Abstract
Sodium taurocholate cotransporting polypeptide (NTCP, encoded by Slc10a1/SLC10A1) deficiency can result in hypercholanemia but no obvious symptoms in both mice and humans. However, the consequence of and response to long-term hypercholanemia caused by NTCP deficiency remain largely unexplored. Here, we analyzed lifelong dynamics of serum total bile acid (TBA) levels in Slc10a1−/− mice, and we also assessed changes of TBA levels in 33 young individuals with SLC10A1 loss-of-function variant p.Ser267Phe. We found that overall serum TBA levels tended to decrease gradually with age in both Slc10a1−/− mice and p.Ser267Phe individuals. Liver mRNA profiling revealed notable transcription alterations in hypercholanemic Slc10a1−/− mice, including inhibition of bile acid (BA) synthesis, enhancement of BA detoxification, and altered BA transport. Members of the sulfotransferase (SULT) family showed the most dramatic increases in livers of hypercholanemic Slc10a1−/− mice, and one of their BA sulfates, taurolithocholic acid 3-sulfate, significantly increased. Importantly, consistent with the mouse studies, comprehensive profiling of 58 BA species in sera of p.Ser267Phe individuals revealed a markedly increased level of BA sulfates. Together, our findings indicate that the enhanced BA sulfation is a major mechanism for BA detoxification and elimination in both mice and humans with Slc10a1/SLC10A1 deficiency.
Keywords: bile acid, sulfotransferase, liver metabolism, mass spectrometry (MS), lipid metabolism, SLC10A1, sulfation, hypercholanemia, TLCA-3-sulfate, Sult2a1
Introduction
Sodium taurocholate cotransporting polypeptide (NTCP,4 SLC10A1) is localized at the basolateral membrane of hepatocytes and mediates uptake of conjugated bile acids (BAs) (1, 2). It was also identified as an entry receptor for hepatitis B virus and hepatitis D virus (3). NTCP/Ntcp deficiency has been reported in humans as well as Ntcp knockout mice (4–8). In humans, reported pediatric cases with NTCP mutation demonstrated more severe hypercholanemia compared with that in adults (4–7), whereas both normocholanemia and hypercholanemia were observed in Slc10a1−/− mice at 2 months of age (8). Slc10a1−/− mice with hypercholanemia presented with increased renal BA excretion, altered expressions of organic anion transporting polypeptide, and reduction of hepatic BA synthesis (8, 9). However, the long-term consequence of Slc10a1/SLC10A1 deficiency is unknown.
BAs are cytotoxic when their concentrations reach to abnormally high levels. High-concentration BA can damage cell membranes, increase reactive oxygen species, and induce both apoptosis and necrosis (1, 10). However, no severe abnormality of liver function has been observed in hypercholanemic subjects with NTCP mutations reported to date. Similarly, no inflammation or hepatocellular damage was detected in Slc10a1−/− mice with hypercholanemia (8). The observations suggest that in both humans and mice with SLC10A1/Slc10a1 deficiency, there are mechanism(s) for detoxification of overload BAs.
Here we combined biochemical assays with RNA profiling studies to investigate the long-term consequence of and the hepatocyte response to the high level BAs in Slc10a1−/− mice. We also utilized high-end ultrahigh-performance LC/multiple-reaction monitoring-MS (UPLC/MRM-MS) analysis to profile BAs in individuals whom we identified to harbor the p.Ser267Phe variant in NTCP.5 Our study reveal that enhanced BA sulfation is a major mechanism for BA detoxification and elimination in both mice and humans with Slc10a1/SLC10A1 deficiency.
Results
Body weight catch-up and serum TBA level reductions in Slc10a1−/− mice
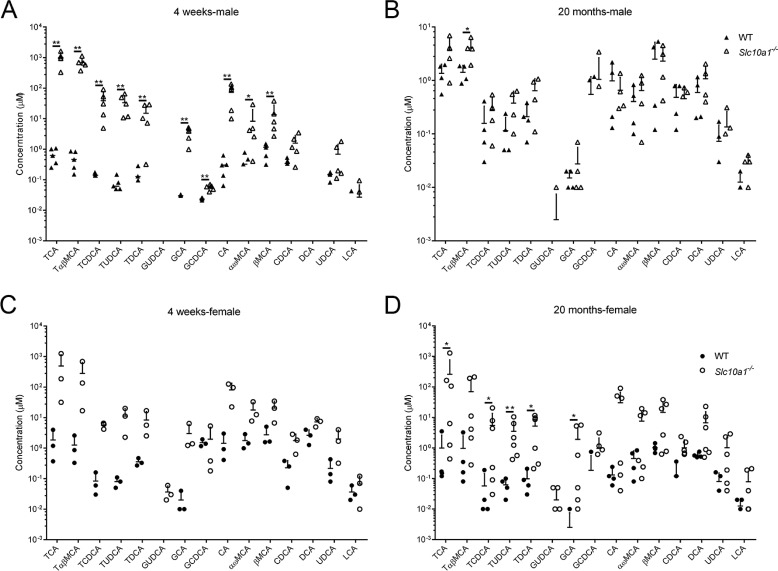
The establishment of Slc10a1−/− mice was described under “Experimental procedures” (Fig. S1). Slc10a1 deficiency resulted in dramatic elevation of serum TBA levels in both male and female mice at 8 weeks, and the body weights were inversely correlated with total serum BA levels (8). Here, we confirmed that most Slc10a1−/− mice had reduced body weights compared with that of WT littermates starting from post-weaning (day 21; 8 of 11 mice); however, surprisingly, we noted that the body weight of Slc10a1−/− mice could catch up with that of WT as age increased (Fig. 1, A and B). Interestingly, Slc10a1−/− mice exhibited various serum TBA levels, from normal (0–20 μm) to extremely high levels (∼2 mm) at early ages, and the overall serum TBA levels tended to decrease with age in Slc10a1−/− mice (Fig. 1C). Nonetheless, some Slc10a1−/− mice had TBA levels >100 μm in serum throughout their lifespans (2 of 15 males and 5 of 39 females); however, importantly, the survival of Slc10a1−/− mice was not affected under this hypercholanemia by 20 months of age, the end point of the present study (Fig. 1C). Profiling of BAs showed that most of the conjugated BAs significantly increased in both male and female mice at 4 weeks (Fig. 2, A and C). However, at 20 months, male Slc10a1−/− mice showed a BA profiling similar to that of WT mice, and some female Slc10a1−/− mice still had significant increases in some conjugated BA species, such as TCA, TUDCA, and TCDCA (Fig. 2, B and D).
Figure 1.
Body weight catch-up and serum TBA level reductions in Slc10a1−/− mice. A and B, individual body weight changes of male (A, No.1–7) and female Slc10a1−/− mice (B, No.1–4) during a 22-week and 24-week follow-up period, respectively. Body weight in the WT group is shown as mean ± S.D. (error bars) of a total of eight mice. C, serum TBA levels at 1–2 months and 10–20 months in male and female Slc10a1−/− mice, respectively. Data are given on a log-10 scale, and mean ± S.D. values are depicted in the figures (15–45 mice/group). Each symbol represents an individual mouse (two-way ANOVA followed by a Bonferroni post hoc test).
Figure 2.
Serum bile acid profiling of the Slc10a1−/− mice. Profiling of serum bile acids in male (A and B) and female (C and D) WT and Slc10a1−/− mice at 4 weeks and 20 months, respectively. BA species were quantified using UPLC-MS/MS. Data are given as mean ± S.D. (error bars) on a log-10 scale for 3–6 mice in each group. A mouse with BA species under the detection limit was not presented. *, p < 0.05; **, p < 0.01, two-tailed Mann–Whitney U test.
TBA levels in individuals with homozygous mutation of p.Ser267Phe in SLC10A1
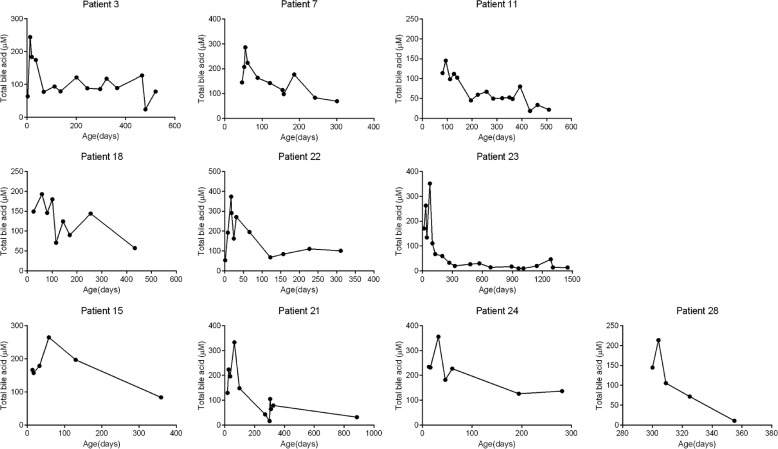
We recently identified 33 individuals with hypercholanemia who have homozygous mutation of p.Ser267Phe in SLC10A1.5 We retrospectively examined the historical data of serum TBA levels of those individuals who had a TBA test before 1 year old to see if their TBA level also declined over time like what we observed in Slc10a1−/− mice. Indeed, TBA levels collected before the age of 100 days were significantly higher than that of following days for those individuals during our follow-ups (Fig. S2A). Specifically, 6 of 23 males and 4 of 10 females showed a clear decline of serum TBA levels compared with their early examinations; nine infants exhibited with a fluctuation of TBA levels during the follow-up examinations (Fig. 3 and Fig. S2B).
Figure 3.
Dynamic analysis of blood bile acid levels in human subjects with p.Ser267Phe variants. Retrospective analysis of blood TBA levels in male (patient IDs: 3, 7, 11, 18, 22, and 23) and female (patient IDs: 15, 21, 24, and 28) p.Ser267Phe variants during their medical processes. Patient ID is indicated above each panel.
Liver mRNA profiling in Slc10a1−/− mice
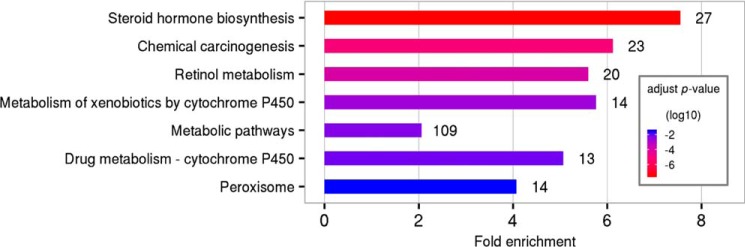
To decipher the biochemical basis for the declining of TBA levels in the late age of Slc10a1−/− mice, we performed a serial liver RNA-Seq analysis of mRNA expression with male mice at 4 weeks, 8 weeks, and 20 months of age, respectively (Fig. S3A). At 4 weeks, WT and Slc10a1−/− mice exhibited clearly different patterns in terms of transcriptome similarity and were well-separated (Fig. S3, A and B). However, the difference was indistinguishable at 20 months (Fig. S3A). KEGG pathway enrichment analysis showed that the most altered pathways included steroid hormone biosynthesis, chemical carcinogenesis, retinol metabolism, and metabolism of xenobiotics by cytochrome P450 (Fig. 4).
Figure 4.
KEGG pathway analysis of differentially expressed gene between male Slc10a1−/− and WT mice. Pathways are ranked according to p value. Numbers on right of each pathway indicate the number of differentially expressed genes. Mice were 4 weeks of age (n = 3).
Hepatic gene signature of hypercholanemia in Slc10a1−/− mice
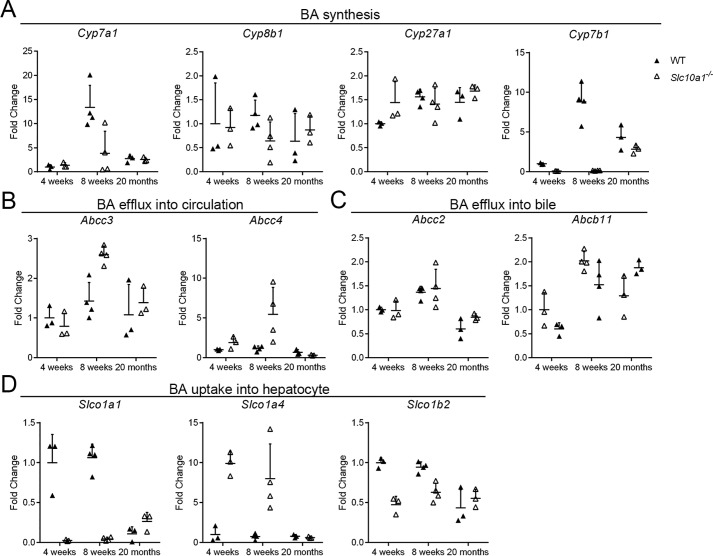
We further conducted quantification analysis of the RNA-Seq data, focusing on transcriptional alterations in pathways of BA synthesis, transport, and metabolism. For BA synthesis, Slc10a1−/− mice with hypercholanemia exhibited strong reductions relative to WT mice in genes involved in classical BA synthesis (e.g. Cyp7a1) and alternative BA synthesis (e.g. Cyp7b1) at 8 weeks (Fig. 5A); there was a slight decrease of the expression of Cyp8b1, which encodes sterol 12α-hydroxylase, an enzyme that directs BA precursors toward hydrophobic BAs at 8 weeks (Fig. 5A). As for BA transport, Slc10a1−/− mice with hypercholanemia exhibited significantly increased expression of genes encoding transporters that pump BAs into systemic circulation, such as Abcc3 and Abcc4, indicating the enhanced clearance of BAs at 8 weeks (Fig. 5B). Abcb11 and Abcc2, which encode transporters responsible for BA efflux into bile, showed no significant differences (Fig. 5C). On the other hand, consistent with a previous study (9), genes involved in BA uptake into hepatocytes, Slco1b2 decreased whereas Slco1a4 increased, reflecting the maintenance of BA circulation even at high levels of BAs with Slc10a1 deficiency at 8 weeks (Fig. 5D). Meanwhile, genes encoding phase I (Cyps) and phase II enzymes (Sults and Ugts) were induced in Slc10a1−/− mice with hypercholanemia at 4 weeks and 8 weeks (Fig. S4, A–C), consistent with a compensatory mechanism to decrease the overall BA load and to enhance the detoxification reactions in mice. In line with previous findings of BA hydroxylation as a pathway to detoxify overload BAs (11), the mRNA expression level of Cyp2b10 showed significant increase at 8 weeks (Fig. S5, A and B), and at the protein level, CYP2B10 significantly increased in half of Slc10a1−/− mice (5 of 10 males; Fig. S5D); whereas Sult2a8, the newly identified hepatic BA sulfotransferase with high activity toward 7α-hydroxylated BAs in mice was significantly decreased in the hypercholanemia mice with Slc10a1 deficiency at 4 and 8 weeks (12) (Fig. S5C).
Figure 5.
Transcriptional alterations in BA synthesis and transport pathways in male Slc10a1−/− mice. Total RNA (n = 3–4) for each time point was prepared, and -fold changes of gene expression with respect to WT at 4 weeks were analyzed using RNA-Seq data. Genes involved in classical and acidic synthesis of BA (Cyp7a1, Cyp8b1, Cyp27a1, and Cyp7b1) (A), BA efflux into circulation (Abcc3 and Abcc4) (B), BA efflux into bile (Abcc2 and Abcb11) (C), and BA uptake into hepatocyte (Slco1a1, Slco1a4, and Slco1b2) (D) were analyzed. Data are given as mean ± S.D. (error bars) of -fold changes to WT at each time point. Data at 8 weeks are a representative result from three independent repeats of RNA-Seq analysis.
Regardless of all of these transcription alterations, strikingly, the top five ranked up-regulated genes in male Slc10a1−/− mice all belonged to the sulfotransferase (Sult) gene family, including Sult2a1, Sult2a2, Sult1e1, Sult2a6, and Sult2a4 (Table 1). Among them, the most up-regulated gene was Sult2a1, and its expression was increased more than 1000-fold in Slc10a1−/− mice (Table 1). Nuclear receptors have been implicated in orchestrating the various steps involved in BA detoxification, conjugation, and elimination (13, 14). We found that expressions of farnesoid X receptor (Fxr), constitutive androstane receptor (Car), retinoid X receptor (Rxra), and pregnane X receptor (Pxr) were increased to different degrees, indicating their potential involvement in regulating of phase I and II detoxification in NTCP-deficient mice (Fig. S6).
Table 1.
Top five up-regulated genes in Slc10a1−/− mice
Mice were male and 4 weeks of age; TBA > 20 μm. n: read counts normalized by library size.
| Gene name | WT mean (n) | Slc10a1−/− mean (n) | Fold change (log2) | Adjusted p |
|---|---|---|---|---|
| Sult2a1 | 5 | 13,069 | 10.9 | 6.7E−08 |
| Sult2a2 | 1 | 2560 | 10.1 | 9.0E−08 |
| Sult1e1 | 26 | 14,259 | 9.0 | 1.9E−10 |
| Sult2a6 | 0 | 432 | 8.4 | 4.1E−06 |
| Sult2a4 | 0 | 149 | 7.2 | 1.6E−03 |
Increased taurine-conjugated lithocholic acid-3-sulfate in Slc10a1−/− mice
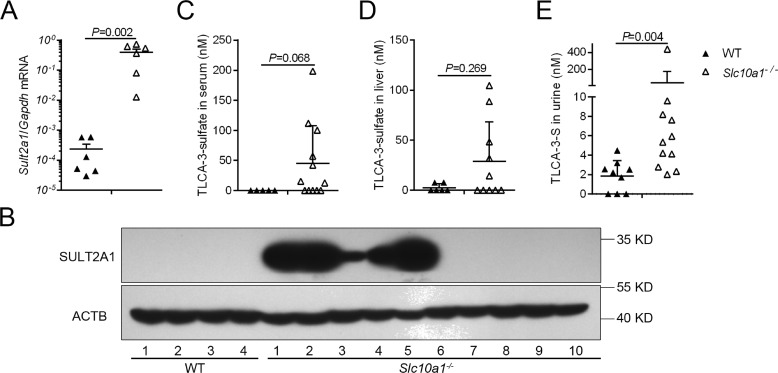
We verified the RNA-Seq results for Sult2a1 expression using quantitative PCR (qPCR) analysis and Western blotting. Again, qPCR confirmed a more than 1000-fold increase of the Sult2a1 gene in male Slc10a1−/− mice, and at the protein level, Slc10a1−/− mice with no CYP2B10 expression showed dramatically increased SULT2A1 (Fig. 6, A and B). Although Sult2a1 is one of the most markedly female-predominant Sult in mouse liver, the expression of Sult2a1 also further increased in female Slc10a1−/− mice compared with that of WT mice (Fig. S7A). SULT2A1 is responsible for the sulfation of BAs, a biochemical process known to reduce BA toxicity and promote the elimination of BAs in humans (13, 15). SULT2A1 plays a significant and unique role in 3-hydroxyl group sulfation, particularly in sulfation of LCA and TLCA (16). To verify whether BA sulfation activity was activated under hypercholanemia in Slc10a1−/− mice, we measured serum LCA-3-sulfate and TLCA-3-sulfate levels in Slc10a1−/− and WT mice with UPLC/MS/MS analysis. TLCA-3-sulfate levels were increased in male Slc10a1−/− mice (p = 0.068; Fig. 6C). We also observed slightly increased TLCA-3-sulfate levels in the sera of female Slc10a1−/− mice compared with that of WT mice (p = 0.321; Fig. S7B). LCA-3-sulfate was not detected in either WT or Slc10a1−/− mice. To exclude the possibility that the observed increased TLCA-3-sulfate levels in the serum could have been caused by altered hepatic uptake of this sulfate, we also examined the TLCA-3-sulfate levels in the liver. Compared with WT mice, the liver TLCA-3-sulfate levels increased in both male and female Slc10a1−/− mice, which further confirmed the increased BA sulfation under conditions of Slc10a1 deficiency (Fig. 6D and Fig. S7C). BA sulfates are more water-soluble and therefore are more readily excreted in urine (13). Indeed, the urinary excretion of TLCA-3-sulfate was significantly increased in Slc10a1−/− mice (p = 0.004; Fig. 6E).
Figure 6.
Increased TLCA-3-sulfate in male Slc10a1−/− mice. Hepatic SULT2A1 expression was determined by qPCR (A) and Western blotting (B), respectively. Relative mRNA expression levels were calculated for WT mice and Slc10a1−/− mice with high TBA concentrations using the geometric mean of housekeeping gene Gapdh. UPLC-MS/MS analysis of TLCA-3-sulfate concentrations in sera (C), liver (D), and urine (E) of Slc10a1−/− mice. All male mice are 4 weeks of age. Each symbol represents an individual mouse. Two-tailed Mann–Whitney U test was used. Values are given as mean ± S.D. (error bars) for 5–12 mice/group. In B, mice 6–10 expressed CYP2B10 protein (see Fig. S5D).
Elevated BA sulfates in human plasma and urine of homozygous p.Ser267Phe mutation in SLC10A1
Based on the findings in mice, we asked whether the BA sulfates also increased in human individuals with p.Ser267Phe mutation in SLC10A1. Comprehensive plasma BA profiling of 58 BA species in these individuals was conducted by UPLC/MRM-MS. The concentrations of main primary BAs, including CA, CDCA, and their taurine or glycine conjugates, significantly increased compared with those of healthy controls (Table 2), suggesting hypercholanemia in those p.Ser267Phe variants. For the secondary BAs, DCA and LCA, and their taurine or glycine conjugates, no significant differences were observed (Table 2). We thus could confirm elevated BA sulfates in plasma of homozygous p.Ser267Phe individuals. In particular, the most predominant BA sulfates in the plasma of healthy controls GUDCA-3-sulfate and GLCA-3-sulfate increased from 131.02 ± 132.24 and 130.94 ± 261.36 nm to 1679.92 ± 3229.69 and 400.84 ± 1479.65 nm in p.Ser267Phe variants, respectively (Table 3). Interestingly, some representative atypical BAs detected in these variants were also changed significantly, such as 7-keto-DCA, 7-keto-LCA, Glycine-conjugated hyodeoxycholic acid (GHDCA), TUDCA, nor-UDCA, TβMCA, TλMCA, GλMCA, etc. (Table S2). The p.Ser267Phe individuals also showed a modestly increased ratio of BA sulfates/TBA in urine (Fig. S8), indicating the enhanced urinary clearance of BA sulfates in those individuals with the p.Ser267Phe mutation.
Table 2.
Plasma concentrations of main primary and secondary bile acid species in healthy controls and p.Ser267Phe variants
Concentration values are mean ± S.D.
| Healthy controls (n = 43) | p.Ser267Phe (n = 15) | p value, healthy controls/p.Ser267Phe | |
|---|---|---|---|
| nm | nm | ||
| CA | 86.9 ± 135.2 | 819.2 ± 1088.1 | 0.000 |
| TCA | 263.8 ± 614.5 | 17,434.1 ± 15,473.8 | 0.000 |
| GCA | 1176.7 ± 3165.5 | 57,694.9 ± 48,102.9 | 0.000 |
| CDCA | 301.2 ± 414.8 | 615.2 ± 426.1 | 0.000 |
| TCDCA | 687.3 ± 1023.8 | 7606.3 ± 8174.4 | 0.000 |
| GCDCA | 3581.3 ± 3957.4 | 67,109.77 ± 44,185.9 | 0.000 |
| DCA | 274.3 ± 491.7 | 210.4 ± 238.8 | 0.576 |
| TDCA | 43.2 ± 72.1 | 81.2 ± 193.2 | 0.776 |
| GDCA | 248.1 ± 435.8 | 1556.5 ± 4825.7 | 0.852 |
| LCA | 3.98 ± 4.07 | 3.64 ± 6.47 | 0.093 |
| TLCA | 1.21 ± 1.99 | 0.43 ± 0.58 | 0.192 |
| GLCA | 14.42 ± 27.68 | 31.94 ± 98.7 | 0.299 |
Table 3.
Plasma concentrations of some sulfated bile acids species in healthy controls and p.Ser267Phe variants
Concentration values are mean ± S.D.
| Healthy controls (n = 43) | p.Ser267Phe (n = 15) | p value, healthy controls/p.Ser267Phe | |
|---|---|---|---|
| nm | nm | ||
| CA-3–sulfated | 1.4 ± 1.89 | 3.3 ± 2.2 | 0.000 |
| GCA-3–sulfated | 1.82 ± 2.49 | 9.77 ± 10.31 | 0.000 |
| DCA-3–sulfated | 1.49 ± 2.54 | 0.95 ± 2.62 | 0.023 |
| GDCA-3–sulfated | 77.99 ± 117.99 | 166.04 ± 574.13 | 0.086 |
| TDCA-3–sulfated | 12.86 ± 28.58 | 0.1 ± 0.11 | 0.233 |
| TCDCA-3–sulfated | 98.19 ± 205.54 | 0.8 ± 1.08 | 0.136 |
| TLCA-3–sulfated | 0.68 ± 1.28 | 0.06 ± 0.07 | 0.025 |
| GLCA-3–sulfated | 130.94 ± 261.36 | 400.84 ± 1479.65 | 0.011 |
| GUDCA-3–sulfated | 131.02 ± 132.24 | 1679.92 ± 3229.69 | 0.001 |
| TUDCA-3–sulfated | 16.2 ± 27.76 | 0.64 ± 0.74 | 0.435 |
Discussion
We found in this study apparent renormalization of TBA levels over time in mice and humans with Slc10a1/SLC10A1 deficiency, and we report that the renormalization of TBA level is accompanied with increased BA sulfation, suggesting that BA sulfation may underlie the detoxification and elimination of overload BAs both in mice and humans with Slc10a1/SLC10A1 deficiency.
Both in mice and in humans, Slc10a1/SLC10A1 deficiency–related hypercholanemia tends to attenuate as age increases. Indeed, we show that the overall serum TBA levels decrease with age in Slc10a1−/− mice, especially in the males, whereas in female Slc10a1−/− mice, ∼50% persist with moderate hypercholanemia. Nonetheless, the overall survival rate of those hypercholanemic Slc10a1−/− mice was not affected (data not shown). The body weights of Slc10a1−/− mice were inversely correlated with serum TBA levels, consistent with a previous report (8). Interestingly, we noticed a significant catch-up of body weight gain of Slc10a1−/− mice starting from 8 weeks of age, in parallel with TBA declining in these mice. In humans, SLC10A1 with p.Arg252His variation was the first reported case with NTCP deficiency. The affected individual presented with a relatively mild clinical phenotype at about 2 years old (TBA up to 1500 μm), and her TBA declined (∼500 μm) when she was 8 years old (4, 17). However, in adulthood, humans with NTCP deficiency had a relative mild to normal TBA level (6, 7). In our study, 10 of 33 children with NTCP deficiency exhibited clearly declined TBA levels during the follow-up period; four children still had a TBA level above 100 μm when they were at least 7 years old (data not shown). Long-term follow-up of TBA levels of these individuals is warranted.
A previous study showed that hypercholanemic Slc10a1−/− mice displayed changes in expression of hepatic, ileal, and renal BA transporters and enhanced renal BA excretion to attenuate the circulatory BA overload (8). Expression of hepatic Cyp7a1 was found to be suppressed via induction of ileal Fgf15 (fibroblast growth factor 15) in the hypercholanemic Slc10a1−/− mice (9). In this study, we conducted comprehensive mRNA deep sequencing analysis of Slc10a1−/− mice, which provides unbiased information on the metabolism alteration in NTCP-disrupted animals. Remarkably, our results revealed, for the first time, changes of pathways involved in the metabolism of xenobiotics. In particular, the dramatically increased expressions of Sult genes implicated enhanced phase II (sulfation) detoxification of BAs in Slc10a1−/− mice. Importantly, BA sulfation could be confirmed by UPLC-MS/MS analysis with the presence of TLCA-3-sulfate both in circulation and liver of Slc10a1−/− mice. CYP2B10, a phase I hydroxylation enzyme has been indicated to be associated with hydroxylated BAs (18–21). Expression of CYP2B10 was also changed significantly in a subtype of Slc10a1−/− mice, suggesting the increased BA hydroxylation in Slc10a1−/− mice. The enhanced ability of phase I and II detoxification in conditions of NTCP deficiency could be an adaptive response to the overload BAs in the liver (11, 20, 22). Nuclear receptors, such as Fxr, Car, Rxr, and Pxr increased and could be responsible for the enhanced BA detoxification (i.e. SULT2A1 and CYP2B10) in male Slc10a1−/− mice at 4 weeks. However, the molecular mechanism of SULT2A1 and CYP2B10 regulation may be different in Slc10a1−/− mice, as those Slc10a1−/− mice with high SULT2A1 expression demonstrated a relatively low CYP2B10 expression.
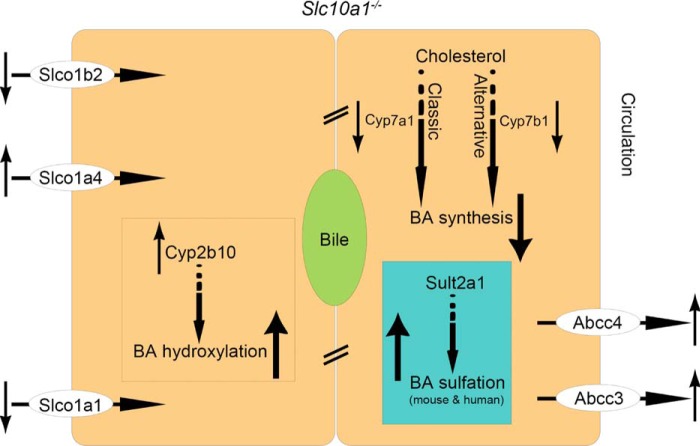
Notably, the increased BA sulfates were also presented in blood and urine of human individuals with the p.Ser267Phe mutation in SLC10A1. Therefore, sulfation probably serves as a critical mechanism to decrease BAs' toxic effect and clear the overload BAs both in mice and humans with Slc10a1/SLC10A1 deficiency. Importantly, from the analysis of BA metabolites by UPLC/MRM-MS, we noted that, whereas the concentrations of sulfates of DCA, LCA, and their conjugates were increased in individuals with the p.Ser267Phe variant, the concentrations of DCA, LCA, and their corresponding conjugates were not changed significantly, suggesting that the overall enterohepatic circulation of BAs in those p.Ser267Phe variants was intact. Our study highlighted enhanced BA sulfation in Slc10a1/SLC10A1 deficiency; in such a condition, the BA levels are controlled by coordinated adaptive changes of a phase I bile acid-hydroxylating enzyme, Cyp2b10, and a phase II bile acid-sulfating enzyme, Sult2a1, in BA metabolism and transportation with transporters of Abcc3 and Abcc4 (Fig. 7).
Figure 7.
Bile acid sulfation contributes to detoxification of BAs in mice and human with Ntcp/NTCP deficiency. In the status of hypercholanemia with Ntcp (Slc10a1) deficiency, BA synthesis from both the classic (Cyp7a1) and alternative (Cyp7b1) pathways is decreased. BA sulfation (Sult2a1) highlighted in blue box, along with BA hydroxylation (Cyp2b10) both are increased to augment the detoxification of overload BAs. Transporters (Abcc3 and Abcc4) effluxing BAs into circulation are up-regulated to facilitate export.
Experimental procedures
Mice studies
Two transcription activator-like effector nuclease (TALEN) constructs targeting 5′-TACGGTATCATGCCCCTCAG-3′ and 5′-TGGTCAGATGAAAGACCTTG-3′ in exon 1 of Slc10a1 were assembled as described previously (23). C57BL/6 zygotes injected with the TALEN plasmid were transplanted to the ovarian ducts of pseudopregnancy C57BL/6 mice to generate Slc10a1−/− mice. A mouse with a 19-bp deletion (5′-TTCTGGGCAAGGTCTTTCA-3′) in exon 1 of Slc10a1 was chosen as the founder, and all mice used in this study were offspring of the founder mouse. Mice were housed in specific pathogen-free conditions in the animal facility of the National Institute of Biological Sciences (NIBS), Beijing. The current study was approved by the NIBS Institutional Animal Care and Use Committee. Quantitative analysis of serum TBA was conducted commercially by the Beijing Lawke Health Laboratory Center for Clinical Laboratory Development, Inc. The uptake assay of [3H]taurocholate was described previously (24).
RNA profiling and bioinformatics analysis
Liver mRNA extraction for RNA-Seq and qPCR analysis was described previously (25, 26). Clean mRNA-Seq reads were aligned to the mouse reference genome (GRCm38) with TopHat (version 2.0.13), guided by Ensembl gene annotation v83. Only uniquely aligned and properly paired reads were retained for further analysis. Gene-level read counts were calculated with HTseq (v0.6.1p1) with the union mode. Genes with less than a total of 11 nonnormalized reads across all samples were excluded. Data normalization and statistical analysis of differential expression were performed with the DEseq2 (v1.10.0) R package. Pair-wise Spearman correlation between samples across all quantified genes was used for hierarchical clustering. KEGG pathway enrichment analysis of differentially expressed genes was performed with KOBAS 2.0. Real-time qPCR was performed on CFX96 TouchTM Real-Time PCR Detection System. Actb and Gapdh were used for internal normalization. Primer sequences used in this study are listed in Table S1.
Biochemical and UPLC/MS/MS analysis
BAs, including αMCA, βMCA, ωMCA, TαMCA, and TβMCA, were purchased from Steraloids. CA, CDCA, DCA, LCA, UDCA, TCA, TCDCA, TDCA, TLCA, TLCA-3-sulfate, TUDCA, GCA, GCDCA, and GUDCA were purchased from Sigma-Aldrich. BAs in serum samples were extracted with the L3 protocol reported by Humbert et al. (27). BA extraction from liver samples was conducted using the method described by Alnouti et al. (28). For the profiling analysis, the UPLC system was coupled to a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a heated electrospray ionization (HESI) probe. BA extracts were separated by a BEH C18 100 × 2.1-mm column (Waters). A binary solvent system with mobile phase A containing 100% H2O, 7.5 mm ammonium acetate (pH 4) and mobile phase B containing MeOH/ACN (95:5) was used over a 25-min gradient with a flow rate of 300 μl/min. Because we were not able to separate the analytical signals for αMCA from ωMCA, their concentrations were calculated together (termed αωMCA); TαMCA and TβMCA are termed TαβMCA. Mouse BA profiling with UPLC-MS/MS was conducted by the National Protein Science Technology Center, Tsinghua University (Beijing, China). MS analysis of TLCA-3-sulfate in urine was conducted by Metabo-Profile (Shanghai, China).
Immunoblotting
Equal amounts of protein from mouse liver were separated by SDS-PAGE and blotted onto polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies overnight at 4 °C. Appropriate peroxidase-conjugated secondary antibodies were applied, and the membranes were visualized by Super Signal Chemiluminescence (Thermo Scientific). The anti-CYP2B10 mAb (sc-53242) and anti-SULT2A1/2/5 mAb (sc-398965) were purchased from Santa Cruz Biotechnology, Inc. Anti-tubulin (BE3312) and anti-ACTB (BE0033) were purchased from EASYBIO.
Human study
This study was approved by Children's Hospital and Jinshan Hospital of Fudan University (Shanghai, China). Each participant provided written informed consent prior to participation in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Plasma was separated from blood by centrifugation, and urine was collected, aliquoted (50 μl), freeze-dried, and stored at −80 °C until BA profiling. BA analysis was performed by reversed-phase UPLC/MRM-MS with negative ion detection at the UVic-Genome British Columbia Proteomics Centre as described previously (29). The quantitation of BAs utilized standard substances of 58 BAs and 14 deuterium-labeled analogues of BAs as internal standards. These targets, described previously (29, 30), included all of the major BAs.
Statistical analysis
All data are expressed as mean ± S.D. For analysis in mice experiments, inferential statistical significance between two groups was examined using two-tailed Mann–Whitney U tests. Two-way ANOVA followed by a Bonferroni post hoc test was used to determine the effect of age or gender interactions. Statistical analyses were conducted using GraphPad Prism version 6.0 (GraphPad Software). For analysis in humans, statistical analysis using the software package IBM SPSS 19.0 was performed to determine differences in the BA profiles between p.Ser267Phe variants and healthy controls. Student's t test was performed when the data showed a normal distribution. A nonparametric test, the Mann–Whitney U test, was performed when the data did not show a normal distribution. One-way ANOVA followed by a Dunnett's post-hoc test was used to examine the change of TBA levels in infants. p values < 0.05 were considered statistically significant.
Author contributions
F. M., X. H., J.-S. W., and W. L. conceptualization; F. M., T. L., and X. H. data curation; F. M., T. L., X. H., H. Z., and W. L. formal analysis; F. M., X. H., J.-S. W., and W. L. validation; F. M., T. L., X. H., and F. W. investigation; F. M., X. H., and Z. J. visualization; F. M., T. L., X. H., W. H., C. L., X. L., J. H., and C. H. B. methodology; F. M. and X. H. writing-original draft; F. M., T. L., J.-S. W., and W. L. writing-review and editing; H. Z. and Z. J. software; J. S., J. H., and C. H. B. resources; J. S., J. H., C. H. B., J.-S. W., and W. L. funding acquisition; J.-S. W. and W. L. supervision; W. L. project administration.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grants 81525018 (to W. L.) and 81570468 and 81741056 (to J.-S. W.); Science and Technology Major Project of Beijing Grant D171100003117003 (to W. L.); China Scholarship Council Grant 201606100226 (to T. L.); the Science and Technology Bureau of Beijing Municipal Government (to W. L. and J. S.); the Metabolomics Innovation Centre (TMIC) through the Genome Innovations Network (GIN) from Genome Canada, Genome BC, and Genome Alberta for operations (205MET and 7203) and technology development (215MET and MC3T) (to J. H. and C. H. B.); the Leading Edge Endowment Fund (University of Victoria) and the Segal McGill Chair in Molecular Oncology at McGill University (Montreal, Canada) (to C. H. B.); and the Warren Y. Soper Charitable Trust and the Alvin Segal Family Foundation to the Jewish General Hospital (Montreal, Canada) (to C. H. B.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2 and Figs. S1–S8.
Y. Yan, unpublished data.
- NTCP
- sodium taurocholate cotransporting polypeptide
- TBA
- total bile acids
- αMCA
- α-muricholic acid
- βMCA
- β-muricholic acid
- ωMCA
- ω-muricholic acid
- TαMCA
- tauro-α-muricholic acid
- TβMCA
- tauro-β muricholic acid
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- DCA
- deoxycholic acid
- LCA
- lithocholic acid
- UDCA
- ursodeoxycholic acid
- TCA
- taurocholic acid
- TCDCA
- taurochenodeoxycholic acid
- TDCA
- taurodeoxycholic acid
- TLCA
- taurolithocholic acid
- TUDCA
- tauroursodeoxycholic acid
- GCA
- glycocholic acid
- GCDCA
- glycochenodeoxycholic acid
- GUDCA
- glycoursodeoxycholic acid
- TLCA-3-sulfate
- taurolithocholic acid 3-sulfate
- UPLC/MRM-MS
- ultra-high performance liquid chromatography/multiple-reaction monitoring–mass spectrometry
- UPLC-MS/MS
- ultra-performance liquid chromatography-tandem mass spectrometer
- TALEN
- transcription activator-like effector nuclease.
References
- 1. Meier P. J., and Stieger B. (2002) Bile salt transporters. Annu. Rev. Physiol. 64, 635–661 10.1146/annurev.physiol.64.082201.100300 [DOI] [PubMed] [Google Scholar]
- 2. Dawson P. A., and Karpen S. J. (2015) Intestinal transport and metabolism of bile acids. J. Lipid Res. 56, 1085–1099 10.1194/jlr.R054114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan H., Zhong G., Xu G., He W., Jing Z., Gao Z., Huang Y., Qi Y., Peng B., Wang H., Fu L., Song M., Chen P., Gao W., Ren B., et al. (2012) Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1, e00049 10.7554/eLife.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vaz F. M., Paulusma C. C., Huidekoper H., de Ru M., Lim C., Koster J., Ho-Mok K., Bootsma A. H., Groen A. K., Schaap F. G., Oude Elferink R. P. J., Waterham H. R., and Wanders R. J. A. (2015) Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology 61, 260–267 10.1002/hep.27240 [DOI] [PubMed] [Google Scholar]
- 5. Van Herpe F., Waterham H. R., Adams C. J., Mannens M., Bikker H., Vaz F. M., and Cassiman D. (2017) NTCP deficiency and persistently raised bile salts: an adult case. J. Inherit. Metab. Dis. 40, 313–315 10.1007/s10545-017-0031-9 [DOI] [PubMed] [Google Scholar]
- 6. Deng M., Mao M., Guo L., Chen F. P., Wen W. R., and Song Y. Z. (2016) Clinical and molecular study of a pediatric patient with sodium taurocholate cotransporting polypeptide deficiency. Exp. Ther. Med. 12, 3294–3300 10.3892/etm.2016.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu R., Chen C., Xia X., Liao Q., Wang Q., Newcombe P. J., Xu S., Chen M., Ding Y., Li X., Liao Z., Li F., Du M., Huang H., Dong R., et al. (2017) Homozygous p.Ser267Phe in SLC10A1 is associated with a new type of hypercholanemia and implications for personalized medicine. Sci. Rep. 7, 9214 10.1038/s41598-017-07012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slijepcevic D., Kaufman C., Wichers C. G. K., Gilglioni E. H., Lempp F. A., Duijst S., de Waart D. R., Elferink R. P., Mier W., Stieger B., Beuers U., Urban S., and van de Graaf S. F. J. (2015) Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+)-taurocholate cotransporting polypeptide knockout mice. Hepatology 62, 207–219 10.1002/hep.27694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Slijepcevic D., Roscam Abbing R. L. P., Katafuchi T., Blank A., Donkers J. M., van Hoppe S., de Waart D. R., Tolenaars D., van der Meer J. H. M., Wildenberg M., Beuers U., Oude Elferink R. P. J., Schinkel A. H., and van de Graaf S. F. J. (2017) Hepatic uptake of conjugated bile acids is mediated by both sodium taurocholate cotransporting polypeptide and organic anion transporting polypeptides and modulated by intestinal sensing of plasma bile acid levels in mice. Hepatology 66, 1631–1643 10.1002/hep.29251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perez M. J., and Briz O. (2009) Bile-acid-induced cell injury and protection. World J. Gastroenterol. 15, 1677–1689 10.3748/wjg.15.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marschall H. U., Wagner M., Bodin K., Zollner G., Fickert P., Gumhold J., Silbert D., Fuchsbichler A., Sjövall J., and Trauner M. (2006) Fxr(-/-) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J. Lipid Res. 47, 582–592 10.1194/jlr.M500427-JLR200 [DOI] [PubMed] [Google Scholar]
- 12. Feng L., Yuen Y. L., Xu J., Liu X., Chan M. Y., Wang K., Fong W. P., Cheung W. T., and Lee S. S. (2017) Identification and characterization of a novel PPARα-regulated and 7α-hydroxyl bile acid-preferring cytosolic sulfotransferase mL-STL (Sult2a8). J. Lipid Res. 58, 1114–1131 10.1194/jlr.M074302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alnouti Y. (2009) Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol. Sci. 108, 225–246 10.1093/toxsci/kfn268 [DOI] [PubMed] [Google Scholar]
- 14. Runge-Morris M., Kocarek T. A., and Falany C. N. (2013) Regulation of the cytosolic sulfotransferases by nuclear receptors. Drug Metab. Rev. 45, 15–33 10.3109/03602532.2012.748794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmer R. H. (1967) The formation of bile acid sulfates: a new pathway of bile acid metabolism in humans. Proc. Natl. Acad. Sci. U.S.A. 58, 1047–1050 10.1073/pnas.58.3.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radominska A., Comer K. A., Zimniak P., Falany J., Iscan M., and Falany C. N. (1990) Human liver steroid sulphotransferase sulphates bile acids. Biochem. J. 272, 597–604 10.1042/bj2720597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vaz F. M., Huidekoper H. H., and Paulusma C. C. (2017) Extended abstract: deficiency of sodium taurocholate cotransporting polypeptide (SLC10A1): a new inborn error of metabolism with an attenuated phenotype. Dig. Dis. 35, 259–260 10.1159/000450984 [DOI] [PubMed] [Google Scholar]
- 18. Wagner M., Halilbasic E., Marschall H.-U., Zollner G., Fickert P., Langner C., Zatloukal K., Denk H., and Trauner M. (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42, 420–430 10.1002/hep.20784 [DOI] [PubMed] [Google Scholar]
- 19. Zollner G., Wagner M., Moustafa T., Fickert P., Silbert D., Gumhold J., Fuchsbichler A., Halilbasic E., Denk H., Marschall H.-U., and Trauner M. (2006) Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-α/β in the adaptive response to bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G923–G932 10.1152/ajpgi.00490.2005 [DOI] [PubMed] [Google Scholar]
- 20. Fuchs C. D., Paumgartner G., Wahlström A., Schwabl P., Reiberger T., Leditznig N., Stojakovic T., Rohr-Udilova N., Chiba P., Marschall H.-U., and Trauner M. (2017) Metabolic preconditioning protects BSEP/ABCB11−/− mice against cholestatic liver injury. J. Hepatol. 66, 95–101 10.1016/j.jhep.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 21. Kulkarni S. R., S. C. J. Hagey L. R., Boyer J. L. (2016) Sirtuin 1 activation alleviates cholestatic liver injury in a cholic acid–fed mouse model of cholestasis. Hepatology 64, 2151–2164 10.1002/hep.28826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fickert P., Wagner M., Marschall H. U., Fuchsbichler A., Zollner G., Tsybrovskyy O., Zatloukal K., Liu J., Waalkes M. P., Cover C., Denk H., Hofmann A. F., Jaeschke H., and Trauner M. (2006) 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 130, 465–481 10.1053/j.gastro.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 23. Sanjana N. E., Cong L., Zhou Y., Cunniff M. M., Feng G., and Zhang F. (2012) A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192 10.1038/nprot.2011.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan H., Peng B., Liu Y., Xu G., He W., Ren B., Jing Z., Sui J., and Li W. (2014) Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J. Virol. 88, 3273–3284 10.1128/JVI.03478-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He W., Ren B., Mao F., Jing Z., Li Y., Liu Y., Peng B., Yan H., Qi Y., Sun Y., Guo J.-T., Sui J., Wang F., and Li W. (2015) Hepatitis D virus infection of mice expressing human sodium taurocholate co-transporting polypeptide. PLoS Pathog. 11, e1004840 10.1371/journal.ppat.1004840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He W., Cao Z., Mao F., Ren B., Li Y., Li D., Li H., Peng B., Yan H., Qi Y., Sun Y., Wang F., Sui J., and Li W. (2016) Modification of three amino acids in sodium taurocholate cotransporting polypeptide renders mice susceptible to infection with hepatitis D virus in vivo. J. Virol. 90, 8866–8874 10.1128/JVI.00901-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Humbert L., Maubert M. A., Wolf C., Duboc H., Mahé M., Farabos D., Seksik P., Mallet J. M., Trugnan G., Masliah J., and Rainteau D. (2012) Bile acid profiling in human biological samples: comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 899, 135–145 10.1016/j.jchromb.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 28. Alnouti Y., Csanaky I. L., and Klaassen C. D. (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 873, 209–217 10.1016/j.jchromb.2008.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han J., Liu Y., Wang R., Yang J., Ling V., and Borchers C. H. (2015) Metabolic profiling of bile acids in human and mouse blood by LC-MS/MS in combination with phospholipid-depletion solid-phase extraction. Anal. Chem. 87, 1127–1136 10.1021/ac503816u [DOI] [PubMed] [Google Scholar]
- 30. Qiu Y. L., Gong J. Y., Feng J. Y., Wang R. X., Han J., Liu T., Lu Y., Li L. T., Zhang M. H., Sheps J. A., Wang N. L., Yan Y. Y., Li J. Q., Chen L., Borchers C. H., et al. (2017) Defects in myosin VB are associated with a spectrum of previously undiagnosed low γ-glutamyltransferase cholestasis. Hepatology 65, 1655–1669 10.1002/hep.29020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.