Abstract
Angiogenesis is an essential step in maintaining tumor growth and facilitating metastasis. The regulatory mechanisms of tumor-induced angiogenesis are extremely complicated, and include sophisticated crosstalk between tumors and surrounding microenvironment cells, oncogenic signaling pathway activation and aberrant expression of angiogenesis-related genes. Recently, emerging evidence demonstrated that long noncoding RNAs (lncRNAs) play crucial roles in angiogenesis. However, there are lack of reports to review the progression in this scientific field. Here, we focus on and summarize the latest findings of lncRNA in angiogenesis in various cancers. Firstly, we introduced how lncRNAs in tumor cells to modulate the cellular signaling axis, interact with proteins and serve as competitive endogenous RNAs (ceRNAs) to alter target gene expression, by which induce endothelial cell to form capillaries. Then, we recapitulated the essential functions of lncRNA in endothelial cells, and how lncRNAs in tumor-associated macrophages to mediate angiogenesis. Next, the angiogenesis mechanism of tumor-derived lncRNAs via exosomes were collectively described. At last, the effects of lncRNAs on vasculogenic mimicry were summarized, which showed that malignant tumor cells acquire dedifferentiated and endothelial properties to form vessel-like structures by themselves. This review provides new insights into the complexity of angiogenesis, and suggests that lncRNAs may become promising biomarkers and targets for enhancing the efficacy of anti-angiogenesis therapy in cancer.
Keywords: Angiogenesis, lncRNA, signaling pathway, ceRNA, tumor microenvironment
Introduction
Angiogenesis is one of the most important hallmarks of cancer [1]. During the process of tumor growth, new vasculature is needed to supply ample nutrients and remove metabolic wastes. Tumor-induced angiogenesis frequently displays disordered morphology and is characterized by precocious, tortuous, permeable and dysfunctional vascular structure [1]. These atypical vasculature networks create a hypoxic microenvironment within tumors, which activates hypoxia-induced factors (HIFs) to drive the expression of numerous oncogenes and induce immunosuppression. Additionally, the high permeability and leakage of the neovasculature system facilitate the direct entry of tumor cells into the bloodstream to trigger metastasis. Moreover, this disabled vasculature makes it difficult to deliver drugs efficiently to tumors and results in therapeutic resistance [2]. Therefore, angiogenesis-related factors have become attractive targets for the development of anti-cancer drugs, such as FDA-approved bevacizumab (anti-VEGF) and ramucirumab (anti-VEGFR2) [3]. Though these anti-angiogenic drugs may improve progression-free survival in some patients, the long-term response is disappointing [3]. Extremely intricate mechanisms beyond these well-documented coding genes regulate the angiogenesis process. Further exploration and a better understanding of these mechanisms are required to develop more effective strategies for cancer therapy.
Over the past decades, the development of high-throughput sequencing technology has revealed that more than 70% of the human genome is pervasively transcribed, but only less than 2% of the transcripts are translated into proteins; most of human transcriptome are noncoding RNAs (ncRNAs) [4]. Emerging evidence has demonstrated that instead of being “transcriptional junk”, such as miRNA, siRNA, piRNA, circRNA, snoRNA and the attractive long noncoding RNAs, play essential roles in manipulating gene expression [5]. Long noncoding RNAs (LncRNAs) are collectively defined as transcripts with more than 200 nucleotides that have limited potential to encode proteins; lncRNAs are the primary class of noncoding family [6]. LncRNAs may display diverse functions and participate widely in developmental, physiological and pathophysiological processes, including cancer progression. Mechanistically, lncRNAs can associate with DNA, RNA and proteins to modulate local or global gene expression in a cis or trans manner [7]. For instance, lncRNA HOTAIR can serve as scaffold to recruit various protein complexes to specific genomic regions, thereby reprogramming the chromosomal state to affect gene expression [8]. Additionally, lncRNA may function as a competing endogenous RNA (ceRNA) to sequester miRNA from its targets, and antagonize the repressive effects of miRNAs on mRNAs [9]. Recently, many lncRNAs have been found to participate in tumor-induced angiogenesis by triggering oncogenic signaling pathways, binding to various proteins and miRNAs, encapsuling into exosome for delivery, or reprogramming tumor microenvironment. These findings provide new insights into the complexity of angiogenesis.
In this review, we systematically summarized the functions and underlying mechanisms of lncRNAs in tumor-associated angiogenesis, and proposed that lncRNAs have great potential to be novel biomarkers and targets in cancer therapy.
Aberrant lncRNA expression in cancers drive angiogenesis via multiple manners
LncRNAs trigger angiogenesis through activating oncogenic signaling pathways in tumor cells
The activation of oncogenic signaling pathways, such as STAT3, NF-κb, AKT, mTOR and WNT, plays important roles in driving angiogenesis of tumor. Emerging studies demonstrate that lncRNAs can alter the activation of these pathways to regulate angiogenesis as summarized in Figure 1.
Figure 1.
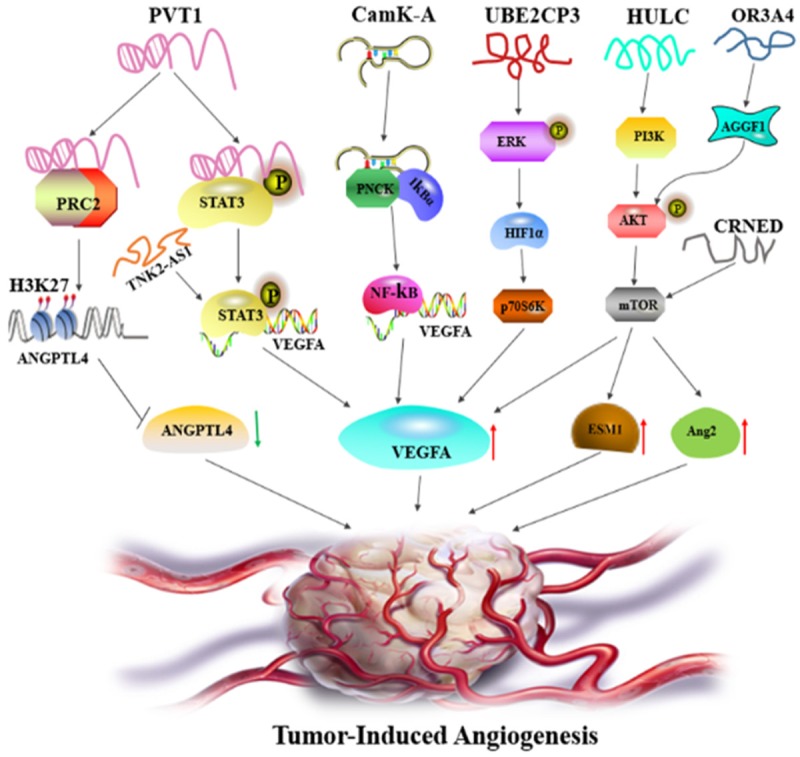
LncRNAs regulate angiogenesis through modulating multiple signaling pathways. LncRNA PVT1 binds to and activates STAT3 pathway to drive VEGFA expression, or interacts with PRC2 complex to induce H3K37 chromosomal modification to repress ANGPTL4 expression. LncRNA TNK-AS1 exerts similar mechanism with PVT1 to elicit STAT3/VEGFA axis. LncRNA CamK-A associate with PNCK and degrade IkBα, thus stimulate NF-κb pathway to increase VEGFA expression. UBE2CP3 triggers ERK/HIF-1α/p70S6K cascade to elevate VEGFA levels. HULC regulates ESM1 expression via the PI3K/AKT/mTOR pathway. LncRNA ORA3A4 mediates angiogenesis by the AGGF1/AKT/mTOR axis. LncRNA CRNDE induce VEGFA and Ang-2 expression through activating mTOR pathway. The red arrows indicate the upregulation and the green arrow represents the downregulation.
Our recent study revealed that lncRNA PVT1 promotes angiogenesis by inducing the STAT3/VEGFA axis in gastric cancer. PVT1 is upregulated in gastric cancer tissues and cells, and is associated with high microvessel density and shorter survival time. Further investigation showed that PVT1 can form a complex with STAT3 in the nucleus, which protects STAT3 from poly-ubiquitination and proteasome-mediated degradation. Reciprocally, STAT3 can occupy the PVT1 promoter to increase PVT1 transcription. The positive feedback loop between PVT1 and STAT3 sustainably increases VEGFA expression to induce angiogenesis [10]. In non-small cell lung cancer (NSCLC), lncRNA TNK2-AS1 also mediates angiogenesis via the STAT3 signaling pathway. Similar to PVT1, TNK2-AS1 binds to and stabilizes STAT3 to enhance VEGFA expression for neovascularization, while STAT3 triggers TNK2-AS1 transcription in turn [11].
LINC01410 is another critical lncRNA involved in the angiogenesis of gastric cancer. Unlike the abovementioned mechanism, LINC01410 acts as a ceRNA that interacts with and depletes miR-532-5p, which increases the expression of the miR-532-5p target NCF2, and subsequently activates the NF-κB pathway by increasing p65 protein levels in the nucleus. Interestingly, NCF2 can also stimulate LINC01410 expression via the NF-κB pathway. As a result, the constitutive activation of the LINC1410/miR-532-5p/NCF2/NF-κB feedback loop aggravates the malignant progression of gastric cancer [12].
In hepatocellular carcinoma (HCC), Lin et al. demonstrated that lncRNA UBE2CP3 expression is upregulated in HCC tissues with higher vessel density. In a co-culture system of cancer cells and human umbilical vein endothelial cells (HUVECs), dysregulated UBE2CP3 expression in the cancer cells can alter endothelial cell proliferation, migration and tube formation. Consistently, gaining UBE2CP3 expression may enhance the arteriole formation and vessel density of xenografts in vivo. A mechanistic study revealed that UBE2CP3 can activate the ERK/HIF-1α/p70S6K signaling cascade to augment VEGFA levels and drive angiogenesis in HCC [13].
Several studies indicate that some lncRNAs can alter the activation of the AKT/mTOR pathway to govern angiogenesis in various cancers. For instance, the oncogenic lncRNA HULC is overexpressed and positively correlated with the expressing of proangiogenic factors VEGF and ESM-1, as well as with high microvessel density in glioma tissues. HULC silencing can decrease proliferation, migration, invasion, and capillary tuber formation in glioma cells. The authors also found that ESM-1 is responsible for HULC-mediated angiogenesis, and that HULC regulates ESM-1 expression via the PI3K/AKT/mTOR signal pathway [14]. Analogously, lncRNA OR3A4 overexpression can mediate proliferation, migration and angiogenesis via the AGGF1/AKT/mTOR pathway in HCC [15]. In hepatoblastoma, upregulation of lncRNA CRNDE is markedly linked to VEGF, ESM-1 and CD34 expression. Silencing CRNDE has anti-proliferative and anti-angiogenic effects via blocking the mTOR/P70S6K signaling. Rapamycin, an inhibitor of the mTOR pathway, can successfully decrease CRNDE-mediated VEGFA and Ang-2 expression [16]. In osteosarcoma, lncRNA MALAT1 exerts a proangiogenic effect via activating the mTOR/HIF-1α pathway. Mutually, activated mTOR/HIF-1α induces MALAT1 expression to sustain oncogenic functions [17]. In lung adenocarcinoma, lncRNA LOC100132354 enables the activation of the VEGFA/VEGFR2/RAF/MEK/ERK signaling pathway to enhance angiogenesis [18].
Several lncRNAs that are not proangiogenic, such as GAS5 and MEG3, have the capacity to suppress angiogenesis. In colorectal cancer (CRC), GAS5 can inactivate the Wnt/β-catenin pathway to block angiogenesis and metastasis [19]. MEG3 is expressed at low levels in breast cancer. MEG3 overexpression can inhibit cell proliferation and invasion, as well as the expression of angiogenesis-related factors, such as VEGFA/B, PGF, bFGF, SDF-1, TGF-β and angiogenin. MEG3 suppresses the capillary tube formation of endothelial cells through inactivating AKT signaling [20].
LncRNAs mediate angiogenesis via binding to various proteins within tumor cells
LncRNAs can associate directly with proteins, which may affect protein secretion, stability, enzymatic activity, guide proteins to specific genomic regions, or decoy transcriptional factors away from target gene promoters [7]. LncRNAs can interact with various proteins to mediate angiogenesis within tumors.
MVIH is the first identified lncRNA accounting for tumor-induced angiogenesis. MVIH is substantially overexpressed in HCC specimens with high microvascular invasion and tumor node metastasis, and these patients frequently suffer from shorter recurrence-free survival and overall survival time. MVIH can associate with the PKG1 protein and inhibit PKG1 secretion to induce angiogenesis in HCC [21].
In cervical cancer, lnc-CCDST acts as a scaffold to enhance the interaction of the E3 ubiquitin ligase MDM2 with the oncogenic protein DHX9. The lnc-CCDST-dependent interplay facilitates DHX9 degradation by MDM2 and inhibits angiogenesis. However, in HPV-infected cervical cancer, the virus-encoded E6 and E7 proteins diminish lnc-CCST expression and elevate DHX9 abundance to induce malignant behaviors [22].
In gastric cancer, the upregulation of lncRNA OR3A4 is associated closely with invasion and lymphatic and distal metastasis. Mechanistically, OR3A4 can trigger a variety of gene expression and form complexes with the PDLIM2, MACC1, NTN4 and GNB2L1 proteins, thus promoting angiogenesis, tumor growth and metastasis both in vitro and in vivo [23]. In contrast to the oncogenic function of OR3A4, NBAT1 is a tumor suppressing lncRNA in gastric cancer, which can inhibit proliferation, migration, invasion and capillary tube formation. Low expression levels of NBAT1 significantly correlate with advanced tumor stage, lymph node metastasis and poor prognosis. NBAT1 interacts with Sox9 to mediate its proteasome-dependent proteolysis. Sox9 degradation leads to a reduction in NBAT1, as Sox9 can bind to the NBAT1 promoter to drive its expression. The negative feedback between NBAT1 and Sox9 continuously aggravates gastric cancer progression [24].
LncRNA PVT1 has proangiogenic properties in more cancer types than gastric cancer. Yu et al. demonstrated that lncRNA PVT1 can control angiogenesis, proliferation and apoptosis in cholangiocarcinoma (CCA) via reprogramming the chromatin state. PVT1 binds to EZH2, the crucial component of PRC2 complexes, and induces H3K27me3 epigenetic modifications within the ANGPTL4 promoter, thereby decreasing ANGPTL4 expression to promote tumorigenesis [25].
There are some oncogenic lncRNAs, such as MALAT1 and HOTAIR, which also drive angiogenesis through pleiotropic regulatory patterns. For example, MALAT1 can induce angiogenesis by affecting Sox2 expression in pancreatic cancer [26], while MALAT1 was found to promote vasculature formation via increasing FGF2 secretion in neuroblastoma cells under hypoxic conditions [27]. In nasopharyngeal carcinoma (NPC), HOTAIR knockdown attenuates tumor growth and angiogenesis both in vitro and in vivo. A subsequent study revealed that HOTAIR can bind directly to the specific DNA motif of VEGFA promoter to modify the genome structure and increase VEGFA transcription. On the other hand, HOTAIR can regulate GRP78 protein levels to indirectly affect VEGFA expression [28].
LncRNAs affect angiogenesis through crosstalk with miRNAs and mRNAs in tumor cells
In addition to interactions with proteins, intricate crosstalk exists among lncRNAs, miRNAs and mRNAs. LncRNA may function as ceRNA, which sponges miRNA via complementary miRNA binding sites, and this sponging function antagonizes miRNA availability to mRNAs and attenuates the suppressive roles of miRNA on its targets [29]. The mechanisms of ceRNA have been investigated widely in tumor angiogenesis.
In colorectal cancer (CRC), lncRNA-ZFAS1 is markedly upregulated in cancer tissues and is associated with shorter survival time. Gain- and loss- of ZFAS1 expression have a great impact on invasion, migration and angiogenesis. The transcriptional factor SP1 can be recruited within ZFAS1 promoter to initiate its expression. A mechanistic study demonstrated that ZFAS1 acts as miRNA sponge to sequester miR-150-5p away from its target VEGFA in an AGO2-dependent manner. ZFAS1-mediated VEGFA elevation activates VEGFR2 and the downstream AKT/mTOR pathway to exacerbate cancer progression [30].
Glioblastoma is a typically vessel-enriched malignant brain tumor. LncRNA TUG1 upregulation is partly responsible for the aggressive phenotype. Depletion of TUG1 notably inhibits tumor-induced endothelial cell proliferation, migration, capillary tube formation and spheroid-based angiogenesis in vitro, as well as retards tumor growth and decreases microvessel density in vivo. TUG1 can directly bind to and repress miR-299 expression. As a result, the miR-299 target VEGFA is upregulated by TUG1 and contributes to angiogenesis in glioblastoma [31]. In addition, Dong et al. reported that high lnRNA TUG1 expression can lead to angiogenesis in hepatoblastoma through ceRNA function. TUG1 may titrate and decrease miR-34a-5p levels, thereby elevating the expression of its target gene VEGFA. VEGFA antibody and miR-34a-5p treatment can block TUG1-induced tumor growth and angiogenesis [32].
LncRNA XIST overexpression not only induces angiogenesis in glioma cells but also modulates the biological properties of glioma endothelial cells (GECs). Cheng et al. found that lncRNA XIST displays significant elevation in glioma samples and that XIST knockdown can repress metastasis and angiogenesis both in vitro and in vivo. A subsequent study revealed that miR-429 can target and silence XIST expression, while XIST can function as a ceRNA to sponge miR-429 in turn. The expression levels of lncRNA XIST and miR-429 are inversely correlated in glioma [33]. Intriguingly, Yu et al. demonstrated that lncRNA XIST is overexpressed in glioma endothelial cells (GECs) co-cultured with glioma cell line U118. Silencing XIST in GECs can effectively increase the permeability of blood-tumor barrier and reduce angiogenesis. A deep investigation showed that XIST binds to miR-137, and they reciprocally inhibit each other’s expression. The tight junction-related proteins ZO-2 and FOXC1 are direct targets of miR-137. XIST regulates blood-tumor barrier permeability and angiogenesis via sequestering miR-137 to increase ZO-2 and FOXC1 expression [34].
In bladder cancer (BC), lncRNA H19 and DNMT3B are highly co-expressed in tissue samples and cells. H19 promotes the rearrangement of cytoskeleton, epithelial-to-mesenchymal transition (EMT), tumorigenesis, angiogenesis and metastasis both in vivo and in vitro. This study also verified that H19 can bind to and co-localize with miR-29b, which relieves the silencing effect of miR-29b on DNMT3B and promotes bladder cancer development [35]. Recently, the same group found that lncRNA RP11-79H23.3 and the tumor suppressor gene PTEN are remarkably downregulated in BC tissues, and this downregulation can inhibit tumorigenesis, angiogenesis and metastasis [36]. Similar to H19, lncRNA RP11-79H23.3 functions as a ceRNA of miR-107 and represses the effect of miR-107 on PTEN. The elevation in PTEN levels consequently blocks PI3K/AKT signaling pathway activity to suppress BC progression [36].
In pancreatic ductal adenocarcinoma (PDAC), Linc00511 serves as a ceRNA to titrate miR-29-3P and subsequently increases the expression of miR-29 target VEGFA to induce angiogenesis. High levels of linc00511 are associated with metastasis and poor prognosis in PDAC [37].
In HCC, lncRNA HULC can act as ceRNA to sequester miR-107, which increases the expression of miR-107 target E2F1. As a result, the transcriptional factor E2F1 binds to the SPHK1 promoter and elevates SPHK1 expression to induce angiogenesis [38].
Taken together, lncRNAs can exert diverse mechanisms to stimulate tumor cells to release angiogenic factors, and these growth factors induce endothelial cells to form capillaries network within tumors. As we described above, there exist many feedback regulation between aberrant lncRNA expression and oncogenic signaling pathway activation, such as the reciprocal interplay loop between PVT1 and STAT3, TNK2-AS1 and STAT3, as well as LINC1410 and NF-κB. These positive feedback loops represent essentially biological switches to consecutively sustain oncogenic properties including angiogenesis, which may be critical intervention targets for cancer therapy.
It should be realized that there are some limits in these present studies. Firstly, the activation of STAT3, NF-κb, AKT, mTOR and WNT pathways may initiate a lot of downstream gene expression, some of which are responsible for activating tumor microenvironment cells. Tumor angiogenesis need the crosstalk among tumor, endothelial and mesenchymal cells. However, most of present studies were directly carried out with co-culture system between tumor cells and endothelial cells in vitro, and took immune deficient mice as the in vivo model, which can’t fully reflect the effects of tumor immune microenvironment on angiogenesis, and the regulatory roles of other cell types such as myeloid cells or fibroblasts on angiogenesis. Secondly, the ceRNA function of lncRNA has been widely studied in angiogenesis. It is noteworthy that lncRNA has different miRNA response elements, and each miRNA has various targets. The interaction among different miRNAs and their targets may synergistically contribute to angiogenesis, but there are lack of further studies. In addition, although a number lncRNAs have been identified as oncogenes to promote the secretion of angiogenic factor from tumor cells into the tumor microenvironment, the underlying mechanisms of how endothelial cells respond to the dysregulation of lncRNAs in tumors are largely unknown, which need to be elucidated in future studies.
LncRNAs are essential for maintaining endothelial cell functions
LncRNAs also play important roles in endothelial cells to sustain specialized endothelial cell phenotypes and functions, including new blood vessel formation. Recently, the functions and underlying mechanisms of some lncRNAs in endothelial cells have been identified.
LncRNA STEEL is an endothelial cell-enriched lncRNA that can increase tube formation capacity. STEEL can transcriptionally increase eNOS and KLF2 expression via activating the epigenetic status of H3K4me3 in their promoters. In addition, STEEL complexes with PARP1 and recruits PARP1 to the KLF2 promoter, which partially accounts for STEEL-mediated KLF2 upregulation. Conversely, eNOS and KLF2 exert feedback inhibition on lncRNA STEEL [39].
In tumor-associated endothelial cells, tumor cells may alter the lncRNA profile in endothelial cells to modulate angiogenesis. Glioma has been extensively reported to control the biological behaviors of glioma vascular endothelial cells through the lncRNA/miRNA axis. Zheng et al. found that when glioma vascular endothelial cells are stimulated with glioma-conditioned medium, PVT1 is highly expressed and increases cell proliferation, migration and angiogenesis in glioma vascular endothelial cells. A subsequent investigation elucidated that PVT1 acts as a ceRNA to sequester miR-186 and blocks the silencing effect of miR-186 on its targets Atg7 and Beclin1, by which increasing Atg7 and Beclin1 expression to enhance protective autophagy [40]. H19 is expressed highly in the microvessels of glioma tissues and endothelial cells cultured with glioma-conditioned medium. Further study indicated that H19 can regulate VASH2 expression to mediate angiogenesis by binding directly to and inhibiting miR-29a in endothelial cells [41]. MCM3AP-AS1 is another lncRNA, that is upregulated in glioma-associated endothelial cells (GECs). Silencing MCM3AP-AS1 reduces GEC cell viability, migration and tube formation potential. MCM3AP-AS1 can increase KLF5 levels by decreasing its negative regulator miR-211. Then, KLF5 is enriched within the promoter region of AGGF1 to initiate AGGF1 expression, thereby triggering the PI3K/AKT and ERK1/2 signaling pathways to enhance glioma angiogenesis [42].
In addition to those in GECs, lncRNAs in vascular endothelial cells have been closely investigated. LncRNA PVT1 upregulation in vascular endothelial cells can trigger proliferation, migration and capillary tube formation as well. Mechanistically, PVT1 binds to and degrades miR-26b to increase the expression levels of CTGF and ANGPT2 in vascular endothelial cells [43]. Another study in human umbilical vein endothelial cells (HUVECs) showed that DNA methylation controls lncRNA MEG3 expression under normoxic conditions, whereas HIF-1α is responsible for MEG3 transcription in hypoxic treatment. MEG3 knock-down diminishes VEGFR2 expression and reduces angiogenesis by exclusively blocking the VEGFA/VEGFR2 pathway [44].
Collectively, the stimuli from tumor cells can alter lncRNA expression profiles in endothelial cells. These deregulated lncRNAs have great impact on biological properties of vascular endothelial cells through various mechanisms, and result in angiogenesis. Now, endothelia-derived lncRNAs have not been extensive studied, and the functions of aberrant lncRNA expression in endothelial cells are still unclear. Meanwhile, what are the determining factors in tumor stimuli, and how these factors to change lncRNA profiles in endothelial cells need deeply investigated as well.
Dysregulation of lncRNAs in tumor-associated macrophages stimulates angiogenesis
Tumor immune microenvironment plays pivotal roles in cancer development. Tumor-associated macrophages (TAMs) are the major component of tumor microenvironment, which can recruit endothelia cell to form capillaries within tumors, induce immune evasion and metastasis [45]. Several studies reported that dysregulation of lncRNAs may endow macrophage with TAM properties and promote angiogenesis.
It has been established that TAMs commonly share many properties with M2 macrophages in that they both modulate tumor growth, angiogenesis and immune escape. Cao et al. used IL-4 and IL-13 induction to construct cell-based M2 polarization models and observed the importance of lncRNA-MM2P in determining M2 differentiation; the lncRNA-MM2P expression levels increase during M2 polarization but decrease in M1 macrophages [46]. This study also showed that silencing lncRNA-MM2P expression in macrophages markedly blocks cytokine-mediated M2 polarization and inhibits M2-induced angiogenesis both in vitro and in vivo by inactivating the STAT6 signaling pathway [46]. Recently, Sang et al. identified a crucial lncRNA, CamK-A, that enables the remodel of cancer cell metabolic and immune microenvironments. LncRNA CamK-A can bind directly to the calmodulin-dependent kinase PNCK to phosphorylate and degrade IkB-α, which in turn stimulates the NF-κB pathway in a calmodulin-dependent manner. Consequently, the NF-κB signaling axis transactivates a number of cytokines, such as VEGF, IL-6, IL-8 and metabolism-related genes, which results in macrophage recruitment, angiogenesis and tumor progression [47].
In thyroid cancer, Huang et al. enriched CD14 positive monocytes of human peripheral blood mononuclear cells (PBMCs) and treated them with LPS, IL-4 and conditioned medium of cancer cell line FTC133 to induce M1, M2 and TAM models respectively. TAMs have properties similar to those of M2, in which IL-10 and FGF2 are increased, whereas TNF-α and IL-12 secretion are decreased. When lncRNA MALAT1 is knocked down in TAMs, the condition medium from the TAMs can suppress the proliferation, migration and invasion of tumor cells, as well as reduce angiogenesis in endothelial cells. These results show that MALAT1 in TAMs mediates angiogenesis through influencing FGF2 secretion [48].
TAMs is believed to play critical roles in mediating immunosuppression. LncRNAs are implicated in the polarization process of macrophage. The number of TAMs infiltrated into tumors is closely related to malignant cancer progression. There are several means to construct TAMs cell models in vitro, including IL-4/IL-13-induced M2 polarization, and cancer cell conditioned medium-treated macrophages and PBMCs. But these cell-based TAM models in vitro are greatly different from the TAMs infiltrated within tumors in vivo. Therefore, isolation and purification of the infiltrated TAMs from solid tumors may provide more convincible evidence. The comparison of LncRNA signature among cell/PBMC-based and infiltrated TAMs is helpful to identify the specific lncRNAs for TAM polarization. Additionally, how lncRNAs in tumor cells educate tumor microenvironment cells to become TAMs, cancer-associated fibroblasts and endothelial cells, which still need further exploration.
Tumor-derived lncRNAs can be delivered into endothelia cells via exosomes to promote angiogenesis
Exosomes are a subset of extracellular vesicles with diameters of 30-150 nm, which serve as an important communication system. Exosomes can load a variety of molecules including DNA, RNA, protein, lncRNA, miRNA and circRNA, carrying these molecules to neighboring cells. Tumor cells can release millions of exosomes to reprogram tumor microenvironment to aggravate tumor progression [49]. Tumor-derived exosomes play crucial roles in inducing angiogenesis [50]. Several studies have demonstrated that exosomal lncRNAs from tumor cells can be taken up by endothelia cell to modulate angiogenesis.
Linc-POU3F3 is highly expressed in glioma tissues and cells. When exosomes from glioma cells are co-incubated with human brain microvascular endothelial cells (HBMECs), the exosomes are internalized by the HBMECs and linc-POU3F3 expression is increased within endothelia cells. The glioma-derived exosomes can markedly promote migration, proliferation, tubular-like structure formation in vitro and arteriole formation in vivo by increasing the levels of angiogenesis-related proteins in HBMECs [51]. Exosomes from another glioma cell line, U87, that has high linc-CCAT2 expression, can transmit linc-CCAT2 to endothelial cells to induce angiogenic phenotypes both in vitro and in vivo, as well as exert anti-apoptosis effects via hypoxia. Linc-CCAT2 upregulates VEGFA, TGF-β and Bcl-2, but downregulates Bax and caspase-3 expression to inhibit hypoxia-mediated apoptosis in endothelial cells [52]. HOTAIR also has the capacity to enhance angiogenesis through increasing VEGFA in glioma cells. Further studies showed that HOTAIR can be encapsulated into glioma cell-derived extracellular vesicles for delivery to endothelial cells [53].
In HCC, exosomes isolated from CD90+ liver cancer stem-like cells can stimulate endothelial cells to form capillary networks and enhance the adhesion of CD90+ cells to monolayer endothelial cells. LncRNA profiling revealed that lncRNA H19 is enclosed into CD90+ cell-derived exosomes and mediates the expression of proangiogenic factors, including VEGF, VEGFR1 and ICAM1 in endothelia cells [54].
In epithelial ovarian cancer (EOC), lncRNA MALAT1 is transported to endothelial cells through cancer cell-secreted exosomes, and then stimulates proangiogenic behaviors through modulating angiogenesis-relevant genes in endothelial cells. Furthermore, serum exosomal MALAT1 levels are tightly correlated with advanced and metastatic outcomes, and they act as an independent predictive factor for overall survival in EOC [55].
In addition to exosome loading, lncRNAs can affect tumor-derived exosome production. During colorectal carcinoma (CRC) development, adenomatous polyposis coli (APC) gene mutations are a critical event. Wang et al. identified an APC-activated lncRNA-APC1 that can weaken tumor growth, metastasis and angiogenesis. A mechanistic study revealed that lncRNA-APC1 associates directly with Rab5b mRNA and decreases its mRNA stability, thereby reducing CRC-derived exosome production. When APC mutations occurs in CRC cells, they may release more exosomes to activate MAPK pathway in endothelial cells to induce angiogenesis [56].
Exosome is an efficient carrier for intercellular information communication. Tumor-derived exosomes may contain specific proteins and RNA repertoires including numerous lncRNAs. The processes of exosome loading, secretion and uptake are precisely regulated. How the specific lncRNAs are sorted into exosomes and how the exosome are specifically uptake by vascular endothelial cells, these detailed mechanisms are warranted to figure out.
LncRNAs remodel cancer cell to form vasculogenic mimicry to aggravate cancer development
Angiogenesis is not the only means of tumor vasculature development. Vasculogenic mimicry (VM) is another common neovascularization process in tumor development, which was first found in melanoma in 1999 [57]. VM refers to highly plastic tumor cells that may dedifferentiate and obtain endothelial properties, which allow aggressive tumor cells to form vessel-like structures by themselves [57]. Similar to angiogenesis, VM channels contain plasma and red blood cells and have the capacity to transport oxygen and nutrients [58]. In addition, VM networks enable detached tumor cells to enter the blood stream directly, thereby facilitating tumor metastasis to distant organs. Hence, VM density frequently correlates with a high risk of metastasis and poor prognosis [59]. Recently, several lncRNAs have been identified to be involved in tumor VM formation.
In gastric cancer, lncRNA MALAT1 is upregulated and closely associated with VM density and endothelial vessels. MALAT1 was found to regulate the expression of the VE-cadherin/β-catenin complex, ERK/MMPs and FAK/paxillin signaling pathways. Consistently, p-ERK inhibitors can effectively inhibit gastric cancer cell from forming VM networks [60]. MALAT1 is also involved in ERβ-mediated VM formation in non-small cell lung cancer (NSCLC), but the mechanism is different from that in gastric cancer. In NSCLC, ERβ occupies the estrogen response elements within the MALAT1 promoter to activate its transcription directly. Then, the upregulation of MALAT1 decreases miR145-5p expression and increases the protein levels of its target NEDD9, thus promoting VM formation [61]. In lung adenocarcinoma, LINC00312 can induce migration, invasion and VM via forming a complex with the transcriptional factor YBX1. LINC00312 upregulation is positively related to VM in lung adenocarcinoma specimens [62].
A previous study reported that cancer stem cells (CSCs) are closely linked to the VM process, as tumor cells capable of VM resemble CSCs with highly undifferentiated and plastic properties [63]. In HCC, lncRNA n339260 is overexpressed in cancer stem-like cells and is associated with VM appearance and shorter survival time in mice xenograft model. LncRNA n339260 can induce VE-cadherin expression, which is a well-established molecular marker of VM [64].
In osteosarcoma, Ren et al. reported that lncRNA n340532 has the capacity to stimulate VM. Knockdown of lncRNA n340532 can suppress VM formation in vitro and reduce VM density in orthotopical graft in vivo. But the mechanism has not been interpreted [65].
Glioma is not only characterized as highly angiogenic malignance but also enriched with VM networks. HOXA-AS2 is upregulated and positively correlated with VM density in glioma specimens. Further study revealed that HOXA-AS2 can bind to miR-373 and diminish its expression. As a result, the target of miR-373, EGFR, is increased, which activates the PI3K pathway to increase VE-cadherin and MMP2/9 expression and leads to VM formation [66]. Similarly, LINC00339 is found to regulate VM in glioma as well. Mechanistically, LINC00339 functions as ceRNA to prevent miR-539-5p from its target Twist1. The upregulation of Twist1 enhances its transcriptional activity to the promoters of MMP2/14, which can degrade extracellular matrix and facilitate VM formation. LINC00339 depletion and miR-539-5p introduction can significantly shrink tumor growth and reduce VM density in vivo xenografts [67].
VM networks have been observed in multiple types of highly aggressive tumors, and regarded as an important factor for the failure of anti-angiogenesis treatment. The malignant tumor cells form extracellular matrix (ECM)-rich vasculogenic-like channels, which can be stained by periodic acid-schiff (PAS). In the present studies, VM densities in tumor tissues were determined by dual staining for endothelial marker CD31 or CD34 and PAS. The lumens with positive CD31 or CD34 staining are endothelial vessels, whereas capillaries with positive PAS but negative CD31 or CD34 staining are defined as VM. Increasing evidence suggests that the properties of cancer stem cells and epithelial mesenchymal transition may contribute to VM formation. How lncRNAs direct the undifferentiated and plastic tumor-cell phenotype need to study in-depth.
Conclusion
In this review, we summarized the versatile mechanisms of lncRNAs in tumor-induced angiogenesis as listed in Table 1, and indicated the complexity of lncRNA-mediated angiogenesis in cancer development. Notably, the same lncRNA, such as PVT1, MALAT1, HULC and TUG1, may act via different mechanisms to trigger angiogenesis. In gastric cancer, we found PVT1 facilitates angiogenesis via eliciting the STAT3/VEGFA axis [10]. In cholangiocarcinoma, PVT1 promotes capillary formation through epigenetically silencing ANGPTL4 expression [25]. In addition to cancer cells, PVT1 affects the vasculatures capacity of glioma microvascular endothelial cells by sponging miR-186 to induce protective autophagy [40]. PVT1 can also enhance the tube network formation of vascular endothelial cells by targeting miR-26b to activate CTGF and ANGPT2 [43]. LncRNA MALAT1 acts via diverse mechanisms to control angiogenesis by activating mTOR/HIF-1α signal pathway in osteosarcoma [17], enhancing Sox2 expression in pancreatic cancer [26], and increasing FGF2 secretion in neuroblastoma [27]. Moreover, MALAT1 can directly remodel cancer cells to induce vasculogenic mimicry through impacting the ERK/MMPs and FAK/paxillin axis [60], or by decoying miR-145-5p to elevate NEDD9 expression [61]. These independent studies demonstrate that lncRNAs are multifunctional regulators, and that distinct mechanisms of controlling angiogenesis may depend on different cellular backgrounds or stimulated contexts. Investigations of these regulatory mechanisms are helpful to develop effective and precise approaches for cancer treatment.
Table 1.
The versatile mechanisms of lncRNAs in tumor-induced angiogenesis
| LncRNAs regulate angiogenesis by proteins | ||||
|
| ||||
| LncRNA Expression | Regulatory Target | Cancer types | Reference | |
|
| ||||
| PVT1 | ↑ | STAT3/VEGFA | Gastric cancer | [10] |
| PVT1 | ↑ | PRC2 | Cholangiocarcinoma | [25] |
| MALAT1 | ↑ | mTOR/HIF-1α | Osteosarcoma | [17] |
| MALAT1 | ↑ | Sox2 | Pancreatic cancer | [26] |
| MALAT1 | ↑ | FGF2 | Neuroblastoma | [27] |
| MALAT1 | ↑ | FGF2 secretion | Thyroid cancer | [48] |
| MALAT1 | ↑ | VE-cadherin/β-catenin, ERK/MMPs, and FAK/paxillin | Gastric cancer | [60] |
| lncRNA-MM2P | ↑ | STAT6 activation | Osteosarcoma | [46] |
| TNK2-AS1 | ↑ | STAT3/VEGFA | Non-small cell lung cancer | [11] |
| LOC100132354 | ↑ | VEGFA/VEGFR2/Raf/MEK/ERK | Lung adenocarcinoma | [18] |
| UBE2CP3 | ↑ | ERK/HIF-1α/p70S6K/VEGFA | Hepatocellular carcinoma | [13] |
| OR3A4 | ↑ | AGGF1/AKT/mTOR | Hepatocellular carcinoma | [15] |
| MVIH | ↑ | PKG1 | Hepatocellular carcinoma | [21] |
| LINC00312 | ↑ | YBX1 | Hepatocellular carcinoma | [62] |
| n339260 | ↑ | VE-cadherin | Hepatocellular carcinoma | [64] |
| H19 | ↑ | VEGF, VEGFR1 and ICAM1 | Hepatocellular carcinoma | [54] |
| CRNDE | ↑ | mTOR/P70S6K/VEGFA and ESM1 | Hepatoblastoma | [16] |
| HULC | ↑ | PI3K/Akt/mTOR/ESM-1 | Glioma | [14] |
| Linc-CCAT2 | ↑ | VEGFA, TGF-β, Bcl-2, Bax, caspase-3 | Glioma | [52] |
| HOTAIR | ↑ | VEGFA | Glioma | [53] |
| OR3A4 | ↑ | PDLIM2, MACC1, NTN4 and GNB2L1 | Gastric cancer | [23] |
| HOTAIR | ↑ | VEGFA promoter/GRP78 | Nasopharyngeal carcinoma | [28] |
| CamK-A | ↑ | PNCK/IkB-α/NF-κB/VEGF, IL-6 and IL-8 | Breast cancer | [47] |
| GAS5 | ↓ | Wnt/β-catenin | Colorectal cancer | [19] |
| lncRNA-APC1 | ↓ | Rab5b | Colorectal cancer | [56] |
| MEG3 | ↓ | AKT/VEGF | Breast cancer | [20] |
| lnc-CCDST | ↓ | MDM2/DHX9 | Cervical cancer | [22] |
| NBAT1 | ↓ | Sox9 | Gastric cancer | [24] |
|
| ||||
| LncRNAs regulate angiogenesis via ceRNA function | ||||
|
| ||||
| LncRNA Expression | miRNA and target | Cancer types | Reference | |
|
| ||||
| LINC01410 | ↑ | miR-532-5p/NCF2/NF-κB | Gastric cancer | [12] |
| TUG1 | ↑ | miR-299/VEGFA | Glioblastoma | [31] |
| TUG1 | ↑ | miR-34a-5p/VEGFA | Hepatoblastoma | [32] |
| lncRNA XIST | ↑ | miR-429 | Glioma | [33] |
| HOXA-AS2 | ↑ | miR-373/EGFR/PI3K/VE-cadherin/MMPs | Glioma | [66] |
| LINC00339 | ↑ | miR-539-3p/Twist1/MMPs | Glioma | [67] |
| Linc00511 | ↑ | miR-29-3P/VEGFA | Pancreatic cancer | [37] |
| HULC | ↑ | miR-107/E2F1/SPHK1 | Hepatocellular carcinoma | [38] |
| ZFAS1 | ↑ | miR-150-5p/VEGFA | Colorectal cancer | [30] |
| MALAT1 | ↑ | miR145-5p/NEDD9 | Non-small cell lung cancer | [61] |
| H19 | ↑ | miR-29b/DNMT3B | Bladder cancer | [35] |
| RP11-79H23.3 | ↓ | miR-107/PTEN | Bladder cancer | [36] |
|
| ||||
| LncRNAs regulate angiogenesis via maintaining endothelial cells function | ||||
|
| ||||
| LncRNA Expression | miRNA and target | Endothelia cell types | Reference | |
|
| ||||
| STEEL | ↑ | STEEL/PARP1/KLF2 negative feedback | Endothelial cells | [39] |
| PVT1 | ↑ | miR-186/Atg7 and Beclin1 | Glioma endothelial cells | [40] |
| H19 | ↑ | miR-29a/VASH2 | Glioma endothelial cells | [41] |
| MCM3AP-AS1 | ↑ | miR-211/KLF5/AGGF1/PI3K/AKT/ERK | Glioma endothelial cells | [42] |
| lncRNA XIST | ↑ | miR-137/ZO-2 and FOXC1 | Glioma endothelia cells | [34] |
| PVT1 | ↑ | miR26b/CTGF and ANGPT2 | Vein endothelial cells | [43] |
| MEG3 | ↑ | VEGFA/VEGFR2 | Vein endothelial cells | [44] |
To the best of our known, although anti-angiogenesis therapy has greatly improved progression-free survival, the overall survival time remains unsatisfactory because of the lack of proper biomarkers to identify sensitive responders [3]. VEGFA alone is inadequate to predict the effects of bevacizumab (anti-VEGF) treatment [68]. Some lncRNAs, such as PVT1 and MALAT1, are very stable in physiological environments and can be detected successfully in serum and exosomes; thus, these lncRNAs have potential diagnostic and prognostic value [55,69]. Nowadays, lncRNA PCA3 has been approved as early diagnostic biomarker for prostate cancer by FDA [70]. Combining lncRNAs and proteins might be a feasible strategy to monitor the efficacy and predict the prognosis of anti-angiogenesis therapy. Additionally, the regulatory signaling pathways of lncRNAs in angiogenesis may be promising therapeutic targets. The clinical application of lncRNAs merits further investigations in the future.
Acknowledgements
This study was supported by the Natural Science Foundation of China (81672378, 81201521 and 81873874) and the National Key Project for Infectious Disease of China (2017ZX10203205-002-004 and 2017ZX10203205-003-003).
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 3.Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518–529. doi: 10.1016/S0140-6736(15)01088-0. [DOI] [PubMed] [Google Scholar]
- 4.ENCODE Project Consortium; Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE, Kuehn MS, Taylor CM, Neph S, Koch CM, Asthana S, Malhotra A, Adzhubei I, Greenbaum JA, Andrews RM, Flicek P, Boyle PJ, Cao H, Carter NP, Clelland GK, Davis S, Day N, Dhami P, Dillon SC, Dorschner MO, Fiegler H, Giresi PG, Goldy J, Hawrylycz M, Haydock A, Humbert R, James KD, Johnson BE, Johnson EM, Frum TT, Rosenzweig ER, Karnani N, Lee K, Lefebvre GC, Navas PA, Neri F, Parker SC, Sabo PJ, Sandstrom R, Shafer A, Vetrie D, Weaver M, Wilcox S, Yu M, Collins FS, Dekker J, Lieb JD, Tullius TD, Crawford GE, Sunyaev S, Noble WS, Dunham I, Denoeud F, Reymond A, Kapranov P, Rozowsky J, Zheng D, Castelo R, Frankish A, Harrow J, Ghosh S, Sandelin A, Hofacker IL, Baertsch R, Keefe D, Dike S, Cheng J, Hirsch HA, Sekinger EA, Lagarde J, Abril JF, Shahab A, Flamm C, Fried C, Hackermüller J, Hertel J, Lindemeyer M, Missal K, Tanzer A, Washietl S, Korbel J, Emanuelsson O, Pedersen JS, Holroyd N, Taylor R, Swarbreck D, Matthews N, Dickson MC, Thomas DJ, Weirauch MT, Gilbert J, Drenkow J, Bell I, Zhao X, Srinivasan KG, Sung WK, Ooi HS, Chiu KP, Foissac S, Alioto T, Brent M, Pachter L, Tress ML, Valencia A, Choo SW, Choo CY, Ucla C, Manzano C, Wyss C, Cheung E, Clark TG, Brown JB, Ganesh M, Patel S, Tammana H, Chrast J, Henrichsen CN, Kai C, Kawai J, Nagalakshmi U, Wu J, Lian Z, Lian J, Newburger P, Zhang X, Bickel P, Mattick JS, Carninci P, Hayashizaki Y, Weissman S, Hubbard T, Myers RM, Rogers J, Stadler PF, Lowe TM, Wei CL, Ruan Y, Struhl K, Gerstein M, Antonarakis SE, Fu Y, Green ED, Karaöz U, Siepel A, Taylor J, Liefer LA, Wetterstrand KA, Good PJ, Feingold EA, Guyer MS, Cooper GM, Asimenos G, Dewey CN, Hou M, Nikolaev S, Montoya-Burgos JI, Löytynoja A, Whelan S, Pardi F, Massingham T, Huang H, Zhang NR, Holmes I, Mullikin JC, Ureta-Vidal A, Paten B, Seringhaus M, Church D, Rosenbloom K, Kent WJ, Stone EA NISC Comparative Sequencing Program; Baylor College of Medicine Human Genome Sequencing Center; Washington University Genome Sequencing Center; Broad Institute; Children’s Hospital Oakland Research Institute. Batzoglou S, Goldman N, Hardison RC, Haussler D, Miller W, Sidow A, Trinklein ND, Zhang ZD, Barrera L, Stuart R, King DC, Ameur A, Enroth S, Bieda MC, Kim J, Bhinge AA, Jiang N, Liu J, Yao F, Vega VB, Lee CW, Ng P, Shahab A, Yang A, Moqtaderi Z, Zhu Z, Xu X, Squazzo S, Oberley MJ, Inman D, Singer MA, Richmond TA, Munn KJ, Rada-Iglesias A, Wallerman O, Komorowski J, Fowler JC, Couttet P, Bruce AW, Dovey OM, Ellis PD, Langford CF, Nix DA, Euskirchen G, Hartman S, Urban AE, Kraus P, Van Calcar S, Heintzman N, Kim TH, Wang K, Qu C, Hon G, Luna R, Glass CK, Rosenfeld MG, Aldred SF, Cooper SJ, Halees A, Lin JM, Shulha HP, Zhang X, Xu M, Haidar JN, Yu Y, Ruan Y, Iyer VR, Green RD, Wadelius C, Farnham PJ, Ren B, Harte RA, Hinrichs AS, Trumbower H, Clawson H, Hillman-Jackson J, Zweig AS, Smith K, Thakkapallayil A, Barber G, Kuhn RM, Karolchik D, Armengol L, Bird CP, de Bakker PI, Kern AD, Lopez-Bigas N, Martin JD, Stranger BE, Woodroffe A, Davydov E, Dimas A, Eyras E, Hallgrímsdóttir IB, Huppert J, Zody MC, Abecasis GR, Estivill X, Bouffard GG, Guan X, Hansen NF, Idol JR, Maduro VV, Maskeri B, McDowell JC, Park M, Thomas PJ, Young AC, Blakesley RW, Muzny DM, Sodergren E, Wheeler DA, Worley KC, Jiang H, Weinstock GM, Gibbs RA, Graves T, Fulton R, Mardis ER, Wilson RK, Clamp M, Cuff J, Gnerre S, Jaffe DB, Chang JL, Lindblad-Toh K, Lander ES, Koriabine M, Nefedov M, Osoegawa K, Yoshinaga Y, Zhu B, de Jong PJ. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–181. doi: 10.1016/j.cell.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172:393–407. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Du P, Cui P, Qin Y, Hu C, Wu J, Zhou Z, Zhang W, Qin L, Huang G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37:4094–4109. doi: 10.1038/s41388-018-0250-z. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Han D, Pan L, Sun J. The positive feedback between lncRNA TNK2-AS1 and STAT3 enhances angiogenesis in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507:185–192. doi: 10.1016/j.bbrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JX, Chen ZH, Chen DL, Tian XP, Wang CY, Zhou ZW, Gao Y, Xu Y, Chen C, Zheng ZS, Weng HW, Ye S, Kuang M, Xie D, Peng S. LINC01410-miR-532-NCF2-NF-kB feedback loop promotes gastric cancer angiogenesis and metastasis. Oncogene. 2018;37:2660–2675. doi: 10.1038/s41388-018-0162-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Lin J, Cao S, Wang Y, Hu Y, Liu H, Li J, Chen J, Li P, Liu J, Wang Q, Zheng L. Long non-coding RNA UBE2CP3 enhances HCC cell secretion of VEGFA and promotes angiogenesis by activating ERK1/2/HIF-1alpha/VEGFA signalling in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:113. doi: 10.1186/s13046-018-0727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Zhang X, Qi L, Cai Y, Yang P, Xuan G, Jiang Y. HULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomas. Oncotarget. 2016;7:14429–14440. doi: 10.18632/oncotarget.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Fu Q, Man W, Guo H, Yang P. LncRNA OR3A4 participates in the angiogenesis of hepatocellular carcinoma through modulating AGGF1/akt/mTOR pathway. Eur J Pharmacol. 2019;849:106–114. doi: 10.1016/j.ejphar.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Dong R, Liu XQ, Zhang BB, Liu BH, Zheng S, Dong KR. Long non-coding RNA-CRNDE: a novel regulator of tumor growth and angiogenesis in hepatoblastoma. Oncotarget. 2017;8:42087–42097. doi: 10.18632/oncotarget.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang ZC, Tang C, Dong Y, Zhang J, Yuan T, Tao SC, Li XL. Targeting the long noncoding RNA MALAT1 blocks the pro-angiogenic effects of osteosarcoma and suppresses tumour growth. Int J Biol Sci. 2017;13:1398–1408. doi: 10.7150/ijbs.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang F, Wang J, Hu L, Jiang F, Chen J, Chen J, Wang L. lncRNA LOC100132354 promotes angiogenesis through VEGFA/VEGFR2 signaling pathway in lung adenocarcinoma. Cancer Manag Res. 2018;10:4257–4266. doi: 10.2147/CMAR.S177327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song J, Shu H, Zhang L, Xiong J. Long noncoding RNA GAS5 inhibits angiogenesis and metastasis of colorectal cancer through the Wnt/beta-catenin signaling pathway. J Cell Biochem. 2019 doi: 10.1002/jcb.27743. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Zhang CY, Yu MS, Li X, Zhang Z, Han CR, Yan B. Overexpression of long non-coding RNA MEG3 suppresses breast cancer cell proliferation, invasion, and angiogenesis through AKT pathway. Tumour Biol. 2017;39:1010428317701311. doi: 10.1177/1010428317701311. [DOI] [PubMed] [Google Scholar]
- 21.Yuan SX, Yang F, Yang Y, Tao QF, Zhang J, Huang G, Yang Y, Wang RY, Yang S, Huo XS, Zhang L, Wang F, Sun SH, Zhou WP. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–2241. doi: 10.1002/hep.25895. [DOI] [PubMed] [Google Scholar]
- 22.Ding X, Jia X, Wang C, Xu J, Gao SJ, Lu C. A DHX9-lncRNA-MDM2 interaction regulates cell invasion and angiogenesis of cervical cancer. Cell Death Differ. 2018 doi: 10.1038/s41418-018-0242-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo X, Yang Z, Zhi Q, Wang D, Guo L, Li G, Miao R, Shi Y, Kuang Y. Long noncoding RNA OR3A4 promotes metastasis and tumorigenicity in gastric cancer. Oncotarget. 2016;7:30276–30294. doi: 10.18632/oncotarget.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J, Huang W, Huang X, Xiang W, Ye C, Liu J. A negative feedback loop between long noncoding RNA NBAT1 and Sox9 inhibits the malignant progression of gastric cancer cells. Biosci Rep. 2018;38 doi: 10.1042/BSR20180882. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Yu Y, Zhang M, Liu J, Xu B, Yang J, Wang N, Yan S, Wang F, He X, Ji G, Li Q, Miao L. Long non-coding RNA PVT1 promotes cell proliferation and migration by silencing ANGPTL4 expression in cholangiocarcinoma. Mol Ther Nucleic Acids. 2018;13:503–513. doi: 10.1016/j.omtn.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao F, Hu H, Han T, Yuan C, Wang L, Jin Z, Guo Z, Wang L. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci. 2015;16:6677–6693. doi: 10.3390/ijms16046677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tee AE, Liu B, Song R, Li J, Pasquier E, Cheung BB, Jiang C, Marshall GM, Haber M, Norris MD, Fletcher JI, Dinger ME, Liu T. The long noncoding RNA MALAT1 promotes tumor-driven angiogenesis by up-regulating pro-angiogenic gene expression. Oncotarget. 2016;7:8663–8675. doi: 10.18632/oncotarget.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu WM, Lu YF, Hu BG, Liang WC, Zhu X, Yang HD, Li G, Zhang JF. Long noncoding RNA Hotair mediated angiogenesis in nasopharyngeal carcinoma by direct and indirect signaling pathways. Oncotarget. 2016;7:4712–4723. doi: 10.18632/oncotarget.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer Discov. 2013;3:1113–1121. doi: 10.1158/2159-8290.CD-13-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, He B, Pan Y, Sun H, Wang S. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 2018;9:982. doi: 10.1038/s41419-018-0962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H, Liu X, Zheng J, Xue Y, Ma J, Li Z, Xi Z, Li Z, Bao M, Liu Y. Long non-coding RNA taurine upregulated 1 enhances tumor-induced angiogenesis through inhibiting microRNA-299 in human glioblastoma. Oncogene. 2017;36:318–331. doi: 10.1038/onc.2016.212. [DOI] [PubMed] [Google Scholar]
- 32.Dong R, Liu GB, Liu BH, Chen G, Li K, Zheng S, Dong KR. Targeting long non-coding RNA-TUG1 inhibits tumor growth and angiogenesis in hepatoblastoma. Cell Death Dis. 2016;7:e2278. doi: 10.1038/cddis.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng Z, Li Z, Ma K, Li X, Tian N, Duan J, Xiao X, Wang Y. Long non-coding RNA XIST promotes glioma tumorigenicity and angiogenesis by acting as a molecular sponge of miR-429. J Cancer. 2017;8:4106–4116. doi: 10.7150/jca.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Xue Y, Wang P, Liu X, Ma J, Zheng J, Li Z, Li Z, Cai H, Liu Y. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis. 2017;6:e303. doi: 10.1038/oncsis.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv M, Zhong Z, Huang M, Tian Q, Jiang R, Chen J. lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res. 2017;1864:1887–1899. doi: 10.1016/j.bbamcr.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Chi H, Yang R, Zheng X, Zhang L, Jiang R, Chen J. LncRNA RP11-79H23.3 functions as a competing endogenous RNA to regulate PTEN expression through sponging hsa-miR-107 in the development of bladder cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Liu Y, Li Z, Zheng S, Wang Z, Li W, Bi Z, Li L, Jiang Y, Luo Y, Lin Q, Fu Z, Rufu C. Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J Cell Mol Med. 2018;22:655–667. doi: 10.1111/jcmm.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Z, Xiao Z, Liu F, Cui M, Li W, Yang Z, Li J, Ye L, Zhang X. Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1) Oncotarget. 2016;7:241–254. doi: 10.18632/oncotarget.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Man HSJ, Sukumar AN, Lam GC, Turgeon PJ, Yan MS, Ku KH, Dubinsky MK, Ho JJD, Wang JJ, Das S, Mitchell N, Oettgen P, Sefton MV, Marsden PA. Angiogenic patterning by STEEL, an endothelial-enriched long noncoding RNA. Proc Natl Acad Sci U S A. 2018;115:2401–2406. doi: 10.1073/pnas.1715182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Wang P, Xue Y, Qu C, Zheng J, Liu X, Ma J, Liu Y. PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol. 2017;39:1010428317694326. doi: 10.1177/1010428317694326. [DOI] [PubMed] [Google Scholar]
- 41.Jia P, Cai H, Liu X, Chen J, Ma J, Wang P, Liu Y, Zheng J, Xue Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016;381:359–369. doi: 10.1016/j.canlet.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Zheng J, Xue Y, Yu H, Liu X, Ma J, Liu L, Wang P, Li Z, Cai H, Liu Y. The effect of MCM3AP-AS1/miR-211/KLF5/AGGF1 axis regulating glioblastoma angiogenesis. Front Mol Neurosci. 2017;10:437. doi: 10.3389/fnmol.2017.00437. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zheng J, Hu L, Cheng J, Xu J, Zhong Z, Yang Y, Yuan Z. lncRNA PVT1 promotes the angiogenesis of vascular endothelial cell by targeting miR26b to activate CTGF/ANGPT2. Int J Mol Med. 2018;42:489–496. doi: 10.3892/ijmm.2018.3595. [DOI] [PubMed] [Google Scholar]
- 44.Ruan W, Zhao F, Zhao S, Zhang L, Shi L, Pang T. Knockdown of long noncoding RNA MEG3 impairs VEGF-stimulated endothelial sprouting angiogenesis via modulating VEGFR2 expression in human umbilical vein endothelial cells. Gene. 2018;649:32–39. doi: 10.1016/j.gene.2018.01.072. [DOI] [PubMed] [Google Scholar]
- 45.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J, Meng W, Chen Z, Zhang N, Weng Q, Zhu H, He Q, Ying M, Yang B. LncRNA-MM2P identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol Res. 2018;7:292–305. doi: 10.1158/2326-6066.CIR-18-0145. [DOI] [PubMed] [Google Scholar]
- 47.Sang LJ, Ju HQ, Liu GP, Tian T, Ma GL, Lu YX, Liu ZX, Pan RL, Li RH, Piao HL, Marks JR, Yang LJ, Yan Q, Wang W, Shao J, Zhou Y, Zhou T, Lin A. LncRNA CamK-A regulates Ca(2+)-signaling-mediated tumor microenvironment remodeling. Mol Cell. 2018;72:71–83. e77. doi: 10.1016/j.molcel.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Huang JK, Ma L, Song WH, Lu BY, Huang YB, Dong HM, Ma XK, Zhu ZZ, Zhou R. LncRNA-MALAT1 promotes angiogenesis of thyroid cancer by modulating tumor-associated macrophage FGF2 protein secretion. J Cell Biochem. 2017;118:4821–4830. doi: 10.1002/jcb.26153. [DOI] [PubMed] [Google Scholar]
- 49.Tkach M, Thery C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 50.Ludwig N, Whiteside TL. Potential roles of tumor-derived exosomes in angiogenesis. Expert Opin Ther Targets. 2018;22:409–417. doi: 10.1080/14728222.2018.1464141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM, Wu L, Xu GH. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur Rev Med Pharmacol Sci. 2017;21:959–972. [PubMed] [Google Scholar]
- 52.Lang HL, Hu GW, Zhang B, Kuang W, Chen Y, Wu L, Xu GH. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol Rep. 2017;38:785–798. doi: 10.3892/or.2017.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma X, Li Z, Li T, Zhu L, Li Z, Tian N. Long non-coding RNA HOTAIR enhances angiogenesis by induction of VEGFA expression in glioma cells and transmission to endothelial cells via glioma cell derived-extracellular vesicles. Am J Transl Res. 2017;9:5012–5021. [PMC free article] [PubMed] [Google Scholar]
- 54.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, De Leo G, Alessandro R. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu JJ, Lin XJ, Tang XY, Zheng TT, Lin YY, Hua KQ. Exosomal metastas is associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int J Biol Sci. 2018;14:1960–1973. doi: 10.7150/ijbs.28048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang FW, Cao CH, Han K, Zhao YX, Cai MY, Xiang ZC, Zhang JX, Chen JW, Zhong LP, Huang Y, Zhou SF, Jin XH, Guan XY, Xu RH, Xie D. APC-activated long non-coding RNA inhibits colorectal carcinoma pathogenesis through reducing exosome production. J Clin Invest. 2019;129:727–743. doi: 10.1172/JCI122478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishna Priya S, Nagare RP, Sneha VS, Sidhanth C, Bindhya S, Manasa P, Ganesan TS. Tumour angiogenesis-Origin of blood vessels. Int J Cancer. 2016;139:729–735. doi: 10.1002/ijc.30067. [DOI] [PubMed] [Google Scholar]
- 59.Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta. 2010;1806:18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N, Bin J, Liao Y, Liao W. Long non-coding RNA MALAT1 promotes gastric cancer tumorigenicity and metastasis by regulating vasculogenic mimicry and angiogenesis. Cancer Lett. 2017;395:31–44. doi: 10.1016/j.canlet.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 61.Yu W, Ding J, He M, Chen Y, Wang R, Han Z, Xing EZ, Zhang C, Yeh S. Estrogen receptor beta promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene. 2019;38:1225–1238. doi: 10.1038/s41388-018-0463-1. [DOI] [PubMed] [Google Scholar]
- 62.Peng Z, Wang J, Shan B, Li B, Peng W, Dong Y, Shi W, Zhao W, He D, Duan M, Cheng Y, Zhang C, Duan C. The long noncoding RNA LINC00312 induces lung adenocarcinoma migration and vasculogenic mimicry through directly binding YBX1. Mol Cancer. 2018;17:167. doi: 10.1186/s12943-018-0920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011;2:266–272. doi: 10.1007/s13238-011-1041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Sun B, Liu T, Shao B, Sun R, Zhu D, Zhang Y, Gu Q, Dong X, Liu F, Zhao N, Zhang D, Li Y, Meng J, Gong W, Zheng Y, Zheng X. Long noncoding RNA n339260 promotes vasculogenic mimicry and cancer stem cell development in hepatocellular carcinoma. Cancer Sci. 2018;109:3197–3208. doi: 10.1111/cas.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren K, Ni Y, Li X, Wang C, Chang Q, Li Y, Gao Z, Wu S, Shi X, Song J, Yao N, Zhou J. Expression profiling of long noncoding RNAs associated with vasculogenic mimicry in osteosarcoma. J Cell Biochem. 2019;120:12473–12488. doi: 10.1002/jcb.28514. [DOI] [PubMed] [Google Scholar]
- 66.Gao Y, Yu H, Liu Y, Liu X, Zheng J, Ma J, Gong W, Chen J, Zhao L, Tian Y, Xue Y. Long non-coding RNA HOXA-AS2 regulates malignant glioma behaviors and vasculogenic mimicry formation via the MiR-373/EGFR Axis. Cell Physiol Biochem. 2018;45:131–147. doi: 10.1159/000486253. [DOI] [PubMed] [Google Scholar]
- 67.Guo J, Cai H, Liu X, Zheng J, Liu Y, Gong W, Chen J, Xi Z, Xue Y. Long non-coding RNA LINC00339 stimulates glioma vasculogenic mimicry formation by regulating the miR-539-5p/TWIST1/MMPs axis. Mol Ther Nucleic Acids. 2018;10:170–186. doi: 10.1016/j.omtn.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan CL, Li H, Zhu L, Liu Z, Zhou J, Shu Y. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma. 2016;63:442–449. doi: 10.4149/314_150825N45. [DOI] [PubMed] [Google Scholar]
- 70.Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25:770–779. doi: 10.1111/iju.13734. [DOI] [PubMed] [Google Scholar]


