Abstract
Influenza vaccine is considered the most effective way to prevent influenza. Nonetheless, every year vaccine coverage is lower than recommended in the pediatric population. Many factors are supposed to contribute to this phenomenon such as the uncertainty about the indication for vaccination, and the suboptimal vaccine-effectiveness in pediatric age, especially in the youngest children. In this review we discuss the effectiveness, indications, and limits of influenza vaccination in the pediatric population based on the most recent evidences.
Keywords: influenza vaccine, influenza, children, effectiveness, pediatric population
Introduction
Human influenza disease is primarily the result of the infection with influenza A and influenza B viruses. Type A virus can be classified using surface antigens hemagglutinin and neuraminidase. In the last years, the main circulating A strains have been the H1N1 pandemic strain and the H3N2 strain (1). The two major antigenically B viruslineages are B/Victoria and B/Yamagata (2). Each year both B virus strains co-circulate and are responsible for about 25% of influenza disease cases (3). However, the proportion of circulating influenza B strains varies by season and countries (4–6) Different strains have different virulence and prefer to infect different age clusters, probably based on previous exposure to antigenically similar viruses (1). In particular, influenza B virus is likely to affect children, and young adults. Children hospitalizations and fatal influenza cases are mainly associated with B subtypes (1).
Each year, 15–45% of children are infected with an influenza virus and by the age of 6 most children have been infected with influenza virus at least once (7). Viremic titers in children are higher than in adults and shedding of virus goes on for longer periods (8, 9). Therefore, children represent a critical source in the transmission of influenza and sustain annual epidemics (9).
About 870,000 children aged <5 years and about 300,000 children aged <1 year are hospitalized each year all over the world because of influenza and 10–15% of children need medical care for influenza-related diseases (9, 10). It is estimated that between 28,000 and 111,500 children below 5 years of age die each year due to influenza-related causes, most of them in developing countries (9–11) and from 2009, in 8/9 seasons, influenza disease course was reported to be moderate to severe in pediatric population (11).
It is unquestionable that influenza vaccine (IV) is the most effective way to prevent influenza (2). The first vaccine, a live-attenuated monovalent vaccine containing virus A, was developed immediately after the isolation of influenza virus in 1933. Fortunately, during the last decades, much has changed in the prevention of influenza in terms of vaccine manufacture, type of vaccines and strain coverage. Nowadays, two types of IVs are mainly used: an inactivated (IIV), and a live attenuated one (LAIV). The IIV can contain three or four virus strains. The trivalent ones (IIV3) are currently targeted against a H1N1 virus, a H3N2 virus, and a B virus, while the quadrivalent ones (IIV4) are targeted against both viruses A, and both viruses B. IIV3 are currently available in three different formulations: whole-virus vaccines, split-virus vaccines, and subunit vaccines. Two IIV4 vaccines have been developed and marked: a split inactivated vaccine and a subunit one. There are also adjuvanted seasonal IVs, but in most countries, they are not still licensed for children use. The LAIV is a quadrivalent vaccine containing both viruses A and B (12–14).
In this review we discuss the effectiveness, indications, limits, and ongoing direction of IVs in the pediatric population based one the most recent evidences.
Indications of Influenza Vaccination
Although seasonal IVs are available since many decades and recommended since 1960, the first practical indications for children immunization were issued in the early two thousand and only for children with high risk conditions.
After 50 years from the first release, the benefit of the vaccination in children with chronic disorders remains unquestioned. However, the vaccination strategy, which is based only on the direct protection of those subjects at highest risk, has not been proven to be very effective in reducing influenza morbidity, and mortality, as well as not being cost-effective (15). Moreover, children, healthy or chronically ill, –especially those younger than 5 years, are at higher risk for serious influenza-related complications, such as bacterial co-infections (e.g., S. pneumoniae or S. aureus), seizures, influenza-associated encephalitis/encephalopathies, and fulminant myocarditis and pericarditis (16–22).
The extension of the seasonal IV program to all children aims to reduce the public health impact of influenza by providing direct protection and also lowering transmission rates (23). Reducing influenza transmission in the community will avert many cases of severe influenza and influenza-related deaths in older adults and in people with clinical risk factors. Additionally, from an economical perspective, IV was shown to be cost-effective for children in all analyses (24, 25).
Different types of recommendations have been released worldwide. The World Health Organization (WHO) recommends annual vaccination, prioritizing high risk groups including pregnant women, children under 5 years of age, the elderly, and those with underlying health conditions (26).
In the United States (US) the Advisory Committee on Immunization Practices included healthy children aged 6–23 months in the target population of influenza vaccine for the first time in 2004 and it included all healthy children aged more than 6 months only in 2010 (27, 28).
Many countries suggest vaccinating children older than 6 months of age with high-risk conditions only; just few ones universally recommend the vaccination for the whole pediatric population, from 6 months of age, and offer it for free till the age of 5 years (e.g., Canada, Australia) (13, 29, 30). In Europe, the European Center for Disease Control and Prevention (ECDC) has limited power over national IV policies. Therefore, each country establishes its own strategies in recommending vaccination. Not all member states have a formal national action plan for vaccination and in most countries recommendations for seasonal IV only include target or at-risk groups. During the 2017–18 influenza season, only 6/30 states recommended seasonal IVs to healthy children or adolescents (31–33).
Several reasons may explain these different immunization policies: first of all the availability of economic resources. Implementation of resources allocated to influenza vaccination is not always considered a priority for the National Health Authorities. Several economic evaluations are important to assist policymakers defining the costs of influenza vaccination programs and their financial cost-effectiveness. Secondly, influenza vaccination requires additional work that should be efficiently organized at the light of each national immunization schedules: this is of outstanding importance for the success of the influenza vaccine campaign. Despite the awareness of these economical and organizational barriers, in our opinion, the extension of the recommendation for the whole pediatric age could confer great benefits in terms of social equality.
ECDC, Centers for Disease Control and Prevention (CDC), and American Academy of Pediatrics (AAP) indicate that inactivated vaccines should be the primary choice for all children older than 6 months. However, they do not indicate which one between IIV3 and IIV4 should be preferred. Indeed, the type of the formulation is currently debated considering that the prevalence of B viruses is relatively low and varies between seasons (17, 20, 33). LAIV was not recommended in any setting in the past two influenza seasons based on data demonstrating low effectiveness against influenza A(H1N1) (34, 35). However, for the 2018–2019 influenza season, the AAP reintroduced the use of LAIV for healthy children aged older than 2 years who would not otherwise receive an influenza vaccine (13, 35). AAP supports the use of LAIV with the aim of achieving the best vaccination coverage and optimal protection in children of all ages (13).
Concerning IV's schedule, IV should be repeated every year, as recent studies suggested that there was no strong evidence of protection extended for more than one influenza season and vaccine effectiveness seems not to diminish with frequent vaccinations (36–42).
Influenza VACCINE Effectiveness in Pediatric Age
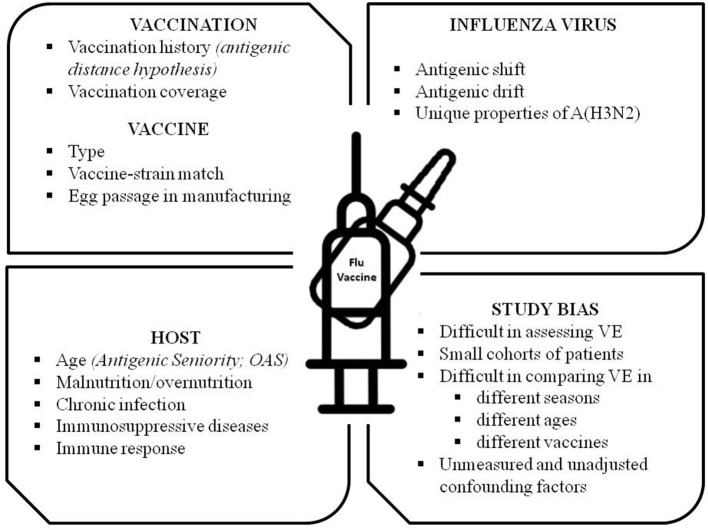
An interesting argument of debate is the vaccine effectiveness (VE) of the available IV. As randomized controlled trials are not suitable for monitoring VE across the seasons, the test-negative design (TND), a modified case-control study, has been introduced since 2004 (43, 44). Based on the results of TND studies, the VE appears to vary from season to season, by age group, with vaccination history, and by country. Many theories and factors have been proposed over the years to explain these discrepancies, such as the suboptimal vaccine-strain match, the different types of vaccine (inactivated vs. LAIV), the vaccine manufacturing (e.g., generating egg-induced mutations in the hemagglutinin that affect antigenicity), the age-dependent patterns in protection (e.g., “Original Antigenic Sin”–OAS–and the more recent model of OAS: the “Antigenic Seniority”), the nutritional status, the unresponsiveness of some hosts to influenza vaccine, the vaccination coverage rates in the community, the prior influenza vaccination (e.g., “the antigenic distance hypothesis,” a theoretical framework explaining the variable effect of repeated vaccination) and the difficulty of measuring VE accurately (45–54). A list of possible factors affecting VE is reported in Figure 1. The contribution and the relative importance of each factor in determining the VE is largely unknown and it is an intriguing field of future research.
Figure 1.
Factors and conditions affecting Influenza Vaccine Effectiveness (VE). OAS, Original Antigenic Sin.
In addition, combining and interpreting differences in VE estimates from available studies is extremely challenging because VE is assessed annually (due to the frequently changing vaccine) and because of the difference in study designs, age of recruited children, influenza seasons, and countries where the studies were conducted.
Given these observations, it is extremely difficult to give data about VE in pediatric age. However, to the best of our knowledge, we summarized the available data as follows:
Children older than 2 years: trivalent inactivated IV showed a higher VE against A/H1N1pdm09 (up to 70%) when compared to LAIV (up to 39%). However, they had similar effectiveness against influenza A/H3N2 and B (55, 56). Quadrivalent inactivated subunit-antigen vaccine showed a VE in preventing influenza illness ranging from 45 to 65% against any type of influenza, 51–71% against influenza A, and 32–34% against B. According to some authors, for this vaccine the VE seems to be highest in the younger children aged 1–5 years old (57–59).
Children between 6 months and 2 years: there are few studies specifically assessing the VE of the inactivated vaccines between 6 and 24 months of age. The great majority of studies performed efficacy analysis or VE pooled analysis considering most often children aged from 2 to 59 months or from 2 to 7–9 years old. Based on the available data, VE in children from 6 to 24 months of age range from 18 to 85% for trivalent inactivated vaccine (60–64).
Efficacy studies showed that the recently quadrivalent split-virion inactivated vaccine was effective against influenza vaccine-like strains (50.9% efficacy against any A or B type and 68.4% against influenza caused by vaccine-like strains) in children aged 6–35 months (65). VE data are not currently available, however effectiveness is expected to be similar to those reported in efficacy studies.
Limits of Influenza Vaccination in Pediatric Age
One of the major limits of IV in pediatric age is the absence of recommendation in infants younger than 6 months of age. The younger infants have a greater risk of severe influenza infection and a higher rate of hospitalization than older infants (66). The risk is even greater if they have chronic conditions (10). Up to now, IV has not been approved by regulators for use in infants and its use is currently off-label and arbitrary. Apart from the well-known cocoon strategy which is extremely difficult to perform, maternal immunization during pregnancy could overcome this limit and is the only measure approved by the health authorities (67). In this field, evidences about immunogenicity and efficacy are rapidly accumulating. IIV or A(H1N1) MF59-adjuvanted vaccine during pregnancy result in transplacental transfer of the generated antibodies (68). The efficacy of pregnant women's vaccination in preventing the disease and influenza-related hospitalization in infants was estimated to be around 50–60% according to different studies (69–71). However, several questions remain unanswered as which should be the best time for maternal immunization to ensure the best and the longest protection for the newborn and young infants and which is the immunological role of breastfeeding. In particular some authors reported the absence of protective antibody levels at birth and limited immunological and clinical protection up to the 3rd month of life (72, 73). Others showed that clinical protection against influenza and influenza–associated pneumonia persists up to the 6th month of life (74, 75). Considering the available data, determining acquired protection duration is imprecise, with few immunological, and effectiveness data between the third and 6th months of infant life. Moreover, long-term adverse effects of maternal immunization on infants have not been reported, and more safety studies are needed. Nonetheless, maternal immunization remains the best practice for protecting children against influenza in the 1st months of life, and should be encouraged.
Another potential limit of IV in pediatric age is the need for 2 shots in the younger naïve children, especially in infants who have a very full immunization schedule. Studies showed that the second dose was not always received or delayed far beyond the recommended interval of 28 days (76, 77). There were probably several reasons for incomplete vaccinations, such as schedule complexity, in between-doses frequent infections, difficulties in scheduling a doctor appointment, financial barriers, and lack of provider–parent discussions on the importance of the second dose (78). Efforts should be made in order to overcome the two shots with just one effective single dose for every age. This change can potentially simplify IV procedures and improve the adherence to IV schedule.
The IVs remain the primary choice for all children, even though LAIV, besides its capacity of inducing mucosal IgA antibodies, providing protection at the site of viral entry against subsequent infection, and eliciting both humoral, and cellular immune responses, may also improve the compliance thanks to its non-invasive administration (endonasal spray) (79).
Ongoing Discussion and Future Perspectives
The characteristics of the immune system in young children on one side and the presence of an immunosuppressive disease on the other have shown to affect IV response (12, 80, 81). This raised debates about the best approach to enhance the immune response to IV in these two specific different groups. Some strategies have been tested, such as the use of higher doses of antigen, and adjuvants (82). In 2009 the high-dose (containing four times as much hemagglutinin as in standard-dose vaccines) trivalent inactivated IV was licensed for use in the elderly on the basis of its safety profile and superior immunogenicity (83). In pediatric population, recent studies showed that the high-dose IV was more immunogenic than the standard one in children with leukemia or solid tumors and in solid organ transplant patients but not in children with HIV (84), with good reported safety profile (84–86). Given the relatively small studied population, despite the evidences that immunocompromised children generate a lower immune response to standard-dose IV compared to healthy subjects (87), no definitive recommendation about the use of the high-dose IV can be drawn. Notably, no data about the use of this high dose IV are available in immunocompromised children, and younger than 2 years. Further studies are needed.
Another approach to enhance the immune response was the use of oil-in-water adjuvant MF59®, firstly approved in 1990 for adults older than 65 years of age. In 2018, a first trial assessed the relative efficacy, immunogenicity, and safety of an MF59-adjuvanted quadrivalent inactivated subunit IV (aQIV) compared to a US-licensed non-adjuvanted influenza vaccine in a large cohort of children aged between 6 months and 5 years in 2 consecutive seasons (88). The authors showed that in the youngest children (6–23 months) aQIV provided greater protection against influenza than a non-adjuvanted vaccine. The clinical benefit was demonstrated since the first vaccination in vaccine-naïve children. The efficacy and vaccine safety profiles of aQIV were similar to the non-adjuvanted comparator vaccine, with the exception of major Solicited Adverse Events. Recently, Daily and colleagues assessed the impact of repeated vaccination on immunogenicity and safety of aQIV in children aged between 6 months to 5 years. This study confirmed an enhanced immunogenicity and a similar safety profile after repeated aQIV vaccination compared to repeated non-adjuvanted influenza vaccination (89). Given the promising results, if confirmed in the ongoing trials, aQIV could be a valid option for the routine use in pediatric population in the near future.
A quadrivalent recombinant vaccine, currently available in adults, was recently studied in children (6–17 years old), and it was found to be comparable to the IIV in terms of safety, and immunogenicity (90).
The Committee for Medicinal Products for Human Use of the European Medicines Agency and the European Commission (in October 2018 and in January 2019 respectively) approved the inactivated cell-based quadrivalent influenza vaccine (QIVc) for the use in patients older than 9 years. The QIVc is supposed to be used as early as in the next influenza season (2019–2020). The different production process of the vaccine (cell-based vaccine vs. embryonated chicken eggs) represent a step forward in avoiding egg-adapted changes, vast amount of eggs, and long manufacturing time, with a comparative or even better efficacy rate (91).
Conclusions
Influenza immunization is the best available strategy to reduce influenza-related morbidity, and mortality and virus spread. Nonetheless, questions and limits about influenza vaccine in pediatric population remain open (Table 1). Firstly, the vaccine effectiveness in children is variable and suboptimal, with reported differences according to vaccine types, seasons, and child age. Estimating the mean effectiveness remains challenging. Secondly, influenza vaccine is currently the only vaccine requiring yearly immunization, with two shots in naïve children which could influence the vaccine uptake especially in younger children. Thirdly, there is no influenza vaccine that directly protects infants <6 months of age. The most promising strategy to protect children that are too young to be vaccinated is the maternal immunization, with estimated efficacy of 50–60%. Finally, the promising recombinant, adjuvanted, and high-dose vaccines are still not universally approved in pediatric population. Addressing these issues, together with better understanding the complex immune responses induced by natural influenza infection, will be of outstanding importance to finally design future universal vaccine.
Table 1.
Visual summary.
| Topics | Conclusion |
|---|---|
| Influenza in children |
|
| Indications of influenza vaccination |
|
| Influenza vaccine effectiveness (VE) in pediatric age |
|
| Limits of influenza vaccination in pediatric age |
|
| Ongoing discussion and future perspectives |
|
Data to be interpret with caution. Please, see the limits discussed into the main manuscript. LAIV, Live-attenuated intranasal vaccine; VE, vaccine effectiveness.
Author Contributions
CM, IC, and MF wrote the paper. GZ revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Gavigan P, McCullers JA. Influenza: annual seasonal severity. Curr Opin Pediatrics. (2019) 31:112–8. 10.1097/MOP.0000000000000712 [DOI] [PubMed] [Google Scholar]
- 2.Shaw MW, Xu X, Li Y, Normand S, Ueki RT, Kunimoto GY, et al. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology. (2002) 303:1–8. 10.1006/viro.2002.1719 [DOI] [PubMed] [Google Scholar]
- 3.Baxter D. Evaluating the case for trivalent or quadrivalent influenza vaccines. Human Vacc Immunother. (2016) 12:2712–7. 10.1080/21645515.2015.1091130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health. (2013) 103:e43–e51.3. 10.2105/AJPH.2012.301137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings L, Huang QS, Barr I, Lee PI, Kim WJ, Buchy P, et al. Literature review of the epidemiology of influenza B disease in 15 countries in the Asia Pacific region. Influenza Other Respir Viruses. (2018) 12:383–41. 10.1111/irv.12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adlhoch C, Snacken R, Melidou A, Ionescu S, Penttinen P. Dominant influenza A. (H3N2) and B/Yamagata virus circulation in EU/EEA, 2016/17 and 2017/18 seasons, respectively. Eurosurveillance. (2018) 23:18–00146. 10.2807/1560-7917.ES.2018.23.13.18-00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. (2011) 378:1917–30. 10.1016/S0140-6736(11)61051-9 [DOI] [PubMed] [Google Scholar]
- 8.Paules C, Subbarao K. Influenza. Lancet. (2017) 390:697–708. 10.1016/S0140-6736(17)30129-0 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Background Paper on Influenza Vaccines and Immunization, SAGE Working Group. Available online at: https://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf
- 10.Kondrich J, Rosenthal M. Influenza in children. Curr Opin Pediatrics. (2017) 29:297–302. 10.1097/MOP.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 11.Lafond KE, Nair H, Rasooly MH, Valente F, Booy R, Rahman M, et al. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med. (2016) 13:e1001977. 10.1371/journal.pmed.1002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mameli C, D'auria E, Erba P, Nannini P, Zuccotti GV. Influenza vaccine response: future perspectives. Exp Opin Biol Ther. (2018) 18:1–5. 10.1080/14712598.2018.1391786 [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Committee on infectious diseases. recommendations for prevention and control of influenza in children, 2017–2018. Pediatrics. (2017) 140:e20172550 10.1542/peds.2017-2550 [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Meldgaard TS, Bertholet S. Novel platforms for the development of a universal influenza vaccine. Front Immunol. (2018) 9:600. 10.3389/fimmu.2018.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bambery B, Douglas T, Selgelid MJ, Maslen H, Giubilini A, Pollard AJ, et al. Influenza vaccination strategies should target children. Public Health Ethics. (2017)11:221–34. 10.1093/phe/phx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkinen T, Silvennoinen H, Peltola V, Ziegler T, Vainionpää R, Vuorinen T, et al. Burden of influenza in children in the community. J Infect Dis. (2004) 190:1369–73. 10.1086/424527 [DOI] [PubMed] [Google Scholar]
- 17.Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis. (2008) 6:114–24. 10.1016/j.tmaid.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Reed C, Kallen AJ, Patton M, Arnold KE, Farley MM, Hageman J, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. (2009) 28:572–6. 10.1097/INF.0b013e31819d8b71 [DOI] [PubMed] [Google Scholar]
- 19.Fraaij PL, Heikkinen T. Seasonal influenza: the burden of disease in children. Vaccine. (2011) 29:7524–8. 10.1016/j.vaccine.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 20.Morparia K, Peshkovsk C, Kalyanaraman M. Purulent pericarditis secondary to influenza and community-acquired methicillin-resistant Staphylococcus aureus co-infection. Cardiol Young. (2018) 28:1481–3. 10.1017/S1047951118001580 [DOI] [PubMed] [Google Scholar]
- 21.Cabral M, Brito MJ, Conde M, Oliveira M, Ferreira GC. Fulminant myocarditis associated with pandemic H1N1 influenza A virus. Rev Portug Cardiol. (2012) 31:517–20. 10.1016/j.repc.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 22.Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2018–19 influenza season. MMWR Recommend Rep. (2018) 67:1. 10.15585/mmwr.rr6703a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang TK, Fang VJ, Ip DK, Perera RA, So HC, Leung G M, et al. Indirect protection from vaccinating children against influenza in households. Nat Commun. (2019) 10:106. 10.1038/s41467-018-08036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ting EE, Sander B, Ungar WJ. Systematic review of the cost-effectiveness of influenza immunization programs. Vaccine. (2017) 35:1828–43. 10.1016/j.vaccine.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 25.D'Angiolella LS, Lafranconi A, Cortesi PA, Rota S, Cesana G, Mantovani LG. Costs and effectiveness of influenza vaccination: a systematic review. Ann dell'Istituto Super Sanita. (2018) 54:49–57. 10.4415/ANN_18_01_10 [DOI] [PubMed] [Google Scholar]
- 26.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recommend Rep. (2005) 54:1–41. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines. MMWR. (2010) 59:1–62. [Google Scholar]
- 28.New South Wales Government. Free Flu Shots for Children Aged 6 Months to Under 5 Years. Available online at: https://www.health.nsw.gov.au/immunisation/Pages/kids-flu-shot.aspx (accessed January 18, 2019).
- 29.Canadian Pediatric Society. Vaccine Recommendations for Children and Youth for the 2018/2019 Influenza Season. (2019). Available online at https://www.cps.ca/en/documents/position/vaccine-recommendations-2018-2019-influenza-season (accessed January 15, 2019).
- 30.Rizzo C, Rezza G, Ricciardi W. Strategies in recommending influenza vaccination in Europe and US. Human Vacc Immunother. (2018) 14:693–8. 10.1080/21645515.2017.1367463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Centre for Disease Prevention and Control. ECDC Scientific Advice on Seasonal Influenza Vaccination of Children and Pregnant Women. Stockholm: ECDC; (2012). [Google Scholar]
- 32.European Centre for Disease Prevention and Control. Seasonal Influenza Vaccination and Antiviral Use in EU/EEA Member States – Overview of Vaccine Recommendations for 2017–2018 and Vaccination Coverage Rates for 2015–2016 and 2016–2017 Influenza Seasons. Stockholm: ECDC; (2018). [Google Scholar]
- 33.Caspard H, Gaglani M, Clipper L, Belongia EA, McLean HQ, Griffin MR, et al. Effectiveness of live attenuated influenza vaccine and inactivated influenza vaccine in children 2–17 years of age in 2013–2014 in the United States. Vaccine. (2016) 34:77–82. 10.1016/j.vaccine.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 34.Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR. (2018) 67:643. 10.15585/mmwr.mm6722a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. (2017) 16:723–36. 10.1080/14760584.2017.1334554 [DOI] [PubMed] [Google Scholar]
- 36.McLean HQ, Thompson MG, Sundaram ME, Meece JK, McClure DL, Friedrich TC, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A (H3N2) and B during 8 seasons. Clin Infect Dis. (2014) 59:1375–85. 10.1093/cid/ciu680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, et al. The impact of repeated vaccination on influenza vaccine effectiveness: a systematic review and meta-analysis. BMC Med. (2019) 17:9. 10.1186/s12916-018-1239-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson MG, Clippard J, Petrie JG, Jackson ML, McLean HQ, Gaglani M, et al. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013: the importance of two priming doses. Pediatr Infect Dis J. (2016) 35:299. 10.1097/INF.0000000000001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilca V, De Serres G, Hamelin ME, Boivin G, Ouakki M, Boulianne N, et al. Antibody persistence and response to 2010–2011 trivalent influenza vaccine one year after a single dose of 2009 AS03-adjuvanted pandemic H1N1 vaccine in children. Vaccine. (2011) 30:35–41. 10.1016/j.vaccine.2011.10.062 [DOI] [PubMed] [Google Scholar]
- 40.Fu C, Xu J, Lin J, Wang M, Li K, Ge J, et al. Concurrent and cross-season protection of inactivated influenza vaccine against A (H1N1) pdm09 illness among young children: 2012–2013 case–control evaluation of influenza vaccine effectiveness. Vaccine. (2015) 33:2917–21. 10.1016/j.vaccine.2015.04.063 [DOI] [PubMed] [Google Scholar]
- 41.McLean HQ, Caspard H, Griffin MR, Gaglani M, Peters TR, Poehling KA, et al. Association of prior vaccination with influenza vaccine effectiveness in children receiving live attenuated or inactivated vaccine. JAMA Netw Open. (2018) 1:e183742 10.1001/jamanetworkopen.2018.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. (2017) 35:4796–800. 10.1016/j.vaccine.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 43.Skowronski DM, Gilbert M, Tweed SA, Petric M, Li Y, Mak A. Effectiveness of vaccine against medical consultation due to laboratory-confirmed influenza: results from a sentinel physician pilot project in British Columbia, 2004–2005. Can Commun Dis Rep. (2005) 31:181–91. [PubMed] [Google Scholar]
- 44.Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. (2013) 11:153. 10.1186/1741-7015-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. (2013) 56:1363–9. 10.1093/cid/cit060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stacey H, Barjesteh N, Mapletoft J, Miller M. "Gnothi Seauton”: leveraging the host response to improve influenza virus vaccine efficacy. Vaccines. (2018) 6:23 10.3390/vaccines6020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moser JAS, Galindo Fraga A, Ortiz Hernández AA, Gu W, Hunsberger S, Galán Herrera JF, et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses. (2019) 13:3–9. 10.1111/irv.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewnard J, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines. (2018) 6:28. 10.3390/vaccines6020028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. (1960) 104:572–8. [Google Scholar]
- 50.Henry C, Palm AKE, Krammer F, Wilson PC. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol. (2018) 39:70–9. 10.1016/j.it.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucharski AJ, Gog JR. The role of social contacts and original antigenic sin in shaping the age pattern of immunity to seasonal influenza. PLoS Comput Biol. (2012) 8:e1002741. 10.1371/journal.pcbi.1002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reber AJ, Kim JH, Coleman LA, Spencer SM, Chung JR, Chen J, et al. Seasonal influenza vaccination of children induces humoral and cell-mediated immunity beyond the current season: cross-reactivity with past and future strains. J Infect Dis. (2016) 214010:1477–86. 10.1093/infdis/jiw380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci USA. (1999) 96:14001–6. 10.1073/pnas.96.24.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung JR, Flannery B, Ambrose CS, Bégué RE, Caspard H, DeMarcus L, et al. Live attenuated and inactivated influenza vaccine effectiveness. Pediatrics. (2019) 143:e20182094. 10.1542/peds.2018-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med. (2017) 377:534–43. 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugaya N, Shinjoh M, Nakata Y, Tsunematsu K, Yamaguchi Y, Komiyama O, et al. Three-season effectiveness of inactivated influenza vaccine in preventing influenza illness and hospitalization in children in Japan, 2013–2016. Vaccine. (2018) 36:1063–71. 10.1016/j.vaccine.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 57.Ando S. Effectiveness of quadrivalent influenza vaccine based on the test-negative control study in children during the 2016–2017 season. J Infect Chemother. (2018) 24:782–8. 10.1016/j.jiac.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 58.Poehling KA, Caspard H, Peters TR, Belongia EA, Congeni B, Gaglani M, et al. 2015–2016 vaccine effectiveness of live attenuated and inactivated influenza vaccines in children in the United States. Clin Infect Dis. (2017) 66:665–72. 10.1093/cid/cix869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Z, Dong Z, Fu C. Seasonal influenza vaccine effectiveness among children aged 6 to 59 months in southern China. PLoS ONE. (2012) 7:e30424. 10.1371/journal.pone.0030424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen S, Campitelli MA, Calzavara A, Guttmann A, Kwong JC. Seasonal influenza vaccine effectiveness in pre-and full-term children aged 6–23 months over multiple seasons. Vaccine. (2013) 31:2974–8. 10.1016/j.vaccine.2013.05.011 [DOI] [PubMed] [Google Scholar]
- 61.Su WJ, Chan TC, Chuang PH, Liu YL, Lee PI, Liu M T, et al. Estimating influenza vaccine effectiveness using routine surveillance data among children aged 6–59 months for five consecutive influenza seasons. Int J Infect Dis. (2015) 30:115–21. 10.1016/j.ijid.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buchan SA, Chung H, Campitelli MA, Crowcroft NS, Gubbay JB, Karnauchow T, et al. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among young children during the 2010-11 to 2013-14 influenza seasons in Ontario, Canada. PLoS ONE. (2017) 12:e0187834. 10.1371/journal.pone.0187834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blyth CC, Jacoby P, Effler PV, Kelly H, Smith DW, Robins C, et al. Effectiveness of trivalent flu vaccine in healthy young children. Pediatrics. (2014) 133:e1218–e1225. 10.1542/peds.2013-3707 [DOI] [PubMed] [Google Scholar]
- 64.Pepin S, Dupuy M, Borja-Tabora CFC, Montellano M, Bravo L, Santos J, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6–35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern Hemispheres. Vaccine. (2019) 37:1876–84. 10.1016/j.vaccine.2018.11.074 [DOI] [PubMed] [Google Scholar]
- 65.Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med. (2006) 355:31–40. 10.1056/NEJMoa054869 [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization Immunization, Vaccines and Biologicals. WHO Recommends Seasonal Influenza Vaccination to Pregnant Women as the Highest Priority. Available online at: http://www.who.int/immunization/newsroom/newsstory_seasonal_influenza_vaccination_preg (accessed January 18, 2019).
- 67.Zuccotti G, Pogliani L, Pariani E, Amendola A, Zanetti A. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A (H1N1) MF59-adjuvanted vaccine. JAMA. (2010) 304:2360–1. 10.1001/jama.2010.1729 [DOI] [PubMed] [Google Scholar]
- 68.Manske JM. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: a review of the evidence. Mater Child Health J. (2014) 18:1599–609. 10.1007/s10995-013-1399-2 [DOI] [PubMed] [Google Scholar]
- 69.Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med. (2014) 371:918–31. 10.1056/NEJMoa1401480 [DOI] [PubMed] [Google Scholar]
- 70.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. (2008) 359:1555–64. 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 71.Nunes MC, Cutland CL, Jones S, Downs S, Weinberg A, Ortiz JR, et al. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Infect Dis. (2017) 65:1066–71. 10.1093/cid/cix497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dabrera G, Zhao H, Andrews N, Begum F, Green HK, Ellis J, et al. Effectiveness of seasonal influenza vaccination during pregnancy in preventing influenza infection in infants, England, 2013/14. Eurosurveillance. (2014) 19:20959. 10.2807/1560-7917.ES2014.19.45.20959 [DOI] [PubMed] [Google Scholar]
- 73.Tapia MD, Sow SO, Tamboura B, Tégueté I, Pasetti MF, Kodio M, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. (2016) 16:1026–35. 10.1016/S1473-3099(16)30054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omer SB, Clark DR, Aqil AR, Tapia MD, Nunes MC, Kozuki N, et al. Maternal Influenza Immunization and prevention of severe clinical pneumonia in young infants. Pediatr Infect Dis J. (2018) 37:436–40. 10.1097/INF.0000000000001914 [DOI] [PubMed] [Google Scholar]
- 75.Hu Y, Chen Y, Zhang B. Two-dose seasonal influenza vaccine coverage and timeliness among children aged 6 months through 3 years: an evidence from the 2010–11 to the 2014–15 seasons in Zhejiang province, East China. Hum Vacc Immunother. (2017) 13:75–80. 10.1080/21645515.2016.1225640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin X, Fiebelkorn AP, Pabst LJ. Trends in compliance with two-dose influenza vaccine recommendations in children aged 6 months through 8 years, 2010–2015. Vaccine. (2016) 34:5623–8. 10.1016/j.vaccine.2016.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hofstetter AM, Barrett A, Stockwell MS. Factors impacting influenza vaccination of urban low-income Latino children under nine years requiring two doses in the 2010–2011 season. J Commun Health. (2015) 40:227–34. 10.1007/s10900-014-9921-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohn KGI, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vacc Immunother. (2018) 14:571–8. 10.1080/21645515.2017.1377376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bektas O, Karadeniz C, Oguz A, Berberoglu S, Yilmaz N, Citak C. Assessment of the immune response to trivalent split influenza vaccine in children with solid tumors. Pediatr Blood Cancer. (2007) 49:914–7. 10.1002/pbc.21106 [DOI] [PubMed] [Google Scholar]
- 80.Goossen GM, Kremer LC, van de Wetering MD. Influenza vaccination in children being treated with chemotherapy for cancer. Cochrane Database Syst Rev. (2013) 8:CD006484 10.1002/14651858.CD006484.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. (2014) 371:635–45. 10.1056/NEJMoa1315727 [DOI] [PubMed] [Google Scholar]
- 82.Hakim H, Allison KJ, Van de Velde LA, Tang L, Sun Y, Flynn PM, et al. Immunogenicity and safety of high-dose trivalent inactivated influenza vaccine compared to standard-dose vaccine in children and young adults with cancer or HIV infection. Vaccine. (2016) 34:3141–8. 10.1016/j.vaccine.2016.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.GiaQuinta S, Michaels MG, McCullers JA, Wang L, Fonnesbeck C, O'shea A, et al. Randomized, double blind comparison of standard dose vs. high dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr Transplant. (2015) 19:219–28. 10.1111/petr.12419 [DOI] [PubMed] [Google Scholar]
- 84.McManus M, Frangoul H, McCullers JA, Wang L, O'shea A, Halasa N. Safety of high dose trivalent inactivated influenza vaccine in pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer. (2014) 61:815–20. 10.1002/pbc.24863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beck CR, McKenzie BC, Hashim AB, Harris RC, Zanuzdana A, Agboado G, et al. Influenza vaccination for immunocompromised patients: summary of a systematic review and meta-analysis. Influenza Other Respir Viruses. (2013) 7:72–5. 10.1111/irv.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vesikari T, Kirstein J, Go GD, Leav B, Ruzycky ME, Isakov L, et al. Efficacy, immunogenicity, and safety evaluation of an MF59-adjuvanted quadrivalent influenza. virus vaccine compared with non-adjuvanted influenza vaccine in children: a multicentre, randomised controlled, observer-blinded, phase 3 trial. Lancet Respir Med. (2018) 6:345–56. 10.1016/S2213-2600(18)30108-5 [DOI] [PubMed] [Google Scholar]
- 87.Daly W, Ramsey K, Forsten A, Heijnen E, Leav B, Oberye J, et al. 987. Repeated exposure to an adjuvanted quadrivalent subunit influenza virus vaccine (aQIV): a randomized, observer blind, multicenter study. Open Forum Infect Dis. (2018) 5:S292 10.1093/ofid/ofy210.824 [DOI] [Google Scholar]
- 88.Dunkle LM, Izikson R, Patriarca PA, Goldenthal KL, Cox M, Treanor JJ. Safety and immunogenicity of a recombinant influenza vaccine: a randomized trial. Pediatrics. (2018) 141:e20173021. 10.1542/peds.2017-3021 [DOI] [PubMed] [Google Scholar]
- 89.Boikos T. Effectiveness of the cell culture- and egg-derived, seasonal influenza vaccine during the 2017-2018 Northern hemisphere influenza season. Present CIC. (2018) 3:44 10.1038/s41541-018-0079-z [DOI] [Google Scholar]
- 90.Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis. (2018) 218:347–54. 10.1093/infdis/jiy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.World Health Organization. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Record. (2012) 87:461–76. [PubMed] [Google Scholar]