Abstract
Objective:
Hypertension-associated parenchymal arteriole (PA) dysfunction reduces cerebral perfusion and impairs cognition. This is associated with impaired TRPV4-mediated PA dilation; therefore, we tested the hypothesis that TRPV4 channels are important regulators of cerebral perfusion, PA structure and dilation, and cognition.
Methods:
10–12-month-old male TRPV4 knockout (WKY-Trpv4em4Mcwi) and age-matched control WKY rats were studied. Cerebral perfusion was measured by MRI with arterial spin labeling. PA structure and function were assessed using pressure myography and cognitive function using the novel object recognition test.
Results:
Cerebral perfusion was reduced in the WKY-Trpv4em4Mcwi rats. This was not a result PA remodeling because TRPV4 deletion did not change PA structure. TRPV4 deletion did not change PA myogenic tone development, but PAs from the WKY-Trpv4em4Mcwi rats had severely blunted endothelium-dependent dilation. The WKY-Trpv4em4Mcwi rats had impaired cognitive function and exhibited depressive-like behavior. The WKY-Trpv4em4Mcwi rats also had increased microglia activation, and increased mRNA expression of GFAP and tumor necrosis factor alpha suggesting increased inflammation.
Conclusion:
Our data indicate that TRPV4 channels play a critical role in cerebral perfusion, PA dilation, cognition and inflammation. Impaired TRPV4 function in diseases such as hypertension may increase the risk for the development of vascular dementia.
Keywords: microcirculation, trpv4 channels, endothelium-dependent dilation, vascular dementia
Introduction
Cerebral small vessel disease (cSVD) is a growing public health issue that lacks effective treatments. cSVD is described as an impairment in the structure and function of small arteries and arterioles, including parenchymal arterioles (PAs), capillaries, and venules with a lumen diameter <100μm (18). PAs are critical regulators of blood flow to the cerebral microcirculation and contribute to vascular resistance (27). These arterioles are not connected to each other by collaterals, thus they are considered the weak link or a bottle-neck in the perfusion of the cerebral microcirculation. Importantly, occlusion of a single PA produces a microinfarct and cognitive dysfunction (27). Since cerebral perfusion is regulated by a combination of artery structure and function, alterations in these arterioles can cause cerebral hypoperfusion which leads to the development of vascular cognitive impairment and exacerbation of other forms of dementia. In fact, cSVD accounts for about 50% of all dementia cases including Alzheimer’s disease (15). Hypertension is a risk factor for cSVD and vascular dementia development. We have previously shown that aging is associated with remodeling of PAs (6). Also, that hypertension causes inward hypotrophic remodeling (5) and impairs endothelium-dependent dilation of PAs; these changes were associated with reduced cerebral perfusion and impaired cognitive function (7).
We are interested in the role of the transient receptor potential vanilloid 4 (TRPV4) channel in cSVD and vascular cognitive impairment. TRPV4 channels are non-selective Ca2+ permeable channels expressed in endothelial cells, smooth muscle cells, astrocytes and neurons (17). All of these cell types are included in the neurovascular unit and act together to regulate cerebral perfusion (16). In endothelial cells, TRPV4 channel activation allows for Ca2+ influx that activates intermediate and small-conductance Ca2+-activated potassium channels (IKCa and SKCa) to elicit endothelium-derived hyperpolarization (EDH) dilation in peripheral arteries and large pial cerebral arteries (3, 4, 11, 14, 22, 28, 29, 33). TPRV4 channels are critical regulators of PA endothelium-dependent dilation in normotensive and hypertensive rats and mice (7, 20, 21, 36). AngII-hypertensive mice have impaired TRPV4-mediated dilation (7, 29, 35) and cognition, and treatments that improve TRPV4 mediated dilation also correct the cognitive dysfunction (7). The link between TRPV4 mediated dilation and cognitive function is further strengthened by studies showing that TRPV4-mediated dilation in PAs is impaired in other models of cognitive decline associated with chronic cerebral hypoperfusion (20). Therefore, we hypothesized that genetic disruption of TRPV4 channel signaling would impair endothelium-dependent dilation and cognitive function using middle-aged TRPV4 knockout (WKY-Trpv4em4Mcwi) rats and their appropriate controls.
Materials and Methods
Experimental Model
All experimental protocols were approved by the Michigan State University Animal Care and Use Committee and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. These studies were performed on 10–12-month-old male rats. Control Wistar Kyoto (WKY) rats were purchased from Envigo. WKY-Trpv4em4Mcwi were generated by CRISPR/Cas9 resulting in a 4 base pair deletion in Exon 4 of the Trpv4 gene, these rats were obtained from the Gene Editing Resource Center at the Medical College of Wisconsin. All rats were housed on 12h:12h light/dark cycle with food and water ad libitum.
Tail-cuff plethysmography
Blood pressure was measured in conscious rats by tail-cuff plethysmography using a RTBP1001 tail-cuff blood pressure system (Kent Scientific, Torrington, CT) as described previously(5, 24, 31).
Pressure Myography
The endothelial function of PAs was assessed by pressure myography (5, 21, 24). To isolate the arterioles, a 5 × 3 mm section of the brain containing the middle cerebral artery (MCA) was dissected, then the pia with the MCA was separated from the brain and the PAs branching off the MCA were used for experiments. Isolated arterioles were cannulated with two glass micropipettes in a custom-made cannulation chamber. Arterioles were equilibrated at 37°C in physiological salt solution (PSS) containing 141.9mmol/L NaCl, 4.79mmol/L KCl, 1.12mmol/L KH2PO4, 1.79mmol/L MgSO4•7H2O, 109mmol/L HEPES, and 59mmol/L Dextrose. A servo-null system was used to pressurize the arterioles, and a leak test was performed prior to each experiment. Arterioles were pressurized to 60mmHg (5) until the development of stable myogenic tone (% tone = [1-(active lumen diameter/passive lumen diameter)] x 100. The diameter of the arterioles was recorded using MyoView 2.0 software (Danish Myo Technology, Aarhus, Denmark). After the generation of stable myogenic tone, endothelium-dependent dilation was assessed by incubating the arterioles with increasing concentrations of the muscarinic receptor agonist CCh (10−9-10−5M) or the TRPV4 agonist, GSK1016790A (10−9-10−5M).
To assess structure and biomechanics, the arterioles were incubated in Ca2+ free-PSS and a pressure response curve was constructed. Intralumenal pressure was increased from 3–120mmHg in 20mmHg increments, at each pressure outer and lumen diameter were recorded. The measurements for diameter were used to calculate wall thickness, wall stress, strain and stiffness.
Novel Object Recognition
Rats were acclimated over a course of three days for 10 minutes per day in the empty arena (an open box with dark walls). On the testing day, rats were placed facing away from the objects and allowed to explore the arena for 10 minutes with two identical objects. After a retention time of 90 minutes, rats were returned to the arena in a similar manner and allowed to explore one familiar and one novel object for 5 minutes and tracked using EthoVision XT. Exploration time of novel object and total time exploring were calculated (1). All analyses were performed by an investigator blinded to experimental condition.
Porsolt swim test
Rats were pre-exposed to the test condition 24 hours before the test session. A 30cm swim cylinder was filled with water (23–25°C), the rat was then place into the swim cylinder and allowed to swim for 15 minutes and tracked using the software Ethovision. The amount of time the rats spent moving and the time immobile was calculated. All rats were monitored by the investigator to ensure that they did not drown in the event that they stopped swimming. All analyses were performed by an investigator blinded to experimental condition.
Immunostaining
Brain section were fixed for 48hrs, then washed twice in 1X PBS (24 hrs each) and stored in 20% sucrose-PBS until section. 40μm cryosections of the cortex were made. For microglia quantification, free-floating brain sections were blocked and permeabilized in 0.1% Triton-X with 10% donkey serum PBS, then incubated in 1:200 rabbit Anti-IBA1 (catalog number 019–19741, Wako, Richmond, VA) overnight at 4°C. After washing in 1X PBS, sections were incubated in secondary AlexaFluor 488 donkey anti-rabbit antibody (catalog number ab150073, Abcam, Cambrige, UK) for one hour. For artery and capillary quantification, 40μm cryosections were incubated overnight in 0.01mg/ml isolectin GS-IB4 Alexa Fluo-568 conjugate (catalog number I21412, Invitrogen, Carlsbad, CA) at 4°C. The next day, sections were washed 4× in 1X PBS (5 min each wash) and coverslips were mounted using Prolong antifade reagent (Invitrogen, Carlsbad, CA) (17). All analyses were performed by an investigator blinded to experimental condition.
qRT-PCR
RNA was extracted from brain sections, near the middle cerebral arteries, for qRT-PCR analysis using Trizol. RNA was reverse transcribed using VILO reverse transcriptase (Invitrogen, Carlsbad, CA). TAQMAN-specific probes were used for the PCR to assess the mRNA expression of TRPV4 (Rn00576745_m1), GFAP (Rn01253033_m1), IL-6 (Rn01810330_m1), TNFα (Rn01525859_g1), synaptophysin (Rn01528256_m1), and doublecortin (Rn00670390_m1). mRNA expression is expressed as the fold change from control using the 2-ΔΔCt method. β2-microglobulin was used as control for normalization.
Plasma protein oxidation
Blood was collected by cardiac puncture immediately prior to euthanasia in anesthetized mice. Plasma oxidized proteins were measured by ELISA (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions.
Magnetic Resonance Imaging
Cerebral perfusion was measured by continuous arterial spin labeling (CASL) using a Bruker 7T BioSpec 70/30 USR housed in the Department of Physiology. Rats were initially anesthetized at 3–5% isoflurane in 100% O2 and maintained at 1–2% throughout experimentation. Temperature was monitored (SA Instruments) and maintained at 37°C using a water bath. Breathing was monitored (SA Instruments) and maintained between 45–60 breaths per minute by changing the % of isoflurane. The anatomical slice of interest was determined using Turbo-RARE sequence with an echo time of 33msec, 11msec spacing and RARE factor of 8. Repetition time was 3.8sec using 1mm slice thickness, 35mm x 35mm FOV and 256×256 pixel resolution which yielded voxel sizes of 137 × 137microns. T1 values for the region of interest were determined by generating a T1 mapping sequence (TE=7msec, 7msec echo spacing, RARE factor 2, 6T1s) using a 35×35mm FOV and 128×128 pixels. The true in plane resolution for the T1 map was 273 × 273microns and was kept during the spin labeling experiment. Continuous arterial spin labeling (CASL) perfusion measurements were performed with the following parameters: TE= 12msec, repetition time 2040 msec, 16 averages. The labeling slice was maintained 25mm from the measuring slice and over the carotids (2sec saturation time, 2.5W power). Using the T1 map, control, and labeled brain slice acquisitions the perfusion was calculated according to:
(32).
Drugs and Chemicals
GSK1016790A was purchased from Cayman Chemicals (Ann Arbor, MI). All other drugs and chemicals were purchased from Sigma-Aldrich unless otherwise specified.
Statistical Analysis
All data are presented as mean ± SEM. Myogenic tone, physiological characteristics, blood pressure, mRNA expression, behavioral, and immunofluorescence data were analyzed by Student’s t-test. For analysis of artery vasodilation and structure, two-way analysis of variance with repeated measures in one factor was utilized followed by Bonferroni-adjusted t-tests for post-hoc comparisons. All statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad, San Diego, CA). In all cases, statistical significance was denoted by p<0.05.
Results
TRPV4 channel deletion reduces cerebral perfusion but does not alter blood pressure
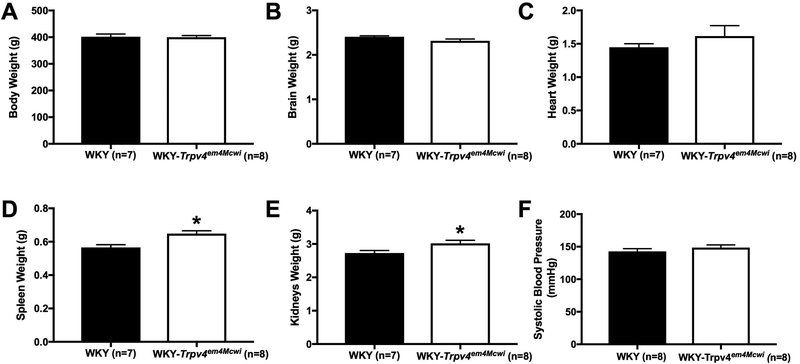
We assessed cerebral perfusion using MRI with continuous arterial spin labeling. The WKY-Trpv4em4Mcwi had reduced cerebral perfusion, p=0.0394 (Fig 1). WKY-Trpv4em4Mcwi had increased spleen (p=0.0331) and kidney (p=0.0057) weights (Fig 2D-E) but no other changes in physiological characteristics were observed (Fig 2 A-C). Deletion of TRPV4 channels did not alter systolic blood pressure, p=0.4668 (Fig 2F).
Figure 1. TRPV4 channel deletion decreases cerebral perfusion.
The role of TRPV4 channels in cerebral perfusion was assessed using MRI with arterial spin labeling. Representative perfusion maps are shown. WKY-Trpv4em4Mcwi rats have reduced cerebral perfusion. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=3/group
Figure 2. TRPV4 channel deletion does not alter blood pressure.
The physiological characteristic and blood pressure were assessed. (A) Body weight, (B) brain weight, (C) heart weight were not changed. WKY-Trpv4em4Mcwi rats have increased (D) spleen and (E) kidneys weight. (F) Systolic blood pressure was not altered by TRPV4 deletion. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=7 for WKY; N=8 for WKY-TRPV4em4Mcwi
TRPV4 channels regulate parenchymal arteriole endothelium-dependent dilation
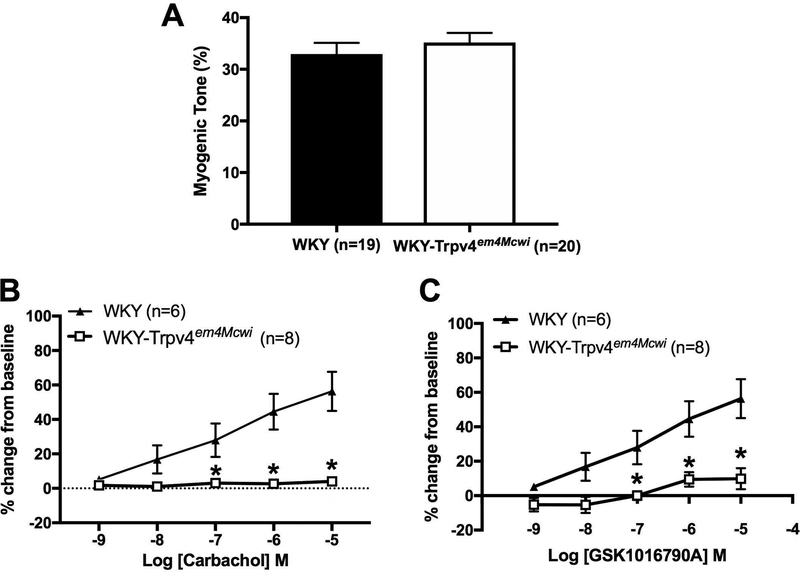
The role of TRPV4 channels in the generation of myogenic tone and endothelium-dependent dilation of PAs was assessed by pressure myography. No significant differences were observed in myogenic tone suggesting that TRPV4 channels do not contribute to tone at the physiological intralumenal pressure of 60mmHg, p=0.4398 (Fig 3A). We also assessed endothelium-dependent dilation in response to CCh. WKY-Trpv4em4Mcwi rats had severely impaired CCh-mediated dilation of PAs, p<0.0001 (Fig 3B). Impaired dilation was also observed in response to the TRPV4 agonist, GSK1016790A, p<0.0001 (Fig 3C).
Figure 3. TRPV4 channel deletion severely impairs endothelium-dependent dilation but does not alter myogenic tone.
The role of TRPV4 channel in PA myogenic tone and endothelium-dependent dilation was assessed using pressure myography. (A) No changes in PA myogenic tone were observed at 60mmHg intralumenal pressure (N=19 for WKY; N=20 for WKY-TRPV4em4Mcwi). WKY-Trpv4em4Mcwi rats did not dilate in response to (B) CCh or (C) the TRPV4 agonist, GSK1016790A (N=6 for WKY; N=8 for WKY-TRPV4em4Mcwi). Data are presented as mean ± SEM. *p<0.05 by Student’s t-test or two-way ANOVA.
TRPV4 channel deletion does not affect parenchymal arteriole structure or cause cerebral vascular rarefaction
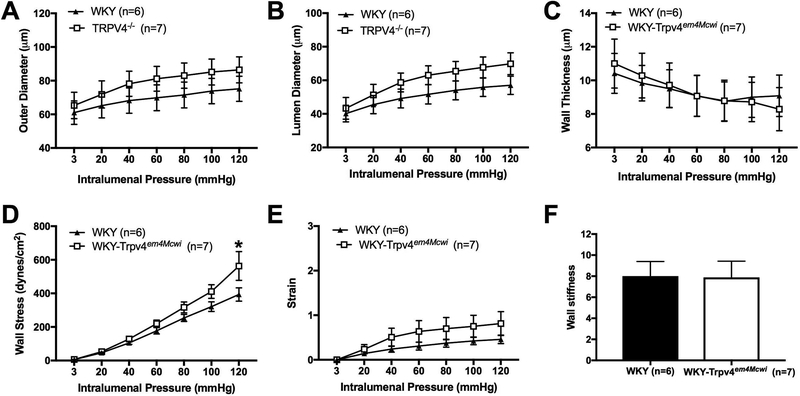
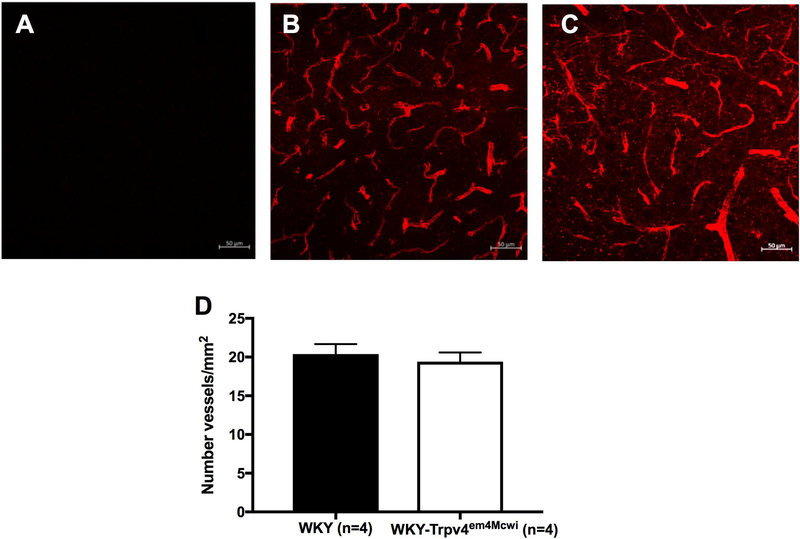
The structure and biomechanical properties of PAs were assessed by pressure myography under calcium free and zero flow conditions. There was a trend to an increase in outer diameter (p>0.9999) (Fig 4A), lumen diameter (p>0.9999) (Fig 4B) but these changes were not statically significant. Wall thickness (p=0.4070) (Fig 4C) was not significantly changed. Wall stress was only statically significantly increased at 120mmHg intralumenal pressure, p=0.0031 (Fig 4D). However, wall strain (p=0.9695) (Fig 4E) and artery wall stiffness (p=0.9578) were not statically significantly changed. (Fig 4F). These data suggest that WKY-Trpv4em4Mcwi rats do not exhibit significant PA remodeling. We also quantified the number of vessels in the cortex using isolectin GS-IB4 as an endothelial cell marker. No statically significant changes in the number of vessels were observed between WKY-Trpv4em4Mcwi rats and the controls, p=0.6206 (Fig 5).
Figure 4. TRPV4 channel deletion does not alter parenchymal arteriole structure causes.
The role of TRPV4 channels in PA structure was assessed using pressure myography. The (A) outer diameter and (B) lumen diameters, and (C) wall thickness were not changed by TRPV4 deletion. (D) Wall stress was significantly increased in WKY-Trpv4em4Mcwi rats at 120mmHg intralumenal pressure. (E) Wall strain and (F) artery wall stiffness were not changed. Data are presented as mean ± SEM. *p<0.05 by two-way ANOVA. N=6 for WKY; N=7 for WKY-TRPV4em4Mcwi
Figure 5. TRPV4 channel deletion does not cause cerebral artery rarefaction.
The number of vessels in the cortex was quantified using the endothelial cell marker isolectin IB-4. (A-C) Representative images are shown. (D) WKY-Trpv4em4Mcwi rats do not have artery rarefaction. Data are presented as mean ± SEM. p>0.05 by Student’s t-test. N=4 for WKY; N=4 for WKY-TRPV4em4Mcwi
TRPV4 knockout rats have impaired cognitive function and depressive-like behavior
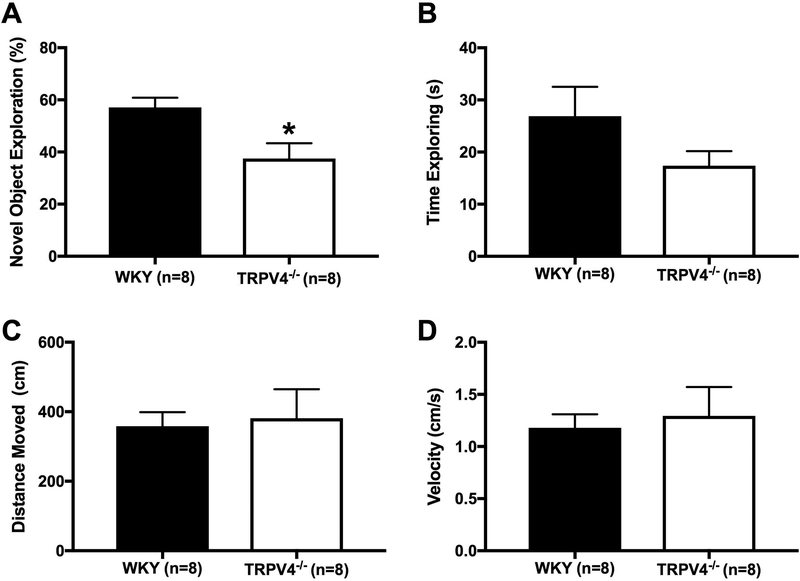
We evaluated non-spatial, short-term memory using the novel object recognition test. The WKY-Trpv4em4Mcwi spent less time exploring the novel object, p=0.0139 (Fig 6A), suggesting the WKY-Trpv4em4Mcwi have impaired cognitive function. The total time of exploration in the knockout rats appeared to be reduced but this did not reach statistical significance (p=0.15, Fig 6B), however the probability that there are differences in mean values as a result of the knockout cannot be completely excluded. The reduction in time spent exploring the novel object was not the result of reduced movement in the WKY-Trpv4em4Mcwi rats, because no significant changes were observed in total distance moved, p=0.7986 (Fig 6C) or the velocity of movement, p=0.7054 (Fig 6D). To explore if the WKY-Trpv4em4Mcwi rats are less motivated and have depressive-like behaviors, we used the Porsolt swim test. The WKY-Trpv4em4Mcwi rats spent less time moving compared to the controls, p=0.0002 (Fig 7). Together these data suggest that TRPV4 channel deletion results in cognitive dysfunction and depressive-like behaviors.
Figure 6. TRPV4 channel deletion impairs cognitive function.
Non-spatial, short-term memory was assessed using the novel object recognition test. (A) WKY-Trpv4em4Mcwi rats spent less time exploring the novel object. (B) There was a trend in reduced total time of exploration in WKY-Trpv4em4Mcwi rats, but the changes were not statistically significant. (C) total distance moved and (D) velocity were not significantly changed. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=8 for WKY; N=8 for WKY-TRPV4em4Mcwi
Figure 7. TRPV4 channel deletion increases depressive-like behaviors.
The Porsolt swim test was used to assess depressive-like behaviors. WKY-Trpv4em4Mcwi rats spent less time swimming compared to control. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=8 for WKY; N=8 for WKY-TRPV4em4Mcwi
WKY-Trpv4em4Mcwi rats have increased cerebral inflammation but not systemic oxidative stress
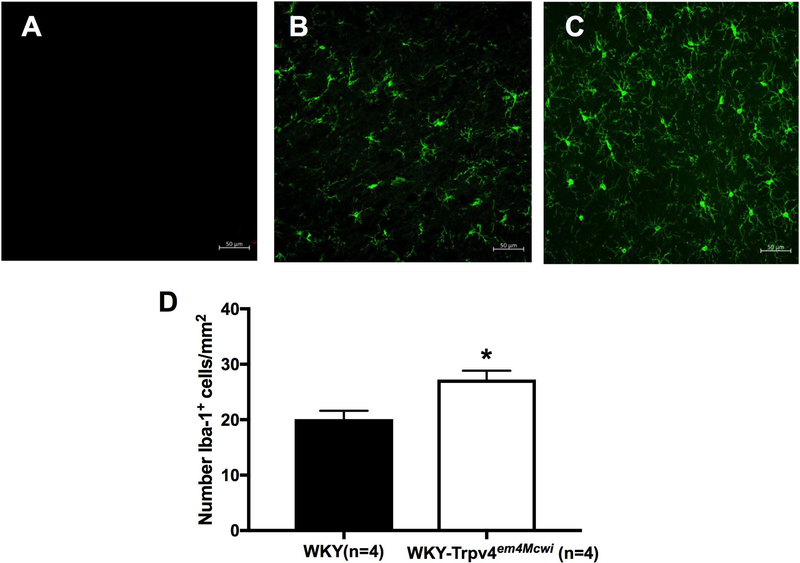
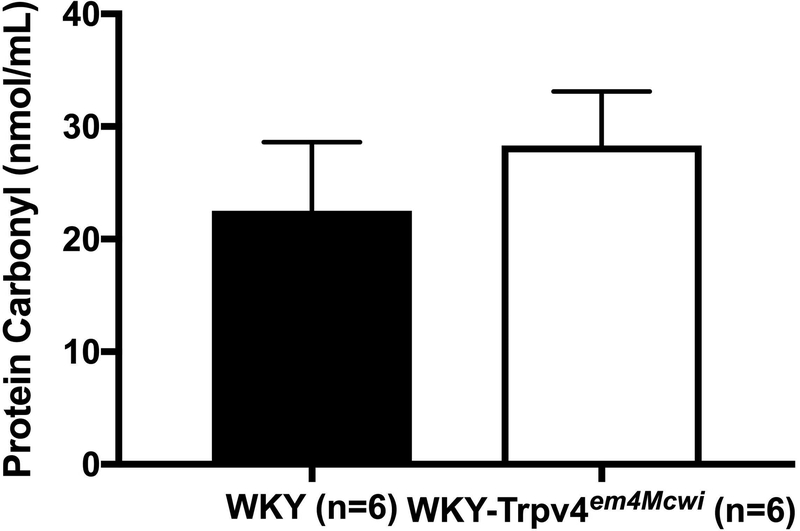
To explore mechanisms associated with the vascular and cognitive changes, we counted activated microglia in the brain as a marker of inflammation and assessed plasma protein oxidation as an index of oxidative stress. Our data show that WKY-Trpv4em4Mcwi rats have increased number of activated microglia, p=0.0058 (Fig 8). However, there was no significant change in plasma oxidized proteins, p=0.4696 (Fig 9).
Figure 8. WKY-Trpv4em4Mcwi rats have increased microglia.
The number of activated microglia were quantified using Iba-1. (A-C) Representative images are shown. (D) WKY-Trpv4em4Mcwi rats have increased number of activated microglia. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=4 for WKY; N=4 for WKY-TRPV4em4Mcwi
Figure 9. WKY-Trpv4em4Mcwi rats do not have increase plasma protein oxidation.
The protein carbonyl content was assessed by ELISA. No changes in plasma oxidized proteins were observed. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=6 for WKY; N=6 for WKY-TRPV4em4Mcwi
TRPV4 channel deletion increases the mRNA expression of markers for inflammation
The gene expression of TRPV4 channels was significantly reduced in the brain of TRPV4 knockout rats, as expected, p=0.0061 (Fig 10A). We measured GFAP mRNA expression in the brain to evaluate changes in astrocytes. The gene expression of GFAP was increased in WKY-Trpv4em4Mcwi rats, p=0.0480 (Fig 10B) suggesting astrogliosis that could contribute to vascular and cognitive dysfunction. Activated microglia can release several inflammatory substances including TNF-α and IL-6. WKY-Trpv4em4Mcwi rats have a significant increase in the mRNA expression of TNF-α (p=0.0025) (Fig 10C) and a small, but not significant, increase in IL-6 (p=0.1717) (Fig 10D). We also evaluated changes in the mRNA expression of markers for neuronal support. There were no significant changes in the mRNA expression of synaptophysin, p=0.0823 (Fig 10E). The mRNA expression of doublecortin was significantly increased in the WKY-Trpv4em4Mcwi rats, p=0.0025 (Fig 10F).
Figure 10. WKY-Trpv4em4Mcwi rats have increased mRNA expression of inflammatory markers.
The brain mRNA expression of markers for inflammation and neuronal support were assessed by qRT-PCR. (A) TRPV4 gene was significantly reduced. (B) GFAP and (C) TNF-α were significantly increased in WKY-Trpv4em4Mcwi rats. (D) IL-6 and (E) synaptophysin were not significantly changed. (F) Doublecortin was significantly increased in WKY-Trpv4em4Mcwi rats. Data are presented as mean ± SEM. *p<0.05 by Student’s t-test. N=5 for WKY; N=7 for WKY-TRPV4em4Mcwi
Discussion
The novel findings in this study are that global TRPV4 channel deletion: 1). reduces cerebral perfusion; 2). causes impaired PA endothelium-dependent dilation without affecting artery structure; 3). impairs cognitive function and increases depressive-like behaviors; 4). causes cerebral inflammation including increased microglia activation; and 5). does not alter blood pressure. One strength of this study is that we used middle-aged rats. These studies improve our understanding of the role of TRPV4 channels in cognition and PA structure and function. This is critical because PA remodeling and impaired dilation with hypertension alters neuronal health and increases the risk of vascular dementia development.
TRPV4 channels are expressed in endothelial cells where they regulate vasodilation, but these channels aid other cell types such as smooth muscle cells, glia and neurons to control vascular tone and cerebral blood flow (10). TRPV4 channel deletion did not alter blood pressure consistent with previous studies in TRPV4−/− mice (12, 29). This lack of change in blood pressure fits with the absence of change in heart weight. Other studies have shown that TRPV4 channels play a role in L-NAME-induced hypertension but not in Angiotensin II (AngII)-hypertension in mice (23). In that study, TRPV4−/− mice were given L-NAME (7 days) or AngII (14 days) and blood pressure was measured. There were no differences in blood pressure in the AngII treated control and TRPV4−/− mice. However, TRPV4−/− mice treated with L-NAME had a significantly higher mean arterial blood pressure than controls. The AngII treated TRPV4−/− and control mice had similar increases in blood pressure (23). That study suggests that TRPV4 channels play a minor role in L-NAME- but not in AngII-induced hypertension. However, TRPV4 channels play an important role in endothelial dysfunction in AngII-hypertension (7, 29).
Vascular and cognitive dysfunction could be associated with reduced cerebral perfusion (8). Therefore, we examined cerebral perfusion in the WKY-Trpv4em4Mcwi rats using MRI with continuous arterial spin labeling. Our data show that the TRPV4 deficient rats have reduced cerebral perfusion. TRPV4 channels are important in amplifying the responses in neurovascular coupling, thus, their absence could change cerebral perfusion (9, 13).
To better understand the role of TRPV4 channels in the cerebral microvasculature, we first evaluated PA function. TRPV4 channel deletion did not change the generation of myogenic tone but resulted in a severe impairment in PA dilation suggesting a critical role for this channel in cerebral arteriolar dilation. This is consistent with previous studies in TRPV4−/− mice (3) and with our previous studies in C57bl/6 mice where inhibition of TRPV4 channels with the pharmacological antagonist GSK2193874 severely blunted PA dilation (7). Studies in the Nelson laboratory also showed that AngII-hypertension alters the coupling of TRPV4 channels to the anchoring protein AKAP-150 and that this impairs the channel activity and endothelium-dependent dilation in mesenteric arteries (22, 29). However, TRPV4 channels may regulate myogenic tone in pathological conditions. Our previous studies show that TRPV4 inhibition with GSK2193874 in normotensive mice did not change myogenic tone of PAs but it resulted in a significant loss of myogenic tone in the PAs from hypertensive mice (7). Preliminary studies suggest that GSK2193874 results in a modest loss of myogenic tone in the WKY-Trpv4em4Mcwi rats (approximately 8% loss of myogenic tone).
Contrary to recent findings in TRPV4−/− mice where the PAs were outward remodeled (3), the structure of the PAs from WKY-Trpv4em4Mcwi rats were not changed. The different findings could be explained by the different species used and the age of the animals. Our studies were conducted in middle aged rats while the TRPV4−/− mice in the other study were young adults. We also evaluated cerebral artery rarefaction. Our data suggest that the TRPV4 deficient rats do not exhibit rarefaction in the cerebral cortex. Therefore, the reduction in blood flow could be associated to the severely blunted PA endothelium-dependent dilation that we observed. We did not assess endothelium-independent dilation in the current study. Thus, we cannot rule out the possibility that smooth muscle cell TRPV4 channels contribute to the reduced cerebral perfusion observed in the WKY-Trpv4em4Mcwi rats.
We assessed the role of TRPV4 channels in non-spatial, short-term memory using the novel object recognition test. Our data show that TRPV4 channel deletion impairs short-term memory as shown by the reduction in the time spent exploring the novel object. No changes were observed in the velocity or the total distance moved between both groups. Although it was not statistically significant, there was a reduction in the total time of exploration in the WKY-Trpv4em4Mcwi rats. This presented the possibility that the WKY-Trpv4em4Mcwi rats might be less motivated than the controls. Therefore, we also assessed depressive-like behaviors using the Porsolt swim test and our data suggest that the WKY-Trpv4em4Mcwi rats have increased depressive-like behaviors. Our data are at odds with studies in the TRPV4−/− mice where no changes in memory were observed and also where the knockout mice had a reduction in depressive-like behaviors (26). The differences could be explained by the different species and also by the age of the animals. Shibasaki et al, used young adult mice (18–24 weeks of age) whereas in our study we used middle age rats (40–48 weeks of age) (26). It is also important to consider that TRPV4 knockout may alter the interactions of other genes, proteins and signaling complexes and this could also contribute to the changes in cognition.
Our data also suggests that the WKY-Trpv4em4Mcwi rats have increased inflammation as shown by the increased number of activated microglia. These activated microglia can release pro-inflammatory cytokines such as IL-6 and TNFα and the mRNA expression of TNFα was increased in the WKY-Trpv4em4Mcwi rats. Astrocytes can also play an important role in inflammation and neurovascular coupling. Importantly, astrocytes express TRPV4 channels. Our data shows that the WKY-Trpv4em4Mcwi rats have increased mRNA expression of the astrocytic marker, GFAP, suggesting that there might be astrogliosis. Similar increases in microglia activation, astrogliosis, IL-6 and TNFα production have been shown to be involved in the pathogenesis of cSVD. Therefore, these pro-inflammatory markers observed in the WKY-Trpv4em4Mcwi rats could be mediating the cognitive decline.
The altered cognition could be associated with changes in the expression of neuronal markers such as synaptophysin, a marker for neuronal function and synaptic plasticity and doublecortin, a marker for new and immature neurons (2, 30). Recent studies show reduced mRNA expression of these markers in AngII-hypertensive mice with cognitive impairment (7). Studies in old rats also show a correlation between reduction in synaptophysin levels and the degree of dementia development (34). However, in the current study the WKY-Trpv4em4Mcwi rats have increased synaptophysin and doublecortin mRNA expression. It is possible that the WKY-Trpv4em4Mcwi rats we used in the current study are at the beginning of the slope of cognitive decline and the increase in the mRNA expression in the markers for neuronal support might be a compensatory response for the reduced perfusion. We also examined protein carbonyl content as a marker for oxidative stress but did not observe any differences. We recognize that this is not a direct measure of oxidative stress, but this is relevant to our studies because oxidized plasma proteins have been observed in the plasma of Alzheimer’s patients (19).
Some limitations in our study must be acknowledged. First, all of our studies were conducted in male rats to match previous studies in our lab on the role of MR signaling in the cerebral vasculature, thus we did not explore sex differences. It is also important to acknowledge that the WKY-Trpv4em4Mcwi rats are global knockouts. Therefore, additional non-endothelial effects TRPV4 deletion, such as neuronal effects, cannot be ruled out. It is also possible that the kidney hypertrophy observed may alter the behavior independent of TRPV4. One way in which kidney function could alter congnition would be through changes in blood pressure, however, this seems unlikely given that there were no differences in blood pressure between the knockout and control rats. We have only studied middle aged rats; thus, more studies should be conducted in younger rats to assess age-associated changes. The MRI studies were performed in a limited number of animals (n=3), therefore the reduction of blood flow in the WKY-Trpv4em4Mcwi rats compared to controls should be confirmed in a larger sample. Also, the cerebral perfusion studies were performed in isoflurane-anesthetized rats and isoflurane is a known cerebral vasodilator (25). Blood pressure was assessed using tail-cuff plethysmography that might not detect smaller differences in blood pressure. As mentioned previously, in the current study, we did not assess endothelium-independent dilation therefore we cannot rule out a possible role for vascular smooth muscle cells in the impaired dilation. We have not assessed anxiety in these rats. In the future, we would also like to expand our studies to hypertensive rats and further explore the role of TRPV4 channels in neurovascular coupling.
Perspectives
TRPV4 channels are important regulators of cerebral perfusion and PA endothelium-dependent dilation. TRPV4 channels also are important regulators of cognitive function. Impaired TRPV4 channel function in diseases like hypertension could increase the risk for the development of vascular cognitive impairment.
Acknowledgments
The authors would like to acknowledge the Gene Editing Center at the Medical College of Wisconsin for providing the WKY-Trpv4em4Mcwi rats.
Sources of Funding
This study was supported by the National Institutes of Health grants R01-HL-137694-01 and PO1-HL-070687 to WF Jackson and AM Dorrance, the National of Institute of Neurological Disorders and Stroke Award F31NS090866 to JM Diaz-Otero and DK950120 to RW Wiseman.
List of Abbreviations
- AngII
angiotensin II
- cSVD
cerebral small vessel disease
- CCh
carbachol
- EDH
endothelium-derived hyperpolarization
- GFAP
glial fibrillary acidic protein
- IL-6
interleukin-6
- IKCa
intermediate conductance calcium-activated potassium channel
- MRI
magnetic resonance imaging
- SKCa
small conductance calcium-activated potassium channel
- PA
parenchymal arteriole
- PSS
physiological salt solution
- TNFα
tumor necrosis factor alpha
- TRP
transient receptor potential
- TRPV4
transient receptor potential vanilloid 4
- WKY
Wistar Kyoto
Footnotes
Conflicts of Interest/Disclosures
None
References
- 1.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borba EM, Duarte JA, Bristot G, Scotton E, Camozzato AL, Chaves MLF. Brain-Derived Neurotrophic Factor Serum Levels and Hippocampal Volume in Mild Cognitive Impairment and Dementia due to Alzheimer Disease. Dement Geriatr Cogn Dis Extra 6: 559–567, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan S-L, Nelson MT, Cipolla MJ. Transient receptor potential vanilloid-4 channels are involved in diminished myogenic tone in brain parenchymal arterioles in response to chronic hypoperfusion in mice. Acta Physiol (Oxf) ( August 28, 2018). doi: 10.1111/apha.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40: 1451–1457, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz-Otero JM, Fisher C, Downs K, Moss EM, Jaffe IZ, Jackson WF, Dorrance AM. Endothelial Mineralocorticoid Receptor Mediates Parenchymal Arteriole and Posterior Cerebral Artery Remodeling During Angiotensin II–Induced Hypertension. Hypertension.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H365–75, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Otero JM, Yen T-C, Fisher C, Bota D, Jackson WF, Dorrance AM. Mineralocorticoid Receptor Antagonism Improves Parenchymal Arteriole Dilation Via a TRPV4-Dependent Mechanism and Prevents Cognitive Dysfunction in Hypertension. Am. J. Physiol. Heart Circ. Physiol. ( August 17, 2018). doi: 10.1152/ajpheart.00207.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin Sci 131: 2451–2468, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 110: 6157–6162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol 305: H609–19, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horsburgh K, Wardlaw JM, van Agtmael T, Allan SM, Ashford MLJ, Bath PM, Brown R, Berwick J, Cader MZ, Carare RO, Davis JB, Duncombe J, Farr TD, Fowler JH, Goense J, Granata A, Hall CN, Hainsworth AH, Harvey A, Hawkes CA, Joutel A, Kalaria RN, Kehoe PG, Lawrence CB, Lockhart A, Love S, Macleod MR, Macrae IM, Markus HS, McCabe C, McColl BW, Meakin PJ, Miller A, Nedergaard M, O’Sullivan M, Quinn TJ, Rajani R, Saksida LM, Smith C, Smith KJ, Touyz RM, Trueman RC, Wang T, Williams A, Williams SCR, Work LM. Small vessels, dementia and chronic diseases - molecular mechanisms and pathophysiology. Clin Sci 132: 851–868, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96: 17–42, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iddings JA, Kim KJ, Zhou Y, Higashimori H, Filosa JA. Enhanced parenchymal arteriole tone and astrocyte signaling protect neurovascular coupling mediated parenchymal arteriole vasodilation in the spontaneously hypertensive rat. J Cereb Blood Flow Metab 35: 1127–1136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joutel A, Faraci FM. Cerebral small vessel disease: insights and opportunities from mouse models of collagen IV-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 45: 1215–1221, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev 2017: 6501046, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matin N, Fisher C, Jackson WF, Diaz-Otero JM, Dorrance AM. Carotid artery stenosis in hypertensive rats impairs dilatory pathways in parenchymal arterioles. Am J Physiol Heart Circ Physiol 314: H122–H130, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matin N, Fisher C, Jackson WF, Dorrance AM. Bilateral common carotid artery stenosis in normotensive rats impairs endothelium-dependent dilation of parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H1321–9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD, Nelson MT, Brayden JE, Santana LF. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol 143: 559–575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishijima Y, Zheng X, Lund H, Suzuki M, Mattson DL, Zhang DX. Characterization of blood pressure and endothelial function in TRPV4-deficient mice with l-NAME- and angiotensin II-induced hypertension. Physiol Rep 2: e00199, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 309: H127–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwinn DA, McIntyre RW, Reves JG. Isoflurane-induced vasodilation: role of the alpha-adrenergic nervous system. Anesth Analg 71: 451–459, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Shibasaki K, Sugio S, Takao K, Yamanaka A, Miyakawa T, Tominaga M, Ishizaki Y. TRPV4 activation at the physiological temperature is a critical determinant of neuronal excitability and behavior. Pflugers Arch 467: 2495–2507, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D. The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16: 55–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 71: 55–61, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilde E, Aubdool AA, Thakore P, Baldissera L, Alawi KM, Keeble J, Nandi M, Brain SD. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J Am Heart Assoc 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 89: 212–216, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Zhan SS, Beyreuther K, Schmitt HP. Synaptophysin immunoreactivity of the cortical neuropil in vascular dementia of Binswanger type compared with the dementia of Alzheimer type and nondemented controls. Dementia 5: 79–87, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br J Pharmacol 170: 661–670, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P, Sun C, Li H, Tang C, Kan H, Yang Z, Mao A, Ma X. TRPV4 (Transient Receptor Potential Vanilloid 4) Mediates Endothelium-Dependent Contractions in the Aortae of Hypertensive Mice. Hypertension ( November 6, 2017). doi: 10.1161/HYPERTENSIONAHA.117.09767. [DOI] [PubMed] [Google Scholar]