Abstract
BACKGROUND AND AIMS:
To compare the utility of the distensibility index (DI) on functional lumen imaging probe (FLIP) topography to other esophagogastric junction (EGJ) metrics in assessing treatment response in achalasia in the context of esophageal anatomy.
METHODS:
We prospectively evaluated 79 patients (ages 17-81, 47% female) with achalasia during follow-up after pneumatic dilation, heller myotomy, or per-oral endoscopic myotomy with timed barium esophagram (TBE), high resolution manometry (HRIM) and FLIP. Anatomic deformities were identified based on consensus expert opinion. Patients were classified based on anatomy and EGJ opening to determine association with radiographic outcome and Eckardt score (ES).
RESULTS:
Twenty-seven patients (34.1%) had an anatomic deformity – 10 pseudodiverticula at myotomy, 7 epiphrenic diverticula, 5 sigmoid, and 5 sinktrap. A 5-min column area of >5 cm2 was best associated with an ES>3, with a sensitivity of 84% (p=.0013). Area-under-the curve for EGJ metrics in association with retention were as follows: DI-.90, MxEGJD-.76, IRP-.64, EGJP-.53. Only FLIP metrics were associated with retention given normal anatomy (DI 2.4 vs 5.2 mm2/mmHg, and MxEGJD 13.1 vs 16.6 mm in patients with vs without retention, p values <.0001 and .002). Using a DI cutoff of <2.8 as abnormal, 40/45 patients with retention (p=.0001) and 23/25 patients with an ES>3 (p=.02) had a combination of a low DI and/or anatomic deformity. With normal anatomy, 21/22 patients with retention had a low or borderline low DI.
CONCLUSIONS:
The FLIP DI is most useful metric for assessing the effect of achalasia treatment on EGJ opening. However, abnormal anatomy is an important mediator of outcome and treatment success will be modulated by anatomic defects that impede bolus emptying.
Keywords: achalasia, esophagogastric junction, myotomy, anatomy, diverticulum
INTRODUCTION
Achalasia is a chronic disease defined by failure of lower esophageal sphincter (LES) relaxation and absence of normal peristalsis 1. The natural history of the disease is variable, with many patients requiring multiple interventions over time for effective symptom control 2–4. The development of newer therapeutic modalities such as per-oral endoscopic myotomy (POEM) has generated interest in accurately measuring esophagogastric junction (EGJ) opening related to initial and any subsequent interventions 5, 6. In years past, the degree of esophageal retention on timed barium esophagram (TBE) had served as the surrogate for the adequacy of EGJ opening 7–9. The widespread use of high-resolution manometry (HRM) has led to the study of its derived EGJ metrics for the same purpose. Unfortunately, the most studied HRM EGJ metrics – integrated relaxation pressure (IRP) and basal EGJ pressure (EGJP), have not correlated strongly with treated outcome in prior investigations 10, 11.
Functional lumen imaging probe (FLIP) topography is a newer technology that uses impedance planimetry to measure the change in luminal area in response to distensive pressure during controlled volumetric distension 12. The FLIP is well tolerated and can be performed in less than 5 minutes during a sedated upper endoscopy. In treated achalasia, it offers the additional vantage of allowing for a simultaneous assessment of esophagitis and anatomic features such as a displaced / obstructed fundoplasty when relevant during endoscopy. The distensibility index (DI) derived from FLIP has been shown to be abnormally low in treatment-naïve achalasics. However, only small series have looked at DI post-treatment and have had mixed results using smaller distension volumes used in older FLIP protocols 13–15.
Esophageal dilatation and anatomy are frequently factored into treatment modality selection and in interpreting treatment response. However, the specific impact of anatomical deformity has not been accounted for in most achalasia studies 16–18. Patients with severely dilated esophagi and advanced deformities such as mega-esophagus have been excluded from some larger randomized treatment trials 19. The aims of this study were to determine whether EGJ metrics on FLIP are useful in evaluating treatment outcomes in achalasia; and to also assess the effectiveness of these metrics in the context of esophageal anatomy.
METHODS
Subjects
We prospectively recruited patients with achalasia and previous treatment with pneumatic dilation (PD), laparoscopic heller myotomy (LHM) [often with Dor or Toupet fundoplasty), or per-oral endoscopic myotomy (POEM) at our institution or as referred from elsewhere. We included and evaluated studies in consecutive patients who underwent full post-treatment assessment consisting of HRIM, upper endoscopy with FLIP, TBE, and Eckardt Score (ES) within a 3-month period at least 3 months after intervention. Patients with known type 3 achalasia pre-intervention were excluded. If multiple interventions were performed, the most recent intervention was used for study classification purposes and follow-up interval.
Timed barium esophagram
TBEs were performed in the upright position with x-ray images of the esophagus obtained at a minimum of 1, 2, and 5 minutes after ingestion of 200-ml of low density (45% weight to volume) barium sulfate. Analysis was performed by a single reader while blinded to the physiologic testing profile using Centricity PACS RA v4.0.3 (GE Healthcare, Barrington, IL, USA).
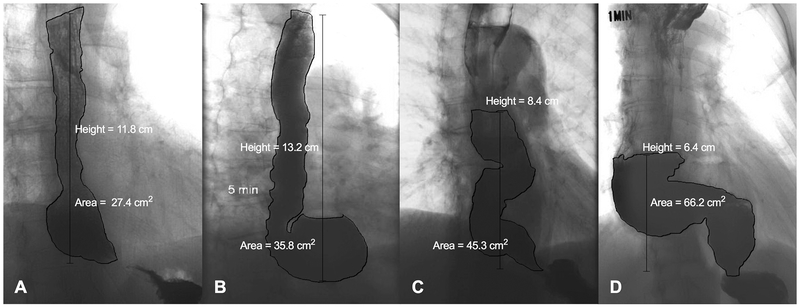
Anatomy was classified as either normal or with deformity present. The relevant deformities based on a consensus opinion of four esophageal specialists and a gastrointestinal radiologist were identified as: 1) pseudodiverticulum at myotomy site, 2) epiphrenic diverticulum, 3) sigmoid deformity, or 4) sinktrap deformity. Examples of each deformity are shown in Figure 1. A pseudodiverticulum was defined as an asymmetric outpouching along the aspect of the myotomy. Sigmoid esophagus was defined as at least two turns greater than 45 degrees in the contour of the esophageal body. A sinktrap deformity was defined as a dilated distal esophagus which was dependent with respect to the EGJ. Our technique of measuring column height and area is shown in Figure 1. Column height (cm) was measured as the vertical distance from the EGJ to the top of the solid contrast column. Column area (cm2) was measured by manually tracing the contour of the esophagus in the same region using the Polygon ROI tool available in PACS.
Figure 1.
Anatomical deformities noted in treated achalasia. A) pseudodiverticulum at the myotomy site, B) epiphrenic diverticulum, C) sigmoid deformity D) sinktrap deformity. Measurement of column height and area is shown for each figure.
Physiologic Testing
HRIM
HRIM studies were completed using a 4.2 outer diameter solid-state assembly with 36 circumferential pressure sensors at 1 cm intervals and 18 impedance segments at 2 cm intervals (Medtronic Inc, Shoreview, MN) after a minimum 6 h fast. The HRIM catheter was passed transnasally and positioned to record from the hypopharynx to the stomach with approximately 3 intragastric pressure sensors. The HRIM protocol included a 5 min baseline recording, 10 and 5 supine and upright 5 mL swallows respectively at 20-30s intervals using 50% saline.
FLIP
The FLIP assembly consisted of a 240-cm long, 3-mm outer diameter catheter with an 18-CM, infinitely compliant balloon (up to a distension volume of 60 ml) mounted near the distal end of the catheter (Medtronic Inc, Shoreview, MN). The balloon tapered at both ends to assume a 16-cm long cylindrical shape in the center that housed 17 impedance planimetry ring electrodes spaced at 1-cm intervals and a solid-state pressure transducer positioned at the distal end to provide simultaneous measurement of 16 channels of cross-sectional area (CSA) converted to diameter based on the assumption of circular geometry and intraballoon pressure. The impedance planimetry segment had a range of measurable diameters of 5.2-22 mm within the infinitely compliant limits of the balloon. Diameter and pressure values could be measured when the balloon was distended beyond a 22-mm diameter, but mechanical properties of the balloon would be engaged. Measurements from the impedance planimetry electrode pairs and the pressure transducer were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit. Subjects underwent sedated upper endoscopy in the left lateral decubitus position. The endoscope was withdrawn before initiation of the FLIP study protocol. The FLIP probe was placed trans-orally and positioned with the distal 2-3 impedance sensors beyond the EGJ as confirmed by demonstration of a waist in the impedance planimetry segment at a balloon distension volume of 20-30 ml. The FLIP assembly position was adjusted by the endoscopist during the study to maintain placement relative to the EGJ as visualized on real-time output. Simultaneous CSAs and intra-balloon pressures were measured during 5-10 ml stepwise distensions beginning with 5 ml and increasing to target volume of 60 or 70 ml; each incremental distension volume was maintained for 10-30 s. The distension protocol was modified during the course of the evaluation period, initially with a limit of 60 ml and later to a limit of 70 ml, as well as initially with 5-ml stepwise distensions and later with 10-ml stepwise distensions.
Data Analysis
Manometry studies were analyzed using ManoView version 3.0 (Medtronic, Shoreview, MN) analysis software to measure the IRP, EGJP, pressurization pattern, presence of peristalsis, and distal latency. Esophageal motility diagnoses were generated from the 10 supine swallows according to the Chicago Classification v3.0, using a median IRP of 15 mm Hg as the upper-limit of normal 1.
FLIP data including distension volume, intra-balloon pressure, and 16 channels of luminal diameter for each subject were exported to MATLAB (Math Works, Natick, MA) for analysis using a customized MATLAB program 20. This program applied a filter to minimize vascular and respiratory artifact and then generated tracings of each channel’s luminal diameter. Interpolation between channels was applied to generate color-coded topography plots by time with corresponding plots of volume distension and intra-balloon pressure. The program identified the EGJ-midline by searching for the minimal diameter of the distal impedance planimetry channels. The maximal EGJ diameter (MxEGJD) during the study was recorded. The EGJ-DI was calculated by measuring the narrowest EGJ CSA and intra-balloon pressure at each data sample obtained during the time course at the 60-ml distension volume. The median values for narrowest EGJ CSA and intra-balloon pressure were then divided to calculate the EGJ-DI (CSA/pressure; mm2/mmHg).
Statistical Analysis
Descriptive statistics for all continuous and ordinal measures were presented as median and interquartile ratio (IQR) unless otherwise stated. Receiver-operating-characteristic (ROC) curves were generated by plotting the sensitivity by false positive rate (1 – specificity) to determine positive associations for incremental value increases of studied variables. The optimal threshold value for each metric was chosen using Youden’s index. Outcome groups were compared using the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Correlations between independent variables were assessed using Spearman’s Rho. Analyses assumed a 5% level of statistical significance.
RESULTS
Subjects
Table 1 summarizes demographic and clinical data of the 79 included patients (age 17-81; 47% female). Median (range) follow-up interval was 13 months (4-204) after most recent intervention with PD, LHM, or POEM. Sixty-two (78.4%) of patients had the most recent intervention at our institution. Table 1 also includes achalasia subtype based on HRM pre-intervention. The 17 patients with unavailable or conventional manometry pre-intervention were termed unclassified achalasia. Twenty-four (70.6%) of patients with LHM, 25 (80.6%) with POEM, and 5 (35.7%) with PD had a DI > 2.8 mm2/mmHg. Ten (12.8%) of patients had documented prior interventions (characteristics are shown in Supplementary Table 1).
Table 1:
Study population (N = 79)
| Age | 48 [17-81] |
| No. female | 37 (47%) |
| Body-mass index | 28 [17-48] |
| Achalasia subtype pre-intervention | |
| Type I | 26 (34%) |
| Type II | 36 (46%) |
| Not specified | 17 (22%) |
| Intervention | |
| Laparoscopic Heller myotomy | 34 (43%) |
| Per-oral endoscopic myotomy | 31 (39%) |
| Pneumatic dilation | 14 (18%) |
| Length of follow-up (months) | 13 [4-204] |
Medians with [range] reported
Radiographic correlates of symptomatic outcome
Area-under-the curve (AUC) for 5-minute column height in association with ES >3 was .70; AUC for 5-minute column area was .73. Results using a column height threshold of 5 cm were as follows: sensitivity 36% and specificity 72.2%, p = .003. The same analysis using a threshold of 3 cm showed a sensitivity 76% and specificity 44.4%, p = .13. A 5-minute column area of >5 cm2 had a sensitivity of 84% and specificity of 55.6%, p = .0013. We used the 5-minute column area with a cutoff of 5 cm2 for further analysis of specific EGJ metrics.
Comparison of anatomical groups
Table 2 summarizes radiographic measures, EGJ metrics, and symptoms in the 52 patients with normal anatomy and 27 patients with anatomic deformity. The breakdown of deformities was as follows: 10 pseudodiverticula at the myotomy site, 7 epiphrenic diverticula, 5 sigmoid, and 5 sinktrap.
Table 2:
Comparison of anatomical groups
| Normal Anatomy (N = 52) | Anatomic Deformity (N = 27) | P value | |
|---|---|---|---|
| Radiographic | |||
| 5-min column height (cm) | 3.3 [1.6-6.9] | 6.1 [3.4-9.0] | .05 |
| 5-min column area (cm2) | 4.2 [2.3-15.2] | 20.4 [12.4-37.3] | .0004 |
| Max esophageal width (cm) | 2.7 [2.2-3.3] | 3.8 [2.9-5.9] | .00001 |
| EGJ | |||
| DI (mm2/mmHg) | 3.4 [2.3-5.0] | 5.2 [3.5-8.7] | .001 |
| IRP (mmHg) | 13 [8-18] | 11 [8-20] | ns |
| EGJP (mmHg) | 10 [6-15] | 7 [5-12] | ns |
| MxEGJD (mm) | 16.1 [12.8-18.0] | 15.8 [14.5-18.3] | ns |
| Symptoms | |||
| Eckardt Score | 3 [2-3] | 3 [2-5] | ns |
| Abnormal (>3) | 28 (23)% | 13 (48)% | .03 |
Abbreviations: DI: distensibility index, EGJ: esophagogastric junction, EGJP: basal esophagogastric junction pressure, IRP: integrated relaxation pressure, MxEGJD: maximal EGJ diameter
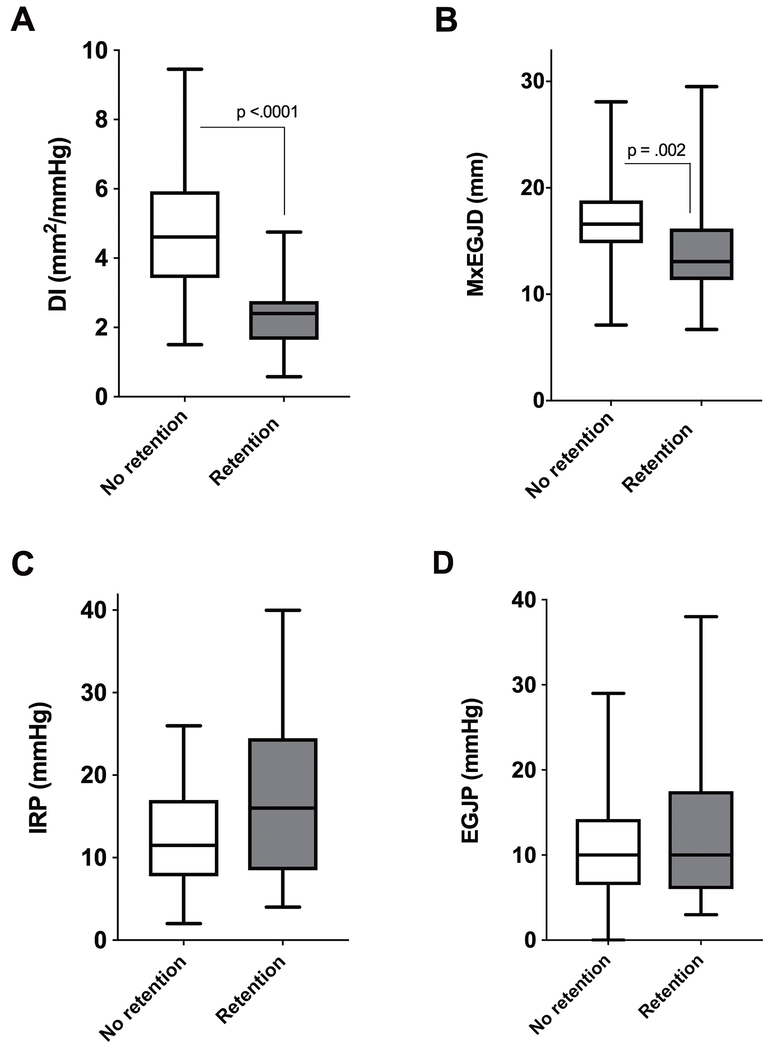
EGJ metrics in normal anatomy
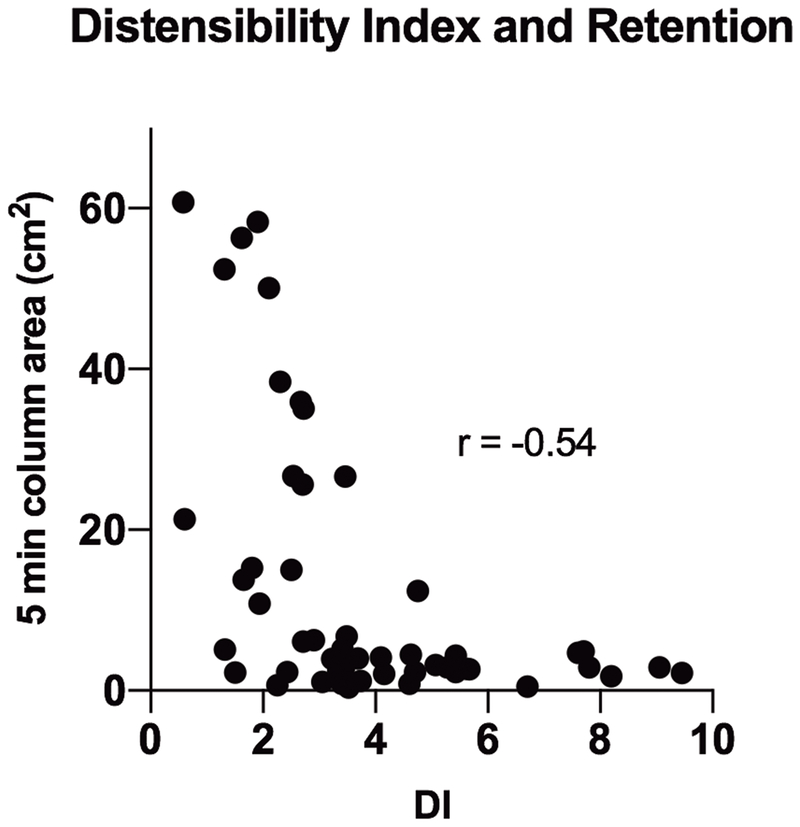
Figure 2 shows FLIP DI and MxEGJD and HRM IRP and EGJP in patients with normal anatomy (n = 52) with and without retention. Only the FLIP DI (2.4 vs 5.2 mm2/mmHg, p <.0001) and MxEGJD (13.1 vs 16.6 mm, p = .002) were significantly different between the two groups. Areas-under-the curve for EGJ metrics in association with 5-minute column area >5 cm2 were as follows: DI - .90, MxEGJD - .76, IRP - .64, EGJP - .53. At a cutoff of < 2.8 mm2/mmHg, the DI was abnormal in 17 / 22 patients (sensitivity 77.3%, p = .0001) with retention; 4 of the remaining 5 cases had a DI < 3.5. The DI correlated (r = −0.54, p < .0001) with the degree of retention as shown in Figure 3. The DI was abnormal in 10 / 12 (sensitivity 83.3%, p = .0005) patients with ES >3; both remaining cases had a DI < 3.5. At a cutoff of < 14 mm, the MxEGJD was abnormal in 13 / 22 patients with retention (sensitivity 59.1%, p = .0008). The MxEGJD was abnormal in 8 / 12 patients with ES >3 (sensitivity 66.7%, p = .01).
Figure 2.
Esophagogastric junction metrics based on retention outcome in 52 patients with normal anatomy. Statistically significant differences are shown.
Abbreviations: DI: distensibility index, EGJP: basal esophagogastric junction pressure, IRP: integrated relaxation pressure, MxEGJD: maximal EGJ diameter
Figure 3.
Degree of retention based on distensibility index (DI) is shown. P value for Spearman’s rho is <.0001.
Outcome based on EGJ opening and anatomy
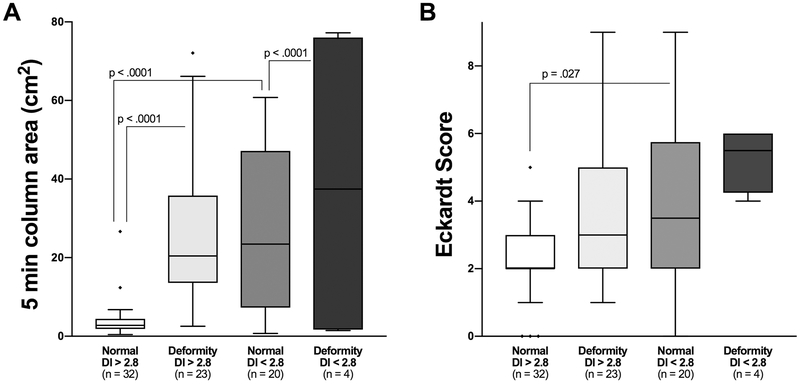
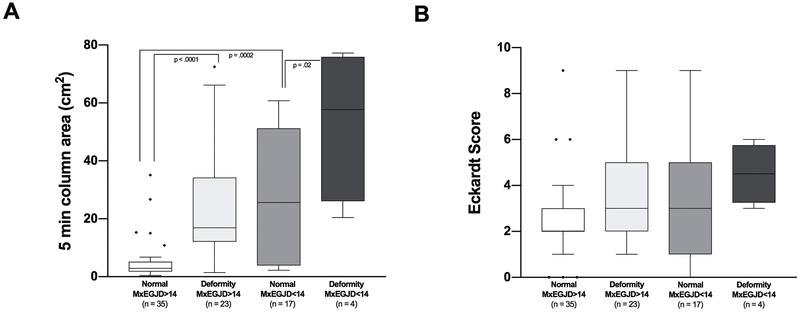
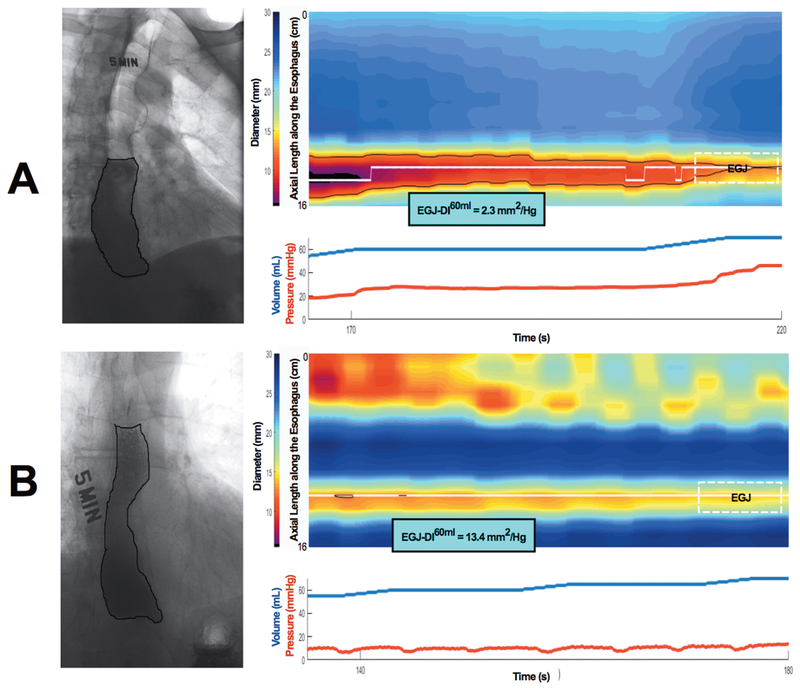
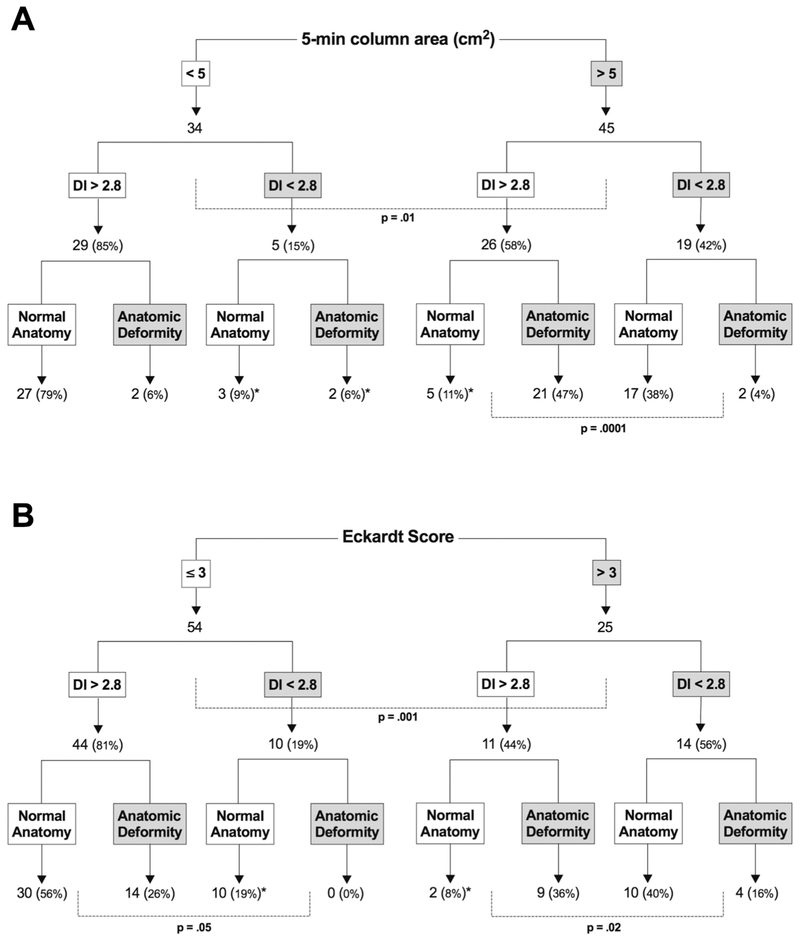
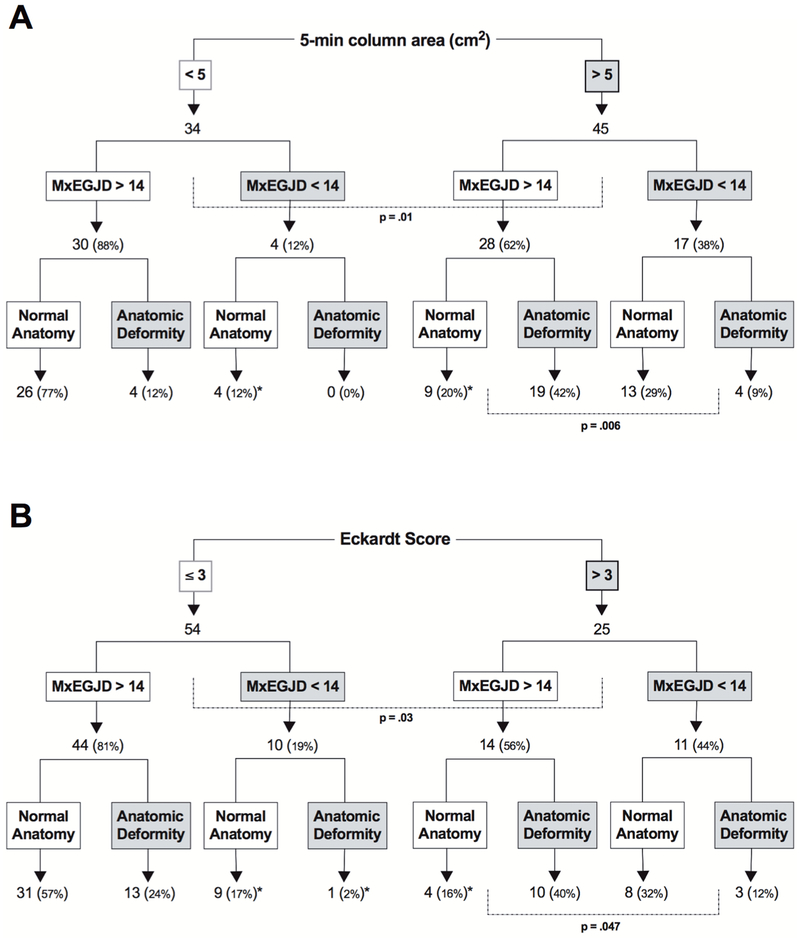
Figures 4 and 5 show retention and ES in 4 groups based on anatomy and EGJ opening measured on FLIP DI and MxEGJD. Case examples of patients with retention with impaired and normal EGJ opening are show in in Figure 6. Outcome analysis diagrams for retention and ES as an effect of DI and anatomy are shown in Figure 7. Out of 45 patients with retention, 40 had a low DI and / or deformity. As mentioned, 4 of the 5 remaining patients with retention had a borderline obstruction with a DI < 3.5. Three of these patients also had repetitive retrograde contractions as defined previously 21. There were 5 patients with a DI < 2.8 yet no retention; all of these patients had contractility on FLIP including repetitive antegrade contractions in 3. The 2 patients that had deformity in this group had epiphrenic diverticula. Similarly, of 25 patients with an Eckardt score >3, 23 had a low DI and / or deformity; the remaining 2 had a borderline DI, with one having repetitive retrograde contractions. A similar outcome analysis as an effect of MxEGJD and anatomy is shown in Figure 8. Of the 45 patients with retention, 36 had a low MxEGJD and / or deformity. Of the 25 patients with ES >3, 21 had a low MxEGJD and / or deformity.
Figure 4.
Outcomes in groups based on distensibility index (DI) and anatomy. Statistically significant differences are shown. Outliers are marked with ◆.
Figure 5.
Outcomes in groups based on maximal EGJ diameter (MxEGJD) and anatomy. Statistically significant differences are shown. Outliers are marked with ◆.
Figure 6.
Case examples of retention driven by A) closed EGJ, and B) anatomic deformity in the presence of an open EGJ. Five-minute timed barium esophagram images are shown on the left. Composite figures with FLIP topography (top) and pressure and volume curves focused on the 60 ml distension volume (bottom) are shown on the right. The EGJ location is marked, and the distensibility index (EGJ-DI60ml) is shown.
Abbreviations: DI: distensibility index, EGJ: esophagogastric junction, FLIP: functional lumen imaging probe
Figure 7.
Outcome analysis tree for retention (A) and Eckardt score (B). A breakdown of patients and % based on distensibility index (DI) and anatomy is shown. Statistically significant differences are indicated. Discordant cases are marked with an *.
3/5 cases a low DI yet no retention had repetitive antegrade contractions, and all 5 had contractility. Both cases of deformity were epiphrenic diverticula.
4/5 cases with retention despite a normal DI had a DI < 3.5 suggesting a borderline obstruction. 3/5 of these cases also had repetitive retrograde contractions.
2/2 cases with an ES >3 despite a normal DI had a DI < 3.5 suggesting a borderline obstruction. 1 of these cases also had repetitive retrograde contractions.
Figure 8.
Outcome analysis tree for retention (A) and Eckardt score (B). A breakdown of patients and % based on maximal EGJ diameter (MxEGJD) and anatomy is shown. Statistically significant differences are indicated. Discordant cases are marked with an *.
Looking at retention in anatomical deformities independently, 8/10 patients with a pseudodiverticulum, 5/7 with epiphrenic diverticulum, 5/5 with sigmoid, and 5/5 with sinktrap esophagus had retention. The DI was normal in all of these subjects except for 2 patients with an epiphrenic diverticulum, and 1 each with sigmoid and sinktrap deformity. The MxEGJD was normal in all patients with anatomical deformity other than in 1 patient with sigmoid and 2 patients with sinktrap deformities.
DISCUSSION
The aims of this study were to determine whether EGJ opening measured on FLIP topography post-treatment of achalasia correlates with outcome in the context of esophageal anatomy. Our main finding was that a combination of an abnormal distensibility index and / or anatomic deformity was present in 89% and 92% of patients with radiographic retention and a high Eckardt score respectively. We also found that the DI has better utility than the IRP or EGJP in association with treatment outcome. The maximal EGJ diameter may be most useful to confirm an open EGJ when there is significant esophageal dilatation rendering the DI less reliable.
Previous attempts to define a measurable surrogate of adequate EGJ opening in treated achalasia have been largely inconclusive. Rohof et al. found that LES pressure had poor correlation with long term radiographic and symptomatic outcome 22. Our group has found the IRP, EGJP, and EGJ-contractile integral to be only 65%, 57%, and 57% sensitive, respectively, for symptomatic outcome and 57%, 57%, and 57% sensitive for radiographic outcome 11. Although a more functional measure on HRIM, bolus flow time, had better correlation, calculation of this metric requires additional analysis which is not universally available. Smaller studies using FLIP with an 8-cm balloon and lower distension volumes have shown mixed results 13, 14.
The superiority of FLIP EGJ indices in correlation with outcomes may be due to the limitations of the IRP related to artifact and pressure measurement variability. However, another possibility is that EGJ opening during volumetric distention may be more physiologically relevant. Which FLIP metric is best is yet to be decisively determined. The DI accounts for the pressure required to open to the EGJ and is thus particularly relevant in diseases where esophageal body contractility is diminished. Thus not surprisingly, the DI was superior overall to the MxEGJD in our achalasia cohort. An additional consideration is that the MxEGJD can be normal if the EGJ opens transiently due to a single contraction, whereas the DI is a median volume over 30 seconds. However, the incorporation of bag pressure predisposes the DI to inaccuracy related to the pressure measurement. The single pressure sensor in the FLIP device may be less reliable, particularly when significant esophageal dilatation is present. Indeed 5 out of 6 patients with a normal DI but a low MxEGJD in our cohort had esophageal dilatation greater than 3.9 cm.
Interestingly, a more open EGJ was suggested by multiple metrics in our anatomic deformity cohort compared to normals. Part of the remodeling process in more advanced achalasia may be decreased hypertonicity of the LES. Furthermore, there could be a tendency towards a more aggressive myotomy when anatomic deformity is present. Our results further highlight the effect of contractility in the treated achalasia population. We have previously postulated that contractility on FLIP in treatment naïve achalasics may represent pathophysiologic variants with implications for prognosis 21. Although assessing contractility was not an aim of this study, we did note that contractility on FLIP, particularly repetitive antegrade contractions, seemed to overcome obstruction at the EGJ in several patients in our cohort. Similarly, uncoordinated contractions including repetitive retrograde contractions may have driven retention in some patients despite an open EGJ and normal anatomy.
Our study is the first to show the impact of anatomy when assessing the role of EGJ functional metrics on treatment response in achalasia. The presence of pre-existing anatomic deformity is a known cause of myotomy failure 18. Thus far, only severe anatomic deformities such as mega-esophagus and sigmoid achalasia have been characterized, and have been excluded from some larger outcome trials 19. We defined additional functionally relevant deformities based on anectodal experience with our complex achalasia referral population. Given the minimal contractility in achalasia, any dilated esophageal segment has the capacity to retain bolus. The more severe deviations in contour are noted with a sigmoid and sinktrap esophagus – this group had esophageal retention universally in our cohort. We grouped pseudodiverticula at the myotomy site, or the “blown out myotomy,” and epiphrenic diverticula with these more severe deformities, which explains the large range in degree of retention in the deformity group. Although not a primary aim of this study, one of our key findings is that these milder deformities may have physiologic relevance – 76% of these patients had retention despite an open EGJ in the majority. Epiphrenic diverticula are more likely to be related to EGJ obstruction. The etiology and consequences of these minor deformities should be investigated further.
Although the role of anatomy and incomplete treatment of the LES on outcomes have been published previously, our work provides an opportunity to create a more precise model to guide management in patients with poor outcomes after myotomy and dilation. A reflexive practice of performing pneumatic dilation or re-do myotomy in the case of treatment failure increases the risk of esophagitis, further development of anatomic deformities and exposure of the patient to a procedural risk with minimal potential to benefit. Rather, management should incorporate an assessment of both the adequacy of EGJ opening using the EGJ-DI and a detailed assessment of anatomy together to target treatment. For example, in cases where EGJ opening is inadequate, intervention with a re-do myotomy or pneumatic dilation is appropriate. However, in many cases the myotomy is adequate; and defective bolus propulsion related to anatomic deformity drives retention. In these cases, additional intervention on the EGJ is unlikely to be of benefit; and an esophagectomy may need to be considered. In the rarer cases of treatment failure despite adequate EGJ opening and normal anatomy, spasm or a functional overlap syndrome should be considered.
Our study has a few limitations. The patient population was not part of a treatment naïve group in a prospective study design. However, the design of this study required poor outcomes which would require many years to collect given the overall successful treatment outcomes in achalasia. Thus, we utilized our referral patient population which had overall worse outcomes than reported in the literature – approximately 1/3 had an abnormal Eckardt score and a similar number had an anatomic deformity. This patient profile is reflective of clinical practice at a tertiary-care institution with a large achalasia referral base. Currently, we only have a small number of patients whom have undergone esophagectomy for severe deformity. A multicenter prospective trial would help further support our findings and also provide the opportunity to test the precision model of treatment failure management.
We use FLIP is a complementary test to endoscopy and esophagram in assessing the treated achalasia population. The advantage of FLIP is that it provides an objective assessment of EGJ opening at the time of endoscopy. Although the endoscopic appearance of a tight LES, or the presence of esophagitis with an open LES may be obvious, EGJ opening is often difficult to assess visually in treated achalasics. We primarily use the distensibility index calculated at the 60 mL volume as our measure of EGJ opening as this is the metric with the most supporting literature. Although our practice is to calculate this using a specialized analysis program, we are currently studying the real-time assessment of the DI and suspect it will be equally valid. We use the maximal EGJ diameter in cases with a borderline DI, or cases where the bag pressures are low (less than approximately 20 mmHg) at higher bag volumes. Notably, these EGJ indices in isolation cannot distinguish between obstruction from a fundoplication vs that from persistent LES hypertonicity, thus a careful endoscopic exam remains imperative. Endoscopic guidance may also be required for proper FLIP positioning in the presence of advanced deformity. Time barium esophagram remains an important test as this better defines anatomy and help correlates symptoms with the presence of retention.
In conclusion, management of recurrent or residual symptoms after intervention in achalasia is complex and should consider the interplay between esophageal anatomy and the degree of EGJ opening. The DI on FLIP is the single-most useful measure of EGJ opening in treated achalasics and the esophagram is an excellent tool to define anatomy and degree of bolus retention. These two diagnostics tests are complementary and crucial to developing treatment approaches for complex referral patients.
Supplementary Material
STUDY HIGHLIGHTS.
What is current knowledge
Metrics predicting outcome in treated achalasia are unclear.
What is new here
Anatomy and EGJ opening determine outcome in achalasia.
The FLIP distensibility index is the best measure of EGJ opening.
Acknowledgments
Financial support: NIH R01 DK079902
Abbreviations:
- AUC
area-under-the-curve
- DI
distensibility index
- EGJ
esophagogastric junction
- EGJP
basal esophagogastric junction pressure
- ES
Eckardt score
- FLIP
functional lumen imaging probe
- HRIM
high resolution impedance manometry
- HRM
high resolution manometry
- IRP
integrated relaxation pressure
- LHM
laparoscopic Heller myotomy
- MxEGJD
maximal EGJ diameter
- PD
pneumatic dilation
- POEM
per-oral endoscopic myotomy
- ROC
receiver-operative characteristic
- TBE
timed barium esophagram
Footnotes
Guarantor of the Article: John E Pandolfino
CONFLICTS OF INTEREST / STUDY SUPPORT
Potential competing interests:
Dustin Carlson, Peter Kahrilas, and John Pandolfino have intellectual property rights surrounding endoFLIP technology
References:
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh CM, Ponds FA, Schijven MP, et al. Efficacy of pneumodilation in achalasia after failed Heller myotomy. Neurogastroenterol Motil 2016;28:1741–1746. [DOI] [PubMed] [Google Scholar]
- 3.Salvador R, Voltarel G, Savarino E, et al. The natural history of achalasia: Evidence of a continuum-”The evolutive pattern theory”. Dig Liver Dis 2017. [DOI] [PubMed] [Google Scholar]
- 4.Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013;144:718–25; quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 5.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc 2015;29:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teitelbaum EN, Sternbach JM, El Khoury R, et al. The effect of incremental distal gastric myotomy lengths on EGJ distensibility during POEM for achalasia. Surg Endosc 2016;30:745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaezi MF. Quantitative methods to determine efficacy of treatment in achalasia. Gastrointest Endosc Clin N Am 2001;11:409–24, viii–ix. [PubMed] [Google Scholar]
- 8.Vaezi MF, Baker ME, Achkar E, et al. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002;50:765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol 1999;94:1802–7. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Xie C, Wang M, et al. Association of High-Resolution Manometry Metrics with the Symptoms of Achalasia and the Symptomatic Outcomes of Peroral Esophageal Myotomy. PLoS One 2015;10:e0139385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Lin Z, Kahrilas PJ, et al. High-Resolution Impedance Manometry Metrics of the Esophagogastric Junction for the Assessment of Treatment Response in Achalasia. Am J Gastroenterol 2016;111:1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon BP, Frokjaer JB, Kunwald P, et al. The functional lumen imaging probe (FLIP) for evaluation of the esophagogastric junction. Am J Physiol Gastrointest Liver Physiol 2007;292:G377–84. [DOI] [PubMed] [Google Scholar]
- 13.Smeets FG, Masclee AA, Keszthelyi D, et al. Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil 2015;27:1495–503. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil 2013;25:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012;143:328–35. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelo F, Petrucciani N, Aurello P, et al. Treatment of achalasia with extreme megaesophagus: heller myotomy or esophagectomy? Am Surg 2011;77:362–4. [PubMed] [Google Scholar]
- 17.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992;103:1732–8. [DOI] [PubMed] [Google Scholar]
- 18.Tsiaoussis J, Athanasakis E, Pechlivanides G, et al. Long-term functional results after laparoscopic surgery for esophageal achalasia. Am J Surg 2007;193:26–31. [DOI] [PubMed] [Google Scholar]
- 19.Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic Heller myotomy. Gut 2016;65:732–9. [DOI] [PubMed] [Google Scholar]
- 20.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP). Neurogastroenterol Motil 2013;25:e765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology 2015;149:1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohof WO, Lei A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol 2013;108:49–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.