Abstract
Many approaches have been taken to identify new biomarkers of pancreatic ductal carcinoma (PDC). Since animal models can be sampled under controlled conditions, better standardization is possible compared with heterogeneous human studies. Transgenic rats with conditional activation of oncogenic RAS in pancreatic tissue develop PDC that closely resembles the biological and histopathological features of human PDC. Using this model, we evaluated the usefulness of leucine-rich α2-glycoprotein-1 (LRG-1) as a serum marker. In this study, we found that LRG-1 was overexpressed in rat PDC compared with normal pancreas tissue of the control rats. Serum levels of LRG-1 were also significantly higher in rats bearing PDC than in controls. Importantly, chronic pancreatitis in male Wistar Bonn/Kobori rats, which is a widely accepted as a model of chronic pancreatitis, did not cause serum levels of LRG-1 to become elevated. These results strongly support serum LRG-1 as a candidate biomarker for noninvasive diagnosis of PDC. Our models of pancreas cancer provide a useful strategy for evaluation of candidate markers applicable to human cancer.
Keywords: pancreas cancer, serum marker, animal model, leucine-rich α2-glycoprotein-1 (LRG-1)
Introduction
Pancreatic ductal carcinoma (PDC) carries the most dismal prognosis of all solid tumors. PDC is one of the most lethal types of cancer, with a five-year survival rate of less than 10% and a mortality rate closely approaching the incidence rate1. The survival rate of pancreas cancer patients can increase 6-fold with early detection2; however, at present biomarkers have limited utility for detecting early-stage PDC. Currently, the best serum marker for pancreatic cancer is carbohydrate antigen 19-9 (CA19-9). The CA19-9 epitope is found on oligosaccharide sialylated Lewis A antigen and on multiple protein carriers including mucin core proteins3. However, the utility of CA19-9 as a PDC biomarker is limited, as its performance varies with disease stage. Unfortunately, CA19-9 may also be positive in patients with nonmalignant diseases including chronic pancreatitis2, 4. Additionally, CA19-9 is not detectable in 5–10% of fucosyl transferase-deficient patients who are negative for the Lewis antigens5.
RAS activation is thought to initiate focal lesions in the pancreatic ducts, which undergo graded histological progression to PDC. We have established transgenic rat lines carrying a human KrasG12V or a human HrasG12V oncogene6, 7 in which the expression of the transgene is regulated by the Cre/loxP system. Targeted activation of the transgene is accomplished by injection of a Cre recombinase-carrying adenovirus (AxCANCre) into the pancreatic ducts through the common bile duct. Importantly, neoplastic lesions in the transgenic rats exhibit morphological and biological similarities to those observed in human pancreas lesions6, 7, 8, 9, 10, 11. Therefore, the transgenic PDC-rat model is suitable for screening for potential biomarkers of human PDC.
Leucine-rich α2-glycoprotein-1 (LRG-1) was identified as a serum protein containing eight leucine-rich repeats12, 13. Increased LRG-1 expression has been demonstrated in ovarian cancer14, lung cancer15, and pancreatic cancer tissue16. In the present study, we present data that supports the use of LRG-1 as a serum marker for pancreas cancer.
Materials and Methods
Animals
Male HrasG12V or KrasG12V transgenic (Hras250, Kras301) rats were obtained from CLEA Japan (Tokyo, Japan); the establishment of these rats has been reported previously6, 7, 8. Male Wistar Bonn/Kobori (WBN/Kob) rats were purchased from Japan SLC (Hamamatsu, Japan). The rats were maintained in plastic cages in an air-conditioned room with a 12-h light/12-h dark cycle. At the end of the experimental period, rats were euthanized by exsanguination from the abdominal aorta under deep anesthesia using isoflurane. All animal experiments were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Nagoya City University Graduate School of Medical Sciences and approved by the Institutional Animal Care and Use Committee (H25M-21).
Tumor induction and pathological examination
Adenovirus vector, AxCANCre, was purified after amplification in HEK293 cells, and pancreas tumors were induced as previously described6, 7, 8, 17, 18. AxCANCre is a recombinant adenovirus vector that expresses Cre recombinase under the control of the CAG promoter. Infection of HrasG12V or KrasG12V transgenic (Hras250, Kras301) rats with AxCANCre results in expression of HrasG12V in infected cells in the Hras250 rat and expression of KrasG12V in infected cells in the Kras301 rat6, 7. AxCANCre was introduced into the pancreatic ducts of Hras250 and Kras301 male rats via injection into the common bile duct. Four weeks after injection of AxCANCre, the rats were euthanized. After sacrifice, tumor nodules present in PDC-bearing pancreas tissue were isolated and frozen in liquid nitrogen for RNA assays or fixed in 4% paraformaldehyde and processed for histological observation. Pancreas tissue from control rats was also frozen in liquid nitrogen for RNA assays or fixed in 4% paraformaldehyde and processed for histological observation. The expression of the KrasG12V transgene, which is expressed as an HA-fusion protein, was confirmed by immunohistochemistry using HA-tagged antibodies8. The expression of the HrasG12V transgene could not be determined directly because the transgene does not have a tag in the Hras250 rat by which it can be distinguished from endogenous Hras. Therefore, expression of total active RAS in the pancreas of these animals was determined using a RAS activation kit as previously described7.
RT-PCR
RT-PCR was performed as previously described6. Total RNA was isolated using ISOGEN (Nippon Gene, Toyama, Japan) and reverse-transcribed using PrimeScript RTase (Takara Bio Inc., Otsu, Japan) with Random Primers (Invitrogen, Carlsbad, CA, USA). The following primers were used for PCR: LRG-1, 5’- TTGGCAGCATCAAGGGAGAA -3’ and 5’-AGCATTGCGAGTCAGATCCA-3’; ribosome 18S, 5’-GTTGGTGGAGCGATTTGTCT-3’ and 5’-GGCCTCACTAAACCATCCAA-3’. The amplification protocol consisted of 32 (LRG-1) or 30 (18S) cycles of denaturation for 30 s at 94°C, annealing for 30 s at 60°C, and extension for 30 s at 72°C.
Serum test
Blood was collected from the tail vein prior to terminal sacrifice and from the abdominal aorta at the time of terminal sacrifice; serum samples were stored at −80°C until use. The serum levels of rat LRG-1 were quantified by ELISA (Code No. 27770, Rat LRG Assay Kit, IBL, Gunma, Japan). For all ELISA experiments, each sample was assayed in duplicate, and the absorbance was measured with a Model 680 microplate reader (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All statistical analyses were done using JMP software (SAS Institute Japan, Tokyo, Japan). The data for the levels of LRG-1 in the serum were compared using the Wilcoxon test for nonparametric data. The data from the same rat were analyzed with the paired t test. Receiver operating characteristic (ROC) curves were constructed, and the area under the curve (AUC) was calculated to evaluate specificity and sensitivity. Cutoffs were defined for LRG-1 as the optimum point (Youden index) at which sensitivity and specificity were maximized. P-values <0.05 were considered to be statistically significant.
Results
Expression of KrasG12V and HrasG12V in the rat pancreas
Four weeks after injection of AxCANCre, whitish nodules were observed throughout the pancreata of both types of RAS transgenic rats, and histological examination showed that these nodules were adenocarcinomas. Neoplastic lesions were not found in other organs. The expression of the HA-tagged human KrasG12V transgene was assessed by immunohistochemistry using HA-tag antibodies. The results were essentially identical to previous results (data not shown; see Tanaka et al.8): the transgene was overexpressed in AxCANCre infected pancreas. The expression of the human HrasG12V transgene was inferred by assessing the expression of activated RAS in control and AxCANCre-infected pancreas. The results were essentially identical to previous results (data not shown; see Ueda et al.7): activated RAS was overexpressed in the AxCANCre-infected pancreas.
Expression of LRG-1 in the rat pancreas
We previously performed transcriptomic analysis of pancreatic tissue by microarray analysis10. We selected genes encoding upregulated secretory proteins because the secreted proteins, including LRG-1, are a potential source of serum biomarkers. Our array analysis indicated that LRG-1 was overexpressed in PDC compared with the pancreata of control rats. Previous reports revealed overexpression of LRG-1 in pancreas carcinomas in humans16. Therefore, we focused on the expression of LRG-1. In the present study, the expression level of the LRG-1 gene was assessed by RT-PCR and found to be higher in PDC compared with normal pancreas tissue of the controls rats in both the Kras301 and Hras250 groups (Fig. 1).
Fig. 1.
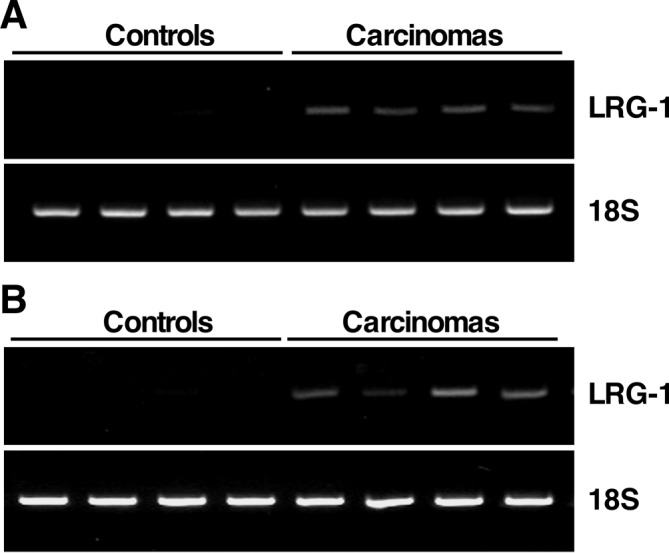
Overexpression of leucine-rich α2-glycoprotein-1 (LRG-1) in pancreatic ductal carcinoma (PDC)-bearing pancreas tissue. RT-PCR for LRG-1 in normal pancreas tissue (controls) and PDC-bearing pancreas tissue (carcinomas) in (A) KrasG12V and (B) HrasG12V transgenic rats. Each lane represents RNA prepared from an individual rat. 18S ribosome serves as an RNA control.
Serum levels of LRG-1 in rats with pancreas carcinoma
To examine whether LRG-1 can be used as a surrogate marker of pancreas carcinoma, we used an ELISA to measure the serum LRG-1 levels in rats with PDC and their controls. The level of LRG-1 in PDC-bearing Kras301 rats was 706.2 ± 35.03 ng/ml (mean ± SE; n=17), and that of the empty adenovirus vector-treated control Kras301 rats was 458.37 ± 28.74 ng/ml (n=14; P<0.001; Fig. 2A). The level of LRG-1 in PDC-bearing Hras250 rats was 599.9 ± 76.83 ng/ml (n=9), and that of the control Hras250 rats was 368.4 ± 28.18 ng/ml (n=10; P<0.001; Fig. 2B).
Fig. 2.
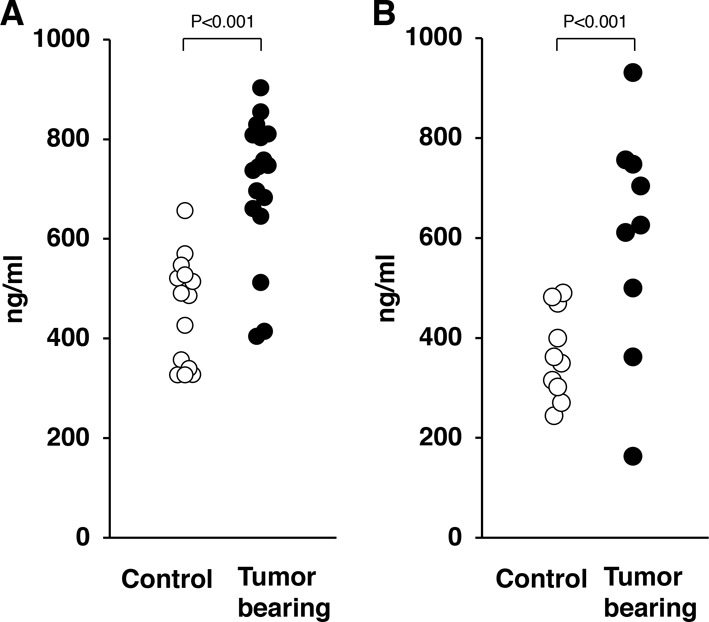
Serum levels of leucine-rich α2-glycoprotein-1 (LRG-1) in RASG12V transgenic rats. The serum levels of LRG-1 in (A) KrasG12V and (B) HrasG12V transgenic rats with pancreatic ductal carcinoma (PDC) were significantly higher than in control rats (P<0.001). Open circles, control rat; closed circle, PDC-bearing rat.
ROC curve analysis
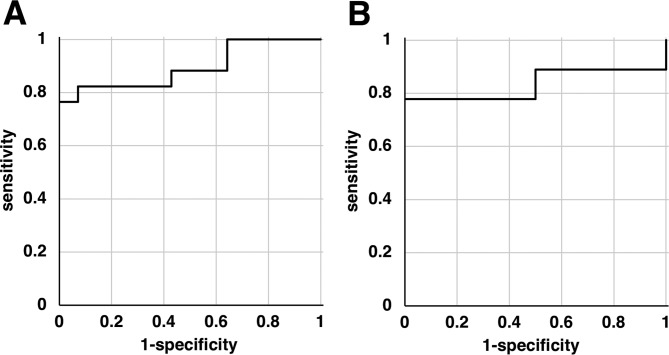
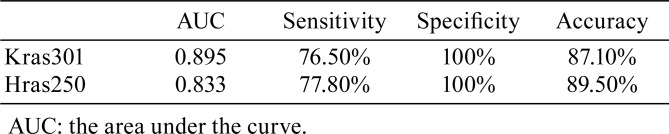
To determine if the changes in the serum levels of LRG-1 could significantly differentiate between pancreatic cancer and controls, ROC curves were constructed (Fig. 3). The AUCs for LRG-1 were 0.895 (95% confidence interval [CI], 0.71–0.97) for Kras301 and 0.833 (95% CI, 0.48–0.96) for Hras250 (Table 1).
Fig. 3.
Receiver operating characteristics (ROC) curve analysis of pancreas cancer-bearing rats versus control rats. ROC curves were constructed to evaluate leucine-rich α2-glycoprotein-1 (LRG-1) as a marker of pancreatic ductal carcinoma (PDC). (A) The area under the curve (AUC) was 0.895 for LRG-1 in KrasG12V transgenic rats. (B) The AUC was 0.833 for LRG-1 in HrasG12V transgenic rats.
Table 1. Performance of Leucine-rich α2-glycoprotein-1 (LRG-1) in Rat Pancreas Cancer Models.
ROC curves were used to evaluate the sensitivities and specificities of serum LRG-1 levels used to distinguish PDC-bearing from PDC-free rats. Using the Youden index-based optimal cut-point of 660.2 ng/ml, the sensitivity and specificity were 76.5% and 100% for LRG-1 in Kras301 rats, and using the Youden index-based optimal cut-point of 499.6 ng/ml, the sensitivity and specificity were 77.8% and 100% for LRG-1 in Hras250 rats. The positive predictive value, negative predictive value, and accuracy of the LRG-1 serum-level cutoff of 660.2 ng/ml in distinguishing rats with pancreatic cancer from controls were 100, 77.8, and 87.1%, respectively, in Kras301 rats (Table 1). The corresponding values in Hras250 rats for an LRG-1 serum-level cutoff of 499.6 ng/ml were 100, 83.3, and 89.5%, respectively (Table 1). These results demonstrate that the serum level of LRG-1 has reasonable capability to differentiate rats with pancreatic cancer from controls.
Serum levels of LRG-1 in WBN/Kob rats
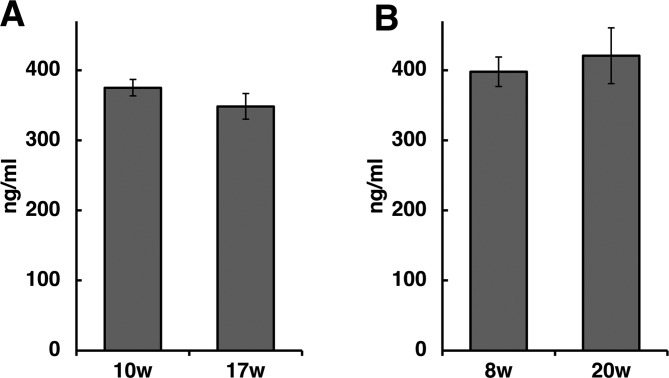
It is reported that LRG-1 is overexpressed during inflammation19. Therefore we evaluated serum levels of LRG-1 in a rat model of chronic pancreatitis. The male Wistar Bonn/Kobori (WBN/Kob) rat is a widely accepted rodent model of chronic pancreatitis20. Chronic pancreatitis-like lesions are observed in 100% of male WBN/Kob rats, which commonly develop chronic pancreatitis by the age of 3 months and diabetes mellitus by 9 months21. In the present study, fibrosis was observed in small areas of the pancreas of 10-week-old WBN/Kob rats, and pancreatitis with marked fibrosis was observed in 17-week-old rats. LRG-1 serum levels were 375.0 ± 11.65 ng/ml in 10-week-old rats (n=4) and did not change significantly in these rats when they were 17 weeks old (348.4 ± 18.35 ng/ml, n=4; Fig. 4A). Because the 10-week-old rats had begun to develop pancreatitis, we examined rats when they were 8 weeks old and 20 weeks old. The pancreata showed no abnormality in 8-week-old male WBN/Kob rats, but there was pancreatitis with marked fibrosis when the rats were 20 weeks old. In the 8-week-old rats, the LRG-1 serum levels were 397.8 ± 20.9 ng/ml (n=4), and these levels did not change significantly when these rats were 20 weeks old (420.6 ± 39.7 ng/ml, n=4; Fig. 4B), indicating that there was no significant difference in LRG-1 serum levels in WBN/Kob rats before and after developing chronic pancreatitis.
Fig. 4.
Serum levels of leucine-rich α2-glycoprotein-1 (LRG-1) in Wistar Bonn/Kobori (WBN/Kob) rats. The WBN/Kob rat strain is an animal model of spontaneous chronic pancreatitis. Male WBN/Kob rats commonly develop chronic pancreatitis by the age of 3 months. (A) Serum levels of LRG-1 were measured in 10- and 17-week-old male WBN/Kob rats: blood was collected from the same rats at the 10- and 17-week time points. (B) Because fibrosis had begun to develop in the 10-week-old rats, serum levels of LRG-1 were also measured in 8- and 20-week-old male WBN/Kob rats: blood was collected from the same rats at the 8- and 20-week time points. The serum levels of LRG-1 showed little change during progression of chronic pancreatitis.
Discussion
In this study, we demonstrated that LRG-1 gene expression in PDC-bearing pancreata was higher than in controls (Fig. 1) and that serum levels of LRG-1 were significantly higher in PDC-bearing rats compared with control rats (Fig. 2). Serum and plasma LRG-1 levels have been reported to be elevated in pancreas cancer patients22, 23, and our data strongly support the postulation that serum LRG-1 is a useful marker for cancer detection in pancreas cancer patients.
In humans, the sensitivity of serum LRG-1 for predicting PDC is slightly lower than the specificity of serum LRG-1 for predicting PDC22, 23. In the PDC-rat model, we obtained a similar result: the optimal predictive serum level of LRG-1 was 100% specific with a sensitivity of approximately 76% to 78%. This suggests that there is potential for false negative results, and therefore, the diagnostic results using LRG-1 should be interpreted with caution. Detection of PDC will be improved by combination with another marker with high sensitivity. For example, it is reported that a three-marker panel (LRG-1, TIMP1, and CA19-9) improved detection of early-stage PDC in humans compared with CA19-9 alone23.
The basal level of LRG-1 in serum was significantly different between Kras301 and Hras250 rats. In this study, we used homozygous Kras301 rats and heterozygous Hras250 rats. The Hras250 rats were maintained by breeding heterozygous male Hras250 rats with female SD rats. In contrast, the Kras301 rats were maintained by breeding homozygous male with homozygous female rats. It is likely that Kras301 rats with higher levels of LRG-1 were unintentionally selected in the course of establishing the homozygous rats. Another possible cause of the difference is the integration site of the RAS transgene. Integration sites in the genome may affect the expression of LRG-1.
We also investigated the expression of LRG-1 in the WBN/Kob rat, which develops spontaneous chronic pancreatitis20. The pancreatitis that develops in this rat mimics the pathophysiological processes of chronic inflammation and fibrosis in humans20, 21. In our study, 8-week-old rats had no discernable pancreatic pathologies; the first pathologic changes in the pancreas were inflammatory cell infiltration and fibrosis in 10-week-old rats, and extensive fibrosis and parenchymal destruction were present in 17- and 20-week-old rats. Importantly, there were no significant differences in serum LRG-1 in these rats between before and after developing pancreatitis, indicating that elevated LRG-1 is specific to malignancy.
LRG-1 was first identified as a highly conserved member of the leucine-rich repeat (LRR) family of proteins12, 13. The LRR structural motif has been identified in a wide variety of proteins and participates in processes such as ligand–receptor interactions, enzyme inhibition, and cell adhesion24, 25. It has been suggested that the major function of the LRR motif is to provide a structural framework that enables protein–protein interactions24, 25. LRR proteins are overexpressed in several types of cancer, where they are proposed to have roles in processes such as cell signaling and metastasis26, 27, 28, 29, 30, 31, 32, 33, 34.
LRG-1 has been proposed as a possible serum and/or plasma biomarker of pancreatic cancer22. Serum/plasma LRG-1 levels are also elevated in patients with several other types of cancer, including lung15, 35, ovarian14, 36, gastric37, colon38, brain39, and biliary tract cancers40. LRG-1 is also detected and upregulated in urine samples from lung41, 42 and ovarian43 cancer patients. Therefore, it is difficult to identify the tumor location using LRG-1 in humans. Animal models can be induced to specifically develop only pancreas tumors. Hence, the elevated serum levels of LRG-1 in the present study were derived from PDC. The serum level of LRG-1 was higher in rats with relatively large pancreas tumors. Although quantitative analysis was not done in this study, it seems likely that there is a relationship between the serum level of LRG-1 and tumor size, and it is possible that the relative tumor size could be estimated from the serum level of LRG-1. Consequently, it is possible that LRG-1 may also be able to be used to screen for candidate chemotherapeutic agents, which could be evaluated for human use. Further studies need to be performed to assess whether LRG-1 can be used for evaluation of the effectiveness of chemotherapy.
The function of LRG-1 remains unknown. Expression of LRG-1 is regulated by IL-6 synergistically with either IL-1β or TNFα19, and a recent study identified LRG-1 as a direct downstream target of PPARβ/δ44. Due to its leucine-rich repeats, it is predicted that LRG-1 could have a role in cell adhesion13, 25. A role for LRG-1 in granulocyte differentiation has also been suggested45. LRG-1 was reported to be coordinately expressed with the TGF-β type II receptor46, and LRG-1 activates the TGF-β angiogenic switch by binding directly to the TGF-β accessory receptor endoglin47. Furthermore, LRG-1 promotes TGF-β-mediated growth suppression of Lewis lung carcinoma cell lines48. In addition, overexpression of LRG-1 enhances tumor migration in gastric cancer37, and LRG-1 promotes proliferation and inhibits apoptosis of colorectal cancer cells via RUNX1 activation49. These reports suggest a potential role of LRG-1 in cancer progression.
A variety of approaches have been taken in an attempt to identify biomarkers of PDC in humans, including analysis of cell-free nucleic acids (e.g., mutant DNA, methylated DNA, and noncoding RNAs), metabolites, autoantibodies, glycosylated antigens, tumor-derived exosomes, and circulating tumor cells50, 51, 52, 53, 54, 55, 56. In contrast to human studies, animal models offer uniform environmental conditions, standardized blood and tissue sampling, and defined stages of tumor development, thereby reducing biological and nonbiological heterogeneity. The rat pancreas cancer model provides a controlled model system for the preliminary identification of possible PDC biomarkers. We have identified several candidate biomarkers by proteome, metabolome, and transcriptome analysis using the rat pancreas cancer model9, 10, 11, and in the present study, we confirmed LRG-1 as a strong candidate for a serum biomarker of PDC. LRG-1 is also upregulated in the serum of the rat colon cancer model ApcPirc/+ rat, which like humans develops adenomas and localized adenocarcinomas preferentially in the colon57; increased serum LRG-1 has also been reported in human colon cancer patients38. A final goal of cancer biomarker research is the development of noninvasive tests that enable early cancer detection. Our findings indicate that the rat models of pancreas cancer used in the present study provide a useful strategy to identify candidate markers applicable to human cancer.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Numbers 26460476 and 17K08767 (K.F.), the Ichihara International Scholarship Foundation (K.F.), the Aichi Cancer Research Foundation (K.F.), and the Project for Development of Innovative Research on Cancer Therapeutics (15cm0106113h0002; H.T.) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Siegel RL, Miller KD, and Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 67: 7–30. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Chari ST, Kelly K, Hollingsworth MA, Thayer SP, Ahlquist DA, Andersen DK, Batra SK, Brentnall TA, Canto M, Cleeter DF, Firpo MA, Gambhir SS, Go VL, Hines OJ, Kenner BJ, Klimstra DS, Lerch MM, Levy MJ, Maitra A, Mulvihill SJ, Petersen GM, Rhim AD, Simeone DM, Srivastava S, Tanaka M, Vinik AI, and Wong D. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 44: 693–712. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akagi J, Takai E, Tamori Y, Nakagawa K, and Ogawa M. CA19-9 epitope a possible marker for MUC-1/Y protein. Int J Oncol. 18: 1085–1091. 2001. [DOI] [PubMed] [Google Scholar]
- 4.Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, Nicolini A, Topolcan O, and Heinemann V. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 21: 441–447. 2010. [DOI] [PubMed] [Google Scholar]
- 5.Scarà S, Bottoni P, and Scatena R. CA 19-9: Biochemical and clinical aspects. Adv Exp Med Biol. 867: 247–260. 2015. [DOI] [PubMed] [Google Scholar]
- 6.Fukamachi K, Tanaka H, Hagiwara Y, Ohara H, Joh T, Iigo M, Alexander DB, Xu J, Long N, Takigahira M, Yanagihara K, Hino O, Saito I, and Tsuda H. An animal model of preclinical diagnosis of pancreatic ductal adenocarcinomas. Biochem Biophys Res Commun. 390: 636–641. 2009. [DOI] [PubMed] [Google Scholar]
- 7.Ueda S, Fukamachi K, Matsuoka Y, Takasuka N, Takeshita F, Naito A, Iigo M, Alexander DB, Moore MA, Saito I, Ochiya T, and Tsuda H. Ductal origin of pancreatic adenocarcinomas induced by conditional activation of a human Ha-ras oncogene in rat pancreas. Carcinogenesis. 27: 2497–2510. 2006. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Fukamachi K, Futakuchi M, Alexander DB, Long N, Tamamushi S, Minami K, Seino S, Ohara H, Joh T, and Tsuda H. Mature acinar cells are refractory to carcinoma development by targeted activation of Ras oncogene in adult rats. Cancer Sci. 101: 341–346. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabushita S, Fukamachi K, Kikuchi F, Ozaki M, Miyata K, Sukata T, Deguchi Y, Tanaka H, Kakehashi A, Kawamura S, Uwagawa S, Wanibuchi H, Suzui M, Alexander DB, and Tsuda H. Twenty-one proteins up-regulated in human H-ras oncogene transgenic rat pancreas cancers are up-regulated in human pancreas cancer. Pancreas. 42: 1034–1039. 2013. [DOI] [PubMed] [Google Scholar]
- 10.Yabushita S, Fukamachi K, Tanaka H, Fukuda T, Sumida K, Deguchi Y, Mikata K, Nishioka K, Kawamura S, Uwagawa S, Suzui M, Alexander DB, and Tsuda H. Metabolomic and transcriptomic profiling of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Carcinogenesis. 34: 1251–1259. 2013. [DOI] [PubMed] [Google Scholar]
- 11.Yabushita S, Fukamachi K, Tanaka H, Sumida K, Deguchi Y, Sukata T, Kawamura S, Uwagawa S, Suzui M, and Tsuda H. Circulating microRNAs in serum of human K-ras oncogene transgenic rats with pancreatic ductal adenocarcinomas. Pancreas. 41: 1013–1018. 2012. [DOI] [PubMed] [Google Scholar]
- 12.Haupt H, and Baudner S. [Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author’s transl)]. Hoppe Seylers Z Physiol Chem. 358: 639–646. 1977. [PubMed] [Google Scholar]
- 13.Takahashi N, Takahashi Y, and Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci USA. 82: 1906–1910. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen JD, Boylan KL, Jemmerson R, Geller MA, Misemer B, Harrington KM, Weivoda S, Witthuhn BA, Argenta P, Vogel RI, and Skubitz AP. Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients. J Ovarian Res. 3: 21 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okano T, Kondo T, Kakisaka T, Fujii K, Yamada M, Kato H, Nishimura T, Gemma A, Kudoh S, and Hirohashi S. Plasma proteomics of lung cancer by a linkage of multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis. Proteomics. 6: 3938–3948. 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kakisaka T, Kondo T, Okano T, Fujii K, Honda K, Endo M, Tsuchida A, Aoki T, Itoi T, Moriyasu F, Yamada T, Kato H, Nishimura T, Todo S, and Hirohashi S. Plasma proteomics of pancreatic cancer patients by multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis (2D-DIGE): up-regulation of leucine-rich alpha-2-glycoprotein in pancreatic cancer. J Chromatogr B Analyt Technol Biomed Life Sci. 852: 257–267. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukamachi K, Iigo M, Hagiwara Y, Shibata K, Futakuchi M, Alexander DB, Hino O, Suzui M, and Tsuda H. Rat N-ERC/mesothelin as a marker for in vivo screening of drugs against pancreas cancer. PLoS One. 9: e111481 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukamachi K, Tanaka H, Sakai Y, Alexander DB, Futakuchi M, Tsuda H, and Suzui M. A novel reporter rat strain that expresses LacZ upon Cre-mediated recombination. Genesis. 51: 268–274. 2013. [DOI] [PubMed] [Google Scholar]
- 19.Shirai R, Hirano F, Ohkura N, Ikeda K, and Inoue S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun. 382: 776–779. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K, Kim JH, Hara H, Aso R, Akimoto T, and Nakama K. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol. 6: 231–247. 1990. [PubMed] [Google Scholar]
- 21.Mori Y, Yokoyama J, Nishimura M, Kurata H, Miura J, and Ikeda Y. Diabetic strain (WBN/Kob) of rat characterized by endocrine-exocrine pancreatic impairment due to distinct fibrosis. Pancreas. 5: 452–459. 1990. [DOI] [PubMed] [Google Scholar]
- 22.Furukawa K, Kawamoto K, Eguchi H, Tanemura M, Tanida T, Tomimaru Y, Akita H, Hama N, Wada H, Kobayashi S, Nonaka Y, Takamatsu S, Shinzaki S, Kumada T, Satomura S, Ito T, Serada S, Naka T, Mori M, Doki Y, Miyoshi E, and Nagano H. Clinicopathological significance of leucine-rich α2-glycoprotein-1 in sera of patients with pancreatic cancer. Pancreas. 44: 93–98. 2015. [DOI] [PubMed] [Google Scholar]
- 23.Capello M, Bantis LE, Scelo G, Zhao Y, Li P, Dhillon DS, Patel NJ, Kundnani DL, Wang H, Abbruzzese JL, Maitra A, Tempero MA, Brand R, Firpo MA, Mulvihill SJ, Katz MH, Brennan P, Feng Z, Taguchi A, and Hanash SM. Sequential validation of blood-based protein biomarker candidates for early-stage pancreatic cancer. J Natl Cancer Inst. 109: djw266 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchanan SG, and Gay NJ. Structural and functional diversity in the leucine-rich repeat family of proteins. Prog Biophys Mol Biol. 65: 1–44. 1996. [DOI] [PubMed] [Google Scholar]
- 25.Kobe B, and Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 11: 725–732. 2001. [DOI] [PubMed] [Google Scholar]
- 26.Fukamachi K, Matsuoka Y, Kitanaka C, Kuchino Y, and Tsuda H. Rat neuronal leucine-rich repeat protein-3: cloning and regulation of the gene expression. Biochem Biophys Res Commun. 287: 257–263. 2001. [DOI] [PubMed] [Google Scholar]
- 27.Fukamachi K, Matsuoka Y, Ohno H, Hamaguchi T, and Tsuda H. Neuronal leucine-rich repeat protein-3 amplifies MAPK activation by epidermal growth factor through a carboxyl-terminal region containing endocytosis motifs. J Biol Chem. 277: 43549–43552. 2002. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, and Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 37: 528–533. 2003. [DOI] [PubMed] [Google Scholar]
- 29.Hamano S, Ohira M, Isogai E, Nakada K, and Nakagawara A. Identification of novel human neuronal leucine-rich repeat (hNLRR) family genes and inverse association of expression of Nbla10449/hNLRR-1 and Nbla10677/hNLRR-3 with the prognosis of primary neuroblastomas. Int J Oncol. 24: 1457–1466. 2004. [PubMed] [Google Scholar]
- 30.Looyenga BD, Furge KA, Dykema KJ, Koeman J, Swiatek PJ, Giordano TJ, West AB, Resau JH, Teh BT, and MacKeigan JP. Chromosomal amplification of leucine-rich repeat kinase-2 (LRRK2) is required for oncogenic MET signaling in papillary renal and thyroid carcinomas. Proc Natl Acad Sci USA. 108: 1439–1444. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piepoli A, Palmieri O, Maglietta R, Panza A, Cattaneo E, Latiano A, Laczko E, Gentile A, Carella M, Mazzoccoli G, Ancona N, Marra G, and Andriulli A. The expression of leucine-rich repeat gene family members in colorectal cancer. Exp Biol Med (Maywood). 237: 1123–1128. 2012. [DOI] [PubMed] [Google Scholar]
- 32.Xi HQ, Cai AZ, Wu XS, Cui JX, Shen WS, Bian SB, Wang N, Li JY, Lu CR, Song Z, Wei B, and Chen L. Leucine-rich repeat-containing G-protein-coupled receptor 5 is associated with invasion, metastasis, and could be a potential therapeutic target in human gastric cancer. Br J Cancer. 110: 2011–2020. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh A, Takatori A, Hossain MS, Hasan MK, Tagawa M, Nagase H, and Nakagawara A. Unfavorable neuroblastoma prognostic factor NLRR2 inhibits cell differentiation by transcriptional induction through JNK pathway. Cancer Sci. 107: 1223–1232. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo W, Tan P, Rodriguez M, He L, Tan K, Zeng L, Siwko S, and Liu M. Leucine-rich repeat-containing G protein-coupled receptor 4 (Lgr4) is necessary for prostate cancer metastasis via epithelial-mesenchymal transition. J Biol Chem. 292: 15525–15537. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guergova-Kuras M, Kurucz I, Hempel W, Tardieu N, Kádas J, Malderez-Bloes C, Jullien A, Kieffer Y, Hincapie M, Guttman A, Csánky E, Dezso B, Karger BL, and Takács L. Discovery of lung cancer biomarkers by profiling the plasma proteome with monoclonal antibody libraries. Mol Cell Proteomics. 10: 010298 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J, Yin H, Zhu J, Buckanovich RJ, Thorpe JD, Dai J, Urban N, and Lubman DM. Validation of LRG1 as a potential biomarker for detection of epithelial ovarian cancer by a blinded study. PLoS One. 10: e0121112 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto M, Takahashi T, Serada S, Sugase T, Tanaka K, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Naka T, Mori M, and Doki Y. Overexpression of leucine-rich α2-glycoprotein-1 is a prognostic marker and enhances tumor migration in gastric cancer. Cancer Sci. 108: 2052–2060. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ladd JJ, Busald T, Johnson MM, Zhang Q, Pitteri SJ, Wang H, Brenner DE, Lampe PD, Kucherlapati R, Feng Z, Prentice RL, and Hanash SM. Increased plasma levels of the APC-interacting protein MAPRE1, LRG1, and IGFBP2 preceding a diagnosis of colorectal cancer in women. Cancer Prev Res (Phila). 5: 655–664. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyauchi E, Furuta T, Ohtsuki S, Tachikawa M, Uchida Y, Sabit H, Obuchi W, Baba T, Watanabe M, Terasaki T, and Nakada M. Identification of blood biomarkers in glioblastoma by SWATH mass spectrometry and quantitative targeted absolute proteomics. PLoS One. 13: e0193799 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandanayake NS, Sinclair J, Andreola F, Chapman MH, Xue A, Webster GJ, Clarkson A, Gill A, Norton ID, Smith RC, Timms JF, and Pereira SP. A combination of serum leucine-rich α-2-glycoprotein 1, CA19-9 and interleukin-6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer. 105: 1370–1378. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Cao J, Li L, Liu Y, Zhao H, Li N, Li B, Zhang A, Huang H, Chen S, Dong M, Yu L, Zhang J, and Chen L. Identification of urine protein biomarkers with the potential for early detection of lung cancer. Sci Rep. 5: 11805 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zhang Y, Qiu F, and Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 32: 1976–1983. 2011. [DOI] [PubMed] [Google Scholar]
- 43.Smith CR, Batruch I, Bauça JM, Kosanam H, Ridley J, Bernardini MQ, Leung F, Diamandis EP, and Kulasingam V. Deciphering the peptidome of urine from ovarian cancer patients and healthy controls. Clin Proteomics. 11: 23 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sng MK, Chan JSK, Teo Z, Phua T, Tan EHP, Wee JWK, Koh NJN, Tan CK, Chen JP, Pal M, Tong BMK, Tnay YL, Ng XR, Zhu P, Chiba S, Wang X, Wahli W, and Tan NS. Selective deletion of PPARβ/δ in fibroblasts causes dermal fibrosis by attenuated LRG1 expression. Cell Discov. 4: 15 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Donnell LC, Druhan LJ, and Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 72: 478–485. 2002. [PubMed] [Google Scholar]
- 46.Sun D, Kar S, and Carr BI. Differentially expressed genes in TGF-beta 1 sensitive and resistant human hepatoma cells. Cancer Lett. 89: 73–79. 1995. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Abraham S, McKenzie JAG, Jeffs N, Swire M, Tripathi VB, Luhmann UFO, Lange CAK, Zhai Z, Arthur HM, Bainbridge J, Moss SE, and Greenwood J. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 499: 306–311. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takemoto N, Serada S, Fujimoto M, Honda H, Ohkawara T, Takahashi T, Nomura S, Inohara H, and Naka T. Leucine-rich α-2-glycoprotein promotes TGFβ1-mediated growth suppression in the Lewis lung carcinoma cell lines. Oncotarget. 6: 11009–11022. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Zhang X, Zhang J, Fang J, Ge Z, and Li X. LRG1 promotes proliferation and inhibits apoptosis in colorectal cancer cells via RUNX1 activation. PLoS One. 12: e0175122 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandra Gupta S, and Nandan Tripathi Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int J Cancer. 140: 1955–1967. 2017. [DOI] [PubMed] [Google Scholar]
- 51.Dumstrei K, Chen H, and Brenner H. A systematic review of serum autoantibodies as biomarkers for pancreatic cancer detection. Oncotarget. 7: 11151–11164. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halbrook CJ, and Lyssiotis CA. Employing Metabolism to Improve the Diagnosis and Treatment of Pancreatic Cancer. Cancer Cell. 31: 5–19. 2017. [DOI] [PubMed] [Google Scholar]
- 53.Kosaka N, Iguchi H, and Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 101: 2087–2092. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis AR, Valle JW, and McNamara MG. Pancreatic cancer: Are “liquid biopsies” ready for prime-time? World J Gastroenterol. 22: 7175–7185. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nuzhat Z, Kinhal V, Sharma S, Rice GE, Joshi V, and Salomon C. Tumour-derived exosomes as a signature of pancreatic cancer - liquid biopsies as indicators of tumour progression. Oncotarget. 8: 17279–17291. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa CM, and Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 35: 1441–1450. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivancic MM, Irving AA, Jonakin KG, Dove WF, and Sussman MR. The concentrations of EGFR, LRG1, ITIH4, and F5 in serum correlate with the number of colonic adenomas in ApcPirc/+ rats. Cancer Prev Res (Phila). 7: 1160–1169. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]