, neutral) words are presented in the center of the screen, one at a time, for three seconds each. Each word is presented only once. A crosshair appeared in the center of the screen for one second between each word. Participants were instructed to say each word to themselves and press a key afterward. Following the presentation of all of the words, there is a surprise delayed free recall and cued yes/no recognition component. Scores are obtained by performance on recall and recognition components for each valence. Prior work has suggested that individuals with active MDD have greater negative recognition memory bias (more negative than neutral words recognized; Hsu et al., 2010); in the present study, we investigated the degree to which negative recognition and recall bias would characterize remitted MDD.
Self-Report Measures.
For comparison with objectively measured indices of bias, a battery of self-report measures was administered to measure individuals’ perceptions of their own biases. Participants completed the Automatic Thoughts Questionnaire (ATQ; Hollon & Kendall, 1980), which measures automatic thoughts that occur in individuals with depression and was shown to strongly correlate with therapists’ ratings of depression, the Beck Depression Inventory-II (BDI-II; Beck et al., 1996), and the Depression scale of the Minnesota Multiphasic Personality Inventory-2 (MMPI-2; Harrell & Ryon, 1983); internal consistency was excellent (α = .98). Rumination was measured using the brooding subscale from the Ruminative Response Scale (RRS; Treynor et al., 2003; Nolen-Hoeksema, 1991), which is the component of rumination that is most reliably associated with risk for depression. The RRS was included because previous studies have shown performance on the WCST (the model on which the ECST was based) to be associated with both depressed mood (Martin et al., 1991) and presence of rumination (Davis & Nolen-Hoeksema, 2000); internal consistency was excellent (α = .89). The Neuroticism trait from the NEO PI-R (Costa & McCrae, 1992) was included, as high neuroticism correlates with vulnerability to depression (Widiger & Trull, 1992) and negative affective bias (Rusting, 1998; Robinson et al., 2006); internal consistency was excellent (α = .94). Subjects also completed the Attentional Control Scale (Derryberry & Reed, 2002), a validated measure of problems with attentional control that signal negative attentional bias (Derryberry & Reed, 2002; Judah et al., 2014); internal consistency was good for the focusing and shifting subscales (αs = .86 and .79, respectively).
Current Symptoms of Depression.
Symptoms of depression were measured using the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), a seventeen-item observational measure that was administered by trained interviewers at baseline. The HDRS is one of the most widely used measures of depression and was chosen because it has been demonstrated to be sensitive to sub-threshold depressive symptoms (Judd et al., 2000; Zimmerman et al., 2013). Detection of sub-threshold symptoms and modest residual symptoms is especially useful in a study examining individuals with rMDD.
Statistical Analyses
2 group (HC, rMDD) x 3 condition (negative, neutral, positive) ANOVAs were conducted for each of the primary outcome variables: (1) ECST PEs; (2) EWT recall; and (3) EWT recognition. Given evidence of negative affective biases in depression, we also planned a priori to examine group differences in negative affective biases (negative minus neutral) for each of these three outcome variables using one-tailed t-tests. Self-report measures of negative affective bias were tested using independent samples t-tests.
Results
Demographic and descriptive statistics are displayed separately by group in Table 1.
Emotion Card Sort Test
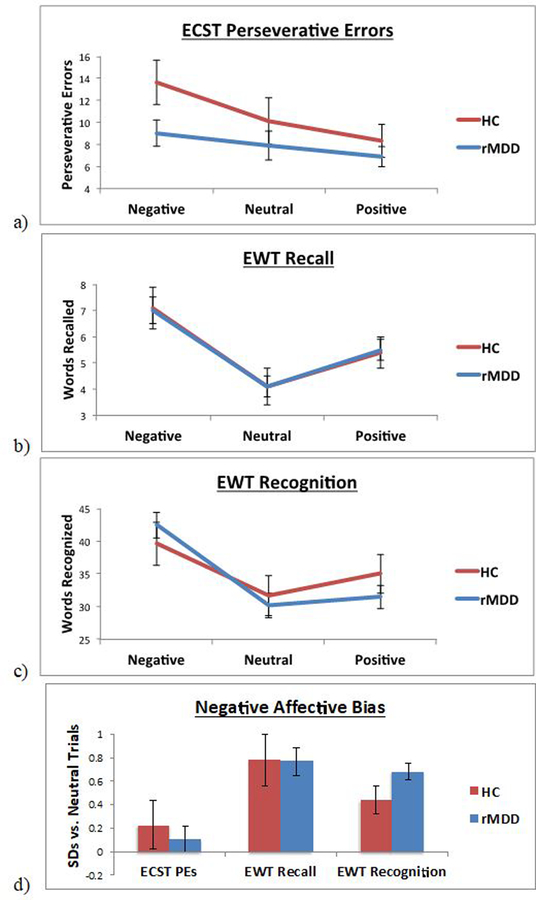
On the ECST, a 2 group (HC, rMDD) x 3 condition (negative, neutral, positive) ANOVA yielded a significant main effect of condition (F(2, 210) = 5.80, p = .004; ηp2 = .05; Figure 1a). Post-hoc pairwise contrasts across groups indicated that all individuals exhibited more PEs for negative relative to positive stimuli (p = .001); PEs did not differ between negative and neutral stimuli (p = .06), or between neutral and positive stimuli (p = .19). However, the main effect of group was not significant (F(1, 105) = 2.31, p = .13, ηp2 = .02), nor was the group x condition interaction significant (F(2, 210) = 1.06, p = .35, ηp2 = .01).
Figure 1.
Performance on Emotional Word Task (EWT) and Emotion Card Sort Test (ECST) based on valence and group, for (a) EWT recall trials, (b) EWT recognition trials, and (c) ECST perseverative errors; and (d) negative affective bias (negative trials minus neutral trials, in Z-standardized units based on neutral trials scores) on each task.
The ECST PE index of negative affective bias (PEs for negative minus neutral words) did not differ between rMDD and HC groups (HC: M = 2.52, SD = 12.64; rMDD: M = 1.15, SD = 10.54; t = −0.53, p = .60, d = −0.12; Figure 1d). Among our rMDD participants, HDRS scores were not significantly correlated with our index of ECST PE negative affective bias (ρ = −.158, p = .165).
Emotion Word Stimulus Test
For recall on the EWT, the main effect of group was not significant (F(1, 121) = 0.001, p = .98; ηp2 < .01). There was a significant main effect of condition (F(2, 242) = 21.64, p < .001; ηp2 = .15; Figure 1b), such that across groups, individuals recalled more negative words than neutral (p < .001) or positive (p = .001) words, and more neutral words than positive words (p = .002). However, the group x condition interaction was not significant (F(2, 242) = 0.02, p = .98; ηp2 < .01).
For recognition, there was a similar significant main effect of condition (F(2, 242) = 48.84, p < .001; ηp2 = .29), such that across groups, individuals recognized more negative words than neutral (p < .001) or positive (p < .001) words; however, individuals recognized more positive words than neutral words (p = .02). The main effect of group was not significant (F(1, 121) = 0.04, p = .85; ηp2 < .01). The group x condition interaction was significant (F(2, 242) = 4.65, p = .01; ηp2 = .04; Figure 1c); however, post-hoc tests indicated that HC and rMDD did not differ on performance for any of the 3 word types (negative: p = .44; neutral: p = .69; positive: p = .32).
The EWT index of negative affective bias (Figure 1d) did not differ between rMDD and HC groups for recall (HC: M = 2.95, SD = 4.87; rMDD: M = 2.91, SD = 4.23; t = −0.05, p = .48, d = −0.01). However, groups did differ on negative affective bias for recognition, such that as hypothesized, individuals with rMDD demonstrated greater bias (relatively more words recognized for negative than for neutral words) than HCs (HC: M = 7.96, SD = 12.15; rMDD: M = 12.35, SD = 12.43; t = 1.76, p = .04, d = 0.36). Among our rMDD participants, HDRS scores were not significantly correlated with our indices of negative affective recall bias (ρ = .033, p = .765) or recognition bias (ρ = −.042, p = .706).
Self-Report Measures
Individuals with rMDD exhibited higher levels of negative affective bias than HCs on each of the self-report measures (Table 1), including brooding, neuroticism, focusing and shifting components of attentional control, and negative automatic thoughts.1,2
Discussion
Major depressive disorder is a common, recurrent, and potentially debilitating condition with well-documented impairments in cognitive performance and biases for negative material in the active phase of illness. Because MDD tends to remit and recur, it is important to establish whether or not and which of these impairments and negative affective biases persist during remission. The results of this study suggest that individuals with rMDD displayed a greater negative affective memory bias than HCs when recognizing emotionally valenced words, suggesting a negative memory bias. However, individuals with rMDD performed comparably to HCs on most other performance measures of cognitive affective bias, including recall of affectively valenced stimuli and on a card sorting task involving emotionally valenced words. In contrast, individuals with rMDD self-reported elevated levels of negative affective bias, including neuroticism, rumination, negative automatic thoughts, and greater self-reported problems with attentional control. These data provide evidence that some of the behavioral indices of negative affective bias improve following remission, although individuals continue to perceive their negative cognitive styles and attentional functioning as being problematic during remission.
On the EWT, overall response patterns did not differ across groups for recall and recognition. However, individuals with rMDD displayed greater negative affective bias (more negative words than neutral words) on recognition trials relative to HCs. This performance marker of negative memory bias appears to persist in remission of MDD, consistent with our hypothesis that negative affective bias may be a trait-like index of risk for MDD. These results are consistent with a prior study utilizing the EWT that found evidence for a negative recognition bias in individuals with MDD relative to HCs, but no differences in negative recall bias (Hsu et al., 2010). In that study, negative memory bias was related to negative life events. In our study, negative recognition bias was not correlated with any of our self-report measures. EWT recognition appears to be more sensitive than free recall in detection of negative memory biases, though reasons for these results are not clear and require further exploration. However, given the lack of a significant group x condition interaction for either recall or recognition, the evidence for overall negative affective bias being trait-like, as opposed to improving with remission, is mixed and will require replication.
On the ECST, performance by individuals with rMDD did not differ from HCs. There were no significant group differences on PEs, regardless of valence. Individuals with rMDD also did not display a negative affective bias (performance on negative relative to neutral trials). Our results contrast with a prior study conducted in individuals with current MDD (Deveney & Deldin, 2006). In that study, individuals with current MDD made more perseverative errors on negative words, specifically. The discrepancy between these findings may suggest that cognitive flexibility for emotional information is affected by current symptomatology and that biases may fade when depression remits. Alternatively, the lack of group differences may be due to a descriptively higher number of perseverative errors among the HC group, which contrasts with prior work on non-emotional card sorting tasks (Davis & Nolen-Hoeksema, 2000; Deveney & Deldin, 2006; Cotrena et al., 2016). Speculatively, a possible explanation is that HCs in the current sample may have exerted less effort compared to our rMDD sample and therefore, the lack of group differences is not due to a true absence of cognitive deficits in the rMDD sample.
Compared to HCs, individuals with rMDD self-reported greater levels of affective bias on measures of brooding, neuroticism, and negative automatic thoughts, as well as focus and shifting domains of attentional control. These results are interesting given the lack of significant difference between groups on most performance measures. However, these results are consistent with those of Joormann and Gotlib (2007) who found evidence of negative attentional biases in euthymic subjects with rMDD. Our results are also consistent with those of Harmer et al. (2009) who examined cognitive biases in individuals with active MDD. The authors found that it is possible to reduce negative cognitive biases independent of a reduction in depressed mood. Following acute administration of reboxetine, individuals with MDD exhibited reductions in negative cognitive biases without alteration of depressed mood (see Harmer, 2008). A possible explanation could be that depressed mood may remit over time as a bottom-up consequence of reduction of negative cognitive biases (e.g. Harmer, 2008). Another potential explanation could be that domains measured by our self-report instruments are more trait-like and may persist in remission. This may be supported by previous reports that individuals with active MDD tend to self-report poorer memory performance than is evidenced by neuropsychological testing (Beblo et al., 2010). Self-report measures generally ask individuals to report on how they typically respond outside of the lab in real-life situations in which affective cues may be particularly salient or personally-relevant, and thus may be particularly likely to detect individual differences relevant to depression, such as those relevant to the negative self-schema theorized by Beck (1967), which are theorized to persist in remission. In contrast, laboratory-based tasks are standardized and the affective cues used may be less relevant to individuals’ personal negative self-schemas (decreased salience), and therefore may be less likely to be able to detect negative affective biases that might persist in remitted depression. Additionally, although the self-report questionnaires and behavioral measures were broadly relevant to negative affective bias, a more parsimonious explanation may be that the questionnaires were assessing somewhat separate constructs, such as those that require participants’ own awareness of negative thinking styles, which could be more trait-like in nature. Such self-report questions are posed about the self, perceptions of the self, and self in interactions with others, rendering them more salient than self-referential negative thoughts (Siegel et al., 2002). Longitudinal studies will be needed to determine how individuals’ responses may change from periods before the onset of depression, to a current episode of MDD, to during the remitted state. These designs would allow for the measurement and distinction of vulnerability markers (e.g., Stange et al., 2016) from scar effects (Just, Abramson, & Alloy, 2001).
Strengths of this study included its sizeable sample of rMDD participants, restricted age range, and its inclusion of two performance-based measures of affective bias, one in an executive function task and the other in a word memory test. However, the study was limited by a comparatively small group of HCs; thus, group differences should be replicated before stronger claims can be made. The study also lacked an active MDD sample, which required our interpretation of the results to be based on reports from prior studies instead of an active MDD sample. An additional limitation of this study is its cross-sectional design. This further highlights the need for longitudinal studies to examine the state- or trait-like nature of these negative affective biases in greater detail. Finally, this study did not systematically examine effects of antidepressant medication on negative cognitive biases using a longitudinal design. Prior studies have examined the effects of antidepressant medication on cognitive biases in healthy controls (e.g. Harmer et al., 2004) and individuals with active MDD (e.g. Fu et al., 2004; Fu et al., 2007; Harmer et al., 2009), but more research is needed to explore their utility in individuals with rMDD.
In conclusion, results from the present study provide evidence that some performance-based measures of negative affective bias may improve when individuals remit from depression, whereas individuals’ perceptions of their own negative affective and cognitive functioning may continue to be seen as problematic during remission. Given that impaired performance on several of these measures appears to persist during remission, these impairments might serve as mechanisms of risk for future relapse (e.g., Nolen-Hoeksema, 2000), a possibility that can be examined in future work. To the extent that these factors confer risk for future depressive episodes, future work might examine the malleability of these factors and how they may best be targeted in the context of treatments that aim to reduce these negative affective biases.
Practitioner Points:
This study suggests that self-reported affective biases may persist in remission of Major Depressive Disorder (rMDD).
Affective attentional biases and affective memory biases were not demonstrated in individuals with rMDD, with the exception of a bias for recognizing negatively versus neutrally valenced stimuli.
Cautions or Limitations:
A limitation of this study was its cross-sectional design. Under ideal conditions, the same individuals would be studied in both the active and remitted phases of illness.
Another limitation of this study was the smaller number of healthy controls relative to individuals with rMDD.
Acknowledgements:
This work was supported by NIMH grant MH 101487 (SAL), and UIC Clinical and Translational Science Awards Program NCATS UL1TR000050 and 1S10RR028898. Jonathan P. Stange was supported by grants 5T32MH067631-12 and 1K23MH112769-01A1 from NIMH.
Footnotes
Group differences on behavioral and self-report measures were consistent when controlling for scores on the HDRS, with the exception of the shifting component of attentional control (ACS Shifting), which was reduced to marginal significance (F=3.68, p=.058). Supplemental analyses also examined differences between individuals who were taking antidepressants at the time of the study and those who were not. Individuals who were taking antidepressants did not differ from those who were not on any study measures or demographic characteristics.
Applying false discovery rate correction (Benjamini & Hochberg, 1995) to account for multiple testing for the negative affective bias analyses yielded a corrected significance level of α = .033. Using this more conservative threshold, group comparisons retained significance for self-report measures, but not for negative affective bias for EWT recognition (p = .04).
References
- Alloy LB, Abramson LY, Murray LA, Whitehouse WG, & Hogan ME (1997). Self-referent information-processing in individuals at high and low cognitive risk for depression. Cognition & Emotion, 11(5–6), 539–568. DOI: 10.1080/026999397379854a. [DOI] [Google Scholar]
- Beblo T, Mensebach C, Wingenfeld K, Schlosser N, Rullkoetter N, Schaffrath C, & Driessen M (2010). The impact of neutral and emotionally negative distraction on memory performance and its relation to memory complaints in major depression. Psychiatry Research, 178(1), 106–111. DOI: 10.1016/j.psychres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Beck AT (1967). Depression: Clinical, Experimental, and Theoretical Aspects University of Pennsylvania Press; DOI: 10.1001/archpsyc.1968.01740120126024 [DOI] [Google Scholar]
- Beck AT (1987). Cognitive model of depression. Journal of Cognitive Psychotherapy, 1, 2–27. DOI: 10.1037/0021-843X.96.3.179 [DOI] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck Depression Inventory-II San Antonio, 78(2), 490–498. [Google Scholar]
- Beck AT, & Bredemeier K (2016). A unified model of depression: Integrating clinical, cognitive, biological, and evolutionary perspectives. Clinical Psychological Science, 4(4), 596–619. DOI: 10.1177/2167702616628523 [DOI] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. DOI: 10.2307/2346101. [DOI] [Google Scholar]
- Biringer E, Lundervold A, Stordal K, Mykletun A, Egeland J, Bottlender R, & Lund A (2005). Executive function improvement upon remission of recurrent unipolar depression. European archives of psychiatry and clinical neuroscience, 255(6), 373–380. DOI: 10.1007/s00406-005-0577-7 [DOI] [PubMed] [Google Scholar]
- Bower GH (1981). Mood and memory. American psychologist, 36(2), 129 DOI: 10.1037/0003-066X.36.2.129 [DOI] [PubMed] [Google Scholar]
- Bower GH (1987). Commentary on mood and memory. Behaviour Research and Therapy, 25(6), 443–455. DOI: 10.1016/0005-7967(87)90052-0 [DOI] [PubMed] [Google Scholar]
- Costa PT, & McCrae RR (1992). Neo PI-R Professional Manual Odessa, FL: Psychological Assessment Resources, 396, 653–65. [Google Scholar]
- Cotrena C, Branco LD, Shansis FM, & Fonseca RP (2016). Executive function impairments in depression and bipolar disorder: association with functional impairment and quality of life. Journal of Affective Disorders, 190, 744–753. DOI: 10.1016/j.jad.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Davis RN, & Nolen-Hoeksema S (2000). Cognitive inflexibility among ruminators and nonruminators. Cognitive Therapy and Research, 24(6), 699–711. DOI: 10.1023/A:1005591412406 [DOI] [Google Scholar]
- Dai Q, & Feng Z (2011). Deficient interference inhibition for negative stimuli in depression: an event-related potential study. Clinical Neurophysiology, 122(1), 52–61. DOI: 10.1016/j.clinph.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Derryberry D, & Reed MA (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111(2), 225–236. [DOI] [PubMed] [Google Scholar]
- Deveney CM, & Deldin PJ (2006). A preliminary investigation of cognitive flexibility for emotional information in major depressive disorder and non-psychiatric controls. Emotion, 6(3), 429–437. DOI: 10.1037/1528-3542.6.3.429 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. SCID-I/P
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, & Bullmore ET (2004). Attenuation of the neural response to sad faces in major depression by antidepressant treatment: A prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry, 61(9), 877–889. DOI: 10.1001/archpsyc.61.9.877 [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, Mitterschiffthaler MT, Andrew CM, Pich EM, & Bullmore ET (2007). Neural responses to happy facial expressions in major depression following antidepressant treatment. American Journal of Psychiatry, 164(4), 599–607. DOI: 10.1176/ajp.2007.164.4.599 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. DOI: 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, & Gotlib IH (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry, 63(12), 1155–1162. DOI: 10.1016/j.biopsych.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Shelley NC, Cowen PJ, & Goodwin GM (2004). Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. American Journal of Psychiatry, 161(7), 1256–1263. DOI: 10.1176/appi.ajp.161.7.1256 [DOI] [PubMed] [Google Scholar]
- Harmer CJ (2008). Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology, 55(6), 1023–1028. DOI: 10.1016/j.neuropharm.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, & Cowen PJ (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. American Journal of Psychiatry, 166(10), 1178–1184. DOI: 10.1176/appi.ajp.2009.09020149 [DOI] [PubMed] [Google Scholar]
- Harrell TH, & Ryon NB (1983). Cognitive-behavioral assessment of depression: Clinical validation of the Automatic Thoughts Questionnaire. Journal of Consulting and Clinical Psychology, 51(5), 721 DOI: 10.1037/0022-006X.51.5.721 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, & Kessing LV (2012). Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology, 26, 642–651. DOI: 10.1016/j.jad.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, & Kessing LV (2011). Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. Journal of Affective Disorders, 134(1), 20–31. DOI: 10.1016/j.jad.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Heaton RK (1981). Manual for the Wisconsin Card Sorting Test Odessa. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Hollon SD, & Kendall PC (1980). Cognitive self-statements in depression: Development of an automatic thoughts questionnaire. Cognitive Therapy and Research, 4(4), 383–395. [Google Scholar]
- Hsu DT, Langenecker SA, Kennedy SE, Zubieta JK, & Heitzeg MM (2010). fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Research: Neuroimaging, 183(3), 202–208. DOI: 10.1016/j.pscychresns.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, & Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. DOI: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2007). Selective attention to emotional faces following recovery from depression. Journal of abnormal psychology, 116(1), 80–85. DOI: 10.1037/0021-843X.116.1.80 [DOI] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2008). Updating the contents of working memory in depression: interference from irrelevant negative material. Journal of Abnormal Psychology, 117(1), 182–192. DOI: 10.1037/0021-843X.117.1.182 [DOI] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Mills AC, & Lechner WV (2014). Factor structure and validation of the attentional control scale. Cognition & Emotion, 28(3), 433–451. DOI: 10.1080/02699931.2013.835254 [DOI] [PubMed] [Google Scholar]
- Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Maser JD, Mueller T, Solomon DA, & Keller MB (2000). Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? American Journal of Psychiatry, 157(9), 1501–1504. DOI: 10.1176/appi.ajp.157.9.1501 [DOI] [PubMed] [Google Scholar]
- Just N, Abramson LY, & Alloy LB (2001). Remitted depression studies as tests of the cognitive vulnerability hypotheses of depression onset: A critique and conceptual analysis. Clinical Psychology Review, 21(1), 63–83. [DOI] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, & De Raedt R (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31(1), 138–145. DOI: 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Koster EH, De Raedt R, Goeleven E, Franck E, & Crombez G (2005). Mood-congruent attentional bias in dysphoria: maintained attention to and impaired disengagement from negative information. Emotion, 5(4), 446–455. DOI: 10.1037/1528-3542.5.4.446 [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, & Berent S (2005). Face emotion perception and executive functioning deficits in depression. Journal of Clinical and Experimental Neuropsychology, 27(3), 320–333. DOI: 10.1080/13803390490490515720 [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs RH, & Passarotti AM (2014). Current neural and behavioral dimensional constructs across mood disorders. Current Behavioral Neuroscience Reports, 1(3), 144–153. DOI: 10.1007/s40473-014-0018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DJ, Oren Z, & Boone K (1991). Major depressives’ and dysthymics’ performance on the Wisconsin Card Sorting Test. Journal of Clinical Psychology, 47(5), 684–690. [DOI] [PubMed] [Google Scholar]
- Mathews A, & MacLeod C (2005). Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol, 1, 167–195. DOI: 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- Maxwell ME (1992). Family Interview for Genetic Studies (FIGS): a manual for FIGS Bethesda, MD: Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health. [Google Scholar]
- Murphy FC, Michael A, & Sahakian BJ (2012). Emotion modulates cognitive flexibility in patients with major depression. Psychological Medicine, 42(7), 1373–1382. DOI: 10.1017/S0033291711002418 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, Suzuki T, Mimura M, & Arai H (2008). Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders, 111(1), 46–51. DOI: 10.1016/j.jad.2008.01.027 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, & Reich T (1994). Diagnostic interview for genetic studies: rationale, unique features, and training. Archives of General Psychiatry, 51(11), 849–859. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, & Otto MW (2010). A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety, 27(12), 1135–1142. DOI: 10.1002/da.20755 [DOI] [PubMed] [Google Scholar]
- Ridout N, Dritschel B, Matthews K, McVicar M, Reid IC, & O’Carroll RE (2009). Memory for emotional faces in major depression following judgment of physical facial characteristics at encoding. Cognition and Emotion, 23(4), 739–752. DOI: 10.1080/02699930802121137 [DOI] [Google Scholar]
- Robinson MD, Wilkowski BM, Kirkeby BS, & Meier BP (2006). Stuck in a rut: Perseverative response tendencies and the neuroticism-distress relationship. Journal of Experimental Psychology: General, 135(1), 78–91. DOI: 10.1037/0096-3445.135.1.78 [DOI] [PubMed] [Google Scholar]
- Romero N, Sanchez A, & Vazquez C (2014). Memory biases in remitted depression: The role of negative cognitions at explicit and automatic processing levels. Journal of behavior therapy and experimental psychiatry, 45(1), 128–135. DOI: 10.1016/j.jbtep.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Rusting CL (1998). Personality, mood, and cognitive processing of emotional information: three conceptual frameworks. Psychological Bulletin, 124(2), 165. [DOI] [PubMed] [Google Scholar]
- Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY, & Alloy LB (2016). Inflexible cognition predicts first onset of major depressive episodes in adolescence. Depression and Anxiety, 33(11), 1005–1012. DOI: 10.1002/da.22513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Alloy LB, & Fresco DM (2017). Inflexibility as a vulnerability to depression: A systematic qualitative review. Clinical Psychology: Science and Practice, 24(3), 245–276. DOI: 10.1002/da.22513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. DOI: 10.1023/A:1023910315561 [DOI] [Google Scholar]
- Trivedi MH, & Greer TL (2014). Cognitive dysfunction in unipolar depression: implications for treatment. Journal of Affective Disorders, 152, 19–27. DOI: 10.1016/j.jad.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Woody ML, & Gibb BE (2015). Integrating NIMH research domain criteria (RDoC) into depression research. Current Opinion in Psychology, 4, 6–12. DOI: 10.1016/j.copsyc.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA, & Trull TJ (1992). Personality and psychopathology: an application of the Five‐Factor Model. Journal of Personality, 60(2), 363–393. [DOI] [PubMed] [Google Scholar]
- Xu G, Lin K, Rao D, Dang Y, Ouyang H, Guo Y, Jinxiang M, & Chen J (2012). Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. Journal of Affective Disorders, 136(3), 328–339. DOI: 10.1016/j.jad.2011.11.029 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, & Dalrymple K (2013). Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders, 150(2), 384–388. DOI: 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]