Abstract
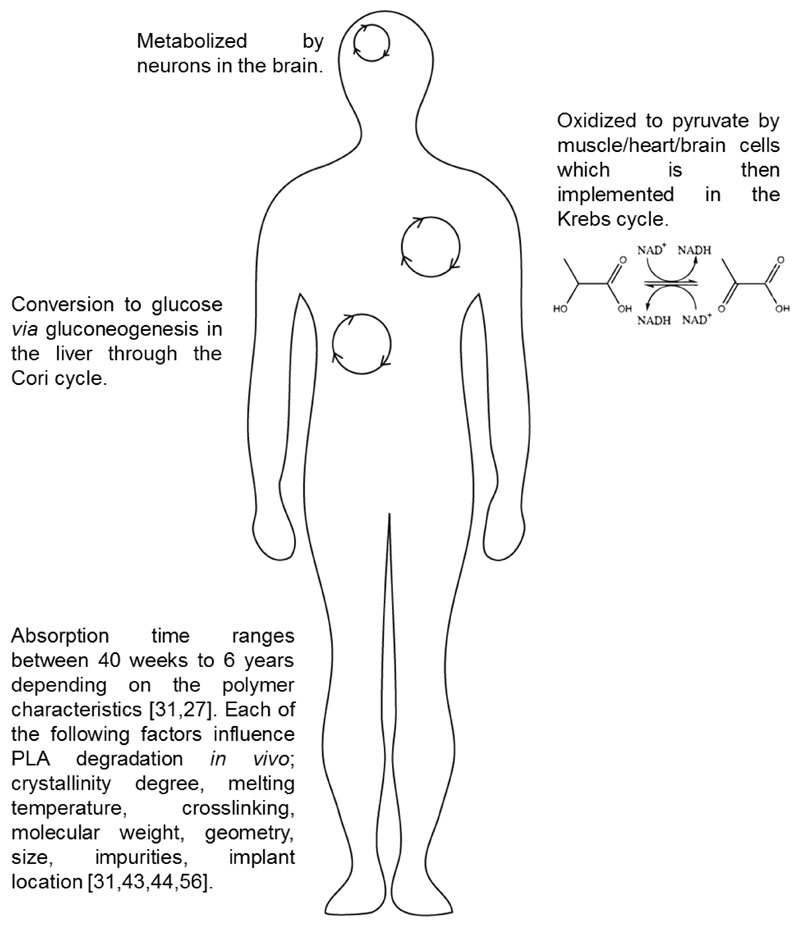
Polylactic acid (PLA) is the most commonly used biodegradable polymer in clinical applications today. Examples range from drug delivery systems, tissue engineering, temporary and long-term implantable devices; constantly expanding to new fields. This is owed greatly to the polymer's favorable biocompatibility and to its safe degradation products. Once coming in contact with biological media, the polymer begins breaking down, usually by hydrolysis, into lactic acid (LA) or to carbon dioxide and water. These products are metabolized intracellularly or excreted in the urine and breath. Bacterial infection and foreign-body inflammation enhance the breakdown of PLA, through the secretion of enzymes that degrade the polymeric matrix.
The biodegradation occurs both on the surface of the polymeric device and inside the polymer body, by diffusion of water between the polymer chains.
The median half-life of the polymer is 30 weeks; however, this can be lengthened or shortened to address the clinical needs. Degradation kinetics can be tuned by determining the molecular composition and the physical architecture of the device. Using L- or D- chirality of the LA will greatly slow or lengthen the degradation rates, respectively.
Despite the fact that this polymer is more than 150 years old, PLA remains a fertile platform for biomedical innovation and fundamental understanding of how artificial polymers can safely coexist with biological systems.
Keywords: poly(lactic) acid, polymer, safety, biodegradation, elimination, excretion, poly(lactic acid), drug delivery, tissue engineering, drug targeting
Introduction
Polymers are probably the most important and widely used class of materials that contributed to the industrial revolution. The ability to tailor a polymer's mechanical properties and bio-degradation kinetics, made these materials extremely appealing for biomedical applications [1–7].
Lactic acid (LA) is a naturally-occurring compound, which is the precursor of downstream metabolic product of pyruvate, through the Cori cycle [8, 9]. LA can also be manufactured synthetically in large scale by the fermentation of corn, beets and carbohydrates from other crops [10]. The monomeric form of LA is approved by the regulatory agencies as a food additive [11].
Polylactic acid (PLA), was first synthesized by the French chemist Theophile-Jules Pelouze in 1845, through the poly-condensation of LA into low molecular weight PLA, ranging from 800-5,000 g/mol [12]. Later, DuPont's chemist Wallace Hume Carothers, inventor of nylon, improved the production process, enabling to increase the average molecular weight of the polymer to 100,000 g/mol [13]. This improved PLA's mechanical properties, making it a promising new candidate to compete with other commercial polymers. However, PLA's costly production process hampered broad implementation, narrowing the polymer's use to biomedical applications. In 1989, Dr. Patrick R. Gruber invented a low-cost commercial process for producing high molecular weight PLA, expanding its use to many additional areas, such as agricultural sheets and biodegradable disposable bags [14]. In fact, today PLA is the second most traded polymer worldwide [15].
The surge in the biomedical use of PLA is evident even after a brief look at the increasing number of publications in the field, averaging above 1,000 research papers per year over the past five years (Pubmed; search term: polylactic acid). The impact of PLA is increasingly growing as new modes for tailoring the polymer's properties to address different biomedical needs are being discovered (Figure 1). It is estimated that the biomedical market of PLA is expanding by more than 10% annually, making it one of the most important polymers in biomedical use [16].
Figure 1. Natural biodegrading pathways of polylactic acid (PLA) in primary sites of medical device implantation.
The main mechanism for PLA degradation inside the body is hydrolysis of the ester-bond backbone. The degradation rate is dependent on pH and temperature in the tissue, on one hand, and on the composition of the polymer, on the other. Therefore the degradation rate in sites of inflammation will be higher compared to healthy tissues. Chirality also affects the degradation rate, D-PLA will degrade faster compared to L-PLA. This enables to tailor the implant to the desired organ and for the desired biomedical application.
This review focuses on the biocompatibility of PLA with special attention to the biodegradation and bio-elimination this class of polymers has inside the body.
Permanent and temporary biomedical systems
Biomedical systems can be divided into two groups: permanent and temporary [17–20]. For example, we would want an artificial knee to be non-degradable and permanent, so that the patient would not need to undergo replacement surgeries every decade [21]. Contrarily, sutures, should ultimately degrade after the sewed tissue heals and regains sufficient physical strength to support itself [22–24]. In another example, in the past, when orthopedic surgeons used screws during repair processes these were permanent, displacing any bone that could have grown back over time [1, 25–29]. New materials, such as PLA, have transient properties, starting strong and supportive, and degrading over time to give space to newly grown bone to take over the space the device took in the body [30]. Table 1 summarizes different PLA-based devices and their degradation profiles.
Table 1. PLA based formulations degradation profiles.
| PLA composition | Chirality | Degradation conditions | Degradation time | Usage | References |
|---|---|---|---|---|---|
| (PEG-5k)2-tartarate-PLA | D,L | In vitro: 0.1 M phosphate buffer, pH 7.4, 37 C° | 60% weight loss after 4 weeks | Drug delivery nanospheres | [3] |
| PLA-octreotide microparticles | D | In vitro: 0.1 M phosphate buffer, pH 7.4, 37 C° | 15-30% weight loss after 40 days | Octreotide drug delivery | [4] |
| PLA-octreotide microparticles | D,L | In vitro: 0.1 M phosphate buffer, pH 7.4, 37 C° | 10-20% weight loss after 40 days | Octreotide drug delivery | [4] |
| PLA fibers | -* | In vivo: rat oral tissue | Full degradation between 42-70 days | Sutures | [23] |
| PLA | D,L | In vitro: 0.13 M phosphate buffer, pH 7.4, 37 C° | Plates: 11 weeks Films: 25 weeks | Size-dependence degradation testing | [31] |
| PLA films - initial Intrinsic viscosity OF 3.24 dL/g | D,L | In vivo: subdermal implantation in rabbits | 58% weight loss over 60 weeks | Experimental degradation rate study | [32] |
| PLA films | L | In vitro: 0.2 M citrate buffer, pH 7, 37 C° | 10% weight loss over 16 weeks | Materials in surgery | [33] |
| PLA microcapsules | D,L | In vivo: Intra-muscular injection to rats | Breakdown first observed after 150 days and total erosion after 420 days | Drug delivery | [34] |
| PLA microspheres | L | In vivo: injection to rats' livers | Implant conserved its geometrical form 14 months after injection | Drug delivery | [35] |
| PLA implant | L | In vivo transplantation to rats | 14% weight loss after 3 months | Implants | [36] |
| PLA sheet | -* | In vivo: transplantation in the infraorbital rim of macace monkeys | Remnants found at the surgical site 38-weeks post implantation | PLA bone implants | [37] |
| PLA-Zn 0.05% (98% L) | D,L | In vitro: 0.13 M phosphate buffer, pH 7.4, 37 C° | 9% weight loss after 12 months | PLA screws | [38] |
| PLA plates | D,L | In vivo: subperiosteal in rabbits | 70% loss of molecular weight after 42 days | PLA bone implants | [39] |
Chirality isn’t indicated.
PLA Chemistry
Lactide, PLA's primary monomeric building block, can have an L-lactide or D-lactide chirality. Selecting L-chirality over D will determine the polymer's biodegradability and mechanical properties, as well as if the polymer is semi-crystalline or amorphous, respectively. In general, the D and L/D forms degrade more rapidly than the L form [40–42].
PLA, having L, D or L/D (the former, a blend of both enantiomers) forms are semi-permeable to water and oxygen, making it even more susceptible to biodegradation compared to other biomedical polymers [16, 43]. Increasing the porosity of the polymer, or the surface area-to-volume ratio, will enhance the degradation rate [44]. Moreover, when the device is porous, the degradation will occur both on the outer surface and in the inner core of the polymer [45].
The biodegradation of PLA
ASTM International Standardization Organization (standard #D-5488-94d) defines 'biodegradable' as 'capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, and biomass' [16].
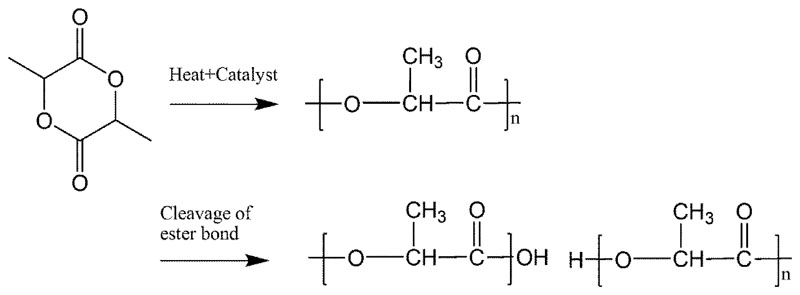
The primary mechanism by which PLA is degraded inside the body is hydrolysis of the ester-bond backbone, Figure 2 [31]. The degradation products can be either monomeric LA or oligomers of LA. The hydrolytic degradation is then further catalyzed by the newly-formed carboxylic groups at the terminal ends of the cleaved PLA chains [32].
Figure 2. The chemical synthesis and natural biodegradation pathway of polylactic acid (PLA) in vivo.
The synthesis of PLA is initiated by poly-condensation of lactic acid into low molecular weight polymer. Biodegradation occurs mostly at the inflammation site and enhanced by acid phosphatase and lactate dehydrogenase secreted by fibroblasts, macrophages and neutrophils.
Degradation occurs on the surface of the polymer and inside the polymer bulk, creating LA monomers and oligomers [46]. Moreover the diffusion of water into the polymer bulk degrades the polymer microstructure through the formation of internal cavities [46]. The cleaved monomers will diffuse out of the polymer over time; however, the diffusion of hydrolyzing water molecules throughout the polymer is far more rapid.
The degradation of PLA is greatly dependent on pH and temperature [33, 47]. Xu and co-workers [47] demonstrated that in physiological pH of 7.4 PLA brushes have a degradation time of 100 hours, in acidic pH of 3 there was no apparent degradation after 400 hours. They also showed that PLA's degradation rate was 4-folds faster in 37°C in comparison to 25°C in which full degradation occurred after 400 hours. Increasing the temperature further to 60°C resulted in an even faster degradation, reaching full degradation after less than 10 hours. The effect of pH on the degradation time can be utilized in order to tailor the half-life of the polymer construct towards the target tissue, as different tissues have different pH ranges [48, 49].
Kulkarni and co-workers [50] measured the degradation kinetics of PLA in biological media. They found that the polymer degrades by random scission of the polymer backbone, obeying second-order kinetics, having an activation energy of 11 kcal/mol. Interestingly, they and others show that L-PLA degrades in a slower manner compared to the D/L-PLA.
Beck and coworkers showed that the half-life of D/L-PLA microspheres injected intramuscularly to rats was 34 weeks [35, 51, 52]. Incorporating other monomers into the PLA matrix, increased the degradation rate; reiterating the concept that neat PLA polymer has slower degradation compared to di-block polymers.
In another study, Reed and Gilding [33], demonstrated that PLA sutures lose 50% of their weight after approx. 14 weeks at pH 7 and 37°C. However, the decline in the suture's tensile strength is far more rapid, declining 50% over 6-8 weeks. This indicates that the degradation process is not only erosion of the suture's surface, but also internal cleavage of molecular bonds inside the suture bulk, without mass loss [31, 53].
Multi-year degradation rates may be less suitable for drug delivery systems. To increase the degradation rate, PLA foams were created [45]. These systems have an extremely high surface area/polymer weight ratio, as well as large internal volumes into which the drugs are loaded. Such systems can release therapeutic doses of the drugs immediately after implantation and over several months [9, 54–58].
Enzymatic biodegradation
In 1981 David Franklin Williams discovered the ability to enzymatically degrade PLA into LA [59]. Surprisingly, he showed that proteinase K (sourced from Tritirachium album) and pronase (sourced from Streptomyces griseus) detached the PLA polymeric matrix at 37 °C. Later, it was shown that PLA-depolymerase, a 24 kDa bacterial enzyme, degrades PLA into monomeric LA [60, 61]. While such enzymes may be associated with infection, also inflammation can catalyze the degradation of PLA. Specifically, when any polymeric object is implanted in the body, a foreign body immune response is triggered [36, 62]. Immune cells swarm to the site of implantation to detect, quarantine and remove the foreign object [63]. These cells, include neutrophils, macrophages and fibroblasts, secrete an array of enzymes, such as acid phosphatase and lactate dehydrogenase, which enhance PLA degradation [57, 64, 65].
Clinical degradation and excretion of PLA
Stener and coworkers examined 77 patients eight years after being implanted L-PLA screws in their tibia and femor during tendon reconstruction [66]. The recovery of the PLA-treated group was similar to patients implanted with metal screws. One advantage a biodegradable screw grants is the ability to be replaced by endogenous tissue, during the healing and reconstruction process [2]. The half-life of L-PLA screws in tissue averages 24 months, verses, 12 months of L/D-PLA screws [2].
Facial reconstruction surgeries after trauma also demonstrated excellent tissue compatibility [37]. Remnants of the PLA implants (sheets) were found at the surgical site 38-weeks post implantation.
Upon tissue implantation, the PLA polymer is coated by phagocytic cells and a fibrous capsule, denoting a foreign body reaction [36]. A close look at radiolabeled PLA degradation products indicated these are secreted from the body, and not retained in any primary organ [36]. It is assumed excretion occurs through kidney filtration and urine or as carbon dioxide.
Cutright and Hunsuck [23], noticed that the diameter of PLA sutures increased slightly after implantation. This is most likely due to the diffusion of water between the polymer chains. They noticed the suture is partially degraded one month post implantation, but remnants of the suture were found in the tissue nearly two months after the procedure [23].
Polylactic acid in theranostics
The favorable biocompatibility of PLA has been utilized for combined drug delivery systems and diagnostics [22, 67]. For example, polymeric micelles (mean size of 20nm) accumulated in glioma tumors in mice 24 hr after intravenous administration [68]. To improve tissue-specific targeting various ligands (such as antibodies and sugars) are conjugated to the corona of the particles [69]. Liu and co-workers loaded PLA-based nanoscale micelles with the anti-cancer agent paclitaxel, together with the MRI contrast agent Gd and a specific cancer marker antibody [70]. In this manner they were able to track the biodistribution and targeting capacity of the nanoparticles to H22 (liver cancer) tumors, as well as observe the therapeutic response to the treatment. In a similar manner, Yang et al. developed PLA-based nanoparticles for siRNA co-delivery together with fluorescent and MRI diagnostic contrast agents [71]. These studies emphasize the modularity of PLA; allowing block polymerization with other materials such as polyethylene glycol (PEG), or complexation with inorganic materials such as iron-oxide and gold nanoparticles [72]. The ability to engineer modular theranostic systems of nanoscale dimensions which are safely biodegraded and secreted, opened the door to new applications that combine therapy and diagnostics [67, 73–77].
Adverse reactions
In the enormous body of literature, and thousands of clinical reports regarding the use of PLA devices since the 1980's, we found only a handful of reports that describe adverse effects of PLA in patients.
L-PLA orthopedic implant' complications may occur due to physical damage of the instrument or migration within joints [78]. For example, a posterior cruciate ligament reconstruction performed on a 16-year-old patient resulted in synovitis due to breakage of an L-PLA screw 2 years post implantation, required the removal of the screw fragments. In another case, 15 months after an anterior cruciate ligament reconstruction, the patient suffered from pain and swelling after the biodegradable screw broke and its head migrated intra-articularly. Awareness to the possible screw breakage and performing arthroscopy if symptoms arise can enable immediate removal of the damaged implant and minimizing further adverse effects [25]. However, these examples are rare, and in general only 0.2% of the procedures involved PLA implants present a foreign body reaction [79], as a result from the new interaction of the tissue and device at the implementation site and the device breakdown. It includes protein absorption, recruitment of macrophages and foreign body giant cells [80, 81]. A foreign body reaction is manifested as a cyst-like mass and can be addressed by the removal of device fragments [81, 82].
In one report a systemic allergic reaction occurred in a 30-year-old patient, after being implanted a PLA screws for anterior cruciate ligament reconstruction [83]. The patient suffered from a rash in the right femur, chronic fatigue, and localized alopecia. A skin end-point titration revealed that the PLA screws had allergenicity in this patient, by evaluating the skin reaction towards different concentration of PLA antigen solution [84].. After total removal of the screws, the symptoms disappeared. When PLA was injected intra-dermally, mild nodularity at the site of the injection were reported [85]. When an improper injection technique (incorrect depth for example) was applied, intraoral lesions were observed due to the migration of the dermal filler substance [86]. In summary, few patients were reported to have allergic responses to PLA. However, breakage, or wearing-down of the medical implant can induce foreign body reaction and inflammation. Tuning the mechanical properties of the polymer to the application site, is a prerequisite for a successful implant.
PLA in imensional (3D) printing technologies
Polymeric based 3D scaffolds have become a fundamental part of tissue engineering [87]. They are used in vitro as a platform for cell adhesion that simulates the ECM mechanical support, and in vivo as templates for organ regeneration. Table 2 summarizes different PLA based scaffolds printed with a 3D system. Scaffold design has a great impact on scaffolds’ mechanical properties and permeability. PLA High quality and resolution 3D scaffolds can be fabricated using 3D printing techniques [88]. For example, patient-specific scaffold design can be produced according to the anatomical data of the specific patient[89]. PLA is one of the most common biodegradable polymers used for 3D scaffold printing. The biodegradation time of PLA makes it a very attractive candidate for in vivo implantation. Recently, different combinations of PLA with other materials such as glass particles and PEG have been used to better control the scaffolds physical and mechanical properties and to improve the printing process [90–92].
Table 2. PLA based scaffolds printed with a 3D system.
| Material | Experiment | Application | References |
|---|---|---|---|
| PLA/Polydopamine | In vitro - hADSCs | Craniomaxillofacial bone lesion repair | [93] |
| PLLA/PLGA | In vitro – Human skin fibroblasts | Tissue engineering – skin fibroblasts | [94] |
| HA/Collagen/PLA | In vivo – Rabbits | Bone scaffold | [95] |
| PLA/PEG/G5 glass particles | In vitro – rMSC adhesion | Tissue engineering | [96] |
| PLA/β-TCP | In vivo – Rabbits | heterotopic bone formation | [97] |
| PDLA/rhBMP-2 | In vivo – Rats | Mandibular bone repair | [98] |
Summary
Polylactic acid is widely used clinically as a biomedical scaffold for implants, theranostics and drug delivery systems. It is simple to synthesize, and can be tailored for different therapeutic needs. PLA is naturally degraded over time into well-tolerated and safe degradation products, which are secreted from the body. When coming to design new systems with biomedical applications, considering PLA as a scaffold may prove as a wise decision.
Highlights.
-
-
Polylactic acid (PLA) is a biocompatible polymer that is used widely for biomedical applications.
-
-
PLA biodegrades into lactic acid (LA) or to carbon dioxide and water.
-
-
PLA degradation products are metabolized intracellularly or excreted in the urine and breath.
Adverse reactions or foreign body response to PLA are extremely rare.
Acknowledgements
This work was supported by ERC-STG-2015-680242. The authors also acknowledge the support of the Technion Integrated Cancer Center (TICC), the Russell Berrie Nanotechnology Institute, the Lorry I. Lokey Interdisciplinary Center for Life Sciences & Engineering, the Pre-Clinical Research Authority staff and the Biomedical Core Facility at the Rappaport Faculty of Medicine, as well as the Israel Ministry of Economy for a Kamin Grant (52752); the Israel Ministry of Science Technology and Space – Office of the Chief Scientist (3-11878); the Israel Science Foundation (1778/13); the Israel Cancer Association (2015-0116); the German-Israeli Foundation for Scientific Research and Development for a GIF Young grant (I-2328-1139.10/2012); the European Union FP-7 IRG Program for a Career Integration Grant (908049); a Mallat Family Foundation Grant; A.S. acknowledges Alon and Taub Fellowships. Ms. Krinsky wishes to thank the Baroness Ariane de Rothschild Women Doctoral Program for its generous support. Omer Adir acknowledges the support of the Technion Interdepartmental Nanotechnology Program.
References
- [1].Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- [2].Zhang SY, Bellinger AM, Glettig DL, Barman R, Lee YAL, Zhu JH, Cleveland C, Montgomery VA, Gu L, Nash LD, Maitland DJ, et al. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat Mater. 2015;14:1065. doi: 10.1038/nmat4355. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ben-Shabat S, Kumar N, Domb AJ. PEG-PLA block copolymer as potential drug carrier: Preparation and characterization. Macromol Biosci. 2006;6:1019–1025. doi: 10.1002/mabi.200600165. [DOI] [PubMed] [Google Scholar]
- [4].Bishara A, Domb AJ. PLA stereocomplexes for controlled release of somatostatin analogue. J Control Release. 2005;107:474–483. doi: 10.1016/j.jconrel.2005.05.026. [DOI] [PubMed] [Google Scholar]
- [5].Slager J, Domb AJ. Heterostereocomplexes prepared from D-PLA and L-PLA and leuprolide. II. Release of leuprolide. Biomacromolecules. 2003;4:1316–1320. doi: 10.1021/bm0300249. [DOI] [PubMed] [Google Scholar]
- [6].Manzano M, Vallet-Regí M. New developments in ordered mesoporous materials for drug delivery. Journal of Materials Chemistry. 2010;20:5593–5604. [Google Scholar]
- [7].Kar M, Molina M, Calderon M. How are we applying nanogel composites in biomedicine? Nanomedicine (Lond) 2017;12:1627–1630. doi: 10.2217/nnm-2017-0152. [DOI] [PubMed] [Google Scholar]
- [8].Pasteur L. Mémoire sur la fermentation appelée lactique. Comptes Rendus Chimie. 1857;45:913–916. [Google Scholar]
- [9].Cohen S, Yoshioka T, Lucarelli M, Hwang LH, Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm Res. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- [10].Auras RA, Lim L-T, Selke SEM, Tsuji H. Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications. John Wiley & Sons; 2011. [Google Scholar]
- [11].F.S. Agency. 2014. Current EU approved additives and their E Numbers. [Google Scholar]
- [12].Lim LT, Auras R, Rubino M. Processing technologies for poly(lactic acid) Prog Polym Sci. 2008;33:820–852. [Google Scholar]
- [13].Carothers WH, Hill JW. STUDIES OF POLYMERIZATION AND RING FORMATION. XII. LINEAR SUPERPOLYESTERS. Journal of the American Chemical Society. 1932;54:1559–1566. [Google Scholar]
- [14].Drumright RE, Gruber PR, Henton DE. Polylactic acid technology. Adv Mater. 2000;12:1841–1846. [Google Scholar]
- [15].Grand View Research; 2017. Lactic Acid Market Analysis By Application (Industrial, F&B, Pharmaceuticals, Personal Care) & Polylactic Acid (PLA) Market Analysis By Application (Packaging, Agriculture, Transport, Electronics, Textiles), And Segment Forecasts, 2014 - 2025. [Google Scholar]
- [16].Avérous L, Pollet E. Environmental Silicate Nano-Biocomposites. Springer-Verlag; London: 2012. Biodegradable Polymers. [Google Scholar]
- [17].Amini AR, Wallace JS, Nukavarapu SP. Short-term and long-term effects of orthopedic biodegradable implants. J Long Term Eff Med Implants. 2011;21:93–122. doi: 10.1615/jlongtermeffmedimplants.v21.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miceli E, Kar M, Calderón M. Interactions of organic nanoparticles with proteins in physiological conditions. Journal of Materials Chemistry B. 2017;5:4393–4440. doi: 10.1039/c7tb00146k. [DOI] [PubMed] [Google Scholar]
- [19].Fleige E, Quadir MA, Haag R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: concepts and application. Advanced drug delivery reviews. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [20].Khandare J, Calderon M, Dagia NM, Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chemical Society reviews. 2012;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- [21].Kreuz PC, Muller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res Ther. 2009;11:R33. doi: 10.1186/ar2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Racey GL, Wallace WR, Cavalaris CJ, Marguard JV. Comparison of a polyglycolic-polylactic acid suture to black silk and plain catgut in human oral tissues. J Oral Surg. 1978;36:766–770. [PubMed] [Google Scholar]
- [23].Cutright DE, Hunsuck EE. Tissue reaction to the biodegradable polylactic acid suture. Oral Surg Oral Med Oral Pathol. 1971;31:134–139. doi: 10.1016/0030-4220(71)90044-2. [DOI] [PubMed] [Google Scholar]
- [24].Weldon CB, Tsui JH, Shankarappa SA, Nguyen VT, Ma M, Anderson DG, Kohane DS. Electrospun drug-eluting sutures for local anesthesia. J Control Release. 2012;161:903–909. doi: 10.1016/j.jconrel.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shafer BL, Simonian PT. Broken poly-L-lactic acid interference screw after ligament reconstruction. Arthroscopy. 2002;18:E35. doi: 10.1053/jars.2002.32197. [DOI] [PubMed] [Google Scholar]
- [26].Abate JA, Fadale PD, Hulstyn MJ, Walsh WR. Initial fixation strength of polylactic acid interference screws in anterior cruciate ligament reconstruction. Arthroscopy. 1998;14:278–284. doi: 10.1016/s0749-8063(98)70143-4. [DOI] [PubMed] [Google Scholar]
- [27].Barber FA, Dockery WD. Long-term absorption of poly-L-lactic Acid interference screws. Arthroscopy. 2006;22:820–826. doi: 10.1016/j.arthro.2006.04.096. [DOI] [PubMed] [Google Scholar]
- [28].Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nature nanotechnology. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bostman O, Pihlajamaki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000;21:2615–2621. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- [31].Grizzi I, Garreau H, Li S, Vert M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size-dependence. Biomaterials. 1995;16:305–311. doi: 10.1016/0142-9612(95)93258-f. [DOI] [PubMed] [Google Scholar]
- [32].Pitt CG, Gratzl MM, Kimmel GL, Surles J, Schindler A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials. 1981;2:215–220. doi: 10.1016/0142-9612(81)90060-0. [DOI] [PubMed] [Google Scholar]
- [33].Reed A, Gilding D. Biodegradable polymers for use in surgery poly(glycolic)/poly(lactic acid) homo and copolymers: 2. In vitro degradation. Polymer. 1980;22:494–499. [Google Scholar]
- [34].Visscher GE, Robison RL, Maulding HV, Fong JW, Pearson JE, Argentieri GJ. Note - Biodegradation of and Tissue Reaction to Poly(Dl-Lactide) Microcapsules. Journal of Biomedical Materials Research. 1986;20:667–676. doi: 10.1002/jbm.820200510. [DOI] [PubMed] [Google Scholar]
- [35].Zielhuis SW, Nijsen JF, Seppenwoolde JH, Bakker CJ, Krijger GC, Dullens HF, Zonnenberg BA, van Rijk PP, Hennink WE, van het Schip AD. Long-term toxicity of holmium-loaded poly(L-lactic acid) microspheres in rats. Biomaterials. 2007;28:4591–4599. doi: 10.1016/j.biomaterials.2007.07.012. [DOI] [PubMed] [Google Scholar]
- [36].Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93:839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- [37].Cutright DE, Hunsuck EE. The repair of fractures of the orbital floor using biodegradable polylactic acid. Oral Surg Oral Med Oral Pathol. 1972;33:28–34. doi: 10.1016/0030-4220(72)90204-6. [DOI] [PubMed] [Google Scholar]
- [38].Schwach G, Vert M. In vitro and in vivo degradation of lactic acid-based interference screws used in cruciate ligament reconstruction. Int J Biol Macromol. 1999;25:283–291. doi: 10.1016/s0141-8130(99)00043-4. [DOI] [PubMed] [Google Scholar]
- [39].Tschakaloff A, Losken HW, Vonoepen R, Michaeli W, Moritz O, Mooney MP, Losken A. Degradation Kinetics of Biodegradable Dl-Polylactic Acid Biodegradable Implants Depending on the Site of Implantation. Int J Oral Max Surg. 1994;23:443–445. doi: 10.1016/s0901-5027(05)80043-8. [DOI] [PubMed] [Google Scholar]
- [40].Xinteng Z, Weisan P, Ruhua Z, Feng Z. Preparation and evaluation of poly (D, L-lactic acid) (PLA) or D, L-lactide/glycolide copolymer (PLGA) microspheres with estradiol. Pharmazie. 2002;57:695–697. [PubMed] [Google Scholar]
- [41].Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S. Poly-Lactic Acid: production, applications, nanocomposites, and release studies. Comprehensive Reviews in Food Science and Food Safety. 2010;9:551–571. doi: 10.1111/j.1541-4337.2010.00126.x. [DOI] [PubMed] [Google Scholar]
- [42].Fukushima K, Hirata M, Kimura Y. Synthesis and Characterization of Stereoblock Poly(lactic acid)s with Nonequivalent D/L Sequence Ratios. Macromolecules. 2007;40:3049–3055. [Google Scholar]
- [43].Lehermeier H, Dorgan J, Way J. Gas permeation properties of poly(lactic acid) J Membrane Sci. 2001;190:243–251. [Google Scholar]
- [44].Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- [45].Mikos AG, Thorsen AJ, Czerwonka LA, Bao Y, Langer R, Winslow DN, Vacanti JP. Preparation and Characterization of Poly(L-Lactic Acid) Foams. Polymer. 1994;35:1068–1077. [Google Scholar]
- [46].Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- [47].Xu L, Crawford K, Gorman CB. Effects of Temperature and pH on the Degradation of Poly(lactic acid) Brushes. Macromolecules. 2011;44:4777–4782. [Google Scholar]
- [48].Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. Journal of Nuclear Medicine. 2010;51:1167–1170. doi: 10.2967/jnumed.109.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee ES, Gao Z, Bae YH. Recent progress in tumor pH targeting nanotechnology. J Control Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kulkarni R, Moore E, Hegyeli A, AS, Leonard Fred. Biodegradable Poly(1actic acid) Polymers. J Biomed Mat Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- [51].Beck LR, Cowsar DR, Lewis DH, Gibson JW, Flowers CE., Jr New long-acting injectable microcapsule contraceptive system. Am J Obstet Gynecol. 1979;135:419–426. doi: 10.1016/0002-9378(79)90717-8. [DOI] [PubMed] [Google Scholar]
- [52].Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced drug delivery reviews. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- [53].Kulkarni RK, Moore EG, Hegyeli AF, Leonard F. Biodegradable poly(lactic acid) polymers. J Biomed Mater Res. 1971;5:169–181. doi: 10.1002/jbm.820050305. [DOI] [PubMed] [Google Scholar]
- [54].Langer R, Brem H, Falterman K, Klein M, Folkman J. Isolations of a cartilage factor that inhibits tumor neovascularization. Science. 1976;193:70–72. doi: 10.1126/science.935859. [DOI] [PubMed] [Google Scholar]
- [55].Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- [56].Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221:719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- [57].Kost J, Langer R. Responsive polymeric delivery systems. Advanced drug delivery reviews. 2001;46:125–148. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- [58].Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. Journal of biomedical materials research. Part A. 2006;77:351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- [59].Williams D. Enzymic hydrolysis of polylactic acid. Engineering in Medicine. 1981;10:5–7. [Google Scholar]
- [60].Nakamura K, Tomita T, Abe N, Kamio Y. Purification and characterization of an extracellular poly(L-lactic acid) depolymerase from a soil isolate, Amycolatopsis sp. strain K104-1. Appl Environ Microbiol. 2001;67:345–353. doi: 10.1128/AEM.67.1.345-353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cai H, Dave V, Gross RA, McCarthy SP. Effects of physical aging, crystallinity, and orientation on the enzymatic degradation of poly(lactic acid) Journal of Polymer Science. 1996;34:2701–2708. [Google Scholar]
- [62].Schroeder A, Turjeman K, Schroeder JE, Leibergall M, Barenholz Y. Using liposomes to target infection and inflammation induced by foreign body injuries or medical implants. Expert Opin Drug Deliv. 2010;7:1175–1189. doi: 10.1517/17425247.2010.517519. [DOI] [PubMed] [Google Scholar]
- [63].Colilla M, Manzano M, Vallet-Regi M. Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int J Nanomedicine. 2008;3:403–414. doi: 10.2147/ijn.s3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Schakenraad JM, Hardonk MJ, Feijen J, Molenaar I, Nieuwenhuis P. Enzymatic activity toward poly(L-lactic acid) implants. J Biomed Mater Res. 1990;24:529–545. doi: 10.1002/jbm.820240502. [DOI] [PubMed] [Google Scholar]
- [65].Goldbart R, Traitel T, Lapidot SA, Kost J. Enzymatically controlled responsive drug delivery systems. Polymers Adv Tech. 2002;13:1006–1018. [Google Scholar]
- [66].Stener S, Ejerhed L, Sernert N, Laxdal G, Rostgard-Christensen L, Kartus J. A Long-term, Prospective, Randomized Study Comparing Biodegradable and Metal Interference Screws in Anterior Cruciate Ligament Reconstruction Surgery: Radiographic Results and Clinical Outcome. Am J Sports Med. 2010;38:1598–1605. doi: 10.1177/0363546510361952. [DOI] [PubMed] [Google Scholar]
- [67].Swed A, Cordonnier T, Denarnaud A, Boyer C, Guicheux J, Weiss P, Boury F. Sustained release of TGF-beta1 from biodegradable microparticles prepared by a new green process in CO2 medium. Int J Pharm. 2015;493:357–365. doi: 10.1016/j.ijpharm.2015.07.043. [DOI] [PubMed] [Google Scholar]
- [68].Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer science. 2009;100:572–579. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed-Nanotechnol. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- [70].Liu Y, Li J, Liu F, Feng L, Yu D, Zhang N. Theranostic Polymeric Micelles for the Diagnosis and Treatment of Hepatocellular Carcinoma. J Biomed Nanotechnol. 2015;11:613–622. doi: 10.1166/jbn.2015.1945. [DOI] [PubMed] [Google Scholar]
- [71].Yang C, Vu-Quang H, Husum DMU, Tingskov SJ, Vinding MS, Nielsen T, Song P, Nielsen NC, Norregaard R, Kjems J. Theranostic poly(lactic-co-glycolic acid) nanoparticle for magnetic resonance/infrared fluorescence bimodal imaging and efficient siRNA delivery to macrophages and its evaluation in a kidney injury model. Nanomedicine. 2017;13:2451–2462. doi: 10.1016/j.nano.2017.08.007. [DOI] [PubMed] [Google Scholar]
- [72].Perez C, Sanchez A, Putnam D, Ting D, Langer R, Alonso MJ. Poly(lactic acid)-poly(ethylene glycol) nanoparticles as new carriers for the delivery of plasmid DNA. J Control Release. 2001;75:211–224. doi: 10.1016/s0168-3659(01)00397-2. [DOI] [PubMed] [Google Scholar]
- [73].Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics - application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–677. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Stewart MP, Lorenz A, Dahlman J, Sahay G. Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8:465–478. doi: 10.1002/wnan.1377. [DOI] [PubMed] [Google Scholar]
- [75].Fetsch C, Gaitzsch J, Messager L, Battaglia G, Luxenhofer R. Self-Assembly of Amphiphilic Block Copolypeptoids - Micelles, Worms and Polymersomes. Sci Rep. 2016;6 doi: 10.1038/srep33491. 33491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv Mater. 2010;22:4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- [77].Martin DT, Steinbach JM, Liu J, Shimizu S, Kaimakliotis HZ, Wheeler MA, Hittelman AB, Mark Saltzman W, Weiss RM. Surface-modified nanoparticles enhance transurothelial penetration and delivery of survivin siRNA in treating bladder cancer. Mol Cancer Ther. 2014;13:71–81. doi: 10.1158/1535-7163.MCT-13-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Konan S, Haddad FS. A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery. Knee. 2009;16:6–13. doi: 10.1016/j.knee.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [79].Böstman OM, Pihlajamäki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clinical orthopaedics and related research. 2000;371:216–227. [PubMed] [Google Scholar]
- [80].Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Seminars in immunology. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gonzalez-Lomas G, Cassilly RT, Remotti F, Levine WN. Is the Etiology of Pretibial Cyst Formation After Absorbable Interference Screw Use Related to a Foreign Body Reaction? Clinical Orthopaedics and Related Research. 2011;469:1082–1088. doi: 10.1007/s11999-010-1580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kim TK, Jeong TW, Lee DH. Foreign Body Reaction After PLC Reconstruction Caused by a Broken PLLA Screw. Orthopedics. 2014;37:E1129–E1132. doi: 10.3928/01477447-20141124-91. [DOI] [PubMed] [Google Scholar]
- [83].Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732–733. doi: 10.1016/j.arthro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- [84].Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. The Journal of allergy and clinical immunology. 1992;90:256–262. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- [85].Moyle GJ, Brown S, Lysakova L, Barton SE. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV medicine. 2006;7:181–185. doi: 10.1111/j.1468-1293.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- [86].Shahrabi-Farahani S, Lerman MA, Noonan V, Kabani S, Woo SB. Granulomatous foreign body reaction to dermal cosmetic fillers with intraoral migration. Oral surgery, oral medicineoral pathology and oral radiology. 2014;117:105–110. doi: 10.1016/j.oooo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- [87].Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- [88].Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. Journal of biological engineering. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL. 3D printing based on imaging data: review of medical applications. International journal of computer assisted radiology and surgery. 2010;5:335–341. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- [90].Serra T, Mateos-Timoneda MA, Planell JA, Navarro M. 3D printed PLA-based scaffolds: a versatile tool in regenerative medicine. Organogenesis. 2013;9:239–244. doi: 10.4161/org.26048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Serra T, Ortiz-Hernandez M, Engel E, Planell JA, Navarro M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Materials science & engineering. C, Materials for biological applications. 2014;38:55–62. doi: 10.1016/j.msec.2014.01.003. [DOI] [PubMed] [Google Scholar]
- [92].Stoppato M, Carletti E, Sidarovich V, Quattrone A, Unger RE, Kirkpatrick CJ, Migliaresi C, Motta A. Influence of scaffold pore size on collagen I development: A new in vitro evaluation perspective. J Bioact Compat Pol. 2013;28:16–32. [Google Scholar]
- [93].Kao CT, Lin CC, Chen YW, Yeh CH, Fang HY, Shie MY. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Materials science & engineering. C, Materials for biological applications. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028. [DOI] [PubMed] [Google Scholar]
- [94].Yang J, Shi G, Bei J, Wang S, Cao Y, Shang Q, Yang G, Wang W. Fabrication and surface modification of macroporous poly(L-lactic acid) and poly(L-lactic-co-glycolic acid) (70/30) cell scaffolds for human skin fibroblast cell culture. J Biomed Mater Res. 2002;62:438–446. doi: 10.1002/jbm.10318. [DOI] [PubMed] [Google Scholar]
- [95].Liao SS, Cui FZ, Zhang W, Feng QL. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. Journal of biomedical materials research. Part B, Applied biomaterials. 2004;69:158–165. doi: 10.1002/jbm.b.20035. [DOI] [PubMed] [Google Scholar]
- [96].Serra T, Planell JA, Navarro M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta biomaterialia. 2013;9:5521–5530. doi: 10.1016/j.actbio.2012.10.041. [DOI] [PubMed] [Google Scholar]
- [97].Cao L, Duan PG, Wang HR, Li XL, Yuan FL, Fan ZY, Li SM, Dong J. Degradation and osteogenic potential of a novel poly(lactic acid)/nano-sized beta-tricalcium phosphate scaffold. Int J Nanomedicine. 2012;7:5881–5888. doi: 10.2147/IJN.S38127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid--an experimental study in rats. Biomaterials. 2008;29:103–110. doi: 10.1016/j.biomaterials.2007.09.019. [DOI] [PubMed] [Google Scholar]