Abstract
An aldolase from the bile acid-degrading actinobacterium Thermomonospora curvata catalyzes the C–C bond cleavage of an isopropyl-CoA side chain from the D-ring of the steroid metabolite 17-hydroxy-3-oxo-4-pregnene-20-carboxyl-CoA (17-HOPC-CoA). Like its homolog from Mycobacterium tuberculosis, the T. curvata aldolase is a protein complex of Ltp2 with a DUF35 domain derived from the C-terminal domain of a hydratase (ChsH2DUF35) that catalyzes the preceding step in the pathway. We determined the structure of the Ltp2–ChsH2DUF35 complex at 1.7 Å resolution using zinc-single anomalous diffraction. The enzyme adopts an αββα organization, with the two Ltp2 protomers forming a central dimer, and the two ChsH2DUF35 protomers being at the periphery. Docking experiments suggested that Ltp2 forms a tight complex with the hydratase but that each enzyme retains an independent CoA-binding site. Ltp2 adopted a fold similar to those in thiolases; however, instead of forming a deep tunnel, the Ltp2 active site formed an elongated cleft large enough to accommodate 17-HOPC-CoA. The active site lacked the two cysteines that served as the nucleophile and general base in thiolases and replaced a pair of oxyanion-hole histidine residues with Tyr-246 and Tyr-344. Phenylalanine replacement of either of these residues decreased aldolase catalytic activity at least 400-fold. On the basis of a 17-HOPC-CoA -docked model, we propose a catalytic mechanism where Tyr-294 acts as the general base abstracting a proton from the D-ring hydroxyl of 17-HOPC-CoA and Tyr-344 as the general acid that protonates the propionyl-CoA anion following C–C bond cleavage.
Keywords: steroid, cholesterol, bile acid, β-oxidation, actinobacteria, protein evolution, biodegradation, aldolase, bile acid, C–C bond cleavage, cholesterol, thiolase
Introduction
Certain bacteria from the actinobacteria and proteobacteria phyla have the unusual ability to utilize steroids as sole carbon sources (1, 2). This microbial metabolic capacity is important for the removal of steroid waste in the environment, and the associated catabolic pathways have been exploited for the synthesis of steroidal pharmaceuticals (3). More recently, a cholesterol-degradation pathway has been found to be important for the persistence of Mycobacterium tuberculosis in host macrophages. Although drug-susceptible tuberculosis is treatable through a 6-month course of four first-line antibiotics, patient noncompliance in antibiotic use has resulted in the emergence of multidrug-resistant and extensively drug-resistant strains of the bacteria (4). The steroid degradation pathways and associated enzymes have therefore received attention as potential targets for the development of new antibiotics against tuberculosis (5, 6).
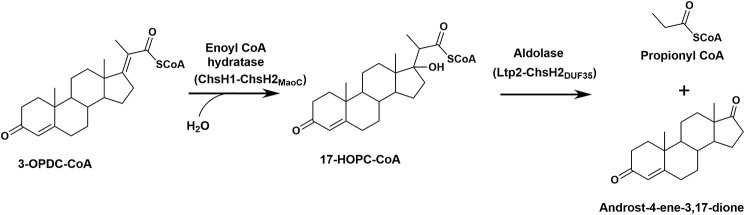
Bacteria degrade the side chains attached to the D-ring of steroids utilizing a series of reactions analogous to fatty acid β-oxidation (7). In a typical β-oxidation cycle, a thiolase is responsible for a C–C bond cleavage, releasing a molecule of acetyl-CoA or propionyl-CoA from a 3-ketoacyl-CoA intermediate, resulting in the shortening of the side chain by 2–3 carbon atoms. However, in the last cycle of β-oxidation of the side chains of cholesterol or bile acids, the hydroxyl substituent on the tertiary D-ring carbon cannot be oxidized to form a 3-ketoacyl-CoA; thus, C–C bond cleavage cannot be performed by a thiolase. Instead, it has been determined recently that in M. tuberculosis a protein named Ltp2 is responsible for catalyzing a retro-aldol cleavage of the isopropyl side chain from the hydroxyl-substituted cholesterol metabolite, 17-HOPC-CoA5 (Fig. 1) (8). Ltp2 is encoded by the last gene in a cluster of five genes within an intracellular growth (igr) operon that is so named because of its importance for intracellular growth of M. tuberculosis in mouse macrophages (9, 10). Ltp2 was also found to associate with the hydratase ChsH1–ChsH2 encoded by upstream genes in the igr operon (8). ChsH1–ChsH2 is a heteromeric enzyme related to the MaoC family of proteins that catalyzes the hydration reaction preceding the retro-aldol reaction in the pathway (11). The hydration reaction has an unfavorable equilibrium, and therefore, coupling of this reaction with the retro-aldol cleavage reaction catalyzed by Ltp2 enables the hydration reaction to proceed to completion. By co-expressing Ltp2 with various fragments of the hydratase, it was determined that Ltp2 associates with the C-terminal domain of ChsH2, a domain designated as DUF35 (domain of unknown function 35; Pfam PF01796) (8). The 3′-DNA fragment of chsH2 encoding the DUF35 domain can be co-expressed with ltp2 in the heterologous host, Rhodococcus jostii RHA1, and the overproduced Ltp2 and ChsH2DUF35 proteins formed a catalytically-competent complex with high aldolase activity. Proteins containing DUF35 domains have also been found in many bacteria and archaea, including those that are not steroid degraders (12). These proteins are typically fused to, or associate with, thiolases, 3-hydroxyacyl-CoA dehydrogenases, crotonases, or acyl transferases, but the function of DUF35 proteins in these enzymes/protein complexes is currently not clear.
Figure 1.
Reactions catalyzed by the hydratase and aldolase in the last round of the β-oxidation of a steroid side chain. The metabolite 3-OPDC-CoA thioester is hydrated to 17-HOPC-CoA thioester that subsequently undergoes C–C bond cleavage catalyzed by an aldolase.
Homologs of ltp2 and chsH2 are found in sterol/bile acid degradation gene clusters of other bacteria, suggesting similar enzymes are used by diverse bacterial species to remove the last isopropyl side chains attached to the D-ring of various steroids (1, 8, 13). Here, we report the first structure of an Ltp2 in complex with the DUF35 domain of ChsH2. The enzyme was derived from the bile acid–degrading thermophilic actinobacterium, Thermomonospora curvata. Ltp2, ChsH1, and ChsH2 from T. curvata shares 79, 65, and 55% sequence similarity with the respective homologous proteins from M. tuberculosis. Together with supporting biochemical data from enzyme variants, the structure revealed the molecular basis for the divergence of functions between Ltp2 and the related SCP-2 family of thiolases.
Results
Expression, purification, and characterization of the Ltp2–ChsH2DUF35 complex of T. curvata
The genes encoding Ltp2 (Tcur3479) and the DUF35 domain of ChsH2 (ChsH2DUF35, Tcur3482) from T. curvata were PCR-amplified, inserted into rhodococcal expression vectors, and transformed into R. jostii RHA1 for co-expression. The overexpressed, untagged ChsH2DUF35 co-purified with the C-terminal His-tagged Ltp2 on a Ni-NTA column, indicating that the two proteins form a complex. Because the 3-carbon side-chain bile acid metabolites are not available commercially, steady-state kinetic parameters were determined for the Ltp2–ChsH2DUF35 complex–catalyzed retro-aldol cleavage of the analogous cholesterol metabolite 17-HOPC-CoA (Table 1). Ltp2 can also be expressed by itself, without the DUF35 domain, in recombinant R. jostii RHA1, in good yield (4.8 mg/liter of culture) and is catalytically competent, albeit with a low specific activity of (4.2 ± 2.6) × 10−4 μmol·min−1·mg−1 toward 17-HOPC-CoA (21 μm). After a 10-min incubation of a 1:5 ratio of Ltp2/ChsH2DUF35, specific activity increased to (1.4 ± 0.079) × 10−1 μmol·min−1·mg−1. DUF35 by itself did not display any aldolase activity.
Table 1.
Kinetic parameters of wildtype and variant Ltp2–ChsH2DUF35 with 17-HOPC-CoA as substrate
The Y294F/Y344F variant has no detectable activity. The Y294F and G82P had very-low activity that precludes the determination of Km and kcat values. At the respective concentrations of 25 and 23 μm 17-HOPC-CoA substrates, Y294F variant had a specific activity of (5.3 ± 2.3) × 10−4 μmol·min−1·mg−1, and G82P variant had a specific activity of (5.8 ± 0.59) × 10−4 μmol·min−1·mg−1. For comparison, the Vmax of the wildtype enzyme is 7.4 ± 0.21 μmol·min−1·mg−1. ND means not determined; NA means no detectable activity.
| Enzyme | Km | kcat | kcat/Km |
|---|---|---|---|
| μm | s−1 | m−1 s−1 | |
| Wildtype | 2.4 ± 0.30 | 7.1 ± 0.20 | (3.0 ± 0.38) × 106 |
| H296A | (7.6 ± 0.98) × 10−1 | (1.7 ± 0.041) × 10−1 | (2.2 ± 0.29) × 105 |
| H346A | (6.2 ± 1.4) × 10−1 | (1.6 ± 0.081) × 10−1 | (2.6 ± 0.60) × 105 |
| Y344F | 2.4 ± 0.34 | (1.7 ± 0.062) × 10−2 | (7.1 ± 1.0) × 103 |
| Y294F | ND | ND | ND |
| Y294F/Y344F | NA | NA | NA |
| G82P | ND | ND | ND |
Structure of the Ltp2–ChsH2DUF35 complex
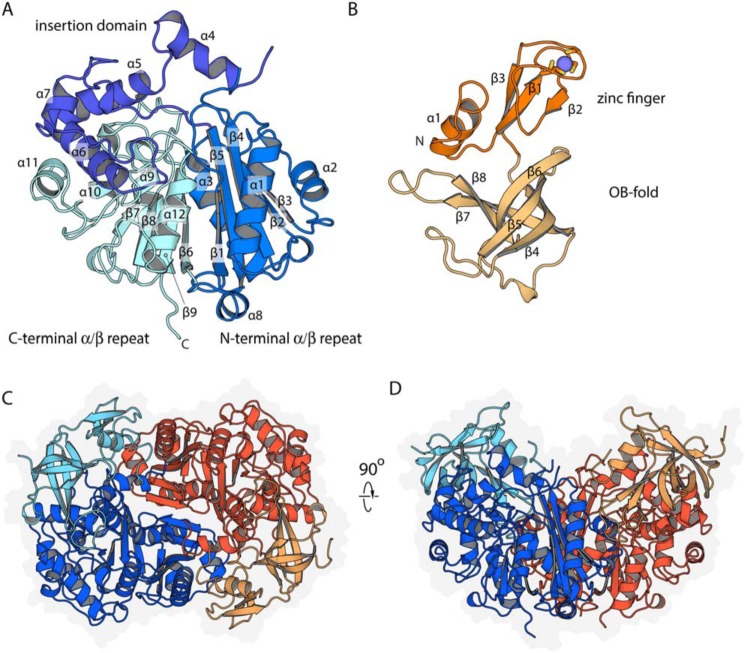
The T. curvata Ltp2–ChsH2DUF35 complex was crystallized, and the structure was determined from a dataset collected at the zinc anomalous peak (to 2.3 Å) using single wavelength anomalous diffraction. This preliminary structure was then used to refine a high-resolution native dataset collected to 1.7 Å. Table 2 lists the data collection and model refinement statistics. As anticipated from the clear sequence homology, Ltp2 structurally resembles thiolases (14). Ltp2 is built around a pair of pseudo-symmetric αβ domains (Fig. 2, A and B). The central mixed β-sheet of the N-terminal domain has topology 1,−5,4,2,3, whereas the C-terminal domain has topology 6,−9,8,7; sandwiched between these two β-sheets are the helices α3 (from the β3–β4 loop) and α11 (from the β7–β8 loop). The β4–β5 loop from the N-terminal domain is elaborated into an extended (∼95 amino acids) subdomain with four α-helices (plus extensive loops); this subdomain packs predominantly on the C-terminal domain.
Table 2.
Table of statistics for the data collection and subsequent model fitting of Ltp2-ChsH2DUF35 determined by Zn-SAD
A preliminary structure determined by Zn-SAD was used to solve (by molecular replacement) a higher resolution structure from a different crystal.
| Crystal | Zn-SAD | Native |
|---|---|---|
| Space group | P212121 | P212121 |
| a, b, c (Å) | 91.84, 110.15, 120.83 | 91.68, 110.56, 120.14 |
| Data collection | ||
| Resolution (Å) | 50–2.3 (2.36–2.3) | 39.94–1.7 (1.76–1.7)a |
| Wavelength (Å) | 1.2824 | 0.97857 |
| Reflections | ||
| Observed | 710,990 | 702,055 |
| Unique | 195,178 | 134,309 |
| Rmerge | 0.074 (0.843) | 0.0485 (0.724) |
| Rmeas | ||
| CC½ | 0.999 (0.807) | 0.999 (0.734) |
| I/σI | 14.0 (2.34) | 19.08 (2.23) |
| Completeness (%) | 100 (100) | 99.92 (99.97) |
| Multiplicity | 6.8 (6.8) | 5.2 (5.2) |
| Wilson B | 58 | 24.5 |
| Refinement | ||
| Resolution (Å) | 1.7 | |
| No. of reflections | 134,294 | |
| Rwork/Rfree | 14.9/16.8 | |
| CCa | 0.968 | |
| B-factors | ||
| Protein | 32.3 | |
| Solvent | 40.5 | |
| r.m.s.d. | ||
| Bond lengths (Å) | 0.003 | |
| Bond angles (degrees) | 0.736 | |
| Ramachandran | ||
| Favored (%) | 96.73 | |
| Allowed (%) | 3.17 | |
| Outliers (%) | 0.10 | |
a Values in parentheses represent statistics for the highest-resolution shell.
Figure 2.
Structure of Ltp2–ChsH2DUF35. A, secondary structure organization of Ltp2, with secondary structure labeled. B, secondary structural organization of the ChsH2DUF35 domain. The zinc ion is depicted as a blue sphere, with the coordinating cysteine residues shown as sticks. C, organization of Ltp2–ChsH2DUF35 heterotetramer. The Ltp2 chains are colored dark orange and blue, and the DUF35 domains of ChsH2 are colored light orange and light blue. A faint gray silhouette depicts the extent of the protein surface. D, Ltp2–ChsH2DUF35 complex depiction is 90° from C.
The ChsH2DUF35 domain is built from two subdomains: an N-terminal zinc finger domain, and a C-terminal domain with an oligonucleotide/oligosaccharide binding (OB)-fold. The zinc finger domain has a three-stranded antiparallel β-sheet (topology 2,−1,3) supplemented by an N-terminal α-helix. The zinc ion is ligated by two pairs of cysteine residues contributed by the β1–β2 and β3–β4 loops. The OB-fold domain forms a five-stranded β-barrel, with topology 4,−5,6,8,−7.
As observed in other thiolase family proteins, the Ltp2 proteins interact extensively and predominantly through their N-terminal αβ domains to form a tight homodimer with an interface area of 2055.9 Å2 (as determined by PISA (15)). The DUF35 domains are positioned at opposite ends of this central Ltp2 dimer, forming an overall αββα heterotetramer. The N-terminal α-helix and the OB subdomain of ChsH2DUF35 interact with α4- and α5-helices of one Ltp2 protomer (interface ∼1500 Å2), whereas the zinc ribbon domain makes limited interactions with α1- and α2-helices of the opposite Ltp2 protomer (interface area ∼460 Å2). These interactions are predominantly hydrophobic in nature.
The observed organization of the Ltp2–ChsH2DUF35 heterotetramer, with both DUF35 domains exposed along one edge of the complex, is consistent with the idea that DUF35 domains act as molecular staples mediating the formation of bifunctional enzyme complexes with thiolase family enzymes as (at least) one key component. The first precedent for this organization is the acetoacetyl-CoA thiolase/3-hydroxy-3-methylglutaryl (HMG)-CoA synthase complex from M. thermolithotrophicus (16). In this complex, a thiolase dimer of dimers occupies the center of the complex, whereas the HMG-CoA synthase occupies the periphery. The DUF35 domain is sandwiched between these two proteins, stabilizing the overall complex. The two enzymes catalyze successive reaction steps, share a single CoA-binding site, and have all active sites opening into a central open chamber; together, this promotes a significant increase in the efficiency of the pathway by substrate channeling. The Ltp2–ChsH2DUF35 complex can be superimposed on the HMG-CoA synthase complex with r.m.s.d. values of 1.8 Å for the thiolase and 2.5 Å for the DUF35 domain. A second precedent is the PhlABC multicomponent acyl synthase/acyltransferase from Pseudomonas protegens (5m3k) (17), with r.m.s.d. values of 2.2 Å for the thiolase, 2.7 Å for the DUF35 domain, and 2.7 Å for the overall complex. The periphery of the structure is composed of two dimers of PhlC, a thiolase family protein that catalyzes a Friedel-Crafts transacylation reaction. The center of the structure contains a dimer–of–dimers of PhlA, a thiolase family protein lacking key thiolase motifs; this protein is of uncertain function but may generate a donor molecule required by PhlC. Linking these domains is PhlB, a DUF35 domain protein. Despite the very different reactions catalyzed, the broad organization of the HMG-CoA synthase and PhlABC complexes is similar.
Although the overall architecture of the Ltp2–ChsH2DUF35 complex resembles these bifunctional complexes, the Ltp2 aldolase itself most closely resembles monofunctional SCP-2 thiolases (18) such as Leishmania mexicana thiolase (PDB 3zbn; r.m.s.d. 1.8 Å). Interestingly, Ltp2 is significantly more distantly related to FadA5, which also degrades steroids, mediating C–C bond cleavage during the β-oxidation of 8- or 5-carbon side-chain cholesterol metabolites in M. tuberculosis (19) (PDB 4ubw; r.m.s.d. 2.45 Å). Together, this suggests that steroid specificity may have evolved separately in these two thiolase superfamily members.
It is also interesting to note that the OB-fold of DUF35 also resembles the C-terminal domain of a thiolase-like protein (TLP) from Mycobacterium smegmatis (PDB 4egv) (20). TLP is a monomeric protein that contains a C-terminal β-barrel domain linked to an N-terminal thiolase-fold. Ltp2 together with the associated OB-fold domain of DUF35 superimpose well to TLP (r.m.s.d. of 2.1 Å). This suggests a possible precursor to the evolution of the DUF35 domain, which so far has been found to only occur as an accessory domain to thiolase family members.
Modeling the full Ltp2–ChsH1–ChsH2 complex
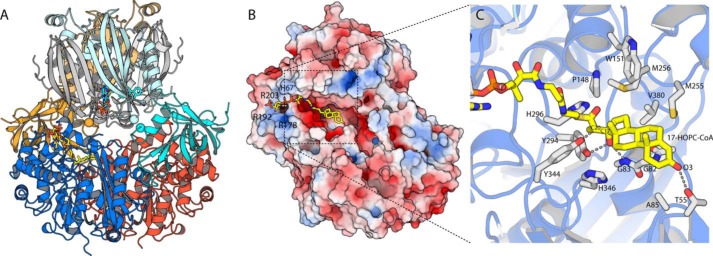
Attempts to crystallize the full Ltp2–ChsH2–ChsH1 complex of T. curvata were unsuccessful. Because the structure of ChsH1–ChsH2 from M. tuberculosis containing the MaoC hydratase domains but lacking the DUF35 domain is available, we modeled the complex using docking. The structure of the M. tuberculosis ChsH1–ChsH2MaoC complex (PDB code 4wnb) shows a heterotetrameric organization, where one ChsH1 and one ChsH2MaoC chain form a functional heterodimer with one catalytic site; these heterodimers then interact through a series of α-helices. The native molecular mass of the T. curvata ChsH1–ChsH2 was determined to be 131 kDa by size-exclusion chromatography (data not shown), consistent with this complex also having a heterotetrameric organization. Symmetry provides a strong constraint on the bifunctional complex as oligomers almost universally interact by aligning symmetry elements so that the interfaces are also symmetric (21); here, both sub-complexes have only a single 2-fold symmetry axis, which is very likely aligned. The ChsH2 DUF35 (ChsH2DUF35) domains are fused to the C terminus of the ChsH2 MaoC hydratase domain (ChsH2MaoC), with an ∼16-amino acid linker separating the domains; this linker dictates which faces of the two sub-complexes can approach one another, and it also constrains how far the respective termini of the two domains can separate. Finally, both proteins have a saddle-like shape along this axis, suggesting that optimal interactions will involve fitting these saddles to one another. We manually docked the Ltp2–ChsH2DUF35 to the ChsH1–ChsH2MaoC complex and then used a customized script to generate a series of variants of this configuration, which conform to the above constraints; Rosettadock was then used to optimize these candidate starting poses and score the refined complexes. The lowest energy solutions all closely resemble that shown in Fig. 3A, where the two protein complexes interact intimately. It should be noted that the ChsH2 MaoC–DUF35 linker and Ltp2 groove-covering loop may also pack between these domains and influence optimal packing, whereas the exposed, extended loops β4–β5 and β7–β8 within the DUF35 domain are also likely to shift so as to maximize interactions. Nevertheless, this complex as depicted buries a respectable total surface of 1370 Å2 with a total solvation energy change of −12.1 kcal/mol (PISA). We therefore anticipate that this model is reasonably accurate in terms of the overall complex geometry, although details including side chain and loop packing are likely not fully captured.
Figure 3.
Modeling the Ltp2 multiprotein and substrate complexes. A, Rosettadock model of the Ltp2–ChsH2DUF35 complex (colored as in Fig. 2) docked onto the M. tuberculosis ChsH1–ChsH2MaoC complex (ChsH2 in pale cyan and pale orange, and ChsH1 in gray shades). Docking is constrained by the high probability of the proteins docking with their 2-fold axis aligned, plus the limited length of the linker joining the C terminus of the ChsH2MaoC and N terminus of the ChsH2DUF35 (marked with spheres). Both proteins have a distinctly saddle-like shape, drastically restricting the plausible conformations. Note that this complex omits two extended loops (joining the ChsH2 domains and covering the Ltp2-active site). B, electrostatic surface (generated using APBS) for Ltp2 with 17-HOPC-CoA manually docked. C, close-up of the model, showing details of 17-HOPC-CoA binding (yellow sticks) and key proposed binding and catalytic residues (white sticks; note: protein residues are shown in their experimentally observed positions and were not shifted during docking).
The generated model suggests that the active sites of ChsH1–ChsH2MaoC and Ltp2–ChsH2DUF35 aldolase complexes face one another and may make minor contributions to one another's active site. In particular, the N-terminal helix and the β7–β8 loop of the DUF35 domain may directly contribute to binding the A-ring of 3-OPDC-CoA by ChsH1–ChsH2MaoC. In return, ChsH1–ChsH2MaoC may possibly help organize the substrate groove-covering loop of Ltp2. It is worth noting is that the CoA-binding site of ChsH1–ChsH2MaoC is too distant to allow the steroid ring/side chain to reach the Ltp2 active site (Fig. 3A). Ltp2 is therefore suggested to bind CoA independently, as supported by the observation that the hydratase and aldolase can each function efficiently without the partner sub-complex present. This observation also implies that the substrate needs to fully release and rebind between reactions, meaning that this complex will likely not exhibit the robust substrate channeling between active sites observed in the archaeal HMG-CoA synthase complex (16).
Active-site organization and enzymatic mechanism of Ltp2
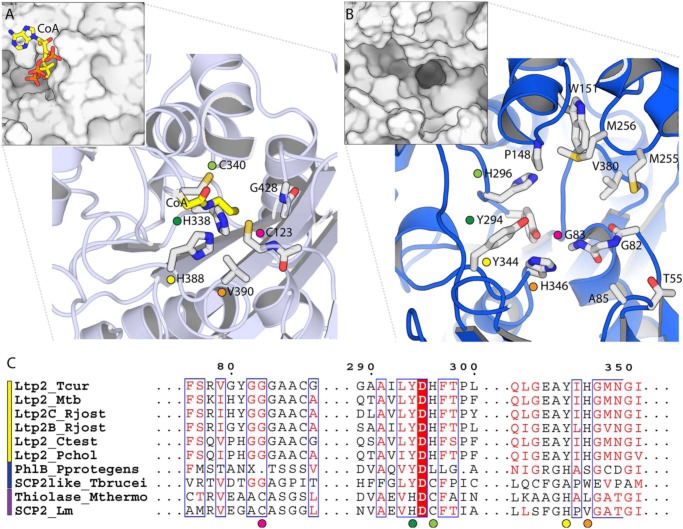
In canonical thiolases, e.g. L. mexicana thiolase, the active site is located at the bottom of a long, relatively narrow tunnel that envelops the substrate and connects to the adenosine moiety on the enzyme surface via the pantetheinyl arm (Fig. 4A). A surface-rendering of Ltp2–ChsH2DUF35, in contrast, shows that the active site takes the form of a long (∼45 Å), deep (∼15 Å), and fairly wide (8–15 Å) groove that spans the full distance between both CoA-binding sites (Fig. 4B). This groove is built entirely from Ltp2 residues (with no contributions with the ChsH2DUF35 domain) and is quite hydrophobic, at least along the bottom of the channel. This groove is less open than it appears as it is bridged by the disordered (in this apo structure) loop composed of residues 119–125. This loop contains several conserved nonpolar residues; we expect that this region becomes ordered either upon substrate binding by packing on the extended nonpolar surface offered by 17-HOPC-CoA or by packing on the ChsH1–ChsH2MaoC hydratase domains. It is worth noting, however, that these disordered motifs are considerably shorter than the equivalent motifs in e.g. L. mexicana thiolase where they form an extended, well-ordered helix (α6) that encloses the active site, whereas the shifting and shortening of other motifs also contribute to the formation of this extended open groove in Ltp2. The openness of this substrate-binding groove is therefore likely an adaptive feature that is needed to accommodate a large sterol substrate, an idea that is buttressed by the similarly open active site of the sterol thiolase, FadA5 (19).
Figure 4.
Comparison of the active site of Ltp2 aldolase with an SCP-2 thiolase. A, active-site structure of the SCP-2 thiolase from L. mexicana (PDB code 3zbg). Larger panel shows key active-site residues, and the smaller inset panel shows a surface view of the same region. CoA (yellow) was positioned from 3zbn. Colored circles denote equivalent residues in A, B, and C. B, equivalent active-site region in Ltp2. C, multiple sequence alignment of Ltp2 from T. curvata and its homologs. The first six sequences (yellow bar) are Ltp2 aldolases; the following two (blue bar) are thiolase-like proteins of unknown activity; and the last two sequences are thiolases. Amino acid sequences of proteins are as follows: T. curvata Ltp2 (Ltp2_Tcur); M. tuberculosis Ltp2 (Ltp2_Mtb); R. jostii RHA1 cholesterol Ltp2 (Ltp2C_Rjost); bile acid Ltp2 (Ltp2B_Rjost); Comamonas testosteroni KF1 Ltp2 (Ltp2_Ctest); Pseudomonas sp. Chol1 Ltp2 (Ltp2_Pchol); PhlB from P. protogens (PhlB_Pprotegens); thiolase-type SCP-2-like from T. brucei (SCP2like_Tbrucei); M. thermolithotrophicus acetoacetyl-CoA thiolase-type SCP-2 (Thiolase_Mthermo); and L. mexicana thiolase-type SCP-2 (SCP2_Lm). Sequences are aligned using Clustal Omega and displayed using ESPRIPT (41, 42).
The thiolase reaction has been intensively studied and is critically dependent upon a pair of conserved catalytic cysteines (22). Considering the reaction in the thiolytic cleavage direction, the nucleophilic cysteine attacks the β-carbonyl carbon of the β-ketoacyl-CoA substrate, forming an acyl-enzyme intermediate. The key to the energetic accessibility of this reaction step is resonance stabilization of the negative charge on the acetyl-CoA leaving group by the thioester. A second conserved cysteine residue then serves as a general acid that donates a proton to the α-carbon of the acyl-CoA anion. Finally, the acyl-enzyme intermediate is resolved by a second CoA molecule attacking the carbonyl group. In addition to the nucleophile and base, the active site also requires two oxyanion holes, which accommodate negative charges that develop on the two carbonyl groups in different steps in the reaction; these oxyanion holes are typically formed by His and Asn residues whose identity varies among thiolase sub-families. Comparison of the Ltp2 catalytic site to L. mexicana thiolase reveals that none of the key residues that mediate the thiolase reaction are conserved (Fig. 4C). In particular, the nucleophilic Cys-123 has been replaced by glycine, Gly-82. The acid/base Cys-340, which protonates the β-carbon of the leaving group, has been replaced by His-296. Finally, His-338 and His-388, which together form the oxyanion hole that stabilizes the CoA-acyl oxygen (O1), are replaced by a pair of tyrosine residues, Tyr-294 and Tyr-344. These radical changes in all elements of the catalytic machinery suggest that the mechanism of the aldolase is likely to utilize the basic thiolase superfamily active-site architecture very differently than thiolases proper.
Extensive attempts to co-crystallize or soak Ltp2–ChsH2DUF35 with the substrate, 17-HOPC-CoA, or products androstenedione and propionyl-CoA did not yield structures containing ligands. The position of 17-HOPC-CoA was therefore manually modeled in the Ltp2 structure to obtain insights into the binding mode and mechanism (Fig. 3, B and C). In most protein families, co-factor binding is quite conservative and so can be reliably modeled where co-structures of other family members serve as a guide. The details of the CoA-binding mode are, however, quite variable among thiolase superfamily members, but the HMG-CoA thiolase structure seems to serve as a reasonable model, placing the negative charges associated with the adenosine group in a positively charged pocket that includes Arg-203 (absolutely conserved) and His-253 (ChsH2DUF35 domain) near the pyrophosphate, and Arg-192 and Arg-178 (conserved as Arg or Lys) adjacent to the 3′-phosphate. There are fewer a priori constraints on the steroid ring placement; in addition, the stereochemistry around C17 and C18 of 17-HOPC-CoA has not been experimentally determined. However, the hydrophobic-binding groove is only just wide enough to accommodate the ring, and the D-ring is constrained to be positioned adjacent to the CoA-binding site. In this orientation, the steroid ring only fits well with its methyl-free side pointing toward the protein surface, packing intimately against two of a trio of absolutely conserved glycine residues, Gly-82 and Gly-83. Puckering the A-ring maximizes nonpolar interactions while placing O3 in hydrogen-bonding distance to Thr-55 (conserved as Ser/Thr in Ltp2 homologs). The B- and D-rings form extensive nonpolar interactions with Met-255, Met-256, and Val-380, which form an extended hydrophobic wall of the pocket. This model places the critical 17-HOPC C17 and C20 carbons in proximity to a set of four absolutely conserved polar residues (Fig. 3C), including Tyr-294 and Tyr-344 (recall that these replace the thiolase oxyanion hole histidine residues). O17 makes hydrogen bonds with Gly-83N and the phenolic hydroxyl of Tyr-294, although the Tyr-344 phenolic hydroxyl interacts closely with the 17-HOPC-CoA C20 hydrogen atom. The edge of the His-346 ring stacks on the C-ring of the modeled substrate, whereas His-296 stacks on the thioester group, with neither residue seeming suitably positioned to form hydrogen bonds (Fig. 3C). Note that this model predicts that 17-HOPC-CoA has a 17(S), 20(S) absolute configuration.
To test the 17-HOPC-CoA–binding model, key amino acid residues in Ltp2 were altered using site-specific mutagenesis, and steady-state kinetics parameters were determined with the substrate 17-HOPC-CoA (Table 1). All Ltp2 variants can be purified as complexes with ChsH2DUF35 indicating proper folding. Our model predicts that the 17-HOPC A-, B-, and C-rings wrap around Gly-82; replacing this residue with proline resulted in activity being reduced at 4 orders of magnitude, consistent with the introduction of significant steric clashes. Replacement of His-296 and His-346 with alanine led to a modest reduction in kcat of about 40-fold, and a decrease in Km by about 3–4-fold, suggesting that these residues, although contributing, are not critical to the mechanism. Replacement of Tyr-344 by phenylalanine led to a 400-fold reduction in specific activity, suggesting that the phenolic oxygen is critical for activity. Replacement of Tyr-294 by phenylalanine reduced catalytic activity by some 4 orders of magnitude. Finally, the Y294F/Y344F double mutant abolished activity.
On the basis of our model and mutagenesis data, we propose that the catalytic mechanism for the Ltp2-catalyzed retro-aldol cleavage of 17-HOPC-CoA revolves around a pair of catalytic tyrosine residues, Tyr-294 and Tyr-344 (Fig. 5). In particular, Tyr-294 acts as a base, abstracting a proton from the hydroxyl group on the D-ring (O17) of the steroid substrate, leading to C–C bond cleavage. Tyr-344 acts as a general acid, donating a proton to the Cβ of propionyl-CoA (C20 of 17-HOPC-CoA). The histidine residues play relatively minor roles, and their exact contribution is unclear; possibly His-296 could reorient to interact with the thioester carbonyl during the enzyme's functional cycle, forming an oxyanion hole to stabilize the carbonyl oxygen. The His-346 imidazole is oriented toward the phenolic ring of Tyr-344, and the resulting cation–π interaction may play a role in positioning this residue, and possibly fine-tuning its pKa. Enzymes that utilize tyrosine residues as general acids/bases include vanillyl alcohol oxidase and fructose-1,6-bisphosphate aldolases. In vanillyl alcohol oxidase, two tyrosine residues are capable of deprotonating the phenolic substrate during catalysis (23). In Thermoproteus tenax fructose-1,6-bisphosphate aldolase, a tyrosyl residue was proposed to be the catalytic base that abstracts the proton from the C4-OH of the substrate (24). In rabbit muscle fructose-1,6-bisphosphate aldolase, however, a tyrosyl residue acts as a general acid to donate a proton to the carbinolamine intermediate (25). The fructose-1,6-bisphosphate aldolases are, however, evolutionarily, structurally, and mechanistically distinct from Ltp2–ChsH2DUF35, and they do not utilize β-hydroxy-CoA thioesters as substrates.
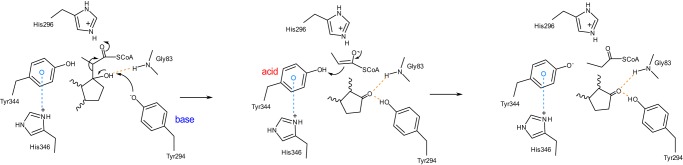
Figure 5.
Proposed retro-aldol cleavage mechanism of Ltp2–ChsH2DUF35. This prediction was made based on the modeling of the 17-HOPC-CoA into the active site and the results obtained from site-directed mutagenesis studies. Tyr-294 deprotonates the C17 hydroxyl group of 17-HOPC-CoA leading to C–C bond cleavage. The second tyrosine residue, Tyr-344, acts as an acid, donating a proton to the enolate anion of the CoA thioester, forming propionyl-CoA and androst-4-ene-3,17-dione. Either Tyr-294 or Tyr-344 could be activated by the nearby histidine residues.
It is also interesting to note that although 17-HOPC-CoA is a functional in vitro substrate, the likely in vivo substrate of this specific enzyme is a closely analogous bile acid degradation metabolite, consistent with the ability of T. curvata to grow on bile acids as sole carbon sources (1). Bile acids differ from cholesterol in the steroid ring by hydroxyl substituents at C7 (B-ring) and/or C12 (C-ring), rendering them significantly more polar. Inspection of the 17-HOPC-CoA docked model suggests that an oxygen atom on C12 may be able to form hydrogen bonds with His-346 and Tyr-344. A hydroxyl group in this position might be expected to improve binding and may explain the conservation of His-346, despite its modest contribution in the 17-HOPC-CoA reaction. A hydroxyl substituent on C7 would, however, point into a shallow, wholly nonpolar pocket. This hydroxyl could possibly be partially accommodated by shifting Met-255 into its observed alternate conformation; while preventing steric clash, this interaction would still exact an enthalpic penalty upon binding, due to the cost of desolvating the hydroxyl group. Interestingly, other Ltp2 homologs often replace this methionine with serine or threonine, residues that could potentially form favorable hydrogen bonds with O7. We therefore predict that T. curvata Ltp2 may prefer deoxycholate metabolites over cholate or chenodeoxycholate metabolites.
Discussion
Ltp2–ChsH2DUF35 shows clear structural and sequence homology to SCP-2 thiolases and seems to have evolved by repurposing the thiolase machinery to catalyze an aldolase reaction. In the canonical thiolase reaction, facile breaking of C–C bonds depends on the stabilization of the anionic thioester intermediate by resonance stabilization within the thioester group. Ltp2 presumably exploits the same stabilization effect to allow an aldolase reaction. Aldolases are generally divided into two groups: class I aldolases stabilize the leaving group by forming an amine adduct, and class II aldolases do so by forming a divalent metal ion complex (26). The Ltp2-type aldolases potentially represent an alternative solution to the problem of aldol leaving group stabilization, using, in this case, the thioester group. Other enzymes that also catalyze retro-aldol cleavage of β-hydroxythioesters include malyl-CoA lyase (27) and citrate synthase (28).
Ltp2 almost certainly evolved from an SCP-2–like thiolase, but this process has substantively remodeled the protein. In particular, not only has every critical catalytic residue been replaced, but the active site has also been dramatically opened up to accommodate the large, rigid steroid substrate. Replacing multiple catalytic residues and remodeling the active site are unlikely to have occurred in a single step; we would suggest that there are some specific features of this transition that suggest that the aldolase reaction evolved first, and sterol specificity second. First, the evolution of aldolase activity in the context of SCP-2 thiolases in particular may have been facilitated on the use of a pair of histidine residues to form the oxyanion hole in this family (18). These histidine residues are necessarily positioned to stabilize the anionic intermediate in SCP-2 thiolases; this also ideally positioned them to potentially initiate an aldolase reaction by deprotonating a similarly positioned hydroxyl group, exploiting the inherent facility of histidine for performing acid/base reactions near neutral pH. The use of histidine in SCP-2 thiolases may therefore have predisposed this family to evolve aldolase activity, with the tyrosines possibly a later optimization. Second, in Ltp2 the nucleophilic cysteine of thiolases has been replaced with a glycine; this glycine is intolerant of bulkier substitutions and appears, in modeling, to stack directly on the steroid ring. This suggests an evolutionary history where aldolase activity originally evolved in context of a different (possibly smaller) substrate, and steroid specificity only arose once the cysteine was dispensable. The alternative evolutionary history, where Ltp2 evolved from a steroid-specific thiolase similar to FadA5, would require that a new steroid-binding site evolved closer to the backbone after the switch to an aldolase reaction permitted by replacement of the cysteine. In this context, it is worth noting that the SCP-2 family includes thiolase-like proteins of unknown function where the nucleophilic cysteine has been lost, but the active site–covering helix appears to be retained (Fig. 4C) (22). The Pseudomonas protegens enzyme has a thiolase ancestor-like histidine in place of Tyr-344 (17), whereas the Trypanosoma brucei protein has an alanine replacing this critical residue, but retains the thiolase acid/base cysteine (29). It will be interesting to determine whether these enzymes represent additional thiolase superfamily aldolases, albeit ones that may differ in the details of their catalytic mechanism, and act on less bulky substrates; if so, these enzymes would be strong candidates from which the sterol aldolases evolved.
Experimental procedures
Chemicals
Ferrocenium hexafluorophosphate was from Sigma-Aldrich (Oakville, Ontario, Canada). 4-Pregnen-3-one-20β-carboxylic acid was from Steraloids Inc. (Newport, RI). CoA was from BioShop Canada, Inc. (Burlington, Ontario, Canada). Restriction enzymes and Pfu polymerase were from Thermo Fisher Scientific (Ottawa, Ontario, Canada). T4 DNA ligase was from New England Biolabs (Pickering, Ontario, Canada). Ni2+-NTA Superflow resin was from Qiagen (Mississauga, Ontario, Canada). 17-HOPC-CoA was synthesized as described previously (8). All other chemicals were purchased from Thermo Fisher Scientific unless otherwise stated.
Bacterial strains and plasmids
T. curvata DSM 43183 was obtained from the Leibniz Institute German Collection of Microorganisms and Cell Cultures. R. jostii RHA1 was obtained from Dr. Lindsay Eltis (Department of Microbiology and Immunology, University of British Columbia, Canada).
DNA manipulation
The genes encoding Ltp2 (Tcur3479) and the DUF35 domain of ChsH2 (Tcur3482) were amplified from T. curvata DSM 43183 genomic DNA using primers indicated in Table 3. The 3′ primer for ltp2 omits the stop codon to append a C-terminal His-tag in the expressed protein. The genes encoding Ltp2 and the ChsH2DUF35 were inserted into the NdeI/HindIII sites of pTIP-Qc2 and pTIP-RT2 (30), respectively. Both plasmids were transformed into R. jostii RHA1 for co-expression. Site-specific mutagenesis was carried out according to the modified QuikChange method (31) using primers listed in Table 3, except for the G82P variant that was created by gene synthesis (Biobasic Inc.).
Table 3.
Sequences of primers used to PCR-amplify ltp2, chsH2DUF35, and to create mutations in ltp2
NdeI and HindIII restrictions sites in primer sequences are underlined. Replaced codons in mutagenic primers are shown in boldface.
| Sequences | |
|---|---|
| ltp2 | GCCCCATATGAGCGTGCTGCCCGGAGCC |
| CGCCAAGCTTTCGGTCCGCTCCCAGGATCA | |
| chsH2DUF35 | CCGGCATATGCGCCCGGCGATCAACCGCGAC |
| CGCCAAGCTTCATCGGTCCGCTCCCAGGATC | |
| H296A | CTGTACGACGCCTTCACCCCCTGGTGCTGCCGCAACTGG |
| GGGGTGAAGGCGTCGTACAGGATGGCCGCGCCGATGTCG | |
| H346A | CCTACATCGCAGGCATGAACGGCATCG CCGAGG |
| TCATGCCTGCGATGTAGGCCTCGCCGAGCTGGC | |
| Y294F | CCATCCTGTTCGACCACTT CACCCCGCTGGTGCTG |
| AAGTGGTCGAACAGGATGG CCGCGCCGATGTCGGA | |
| Y344F | GCGAGGCCTTCATCCACGGCATGAACGGCATCGCCGAGGC |
| CGTGGATGAAGGCCTCGCCGAGCTGGCCGCCGTGG |
Protein expression and purification
FadE28–FadE29, CasI, TTHB247, and ChsH1–ChsH2 were purified from recombinant Escherichia coli BL21 (λDE3) or R. jostii RHA1 according to the methods described previously (8, 32, 33).
Recombinant R. jostii RHA1 containing pTIPQC2/ltp2 and pTIPRT2/chsH2duf35 was grown at 30 °C in 4 liters of LB medium supplemented with chloramphenicol (25 mg/ml) and tetracycline (8 mg/ml). Expression of the recombinant proteins was induced with thiostrepton (1 μg/ml) at mid-log phase (OD600 = 0.4–0.6). Cultures were grown for a further 24 h at 30 °C, and cells were subsequently collected by centrifugation at 9605 × g for 10 min.
R. jostii RHA1 cell pellets were resuspended in 20 mm HEPES, pH 7.5, and cells were lysed through a French press at 20,000 p.s.i. Insoluble fractions were removed by centrifugation at 39,191 × g twice for 15 min. Cell-free extracts were filtered through a 0.45-μm filter and incubated for 1 h at 4 °C with Ni2+-NTA resin in buffer containing 50 mm sodium phosphate, 300 mm sodium chloride, and 20 mm imidazole, pH 8.0. The mixture was poured into a gravity column and washed with the same buffer. The His-tagged proteins were eluted with buffer containing 150 mm imidazole, pH 8.0. The buffer was exchanged into 20 mm sodium HEPES, pH 7.5, by dilution in a stirred cell equipped with a YM10 filter (Amicon). Purified enzymes were stored at −80 °C.
Protein concentration, purity, and molecular weight determination
Concentrations of proteins were determined by the Bradford assay using BSA as the standard (34). Coomassie Blue-stained SDS-PAGE and Image Lab Software (Bio-Rad Inc) was used to assess purity of the enzymes. The native molecular weight of ChsH1–ChsH2MaoC was determined by gel filtration on a Superdex 200 column (GE Biosciences) with 20 mm HEPES buffer pH 7.5 containing 0.15 m NaCl as the equilibration and elution buffer. The standard curve used consisted of the proteins cytochrome c (Mr = 12,400), carbonic anhydrase (Mr = 29,000), BSA (Mr = 66,000), alcohol dehydrogenase (Mr = 150,000), and β-amylase (Mr = 200,000) (all from Sigma).
Steady-state kinetic assays
The retro-aldol activity of the aldolase Ltp2–ChsH2DUF35 toward 17-HOPC-CoA was determined spectrophotometrically using the previously reported coupled assay (8). Briefly, assays were performed at least in triplicate in a total volume of 1 ml at 25 °C, using a Varian Cary 3 spectrophotometer equipped with a temperature-controlled cuvette holder (ϵ = 6200 m−1 s−1). The assay was determined in 100 mm HEPES, pH 7.5, in the presence of 200 μm NADH and 16 μm of the aldehyde dehydrogenase TTHB247 from Thermus thermophilus (8).
Crystallization and structure determination
Conditions for crystallization of Ltp2–ChsH2DUF35 were screened using the JCSG+ kit (Molecular Dimensions Inc.). Crystals used for data collection were grown using the sitting drop method at 10 °C, with 2 μl of reservoir solution (0.2 m sodium thiocyanate, 20% PEG 3350) combined with 2 μl of 16 mg/ml Ltp2–ChsH2DUF35. Crystals were soaked in Paratone N as cryoprotectant prior to freezing. The datasets were collected at the Canadian Light Source, Canadian Macromolecular Crystallography Facility (CMCF-BM), and processed using XDS (35). The structure was initially phased using zinc-single anomalous diffraction (Zn-SAD) in Phaser (36), with an initial experimental figure of merit of 0.147. A native dataset was also collected (from a different crystal) at 1.7 Å and was then refined using the lower resolution Zn-SAD structure as a starting model. Refinements were carried out in PHENIX refine, with manual building in Coot (37). The structure proved to have one full heterotetramer in the asymmetric unit, with the only breaks being in Ltp2 where residues of the active site–covering loop 120–126 (chain A) and 118–128 (chain C) were disordered. Figures of protein structure were generated using PyMOL version 2.0 (38).
Docking and modeling
The structure of the ChsH1–ChsH2MaoC substrate complex (PDB code 4wnb) was initially placed manually on the Ltp2–ChsH2DUF35 complex. Weakly-ordered loops (with high-atomic displacement parameters or that differed significantly between chains) were removed after inspection to avoid steric clashes. Initial molecular placement sought to maximize the overall buried surface area while aligning the two complexes in respective 2-fold symmetry axes. After initial manual placement, variants of this complex were systematically generated by rotations ±5° (in 1° increments) and translating ±2 Å (in 1/3 Å increments) using a perl script previously reported (39). These initial starting poses were then optimized in RosettaDock 3.5 (40), and the lowest energy pose reported. Docking of 17-HOPC-CoA was performed manually in PyMOL version 2.0.
Author contributions
R. A., E. M., K. L. S., and M. S. K. data curation; R. A., E. M., M. S. K., and S. Y. K. S. formal analysis; R. A., E. M., S. E. G., M. A. V., M. S. K., and S. Y. K. S. methodology; R. A., M. S. K., and S. Y. K. S. writing-review and editing; M. S. K. and S. Y. K. S. funding acquisition; M. S. K. and S. Y. K. S. writing-original draft; S. Y. K. S. conceptualization; S. Y. K. S. supervision; S. Y. K. S. project administration.
Acknowledgments
We acknowledge Erin MacIsaac and Cole Heasley for technical assistance in this project. We also thank Shaunivan Labiuk at the Canadian Light source for help in X-ray diffraction data collection.
This work was supported by Grants 2015-05366 and 2015-04045 from the Natural Science and Engineering Research Council of Canada. The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (code 6OK1) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- 17-HOPC-CoA
- 17-hydroxy-3-oxo-4-pregnene-20-carboxyl–coenzyme A thioester
- 3-OPDC-CoA
- 3-oxo-4,17-pregnadiene-20-carboxyl–coenzyme A thioester
- TLP
- thiolase-like protein
- PDB
- Protein Data Bank
- OB
- oligosaccharide binding
- r.m.s.d.
- root mean square deviation
- Ni2+-NTA
- nickel-nitrilotriacetic acid
- Zn-SAD
- zinc-single anomalous diffraction.
References
- 1. Bergstrand L. H., Cardenas E., Holert J., Van Hamme J. D., and Mohn W. W. (2016) Delineation of steroid-degrading microorganisms through comparative genomic analysis. MBio 7, e00865–16 10.1128/mBio.00865-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van der Geize R., Yam K., Heuser T., Wilbrink M. H., Hara H., Anderton M. C., Sim E., Dijkhuizen L., Davies J. E., Mohn W. W., and Eltis L. D. (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 1947–1952 10.1073/pnas.0605728104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. García J. L., Uhía I., and Galán B. (2012) Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb. Biotechnol. 5, 679–699 10.1111/j.1751-7915.2012.00331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dheda K., Gumbo T., Maartens G., Dooley K. E., McNerney R., Murray M., Furin J., Nardell E. A., London L., Lessem E., Theron G., van Helden P., Niemann S., Merker M., Dowdy D., et al. (2017) The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir. Med. 5, 291–360 10.1016/S2213-2600(17)30079-6 [DOI] [PubMed] [Google Scholar]
- 5. Ouellet H., Johnston J. B., and de Montellano P. R. (2011) Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol. 19, 530–539 10.1016/j.tim.2011.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pandey A. K., and Sassetti C. M. (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U.S.A. 105, 4376–4380 10.1073/pnas.0711159105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wipperman M. F., Sampson N. S., and Thomas S. T. (2014) Pathogen roid rage: cholesterol utilization by Mycobacterium tuberculosis. Crit. Rev. Biochem. Mol. Biol. 49, 269–293 10.3109/10409238.2014.895700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilbert S., Hood L., and Seah S. Y. K. (2018) Characterization of an aldolase involved in cholesterol side chain degradation in Mycobacterium tuberculosis. J. Bacteriol. 200, e00512–17 10.1128/JB.00512-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang J. C., Harik N. S., Liao R. P., and Sherman D. R. (2007) Identification of mycobacterial genes that alter growth and pathology in macrophages and in mice. J. Infect. Dis. 196, 788–795 10.1086/520089 [DOI] [PubMed] [Google Scholar]
- 10. Thomas S. T., VanderVen B. C., Sherman D. R., Russell D. G., and Sampson N. S. (2011) Pathway profiling in Mycobacterium tuberculosis: elucidation of cholesterol-derived catabolite and enzymes that catalyze its metabolism. J. Biol. Chem. 286, 43668–43678 10.1074/jbc.M111.313643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang M., Guja K. E., Thomas S. T., Garcia-Diaz M., and Sampson N. S. (2014) A distinct MaoC-like enoyl-CoA hydratase architecture mediates cholesterol catabolism in Mycobacterium tuberculosis. ACS Chem. Biol. 9, 2632–2645 10.1021/cb500232h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krishna S. S., Aravind L., Bakolitsa C., Caruthers J., Carlton D., Miller M. D., Abdubek P., Astakhova T., Axelrod H. L., Chiu H. J., Clayton T., Deller M. C., Duan L., Feuerhelm J., Grant J. C., et al. (2010) The structure of SSO2064, the first representative of Pfam family PF01796, reveals a novel two-domain zinc-ribbon OB-fold architecture with a potential acyl-CoA-binding role. Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 66, 1160–1166 10.1107/S1744309110002514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohn W. W., Wilbrink M. H., Casabon I., Stewart G. R., Liu J., van der Geize R., and Eltis L. D. (2012) Gene cluster encoding cholate catabolism in Rhodococcus spp. J. Bacteriol. 194, 6712–6719 10.1128/JB.01169-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haapalainen A. M., Meriläinen G., and Wierenga R. K. (2006) The thiolase superfamily: condensing enzymes with diverse reaction specificities. Trends Biochem. Sci. 31, 64–71 10.1016/j.tibs.2005.11.011 [DOI] [PubMed] [Google Scholar]
- 15. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 16. Vögeli B., Engilberge S., Girard E., Riobé F., Maury O., Erb T. J., Shima S., and Wagner T. (2018) Archaeal acetoacetyl-CoA thiolase/HMG-CoA synthase complex channels the intermediate via a fused CoA-binding site. Proc. Natl. Acad. Sci. U.S.A. 115, 3380–3385 10.1073/pnas.1718649115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pavkov-Keller T., Schmidt N. G., Żądło-Dobrowolska A., Kroutil W., and Gruber K. (2019) Structure and catalytic mechanism of a bacterial Friedel–Crafts acylase. ChemBioChem. 20, 88–95 10.1002/cbic.201800462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harijan R. K., Kiema T. R., Karjalainen M. P., Janardan N., Murthy M. R., Weiss M. S., Michels P. A., and Wierenga R. K. (2013) Crystal structures of SCP2-thiolases of Trypanosomatidae, human pathogens causing widespread tropical diseases: the importance for catalysis of the cysteine of the unique HDCF loop. Biochem. J. 455, 119–130 10.1042/BJ20130669 [DOI] [PubMed] [Google Scholar]
- 19. Schaefer C. M., Lu R., Nesbitt N. M., Schiebel J., Sampson N. S., and Kisker C. (2015) FadA5 a thiolase from Mycobacterium tuberculosis: a steroid-binding pocket reveals the potential for drug development against tuberculosis. Structure 23, 21–33 10.1016/j.str.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janardan N., Harijan R. K., Wierenga R. K., and Murthy M. R. (2012) Crystal structure of a monomeric thiolase-like protein type 1 (TLP1) from Mycobacterium smegmatis. PLoS ONE 7, e41894 10.1371/journal.pone.0041894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahnert S. E., Marsh J. A., Hernández H., Robinson C. V., and Teichmann S. A. (2015) Principles of assembly reveal a periodic table of protein complexes. Science 350, aaa2245 10.1126/science.aaa2245 [DOI] [PubMed] [Google Scholar]
- 22. Anbazhagan P., Harijan R. K., Kiema T. R., Janardan N., Murthy M. R., Michels P. A., Juffer A. H., and Wierenga R. K. (2014) Phylogenetic relationships and classification of thiolases and thiolase-like proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis. Tuberculosis 94, 405–412 10.1016/j.tube.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 23. Ewing T. A., Nguyen Q. T., Allan R. C., Gygli G., Romero E., Binda C., Fraaije M. W., Mattevi A., and van Berkel W. J. H. (2017) Two tyrosine residues, Tyr-108 and Tyr-503, are responsible for the deprotonation of phenolic substrates in vanillyl-alcohol oxidase. J. Biol. Chem. 292, 14668–14679 10.1074/jbc.M117.778449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorentzen E., Siebers B., Hensel R., and Pohl E. (2005) Mechanism of the Schiff base forming fructose-1,6-bisphosphate aldolase: structural analysis of reaction intermediates. Biochemistry 44, 4222–4229 10.1021/bi048192o [DOI] [PubMed] [Google Scholar]
- 25. Tittmann K. (2014) Sweet siblings with different faces: the mechanisms of FBP and F6P aldolase, transaldolase, transketolase and phosphoketolase revisited in light of recent structural data. Bioorg. Chem. 57, 263–280 10.1016/j.bioorg.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 26. Clapés P., Fessner W. D., Sprenger G. A., and Samland A. K. (2010) Recent progress in stereoselective synthesis with aldolases. Curr. Opin. Chem. Biol. 14, 154–167 10.1016/j.cbpa.2009.11.029 [DOI] [PubMed] [Google Scholar]
- 27. Zarzycki J., and Kerfeld C. A. (2013) The crystal structures of the tri-functional Chloroflexus aurantiacus and bi-functional Rhodobacter sphaeroides malyl-CoA lyases and comparison with CitE-like superfamily enzymes and malate synthases. BMC Struct. Biol. 13, 28 10.1186/1472-6807-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobylarz M. J., Grigg J. C., Sheldon J. R., Heinrichs D. E., and Murphy M. E. (2014) SbnG, a citrate synthase in Staphylococcus aureus. J. Biol. Chem. 289, 33797–33807 10.1074/jbc.M114.603175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harijan R. K., Mazet M., Kiema T. R., Bouyssou G., Alexson S. E., Bergmann U., Moreau P., Michels P. A., Bringaud F., and Wierenga R. K. (2016) The SCP2-thiolase-like protein (SLP) of Trypanosoma brucei is an enzyme involved in lipid metabolism. Proteins 84, 1075–1096 10.1002/prot.25054 [DOI] [PubMed] [Google Scholar]
- 30. Nakashima N., and Tamura T. (2004) Isolation and characterization of a rolling circle type plasmid from Rhodococcus erythropolis and application of the plasmid to multiple recombinant protein expression. Appl. Environ. Microbiol. 70, 5557–5568 10.1128/AEM.70.9.5557-5568.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu H., and Naismith J. H. (2008) An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 8, 91 10.1186/1472-6750-8-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruprecht A., Maddox J., Stirling A. J., Visaggio N., and Seah S. Y. (2015) Characterization of novel acyl coenzyme A dehydrogenases involved in bacterial steroid degradation. J. Bacteriol. 197, 1360–1367 10.1128/JB.02420-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baker P., Hillis C., Carere J., and Seah S. Y. (2012) Protein–protein interactions and substrate channeling in orthologous and chimeric aldolase-dehydrogenase complexes. Biochemistry 51, 1942–1952 10.1021/bi201832a [DOI] [PubMed] [Google Scholar]
- 34. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 35. Kabsch W. (2010) XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 38. DeLano W. (2002) The PyMOL Molecular Graphics System, DeLano Scientific LLC, San Carlos, CA [Google Scholar]
- 39. McGurn L. D., Moazami-Goudarzi M., White S. A., Suwal T., Brar B., Tang J. Q., Espie G. S., and Kimber M. S. (2016) The structure, kinetics and interactions of the carboxysomal carbonic anhydrase, CcaA. Biochem. J. 473, 4559–4572 10.1042/BCJ20160773 [DOI] [PubMed] [Google Scholar]
- 40. Gray J. J., Moughon S., Wang C., Schueler-Furman O., Kuhlman B., Rohl C. A., and Baker D. (2003) Protein–protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J. Mol. Biol. 331, 281–299 10.1016/S0022-2836(03)00670-3 [DOI] [PubMed] [Google Scholar]
- 41. Robert X., and Gouet P. (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320–W324 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., and Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]