Abstract
Pertuzumab is clinically employed in the treatment of cancers over-expressing human epidermal growth factor receptor 2 (HER2). Herein, we developed dual-labeled pertuzumab with a radionuclide (89Zr) and a near-infrared fluorophore (IRDye 800CW) to investigate the feasibility of utilizing dual-labeled monoclonal antibodies (mAbs) with numerous imaging modalities for preoperative imaging and image-guided surgery in ovarian cancer models. MAbs were dually-labeled with 89Zr and IRDye 800CW to generate 89Zr-Df-pertuzumab-800CW or 89Zr-Df-IgG-800CW. Serial positron emission tomography (PET) and near-infrared fluorescence (NIRF) images were acquired up to 72 hours after injection of dual-labeled mAbs to map the tracers’ biodistributions. After the last time point, image-guided tumor resection was executed using different modalities (NIRF, Cerenkov luminescence [CL], and β particle imaging) and ex vivo studies including biodistribution assays and histology analysis were performed to confirm the in vivo imaging data. SKOV3 ovarian cancer cells showed high expression of HER2 and pertuzumab conjugated with Df and IRDye 800CW maintained its binding affinity for these cells. For PET imaging in subcutaneous xenograft ovarian cancer models, 89Zr-Df-pertuzumab-800CW showed a significantly higher tumor-to-muscle ratio compared to the nonspecific 89Zr-Df-IgG-800CW from 24 hours after injection through the last time point (72 h: 30.7 ± 7.4 vs. 7.5 ± 1.8, P < 0.01, n = 3-4). During image-guided surgery, three imaging modalities including NIRF, CL, and β particle imaging could detect ovarian cancer in both subcutaneous and orthotopic models and each exhibited its own imaging characteristics. In addition, ex vivo imaging and biodistribution studies as well as histology analysis corroborated the in vivo imaging results. Therefore, we concluded that this single radiolabeled tracer can provide all-in-one contrast for multiple imaging modalities. The dual-labeled mAbs may hold promise to be employed for image-guided tumor surgery as well as diagnosis and staging through balancing out the strengths and weaknesses of various modalities such as PET/CT, NIRF, CL, and β particle imaging.
Keywords: Human epidermal growth factor receptor 2 (HER2), image-guided surgery, near-infrared fluorescence (NIRF) imaging, ovarian cancer, pertuzumab, positron emission tomography (PET), 89Zr
Introduction
Ovarian cancer is an often-fatal gynecologic disease, ranking at fifth among cancer-related deaths for women [1]. According to a recent report, there will be approximately 22,530 of new cases of ovarian cancer in the United States in 2019, and the estimated number of deaths from ovarian cancer will be 13,980 in the same year [2]. Ovarian cancer can be difficult to recognize because clinical signs and symptoms of ovarian cancer are unclear and non-specific (e.g. abdominal bloating, changes in appetite, and urinary discomfort, etc) [3]. Cancer antigen 125 (CA125) has been used as one of the earliest biomarkers for screening ovarian cancer, but it was discovered that the sensitivity of CA125 is low at early stages and its level can also be increased in various physiological conditions [4]. Besides, ovarian cancer can be developed in any woman even without having high risk factors [3]. For these reasons, many patients who are newly diagnosed already show metastasis in the peritoneal space or outside of the pelvic region [5].
As ovarian cancer spreads mainly through the abdominal cavity [5], complete removal of residual tumor and the minimal safety surgical margin is critical. The standard treatment for advanced ovarian cancer is to remove discernible and palpable tumor tissues and to administer chemotherapy following surgery [6]. However, palpation or visual inspection for removal of tumor burden is not perfect and is subject to the experience of the surgeon. Ataseven et al. confirmed that residual disease following debulking surgery is associated with prognosis in patients with ovarian cancer diagnosed at FIGO (Federation of Gynecology and Obstetrics) stage IV, and complete tumor removal results in the greatest surgical benefits [7]. In this regard, intraoperative image-guided surgery may hold great promise for more accurate and sensitive removal of tumor tissue and potential enhancement of survival rates in patients with ovarian cancer.
To help with this pressing problem, molecular imaging can play a crucial role not only in diagnosis of ovarian cancer but also in staging and treatment. Anatomical imaging can be performed with the help of computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography. On the other hand, radionuclide-based imaging such as positron emission tomography (PET) or single-photon emission computed tomography (SPECT) can provide biological information with high sensitivity on the molecular level. In clinic, 18F-Fludeoxyglucose [8], which exploits glucose metabolism, or 18F-Fluorothymidine [9], which targets proliferating cells, are widely used for cancer imaging. However, these molecular probes remain suboptimal because they are not highly selective for cancerous cells, and the results can be impacted by the physiological conditions of the patients [8]. In this sense, molecular probes targeting specific markers of diseases can complement the information we get from non-specific probes and may provide additional useful information on abnormal lesions.
Optical imaging based on fluorescence is one of the widely-used methods for image-guided surgery and recently has also been employed in clinical trials [10]. Fluorescence imaging has advantages over PET/CT imaging since it is relatively inexpensive and allows real-time high-resolution imaging without using ionizing radiation [11]. In particular, near-infrared (NIR) fluorophores such as indocyanine green or IRDye 800CW absorb light with wavelengths between 650-900 nm and show low nonspecific autofluorescence from the surrounding target tissues and higher signal-to-background ratios due to better tissue penetration than other fluorophores [10]. However, limitations of fluorescence imaging still remain due to low tissue penetration compared to PET imaging [12]. Furthermore, most of the fluorescent agents available in clinic are non-targeted and therefore non-specific for cancerous tissues, such that there is still a great need for scientific development in this area [10].
Another emerging method that can be used for image-guided surgery is Cerenkov luminescence (CL) imaging. CL imaging can detect the Cerenkov photons which are emitted when charged particles (such as positrons or electrons) travel through dielectric media with speeds faster than that of light in the particular medium [13]. Therefore, one of the advantages of CL imaging is that clinically-used radioisotopes, such as 90Y, 68Ga, and 89Zr, for nuclear imaging or therapy can also be used for CL imaging [13]. In addition, various hand-held devices have been developed that may detect charged particles for intraoperative imaging when equipped with electron-multiplying charge-coupled devices [13]. Therefore, in spite of the radiation exposure for patients and surgeons, CL imaging serves as a companion imaging modality to nuclear imaging and presents an alternative to fluorescence imaging for real-time monitoring during surgery.
Pertuzumab (Perjeta), approved by the United States Food and Drug Administration for breast cancer treatment, interferes with the dimerization of human epidermal growth factor receptor 2 (HER2) with other epidermal growth factor receptors and reduces intracellular signaling pathways [14]. Recently, radiolabeled pertuzumab has also been used for imaging of other cancers such as ovarian cancers in preclinical studies [15,16]. Expanding upon previous works, in this study, pertuzumab dual-labeled with 89Zr and IRDye 800CW (89Zr-Df-pertuzumab-800CW) were employed for image-guided ovarian tumor resection following serial PET and NIRF imaging. 89Zr (t1/2: 78.4 h; Emean = 0.396 MeV) allowed for sensitive detection of gross tumors through preoperative PET/CT in both subcutaneous and orthotopic models, while IRDye 800CW enabled nearly real-time visualization for surgical ovarian tumor removal through NIRF. Furthermore, CL and β particle imaging played supportive roles during surgery in addition to NIRF imaging, making the developed monoclonal antibody (mAb) tracer useful for image-guided tumor resection in a multimodal setting. Therefore, through modification of the mAbs with radionuclides and near-infrared fluorophores in this study, dual-labeled pertuzumab enabled the use of many imaging techniques, each of which may complement the merits and augment the vulnerabilities of the others.
Material and methods
Cell culture and animal models
Three human ovarian adenocarcinoma cell lines, SKOV3, Caov3, and OVCAR3, were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) or in RPMI 1640 medium (Gibco), supplemented with 10% fetal bovine serum (Atlanta Biologicals, GA, USA), penicillin (100 U/mL; Gibco), and streptomycin (100 µg/mL; Gibco) at 37°C with 5% CO2.
All animal experiments were implemented under a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee. For subcutaneous xenografts, 1 × 106 tumor cells (SKOV3) were mixed with Matrigel (BD Biosciences, CA, USA) at ratio of 1:1, and 100 µl of the mixture was subcutaneously implanted in the lower right flank of 6-8-week-old female athymic nude mice (Envigo; n = 3-4). Animals were used for experiments when the diameter of their tumors reached 5-10 mm. For orthotopic tumor models, surgical procedures were carried out similarly to those previously reported [15]. To monitor the development of orthotopic tumors, ultrasound imaging (Vevo 2100; FUJIFILM VisualSonics, Toronto, Canada) was performed at 3-4 weeks after implantation. A linear array transducer (MS-400) with 40-MHz center frequency was used to obtain two-dimensional images of mouse ovaries.
Flow cytometry
Cells were harvested and suspended in cold flow cytometry staining buffer (eBioscience; Thermo Fisher Scientific) at a concentration of ~1 × 106 cells/mL. After incubation with pertuzumab or Df-pertuzumab-800CW at a concentration of 25 μg/mL in flow cytometry staining buffer on ice for 30-45 min, cells were washed three times with cold PBS, and incubated with Alexa Fluor 488 IgG goat-anti human antibodies (Invitrogen) on ice for 30-45 min. The samples were analyzed using the MACSQuant cytometer (Miltenyi Biotec, Bergisch Gladbach, Germany), and the results were processed using FlowJo software (TreeStar, Ashland, OR, USA).
Internalization assay
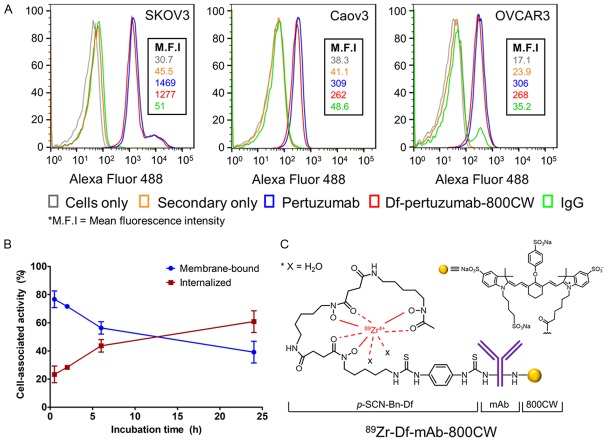
For the internalization assay, ~5 × 104 SKOV3 cells were seeded into each well of 24-well culture plates and incubated in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 U/mL of penicillin, 100 ug/mL of streptomycin). Cells were allowed to sit overnight under standard cell culture conditions and then treated with 18.5 kBq (~0.1 μg) of 89Zr-Df-pertuzumab-800CW in each well. After 30 min, 2 h, 6 h, or 24 h of incubation, an acid wash was performed with 0.2 M sodium acetate for 1-2 min to collect membrane-bound 89Zr-Df-pertuzumab-800CW and followed by trypsinization to obtain internalized dual-labeled mAbs. Collected fractions were measured in a gamma counter. The fraction of membrane-bound or internalized activity (either membrane-bound or internalized activity/(membrane-bound + internalized activity) was plotted against incubation time.
Dual-labeling of monoclonal antibody
Deferoxamine (Df) and IRDye 800CW were conjugated to mAbs as previously reported [17]. Briefly, pertuzumab (Genentech, San Francisco, CA, USA) was mixed with p-SCN-Bn-Df (Macrocyclics, Inc., Dallas, TX, USA) dissolved in DMSO at a mAb:chelator molar ratio of ~1:3 and incubated at room temperature for 2 hours at pH 8.5-9.0. After conjugation, samples were purified with PD-10 desalting columns (GE Healthcare, Chicago, IL, USA) eluted with 1 × PBS. Then, purified Df-pertuzumab was mixed with IRDye 800CW-NHS-ester at a mAb:dye molar ratio of ~1:2.4 for 2 hours at room temperature and pH 8.0-8.5. The mixture was purified using PD-10 columns. Human serum IgG (a non-specific isotype control) was also conjugated with both Df and IRDye 800CW using similar methods. 89Zr was produced by a PETtrace cyclotron (GE Healthcare) via the 89Y (p, n) 89Zr reaction. For 89Zr radiolabeling, 89Zr (~37 MBq) was diluted into 0.5 M HEPES butter (pH 7.0). Df-pertuzumab-800CW or Df-IgG-800CW was added to the diluted solution at a ratio of 150 µg per 37 MBq at 37°C for 1 hour, and purified using a PD-10 column. The number of chelators per antibody was determined by the isotope dilution method reported previously [18]. The amount of IRDye 800CW per antibody was detected by UV measurement at 780 nm.
Serial PET/CT and fluorescence imaging
Longitudinal PET imaging was performed using an Inveon microPET/microCT scanner (Siemens Medical Solutions, Erlangen, Germany). Both subcutaneous and orthotopic tumor-bearing mice (n = 3-5) were intravenously injected with 89Zr-Df-pertuzumab-800CW or 89Zr-Df-IgG-800CW (1.6-8.4 MBq, 13-38 μg per mouse) and scanned at 2, 6, 24, 48 and 72 h after injection. Region-of-interest analysis was performed and decay-corrected whole-body images were reconstructed using vendor software (Inveon Research Workplace). Tissue uptake was represented as mean percent injected dose per gram of tissue ± standard deviation (mean %ID/g ± SD).
Near infrared fluorescence (NIRF) images were acquired (λex/em: 745/800 nm) immediately following each PET scan using the IVIS Spectrum Imaging System (PerkinElmer, Waltham, MA, USA). For image processing and analysis, vendor software (Living Image) was employed.
Image-guided tumor resection
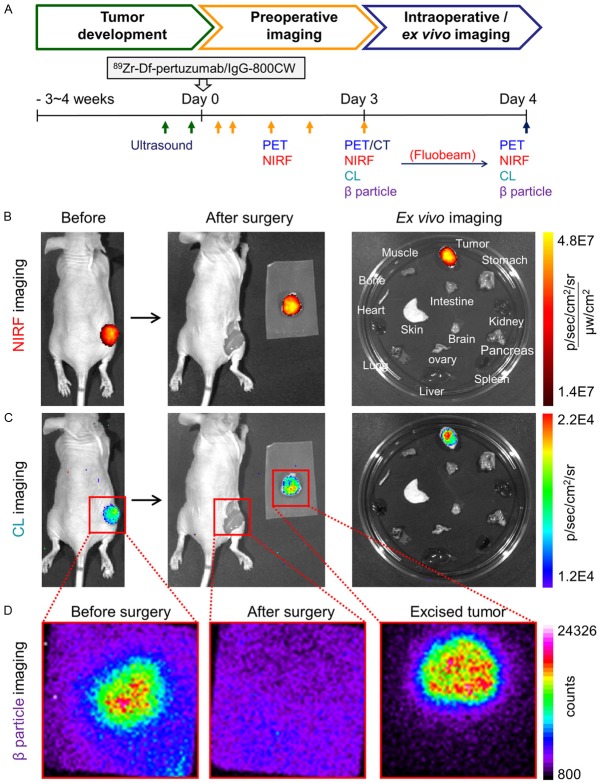
After the last imaging time point, tumor-bearing mice were euthanized by CO2 asphyxiation. Tumor tissues were removed based on the multimodality images obtained from preoperative and intraoperative imaging. After the tumor tissues were removed, surgical sites and the excised tumor tissues were detected with the same modalities to confirm the complete removal of all tumor tissues (Figure 4A).
Figure 4.
Image-guided tumor resection in the subcutaneous SKOV3 tumor model after the last time point (72 h) of serial PET/NIRF imaging. The same mouse shown in Figure 2A is here presented in Figure 4. (A) Schematic diagram of using multimodalities for verification of tumor development, preoperative and intraoperative/ex vivo imaging. The length of the scale does not reflect actual time. (B-D) Image-guided surgery with (B) Fluorescence-based imaging, (C) Cerenkov luminescence (CL) imaging, and (D) β particle imaging. Red boxes are the expected field of view during HARLI imaging. The actual field-of-view was 2.17 × 2.17 cm2.
For fluorescence (λex/em: 745/800 nm) and CL (λex/em: closed/open) imaging, the IVIS Spectrum Imaging System (PerkinElmer) was used. For Fluobeam (Fluoptics, Grenoble, France) imaging, images were taken at a rate of 2 pictures/sec.
To detect emitted β particles, the Hand-Held RadioLuminescence Imaging (HARLI; Lightpoint Medical, MA, USA) system was used. The HARLI system employs a Peltier-cooled electron-multiplying charge-coupled camera (-80°C) to detect charged particles after their interaction with a CsI scintillating film. The exposure time for each image from the HARLI system was 30 seconds and the field-of-view was 2.17 × 2.17 cm2. The vendor software, Andor Solis, was employed for the analysis of data and the scale bar was represented in terms of counts.
Ex vivo imaging and biodistribution studies
Ex vivo imaging of major organs and tissues was performed using PET, NIRF and CL imaging. Following the imaging studies, the organs/tissues were wet weighed and counted using an automated gamma counter. Tracer accumulation was represented as %ID/g (mean ± SD). Normal ovary and tumor tissues were embedded in the paraffin for further hematoxylin and eosin staining.
Immunofluorescence staining
Frozen tissue slices of 7-µm thickness were fixed with 4% paraformaldehyde. To prevent non-specific binding of mAbs, tissue slices were blocked with 5% donkey serum for 1 hour at room temperature and followed by incubation with a mixture of pertuzumab (20 µg/ml) and rat anti-mouse CD31 antibody (BD Biosciences; 2 µg/ml) overnight at 4°C. Next, a mixture of Alexa Fluor 488 goat anti-human (5 µg/ml) and Cy3 donkey anti-rat (Thermo Fisher Scientific; 10 µg/ml) secondary antibodies was incubated with the slices. After DAPI mounting, tissue sections were imaged with a Nikon A1R confocal microscope.
Statistical analysis
Quantitative data is expressed as mean ± SD. The quantitative results were analyzed using a two-tailed unpaired Students’ t-test. P-values less than 0.05 were considered significant.
Results
In vitro characterization
The expression level of HER2 in different ovarian cancer cells and the immunoreactivities of the mAbs were evaluated through flow cytometry (Figure 1A). SKOV3 cells showed high expression of HER2 compared to the two other cell lines, OVCAR3 and Caov3. In addition, there was no significant difference in binding affinity between unconjugated pertuzumab and Df-pertuzumab-800CW in any of the three ovarian cell lines, indicating that conjugation of Df and IRDye 800CW did not compromise the binding affinity of pertuzumab. Therefore, further in vivo and ex vivo assays with dual-labeled mAbs were performed in the HER2-expressing SKOV3 cell line. The internalization assay showed that 89Zr-Df-pertuzumab-800CW presented high internalization kinetics in SKOV3 cell lines during 24 h (Figure 1B). Of all cell-associated mAbs (17.3 ± 3.1% of total incubated activity), 60.8 ± 6.3% were internalized, with a smaller amount remaining on the surface of the cells (39.2 ± 6.3%; n = 3) at 24 h after incubation.
Figure 1.
In vitro characterization and generation of monoclonal antibodies (mAbs) conjugated with 89Zr and IRDye 800CW. (A) Flow cytometry results showing high HER2 expression on SKOV3 cells compared to Caov3 or OVCAR3. (B) Internalization profile of 89Zr-Df-pertuzumab-800CW in SKOV3 cells. Cell-associated activity (%) was calculated as the radioactivity in the bound or internalized fractions over the total radioactivity associated to cells, n = 3. (C) 89Zr-Df-mAb-800CW was prepared as reported previously [17]. Pertuzumab and a non-specific IgG were employed as the mAbs in this study.
Df/IRDye 800CW conjugation and 89Zr labeling
Based on the isotopic dilution experiment, the average number of chelators per antibody in this study was ~2.3. In addition, UV measurement indicated that the ratio of mAbs to IRDye 800CW was ~1:0.7. This ratio is desirable because antibodies conjugated with less than 1 equivalent mole of fluorophore have little chance of losing of fluorescence signal from self-quenching of dye, which can occur via Förster resonance energy transfer [17]. The mAbs conjugated with Df and IRDye 800CW were then radiolabeled with 89Zr (Figure 1C). The radiolabeling yield of the dual-labeled pertuzumab and non-specific IgG with 89Zr was ~70% and ~50% (non-decay corrected), respectively.
Serial PET/NIRF imaging and image-guided tumor resection in subcutaneous tumor models
Time points of 2, 6, 24, 48 and 72 h post-injection (p.i.) were selected for in vivo serial PET imaging of subcutaneous SKOV3 tumor-bearing mice. Subcutaneous models were used as a proof-of-concept imaging study since this model is relatively easy to establish and monitor. Imaging results are presented as maximum intensity projections (MIPs) (Figure 2). The tumor accumulation of 89Zr-Df-pertuzumab-800CW increased until 48 h and remained steady at 72 h. The uptake of 89Zr-Df-pertuzumab-800CW in the heart/blood was highest at 2 h (12.7 ± 3.5 %ID/g, n = 4) and continuously decreased until the last time point (Figure 3A). After 72 h, the tumor/muscle ratio in the 89Zr-Df-pertuzumab-800CW group was significantly higher than that of the 89Zr-Df-IgG-800CW group (30.7 ± 7.4 vs. 7.5 ± 1.8, P < 0.01, n = 3-4; Figure 3C).
Figure 2.
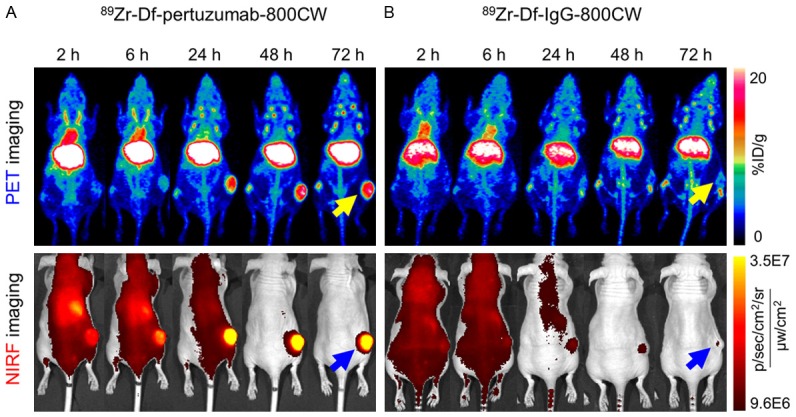
Serial PET and NIRF images of subcutaneous SKOV3 tumor-bearing mice after intravenous injection of the radiolabeled probes. (A) 89Zr-Df-pertuzumab-800CW clearly visualized tumors compared to (B) 89Zr-Df-IgG-800CW as early as 24 h p.i. in both PET and NIRF imaging. For PET imaging, the images are presented as maximum intensity projections (MIPs). The yellow and blue arrows indicate the tumors detected by PET and NIRF imaging, respectively (n = 3-4).
Figure 3.
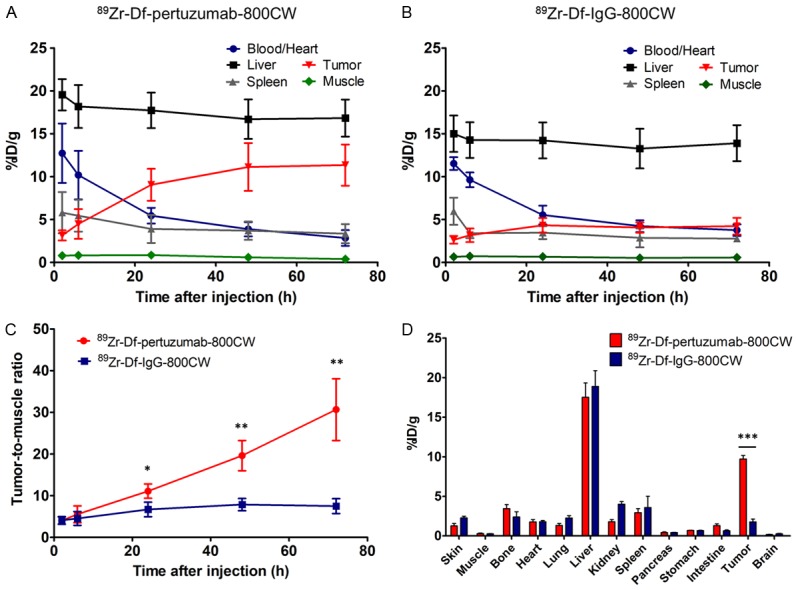
Quantitative region-of-interest analysis of PET imaging over time and ex vivo biodistribution results at 72 h after injection of the tracers. Time-activity curves after administration of (A) 89Zr-Df-pertuzumab-800CW and (B) 89Zr-Df-IgG-800CW in subcutaneous SKOV3 tumor-bearing mice. (C) The tumor-to-muscle ratio was significantly higher in the 89Zr-Df-pertuzumab-800CW group than in the 89Zr-Df-IgG-800CW group after 24 h (*P < 0.05, **P < 0.01; n = 3-4). (D) Ex vivo biodistribution results also confirmed that tumor uptake of 89 Zr-Df-pertuzumab-800CW was significantly higher than that of the control IgG group (***P < 0.001).
NIRF imaging was executed at the same time points as PET imaging. At 24 h p.i. of 89Zr-Df-pertuzumab-800CW, the subcutaneous SKOV3 tumor was clearly outlined through fluorescence imaging (Figure 2A). Since both PET and NIRF imaging could delineate the tumor in an obvious manner at 72 h p.i., and a significant difference in accumulation was noted between the 89Zr-Df-pertuzumab-800CW and 89Zr-Df-IgG-800CW groups in the PET imaging, image-guided tumor resection was performed at this time point.
Surgical removal of subcutaneous tumors was performed immediately following the last time point of serial PET/NIRF imaging. First, preoperative images of tumor-bearing mice were obtained using multimodality imaging (Figure 4). In addition to PET and NIRF imaging (Figure 2), CL and β particle imaging could also delineate subcutaneous tumors in a supportive manner (Figure 4C and 4D). During surgery, tumors were removed based on both the preoperative images and the images acquired from intraoperative imaging. In particular, the Fluobeam instrument allowed tumor resection to be performed with almost real-time image guidance by providing visualization of fluorescence signal. Use of this instrument revealed remaining fluorescence signal sensitively after initial gross tumor surgical removal, leading to a second round of surgical removal in some of the mice (Figure S1). In addition, intraoperative imaging using the Fluobeam demonstrated higher imaging contrast than was observed with post-operative images from other instruments (Figure S1). Postoperative images from the NIRF and CL imaging confirmed that no notable fluorescence or Cerenkov signal was left in the region of the tumor after final tumor resection (Figure 4B and 4C). Additionally, HARLI images indicated that the detected β particles markedly decreased to a background level after tumor removal, and detected counts from the tumors were similar both in vivo before surgery and ex vivo after tissue resection (Figure 4D).
Ex vivo NIRF and CL imaging results indicated that the highest signal from 89Zr-Df-pertuzumab-800CW was in the tumor (Figure 4B and 4C). In addition, the biodistribution results confirmed a statistically significant difference between 89Zr-Df-pertuzumab-800CW and 89Zr-Df-IgG-800CW in tumor uptake (9.7 ± 0.9 vs. 1.8 ± 0.6 %ID/g, P < 0.001, n = 3-4) as well as in tumor-to-muscle ratio (38.7 ± 18.0 vs. 6.6 ± 1.1, P < 0.05, n = 3-4), which correlates with the in vivo PET imaging results (Figure 3D).
Serial PET/NIRF imaging and image-guided tumor resection in orthotopic tumor models
The presence of orthotopic tumors was confirmed by ultrasonography 3-4 weeks after tumor implantation (Figure S2). Even though the tumor cells were injected into the ovary, tumor masses were also observed adjacent to the ovary. The sizes of orthotopic tumors ranged from 1-5 mm in diameter.
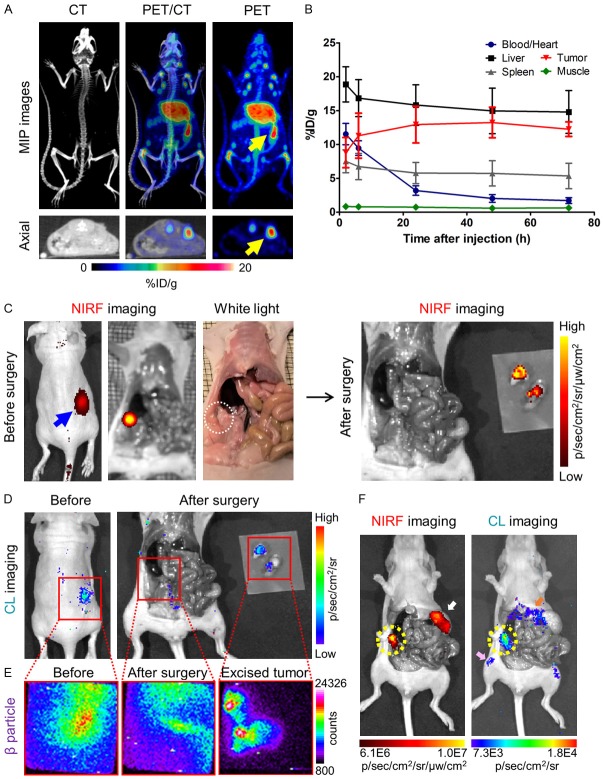
Similar to imaging of subcutaneous tumors, serial PET and NIRF imaging with 89Zr-Df-pertuzumab-800CW were performed for the orthotopic SKOV3 tumor-bearing mice (Figure S3). In addition to ultrasonography, PET/CT images at 72 h after injection of the tracers also confirmed the presence of orthotopic ovarian tumors (Figure 5A). The tumor uptake of the tracer in the orthotopic models peaked at 48 h (13.2 ± 2.2 %ID/g) and slightly reduced at 72 h (12.3 ± 1.1 %ID/g).
Figure 5.
Preoperative/intraoperative imaging and quantitative analysis in orthotopic SKOV3 tumor-bearing mice. (A) PET/CT maximum intensity projection (MIP) and axial images of orthotopic SKOV3 tumor-bearing mice at 72 h after injection of 89Zr-Df-pertuzumab-800CW. The yellow arrows indicate the location of the tumors. (B) Time-activity curve based on PET imaging of orthotopic SKOV3 tumor models after intravenous injection of 89Zr-Df-pertuzumab-800CW (n = 5). The mean tumor uptake (%ID/g) peaked at 48 h after injection of the tracer. (C) Preoperative near-infrared fluorescence (NIRF) imaging at 72 h post-injection of the tracer and image-guided surgery based on white light and NIRF imaging. Both the blue arrow and the white dotted circle indicate the location of the tumor. (D, E) Image guided surgery with (D) Cerenkov luminescence (CL) and (E) β particle imaging. Red boxes are the expected field of view during β particle imaging. The actual field-of-view was 2.17 × 2.17 cm2. (F) Examples of non-target signals detected by NIRF and CL imaging in the orthotopic model. The yellow-dotted circle indicates the SKOV3 orthotopic tumors, while white, pink, and orange arrows represent the stomach, bone, and liver of the SKOV3 tumor-bearing mice, respectively.
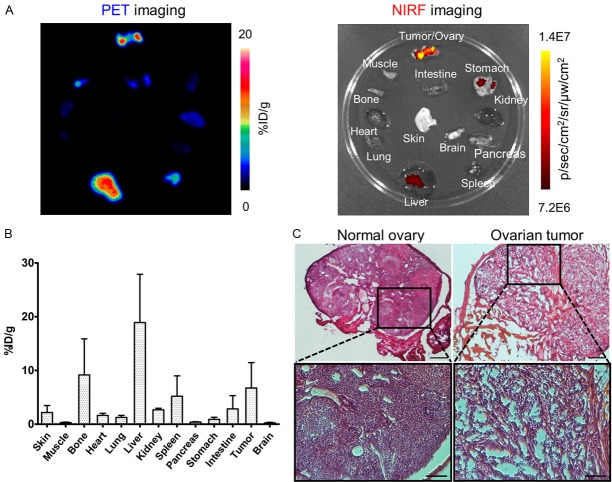
Image-guided orthotopic tumor resection was executed in a similar manner as for the subcutaneous tumor models. During surgery, orthotopic tumors were outlined and removed based on the preoperative NIRF, CL, and β particle imaging (Figure 5C-E). In some of the mice, non-target signals such as fluorescence from food in the stomach and accumulation of free 89Zr in the bones were also observed with NIRF and CL imaging, respectively (Figure 5F). Furthermore, Fluobeam images allowed detection of tumor tissues along the fallopian tubes (Figure S4). Notably, in HARLI imaging, β particle counts remaining in the abdomen near the removed tissue were higher than expected after tumor removal, which could have resulted from relatively wide imaging field-of-view (2.17 × 2.17 cm2) compared to the size of the mice, meaning that the detector might collect signals from organs near the orthotopic tumors (Figure 5E). Ex vivo imaging of harvested organs and the biodistribution study confirmed the uptake of the tracer in the orthotopic tumors (Tumor-to-muscle ratio: 22.2 ± 11.5; n = 5, Figure 6A and 6B). In addition, hematoxylin and eosin staining also verified the malignancy of excised tumor/ovary tissues (Figure 6C).
Figure 6.
Ex vivo imaging and a biodistribution study of 89Zr-Df-pertuzumab-800CW in orthotopic SKOV3 tumor-bearing mice were performed for confirmation of results of preoperative and intraoperative imaging. (A) Ex vivo PET images and near-infrared fluorescence images of the organs collected from the mouse in Figure 5A-E. (B) Biodistribution of 89Zr-Df-pertuzumab-800CW in orthotopic SKOV3 tumor-bearing mice at 72 h post-injection of the tracer (n = 5). (C) Hematoxylin and eosin staining of normal ovary and excised ovarian tumor sections during surgery. The scale bars are 250 μm and 100 μm for low and high magnifications, respectively.
Immunofluorescence staining
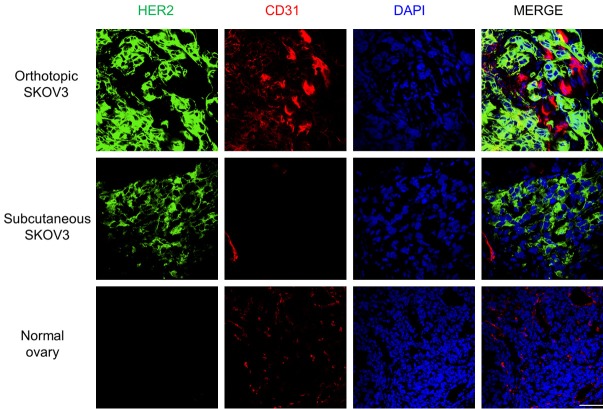
Positive staining of HER2 was pronounced in tumor sections acquired from both subcutaneous and orthotopic models. In addition, the results of immunofluorescence imaging in normal ovaries verified that the in vivo imaging signals came from human HER2 expression on tumor cells, not from intrinsic expression of HER2 in the ovary, since pertuzumab does not cross-react with the mouse antigen (Figure 7). Little signal in organs other than tumor such as the liver suggests that in vivo liver uptake is not due to binding of the tracers but likely from the metabolism of the antibodies (Figure S5).
Figure 7.
Immunofluorescent staining of orthotopic and subcutaneous SKOV3 tumors and a normal mouse ovary. Human HER2 signal is presented in green, and CD31 (red) and DAPI (blue) staining visualize the locations of vasculature and cell nuclei, respectively. Green signal was pronounced in both orthotopic and subcutaneous SKOV3, while it was undetectable in normal ovaries. Scale bar = 50 μm. (For images of other organs [liver, spleen, and kidney], the reader is referred to the supporting information).
Discussion
Ovarian cancer is one of the widely used models for studies of image-guided surgery. In previous studies, molecular probes including fluorophore-conjugated galactosyl serum albumin [19], avidin [20], and folic acids [21] were used for targeting ovarian cancer. Using peptides or analogues of ligands as imaging agents usually requires a short time to reach notable tumor-to-background contrast but can show high false positive rates if they are non-targeted probes [5]. On the other hand, bevacizumab or trastuzumab-based probes have allowed tumor detection with high specificity during intraoperative NIRF imaging [11]. In addition, Holland et al. used 89Zr-DFO-trastuzumab for CL image-guided surgery of HER2-expressing cancers [22]. In this regard, the present study of dual-labeled pertuzumab examined four different modalities (PET/CT, NIRF, CL, and β particle imaging) for ovarian cancer imaging and may suggest effective combinations for image-guided surgery.
Flow cytometry results confirmed high expression of HER2 on SKOV3 cells (Figure 1). Furthermore, serial PET and NIRF imaging with 89Zr-Df-pertuzumab-800CW clearly visualized both subcutaneous and orthotopic ovarian tumors (Figures 2 and S3). In particular, PET imaging results showed that 89Zr-Df-pertuzumab-800CW specifically accumulated in tumors, while 89Zr-Df-IgG-800CW remained at background levels (Tumor-to-muscle ratio: 30.7± 7.4 vs. 7.5 ± 1.8, P < 0.01, n = 3-4; Figure 3C). The significant difference between 89Zr-Df-pertuzumab-800CW and the control group appeared as early as 24 h p.i. in the subcutaneous model. Therefore, 89Zr-Df-pertuzumab-800CW showed promising in vitro and preoperative imaging results, indicating the potential of the same probe for image-guided surgery in ovarian cancer.
Some interesting phenomena were observed during in vivo imaging. First, in the PET/CT imaging, tumor accumulation in the orthotopic model was higher than that in the subcutaneous model at early time points (2 h: 8.8 ± 2.2 vs. 3.2 ± 0.6 %ID/g, P < 0.01; 6 h: 11.3 ± 3.3 vs. 4.5 ± 1.7 %ID/g, P < 0.05, n = 4-5; Figures 3A and 5B), which may be partly explained by the high vascularization of orthotopic tumors [23]. However, after 72 h p.i., the ex vivo biodistribution study showed less accumulation of the tracer in orthotopic tumors than expected, which may have resulted from an increase in the removed tissue mass because of normal tissues from margins being resected together with tumor tissues during surgery (Figure 6B). In addition, while 89Zr4+ is trapped after internalization into cells and contributes to sustained PET signals, dyes will often lose their fluorescence properties over time as a result of their degradation. These phenomena are shown in the serial PET and NIRF imaging, wherein PET signals increased in the tumors over time, while NIRF did not exhibited the same trend and may thus have not reflected the actual concentration of pertuzumab in the tumors (Figures 2 and S3) [24].
In vivo imaging and biodistribution studies herein showed relatively low tumor accumulation and enhanced liver uptake compared to previous studies using 89Zr-labeled pertuzumab [25] or other dual-labeled mAbs [26]. Our flow cytometry studies showed that conjugation of Df and IRDye 800CW did not impair the specificity or affinity of the tracers for their target (Figure 1). In addition, there is a report that ~4 Df groups and ~1 IRDye 800CW molecule can be conjugated to a single antibody, respectively, without compromising of its in vitro and in vivo characteristics [27]. However, since there is still a chance that chelator or dye conjugation can alter the physical characteristics of an antibody or its intermolecular interactions within the body by inducing changes of conformation, charge density or hydrophobicity in the mAb, further investigation may be needed in this regard [28]. Furthermore, both chelator and dye conjugation shared the amine groups of the antibodies resulting in nonspecific conjugation in this study. Therefore, site-specific conjugation could be considered as a future development to optimize the dual-labeling of mAbs.
In this study, we examined the possibility of using dual-labeled pertuzumab for pre/intraoperative imaging with various imaging modalities. Each modality has its own advantages and disadvantages, so researchers and clinicians may be able to choose the proper combination of these modalities depending on their purpose and the availability of the equipment. Based on our study results, we envision a number of possible clinical scenarios. First, for preoperative imaging of ovarian cancer, ultrasonography can be employed as a screening test to look at the uterus, fallopian tubes, as well as ovaries and to find abnormal masses in the ovaries [29]. PET/CT may also play an important role, if suspicious lesions are found, since it allows functional and molecular imaging and quantification of tracer uptake with unlimited tissue penetration, providing information on the location and extent of tumor burden.
Next, for intraoperative imaging, fluorescence, CL, and β particle imaging using hand-held devices can be considered. The use of a fluorescence imaging system using a NIR fluorophore may be the first choice since it is relatively inexpensive and can detect tumor tissues in a sensitive manner without the risk of radiation exposure. In particular, the Fluobeam system was found useful for complete removal of fluorescently-labeled tissues in our study. However, reduced fluorescence signal was observed at the later time points and autofluorescence was also detected in organs other than tumor tissues (Figures 5F and S3). On the other hand, CL imaging was free of autofluorescence or reflection from the excitation light since it did not require an external excitation source. Instead, CL imaging showed signals from bones where free radionuclide (89Zr) can accumulate, pointing to the importance of double-checking imaging signals with multiple imaging modalities to piece apart true and false positives (Figure 5F). Similar to PET imaging, CL imaging could also visualize the uptake of radiotracers with high sensitivity, but risks from radiation exposure to patients and clinicians still remain. The hand-held charged-particle detector, HARLI, has a relatively high spatial resolution of 42 µm as compared to PET with approximately 1-2 mm and 4-6 mm resolution for preclinical and clinical instruments, respectively [30]. Also, the relatively short exposure time (30 sec) of HARLI data acquisition compared to CL imaging (1-5 min) was another merit of using this hand-held device in our study [13]. However, as shown in our experiment, when the field-of-view for imaging is too wide for the subjects, accurate detection of small tumors can be hampered due to signals from other organs. Nonetheless, if this instrument is applied to patients or large animals, the problem of signal interference from other organs with high accumulation of the tracers may be mitigated. Furthermore, a standard camera providing white-light images can also be used as an adjunct with these various modalities to obtain anatomical as well as functional information.
Taken together, pertuzumab conjugated with 89Zr and IRDye 800CW allowed the detection and removal of ovarian tumors in both subcutaneous and orthotopic models. Since ovarian cancer is vulnerable to peritoneal metastasis, this tracer may also find potential in metastatic ovarian cancer models or other cancers overexpressing HER2. In addition, improvement of the dual-labeled mAbs with different radionuclides or fluorophores may further optimize its clinical potential.
Conclusion
In this study, we demonstrated that pertuzumab conjugated with 89Zr and IRDye 800CW could be employed for pre-, and intraoperative imaging of HER2+ cancer in an effective manner. In this regard, development of dual-labeled mAbs conjugated with a radionuclide with a long-half life and a NIR fluorophore may hold promise for diagnosis, staging, image-guided surgery, and even post-operative care in the clinical setting, leading to improved patient outcomes in the future.
Acknowledgements
This work was supported, in part, by the University of Wisconsin-Madison, the National Institutes of Health (P30CA014520, T32GM008505, T32CA009206), and the Brazilian Science Without Borders Program (No. SwB-CNPq). We also would like to acknowledge scientific support from Lightpoint Medical and the Small Animal Imaging and Radiotherapy Facility (SAIRF) at the University of Wisconsin-Madison.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Sharma SK, Nemieboka B, Sala E, Lewis JS, Zeglis BM. Molecular imaging of ovarian cancer. J Nucl Med. 2016;57:827–833. doi: 10.2967/jnumed.115.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35:151–156. doi: 10.1016/j.soncn.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res. 2019;12:28. doi: 10.1186/s13048-019-0503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hekman MCH, Boerman OC, Bos DL, Massuger LFAG, Weil S, Grasso L, Rybinski KA, Oosterwijk E, Mulders PFA, Rijpkema M. Improved intraoperative detection of ovarian cancer by folate receptor alpha targeted dual-modality imaging. Mol Pharm. 2017;14:3457–3463. doi: 10.1021/acs.molpharmaceut.7b00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim MC, Seo SS, Kang S, Kim SK, Kim SH, Yoo CW, Park SY. Intraoperative image-guided surgery for ovarian cancer. Quant Imaging Med Surg. 2012;2:114–117. doi: 10.3978/j.issn.2223-4292.2012.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ataseven B, Grimm C, Harter P, Heitz F, Traut A, Prader S, du Bois A. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol Oncol. 2016;140:215–220. doi: 10.1016/j.ygyno.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Belhocine T, Spaepen K, Dusart M, Castaigne C, Muylle K, Bourgeois P, Bourgeois D, Dierickx L, Flamen P. 18FDG PET in oncology: the best and the worst (Review) Int J Oncol. 2006;28:1249–1261. [PubMed] [Google Scholar]
- 9.Richard SD, Bencherif B, Edwards RP, Elishaev E, Krivak TC, Mountz JM, DeLoia JA. Noninvasive assessment of cell proliferation in ovarian cancer using [18F] 3’deoxy-3-fluorothymidine positron emission tomography/computed tomography imaging. Nucl Med Biol. 2011;38:485–491. doi: 10.1016/j.nucmedbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Keereweer S, Van Driel PB, Snoeks TJ, Kerrebijn JD, Baatenburg de Jong RJ, Vahrmeijer AL, Sterenborg HJ, Lowik CW. Optical image-guided cancer surgery: challenges and limitations. Clin Cancer Res. 2013;19:3745–3754. doi: 10.1158/1078-0432.CCR-12-3598. [DOI] [PubMed] [Google Scholar]
- 11.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, Ntziachristos V, Hollema H, Herek JL, Schroder CP, Kosterink JG, Lub-de Hoog MN, de Vries EG. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011;52:1778–1785. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Chen J, Ma S, Liu Q, Huang L, Chen X, Lou K, Wang W. Recent developments in multimodality fluorescence imaging probes. Acta Pharm Sin B. 2018;8:320–338. doi: 10.1016/j.apsb.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grootendorst MR, Cariati M, Kothari A, Tuch DS, Purushotham A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin Transl Imaging. 2016;4:353–366. doi: 10.1007/s40336-016-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Jiang D, Im HJ, Sun H, Valdovinos HF, England CG, Ehlerding EB, Nickles RJ, Lee DS, Cho SY, Huang P, Cai W. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur J Nucl Med Mol Imaging. 2017;44:1296–1305. doi: 10.1007/s00259-017-3663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam K, Chan C, Reilly RM. Development and preclinical studies of (64) Cu-NOTA-pertuzumab F (ab’) 2 for imaging changes in tumor HER2 expression associated with response to trastuzumab by PET/CT. MAbs. 2017;9:154–164. doi: 10.1080/19420862.2016.1255389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong H, Zhang Y, Severin GW, Yang Y, Engle JW, Niu G, Nickles RJ, Chen X, Leigh BR, Barnhart TE, Cai W. Multimodality imaging of breast cancer experimental lung metastasis with bioluminescence and a monoclonal antibody dual-labeled with 89Zr and IRDye 800CW. Mol Pharm. 2012;9:2339–2349. doi: 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland JP, Divilov V, Bander NH, Smith-Jones PM, Larson SM, Lewis JS. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J Nucl Med. 2010;51:1293–1300. doi: 10.2967/jnumed.110.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn AJ, Hama Y, Koyama Y, Kohn EC, Choyke PL, Kobayashi H. Targeted optical fluorescence imaging of human ovarian adenocarcinoma using a galactosyl serum albumin-conjugated fluorophore. Cancer Sci. 2007;98:1727–1733. doi: 10.1111/j.1349-7006.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hama Y, Urano Y, Koyama Y, Kamiya M, Bernardo M, Paik RS, Krishna MC, Choyke PL, Kobayashi H. In vivo spectral fluorescence imaging of submillimeter peritoneal cancer implants using a lectin-targeted optical agent. Neoplasia. 2006;8:607–612. doi: 10.1593/neo.06268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, Bart J, Low PS, Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 22.Holland JP, Normand G, Ruggiero A, Lewis JS, Grimm J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol Imaging. 2011;10:177–86. 1–3. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Toneri M, Ma H, Yang Z, Bouvet M, Goto Y, Seki N, Hoffman RM. Real-time GFP intravital imaging of the differences in cellular and angiogenic behavior of subcutaneous and orthotopic nude-mouse models of human PC-3 prostate cancer. J Cell Biochem. 2016;117:2546–2551. doi: 10.1002/jcb.25547. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, Yang Y, Cai W. CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics. 2016;6:1918–1933. doi: 10.7150/thno.15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez BV, Ikotun OF, Zheleznyak A, Wright B, Hari-Raj A, Pierce RA, Lapi SE. Evaluation of (89) Zr-pertuzumab in breast cancer xenografts. Mol Pharm. 2014;11:3988–3995. doi: 10.1021/mp500323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Hong H, Severin GW, Engle JW, Yang Y, Goel S, Nathanson AJ, Liu G, Nickles RJ, Leigh BR, Barnhart TE, Cai W. ImmunoPET and near-infrared fluorescence imaging of CD105 expression using a monoclonal antibody dual-labeled with (89) Zr and IRDye 800CW. Am J Transl Res. 2012;4:333–346. [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen R, Stammes MA, de Roos IH, Stigter-van Walsum M, Visser GW, van Dongen GA. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011;1:31. doi: 10.1186/2191-219X-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem. 2010;21:2153–2163. doi: 10.1021/bc100261d. [DOI] [PubMed] [Google Scholar]
- 29.van Nagell JR Jr, Hoff JT. Transvaginal ultrasonography in ovarian cancer screening: current perspectives. Int J Womens Health. 2013;6:25–33. doi: 10.2147/IJWH.S38347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalil MM, Tremoleda JL, Bayomy TB, Gsell W. Molecular SPECT imaging: an overview. Int J Mol Imaging. 2011;2011:796025. doi: 10.1155/2011/796025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.