Abstract
Hexyl acetate (CAS No. 142-92-7) is a naturally occurring ester compound that has a fruity odor. Despite its frequent use as a nature-identical flavoring agent, there are limited repeated dose toxicity data for hexyl acetate. Here we performed a 13-week subchronic toxicity study of hexyl acetate in male and female Crl:CD(SD) rats under GLP regulations. Hexyl acetate was given orally by gavage at doses of 0, 100, 300, or 1,000 mg/kg/day using corn oil as the vehicle. No significant toxicological changes in general condition, body weights, food intake, ophthalmology, hematology, organ weights, and histopathological findings were observed in any groups. Urinalysis revealed occult blood in two male animals treated with 1,000 mg/kg/day hexyl acetate, and one showed red blood cells in the urine sediment. Furthermore, blood biochemistry showed a significant increase in inorganic phosphorus levels in males treated with 1,000 mg/kg/day hexyl acetate. These results indicated that the no-observed-adverse-effect level (NOAEL) of hexyl acetate was 300 mg/kg/day for males and more than 1,000 mg/kg/day for females.
Keywords: hexyl acetate, food additive, flavoring agent, subchronic toxicity, urinalysis, SD rat
Hexyl acetate (CAS No. 142-92-7) is a naturally occurring ester compound that has a fruity odor and is widely used as a nature-identical flavoring agent. It is officially registered on the list of designated additives in Japan and is listed in the Code of Federal Regulations (CFR) as one of the food additives permitted for direct addition to food for human consumption (21 CFR 172.515) in the US.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated hexyl acetate as a flavoring agent categorized as an ester of aliphatic acyclic primary alcohols with aliphatic linear saturated carboxylic acids1. Hexyl acetate was assigned to Class I based on the structural class of flavoring agents. The estimated intake levels of hexyl acetate by humans in the US and Europe were 160 μg/person/day and 3,200 μg/person/day, respectively, and the latter was greater than the Class I threshold of 1,800 μg/person/day. However, the hexyl acetate metabolites hexanoic acid and acetic acid are both endogenously found in physiological metabolisms and were not considered to exceed the physiological range. Therefore, it was concluded that hexyl acetate would not present safety concerns for normal use as a flavoring agent based on the structural class and metabolism1.
As part of risk assessment, hexyl acetate was not mutagenic in the Ames test2. Despite the use of hexyl acetate as a flavoring agent, there are limited data concerning its repeated dose toxicity2. Our laboratory has investigated the toxicity of a number of food additives, including representative flavoring agents of each category, in rodent models3, 4, 5, 6. Here we conducted a 13-week subchronic toxicity study of hexyl acetate orally administered to SD rats by gavage to clarify the toxicological profile and establish a no-observed-adverse-effect level (NOAEL).
Hexyl acetate (lot No. 8633223, purity 99.6%) produced by Takata Koryo Co., Ltd. (Hyogo, Japan) was provided by the Division of Standards and Evaluation, Department of Food Safety, Ministry of Health, Labour and Welfare, Japan, with support of the Japan Flavor & Fragrance Materials Association (Tokyo, Japan). Gavage with corn oil (lot No. V3A0313; Nacalai Tesque, Kyoto, Japan) as the vehicle was chosen as the route of administration because of the insolubility in water and volatility of hexyl acetate. Hexyl acetate solutions were freshly prepared every eight days and kept refrigerated until use. After storage for 8 days at 4°C, the residual ratio of hexyl acetate in the stock solution was confirmed to be more than 90% (94.4–100.9%) by gas chromatography.
A total of 40 male and 40 female specific pathogen-free rats (Crl:CD(SD), 4 weeks old) were purchased from Charles River Laboratories Japan (Yokohama, Japan) and used after acclimation for one week. The animals were individually housed in metallic cages in a room with a barrier system controlled with respect to the light/dark cycle (12 h), ventilation (air-exchange rate of 15 times/h), temperature (23 ± 2°C), and relative humidity (40–75%) during the study. The cages were exchanged once a week. Each animal had free access to tap water and basal diet (CE-2; CLEA Japan, Tokyo, Japan). At the beginning of the experiment, the animals were randomly allocated to four groups of 10 male and 10 female rats each, based on their body weights measured just before starting the test chemical treatment.
In a preliminary 28-day study of hexyl acetate administered at doses of 0, 100, 300, and 1,000 mg/kg/day, no significant toxicity was observed in any of the treated groups (data not shown). Based on these results, we used these four doses for both sexes of rats in the 13-week toxicity study of hexyl acetate.
The study design was in accordance with the Guidelines for Designation for Food Additives and Revision of Standards for Use of Food Additives of Japan (1996) and approved by the Animal Care and Utilization Committee of the National Institute of Health Sciences, Japan. The stability test for hexyl acetate solution in corn oil and the 13-week subchronic toxicity study were conducted in compliance with GLP regulations at the Hatano Research Institute, Food and Drug Safety Center, Japan.
General conditions and mortality were checked daily. Body weights and the amounts of supplied and residual diet were measured once a week during the experimental period.
Ophthalmologic examinations were carried out before the start of the experiment and during the 12th week of hexyl acetate administration. The anterior ocular segment, optic media, and ocular fundus were examined using a binocular indirect ophthalmoscope and a slit lamp after macroscopic observation.
Urine samples for urinalysis were collected from 5 rats/group using metabolic cages over periods of 4 and 24 h at room temperature during the last week of administration. The urine samples were tested for pH, protein, glucose, ketone, bilirubin, occult blood, urobilinogen, color, turbidity, urine sediments (red blood cells, white blood cells, casts, crystals, and epithelial cells), volume, specific gravity, and density and gross volume of electrolytes (Na, K, and Cl).
All rats were fasted overnight at the completion of the treatment, and blood samples for hematology and blood biochemistry were collected from the abdominal vena cava under deep anesthesia with pentobarbital sodium. The following hematological parameters were analyzed using XT-2000iV and CA-1000 automatic hematology analyzers (Sysmex, Kobe, Japan): red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), white blood cell count (WBC), differential leukocytes, and reticulocytes. Plasma biochemical analysis was performed using a JCA-BM6010 clinical chemistry analyzer (JEOL, Tokyo, Japan) to assess the following parameters: total protein (TP), albumin (Alb), albumin/globulin ratio (A/G), glucose, total cholesterol (T-Chol), triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), urea nitrogen (BUN), creatinine (Cre), total bilirubin (Bil), alkaline phosphatase (ALP), inorganic phosphorus (IP), calcium (Ca), sodium (Na), potassium (K), and chlorine (Cl).
A complete necropsy was performed for all animals, and the brain, thymus, heart, lung, liver, kidneys, spleen, testes, ovaries, uterus, prostate (ventral lobe), submandibular glands, seminal vesicles, pituitary gland, thyroid glands, and adrenal glands were weighed. These organs and tissues from the spinal cord, eyes, harderian glad, optic nerve, nasal cavity, Zymbal gland, tongue, esophagus, trachea, aorta, parathyroid glands, pancreas, stomach, small and large intestines, submandibular and mesenteric lymph nodes, vagina, urinary bladder, femur and sternum with bone marrow, skeletal muscle, sciatic nerve, skin, and mammary gland were fixed in 10% neutral-buffered formalin, and paraffin-embedded sections were prepared and stained with hematoxylin and eosin for histopathological examination. Testes and epididymides were fixed in Bouin’s fixative solution. Histopathological assessment was performed on all tissues from animals in the control group and the group given the highest dose, unless any treatment-related lesions were observed.
Variances in the data for body weights during the experimental period as well as for food intake, urinalysis, hematology, blood biochemistry, and organ weights were checked for homogeneity by Bartlett’s test. When the data were homogeneous, one-way analysis of variance was conducted. For heterogeneous data, the Kruskal-Wallis test was applied. When statistically significant differences were indicated, the Dunnett’s multiple comparison test was used to compare the control and treatment groups. Comparisons of histopathological findings, incidences, and grades were analyzed by the Fisher’s exact probability test and Mann-Whitney’s U test, respectively. P values of <0.05 were considered to be statistically significant.
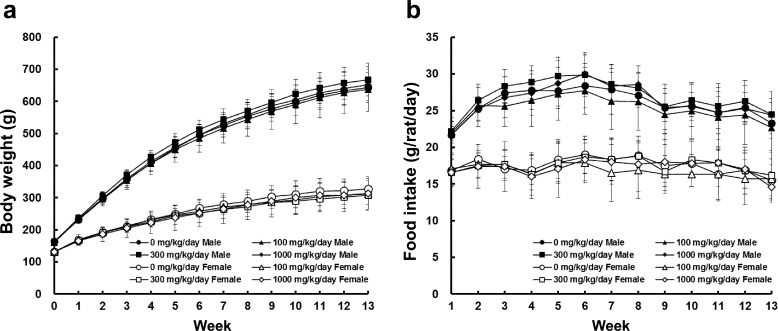
No significant clinical signs were noted throughout the experimental period, and all animals survived until the scheduled necropsy. There were no significant differences in body weights and daily food intake for either sex (Fig. 1). No ophthalmic lesions indicative of toxicity were observed in any of the treatment groups (data not shown).
Fig. 1.
Body weight (a) and daily food intake (b) data for male (close symbols) and female (open symbols) SD rats treated with the indicated dose of hexyl acetate for 13 weeks. Each group had 10 animals. The error bars represent the standard deviation for the experimental groups.
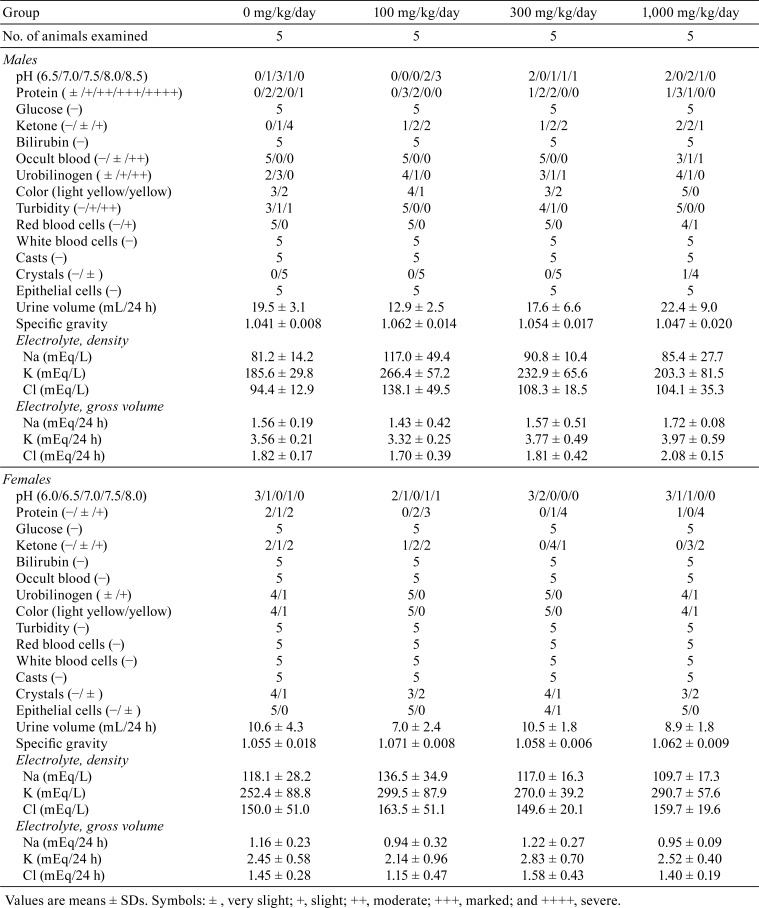
Urinalysis data are summarized in Table 1. Occult blood was detected in two males treated with 1,000 mg/kg/day hexyl acetate, and one had red blood cells in the urine sediment.
Table 1. Urinalysis Data for SD Rats Treated with Hexyl Acetate for 13 Weeks.
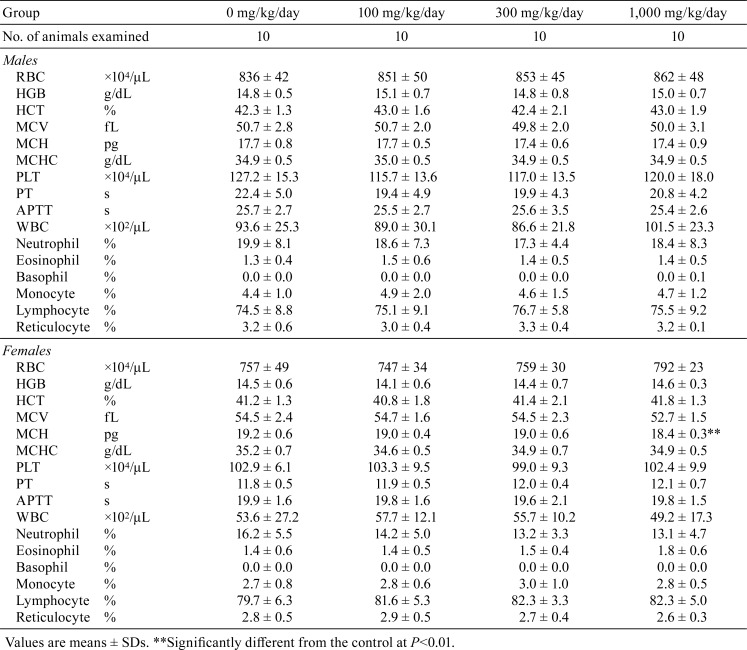
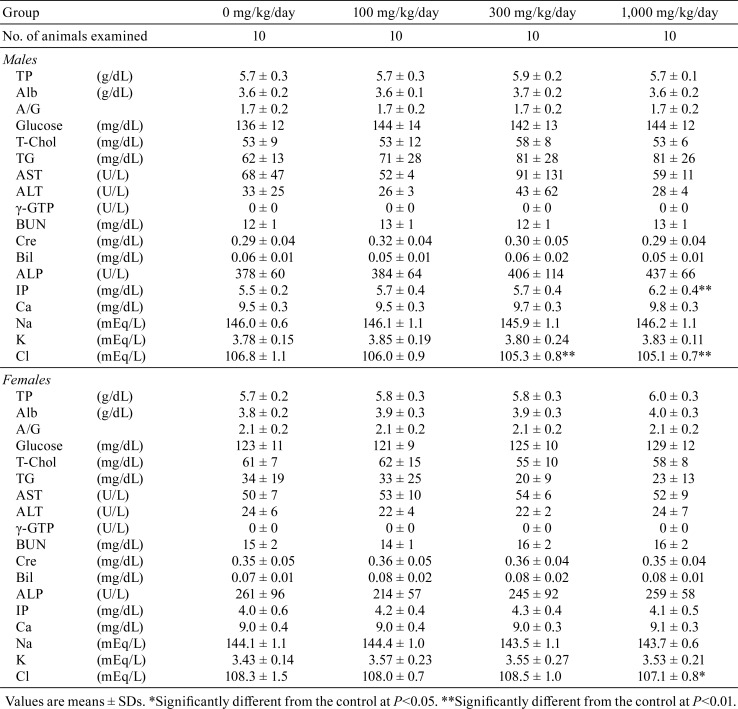
Hematology and blood biochemistry data are shown in Tables 2 and 3, respectively. A significant decrease in MCH was detected in female rats given 1,000 mg/kg/day hexyl acetate (Table 2). In blood biochemistry, a significant increase in IP was noted in males in the 1,000 mg/kg/day group (Table 3). Significant decreases in Cl were observed in males in the 300 mg/kg/day group and in both sexes in the 1,000 mg/kg/day group.
Table 2. Hematology Data for SD Rats Treated with Hexyl Acetate for 13 Weeks.
Table 3. Blood Biochemistry Data for SD Rats Treated with Hexyl Acetate for 13 Weeks.
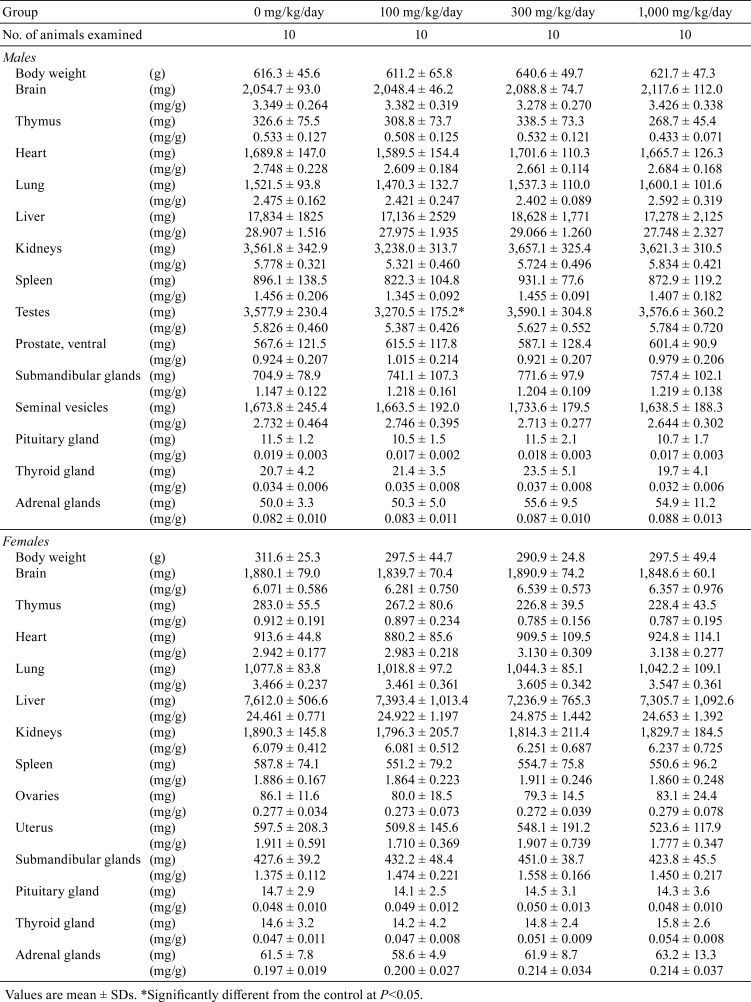
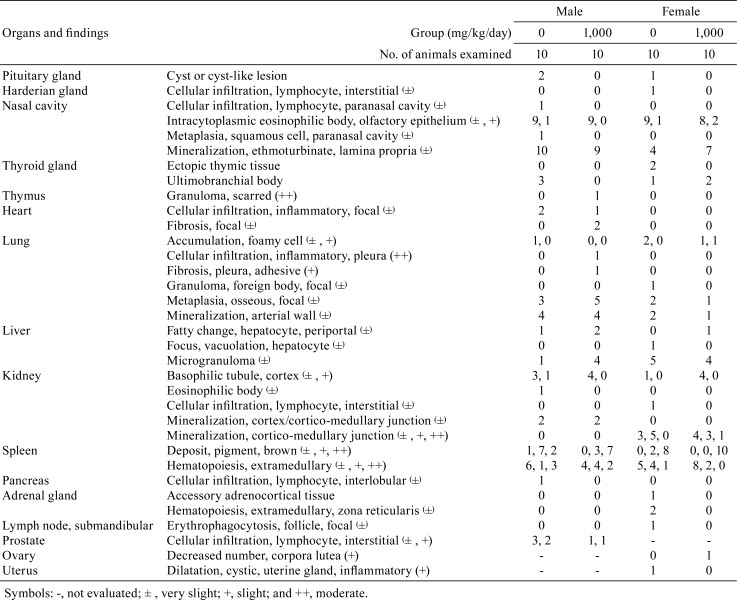
Data for organ weights and histopathological findings are summarized in Tables 4 and 5, respectively. In males, absolute testes weights were significantly decreased in the 100 mg/kg/day group (Table 4). However, the lack of any relationship with dose suggested that this difference was not associated with exposure to the test substance. Although several lesions in various organs were sporadically detected, no significant treatment-dependent alteration in the incidence of these lesions was apparent (Table 5).
Table 4. Organ Weight Data for SD Rats Treated with Hexyl Acetate for 13 Weeks.
Table 5. Histopathological Findings for SD Rats Treated with Hexyl Acetate for 13 Weeks.
In the present 13-week subchronic toxicity study of hexyl acetate, no toxicological changes in general condition, body weights, food intake, ophthalmology, hematology, organ weights, and histopathological analysis were observed. Urinalysis demonstrated that 1,000 mg/kg/day hexyl acetate induced occult blood and red blood cells in the urine sediment of male rats. Although there were no histopathological lesions in the kidney and urinary bladder, obvious hemorrhagic changes in the urinary system should be evaluated as potential toxicologic effects. In males in the 1,000 mg/kg/day group, a significant increase in IP levels was detected in blood biochemistry, and this increase was considered to be associated with the hexyl acetate treatment. Since there were no changes in Ca levels and histopathological lesions in related organs such as the thyroid gland, further investigation is required to clarify the detailed mechanisms involved in this change.
A significant decrease in MCH was detected in females in the 1,000 mg/kg/day group, and this was considered to be incidental due to the lack of changes in other RBC parameters and related histopathological findings. Similarly, decreases in Cl levels in males in the 300 and 1,000 mg/kg/day groups and females in the 1,000 mg/kg/day group were considered to have no toxicological significance, because there were no abnormalities in related parameters and no significant histopathological changes were noted in the kidney. Moreover, fluctuations in Cl levels were within the range of background data for the facility (average ± 2SD: 106.7 ± 3.2 mEq/L for males and 108.5 ± 3.2 mEq/L for females).
A 13-week subchronic toxicity study of octyl acetate (CAS No. 112-14-1), a flavoring agent that has a structure that is closely related to hexyl acetate, was previously performed7. Male and female SD rats were given octyl acetate by gavage (5 days per week) at doses of 0, 100, 500, or 1,000 mg/kg/day for 13 weeks. Reductions in body weight gain, increased relative weights of the liver and kidney, and nephropathy characterized by dilated tubules with granular casts and regenerative tubules were observed with statistical significance in male rats treated with high-dose octyl acetate. There were no significant toxicological changes induced by octyl acetate in hematology and ophthalmological examination. No increases in IP or other parameters related to renal disorders were detected in serum biochemistry, and urinalysis was not performed7. The histopathological lesions observed in the kidney were related to α2u-globulin nephropathy2, which is specific to male rats and thus not relevant for human risk assessment8. The hemorrhagic changes in the urinary system induced by hexyl acetate might be associated with other mechanisms rather than α2u-globulin nephropathy, as there was no increase in eosinophilic droplets in renal tubules, a characteristic change that reflects α2u-globulin accumulation.
The present study demonstrated that toxicological changes induced by oral administration of hexyl acetate could be observed in urinalysis and blood biochemistry in male SD rats given 1,000 mg/kg/day hexyl acetate. These results indicated that the NOAEL of hexyl acetate is 300 mg/kg/day for males and more than 1,000 mg/kg/day for females.
Disclosure of Potential Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by the Ministry of Health, Labour and Welfare, Japan.
References
- 1.The forty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series 40. 1998. from IPCS INCHEM website: http://www.inchem.org/documents/jecfa/jecmono/v040je14.htm.
- 2.Api AM, Belsito D, Botelho D, Bruze M, Burton GA, Jr, Buschmann J, Dagli ML, Date M, Dekant W, Deodhar C, Francis M, Fryer AD, Jones L, Joshi K, La Cava S, Lapczynski A, Liebler DC, O’Brien D, Patel A, Penning TM, Ritacco G, Romine J, Sadekar N, Salvito D, Schultz TW, Sipes IG, Sullivan G, Thakkar Y, Tokura Y, and Tsang S. RIFM fragrance ingredient safety assessment, Hexyl acetate, CAS Registry Number 142-92-7. Food Chem Toxicol. 118(Suppl 1): S103–S113. 2018. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, Toyoda T, Morikawa T, Takahashi M, Inoue K, and Ogawa K. A 13-week subchronic toxicity study of 2-ethylbutanal in F344 rats. Regul Toxicol Pharmacol. 100: 118–126. 2018. [DOI] [PubMed] [Google Scholar]
- 4.Toyoda T, Cho YM, Mizuta Y, Akagi J, and Ogawa K. A 13-week subchronic toxicity study of ferric citrate in F344 rats. Food Chem Toxicol. 74: 68–75. 2014. [DOI] [PubMed] [Google Scholar]
- 5.Akagi J, Cho YM, Mizuta Y, Toyoda T, and Ogawa K. Subchronic toxicity evaluation of 5-hexenyl isothiocyanate, a nature identical flavoring substance from Wasabia japonica, in F344/DuCrj rats. Food Chem Toxicol. 122: 80–86. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Toyoda T, Cho YM, Mizuta Y, Akagi J, Nishikawa A, and Ogawa K. A 13-week subchronic toxicity study of sodium iron chlorophyllin in F344 rats. J Toxicol Sci. 39: 109–119. 2014. [DOI] [PubMed] [Google Scholar]
- 7.Daughtrey WC, Eutermoser M, Thompson SW, and Biles RW. A subchronic toxicity study of octyl acetate in rats. Fundam Appl Toxicol. 12: 313–320. 1989. [DOI] [PubMed] [Google Scholar]
- 8.Swenberg JA. α 2u-globulin nephropathy: review of the cellular and molecular mechanisms involved and their implications for human risk assessment. Environ Health Perspect. 101(Suppl 6): 39–44. 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]