Abstract
A sperm that fertilizes an egg has successfully survived multiple checkpoints within the female reproductive tract, termed pre-fertilization events. The leukocytic response is a pre-fertilization event in which sperm trigger an immune response that promotes homing of circulating leukocytes to the uterine lumen to destroy most sperm. Various glycoconjugates decorate the sperm surface, including sialic acids, which are abundant at the sperm surface where they cap most glycan chains and regulate sperm migration through cervical mucus, formation of the sperm oviductal reservoir, and sperm capacitation. However, the role of sperm-associated sialic acids in the leukocytic reaction remains unknown. The cognate endogenous binding partners of sialic acids, sialic acid-binding immunoglobulin-like lectins (Siglecs) play a pivotal role in regulating many immune responses. Here we investigated whether sperm-associated sialic acids inhibit activation of neutrophils, one of the major immune cells involved in the leukocytic reaction. We used in vitro interactions between sperm and neutrophils as well as binding assays between sperm and recombinant Siglec-Fc chimeric proteins to measure interactions. Moreover, we examined whether Siglecs are expressed on human and mouse endometria, which have a role in initiating the leukocytic reaction. Surprisingly less sialylated, capacitated, sperm did not increase neutrophil activation in vitro. However, we observed expression of several Siglecs on the endometrium and that these receptors interact with sialylated sperm. Our results indicate that sperm sialic acids may interact with endometrial Siglecs and that these interactions facilitate sperm survival in the face of female immunity.
Keywords: spermatozoa, sialic acid, reproduction, innate immunity, lectin, glycan, sialic acid-binding immunoglobulin-like lectins (Siglec)
Introduction
Like all living cells, sperm are covered in a glycocalyx, a complex “sugar coat” consisting of diverse glycan chains conjugated to cell-surface proteins and lipids (Fig. 1a). Unlike most cells, mammalian sperm are introduced into the reproductive tract of a different individual where they have to survive and remain functional until they fertilize an egg in the ampulla of the fallopian tubes (1). This feat is made more difficult because of the presence of large numbers of immune cells and secretions in the female reproductive tract (2–6). The female tract is well-equipped with innate immune receptors including several Toll-like receptors (7) that target potentially harmful, nonself molecules such as pathogen-associated molecular patterns. In contrast, previous work has shown that components of the glycocalyx can function as self-associated molecular patterns (SAMPs)3 by interacting with inhibitory receptors on immune cells (8). Thus, SAMPs defined by constituents of the sperm glycocalyx may facilitate sperm survival in the face of female immunity by binding to glycan-recognizing receptors along the female reproductive tract and thus contribute to successful fertilization (9). A common and well-documented determinant of SAMPs present within the sperm glycocalyx is the terminal monosaccharide sialic acid. Sialic acid is a nine-carbon backbone, amino sugar located at the terminal end of gangliosides, N-glycans and O-glycans and is present in high abundance, tens to hundreds of millions of molecules per cell (10).
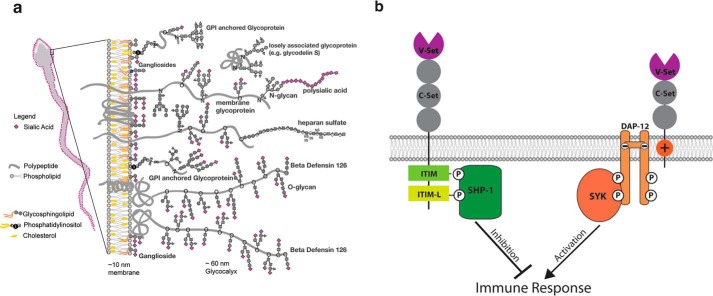
Figure 1.
The sperm glycocalyx and siglec signaling. a, a schematic of the glycocalyx of mammalian ejaculated sperm. Sialic acid is presented as purple diamonds. Modified from Ref. 1. b, Siglecs and downstream signaling. A schematic of an inhibitory Siglec (left) and activating Siglec (right) are shown. After binding a sialic acid ligand, via the V-set domain, Siglecs can modulate an immune response by downstream signaling pathways. Phosphorylation the ITIM and ITIM-L domains of inhibitory Siglecs by SHP-1 initiates a signaling cascade resulting in immune inhibition. Activating Siglecs bind to DAP12 via their transmembrane ITAM domains and initiate a signaling cascade resulting in immune activation.
Sialic acids can modulate immune response by binding to sialic acid-binding immunoglobulin-like lectins (Siglecs), which are primarily expressed by leukocytes (11, 12) and also by binding to factor H of complement (13)(Fig. 1b). All Siglecs contain one or more Ig domains (C2-set) and a N-terminal sialic acid-binding domain (V-set). Inhibitory Siglecs contain cytosolic immunoreceptor tyrosine-based inhibition motif (ITIM) and ITIM-like domains (ITIM-L) (14). The ITIM and ITAM-like domains of inhibitory Siglecs are phosphorylated by a Src family tyrosine kinase allowing for recruitment of SHP-1 (Src homology region 2 domain-containing phosphatase-1) resulting in signaling that inhibits activation of an immune response (15). In contrast, activating Siglecs contain a transmembrane immunoreceptor tyrosine-based activation motif (ITAM) domain. Via the ITAM, activating Siglecs bind to DAP12, which is phosphorylated by a Src family tyrosine kinase allowing for recruitment of SYK resulting in a signaling cascade that promotes activation of an immune response (15).
The female reproductive tract has been previously shown to have immune surveillance that is hormonally regulated to promote fertility while still providing antimicrobial resistance (16). Sperm in the uterus trigger the leukocytic reaction, a rapid-onset immunological response that promotes homing of circulating leukocytes to the uterine lumen (3, 4). This results in the active destruction of the majority of sperm. It is not fully understood how some sperm survive the leukocytic reaction. It is unknown whether Siglecs play a role in the modulation of the immune system during insemination and if Siglecs are present within the female reproductive tract.
However, the presence of sialic acids appears to be important for sperm function. For instance, sialic acids on highly sialylated betadefensin 126 is absolutely required for human sperm to penetrate cervical mucus and for sperm to adhere to the oviductal epithelium before capacitation occurs (17). Capacitation refers to several cellular processes that occur in mammalian sperm before they are competent to fertilize an egg, including an increase in mitochondrial activity to sustain vigorous motility (18–20). Interestingly, previous reports indicate that mammalian sperm partially shed sialic acids during capacitation (19). Although sialic acids are known to be critical for sperm migration, it is unclear whether they also facilitate sperm survival within the female reproductive tract.
In vivo, biologically relevant capacitation is thought to occur in the isthmus of the oviduct. In the isthmus, sperm adhere to the oviductal epithelium in a sialic acid-dependent manner forming the mammalian sperm reservoir (23). Sperm detach from the oviductal epithelium when capacitation is induced and swim toward the egg in the ampulla of the oviduct (23). One successful sperm will acrosome react, digest the protective vestments of the egg, bind to the zona pellucida and upon reaching the perivitelline space, fuse with the egg membrane, resulting in fertilization (21). The oviduct contains a very different immune cell repertoire and it is assumed that sperm, which make it there, are under much reduced immune threat (24). A minority of sperm successfully pass the utero-tubal junction leading to the ovulatory oviduct (21) after they have traveled through the uterus and survived the leukocytic reaction. The leukocytic reaction is a pre-fertilization event in which sperm arriving in the uterus trigger an immunological response that promotes homing of circulating leukocytes to the uterine lumen resulting in the active destruction the majority of sperm (3, 4). Of the few sperm that survive the onslaught of leukocytes in the uterus, even fewer successfully migrate to the isthmus of the oviduct (22).
We hypothesize that sperm sialic acids and female-expressed Siglecs may modulate the leukocytic reaction and promote sperm survival within the female reproductive tract. Indeed, a recent report has shown that sialic acids on sperm are protective against phagocytosis by macrophages, ex vivo (25). To begin to address this, we investigated sialic acid-dependent interactions between sperm and isolated neutrophils in vitro. Neutrophils were chosen because they represent the most abundant immune cell in the human leukocytic reaction following insemination (4) and have documented expression of inhibitory Siglecs-5 and Siglec-9 (26, 27). As capacitation is associated with the loss of sialic acid, which are required to engage inhibitory Siglecs, we investigated via several measures of neutrophil reactivity whether capacitated (C) sperm invoke greater reactivity than noncapacitated (NC) sperm.
Within the female reproductive tract, the endometrium has an established role in responding to and promoting the destruction of pathogens and/or sperm in the uterus (28). However, at least some sperm must survive within the female reproductive tract during the fertile window to achieve fertilization. Therefore, we examined the expression of Siglecs in the human and mouse endometrium. Finally, we tested sialic acid-dependent binding of sperm by Siglecs using recombinant, chimeric Siglec-Fc proteins of Siglecs identified in the endometrium.
Results
Sialidases are immediately activated on sperm exposed to capacitating conditions
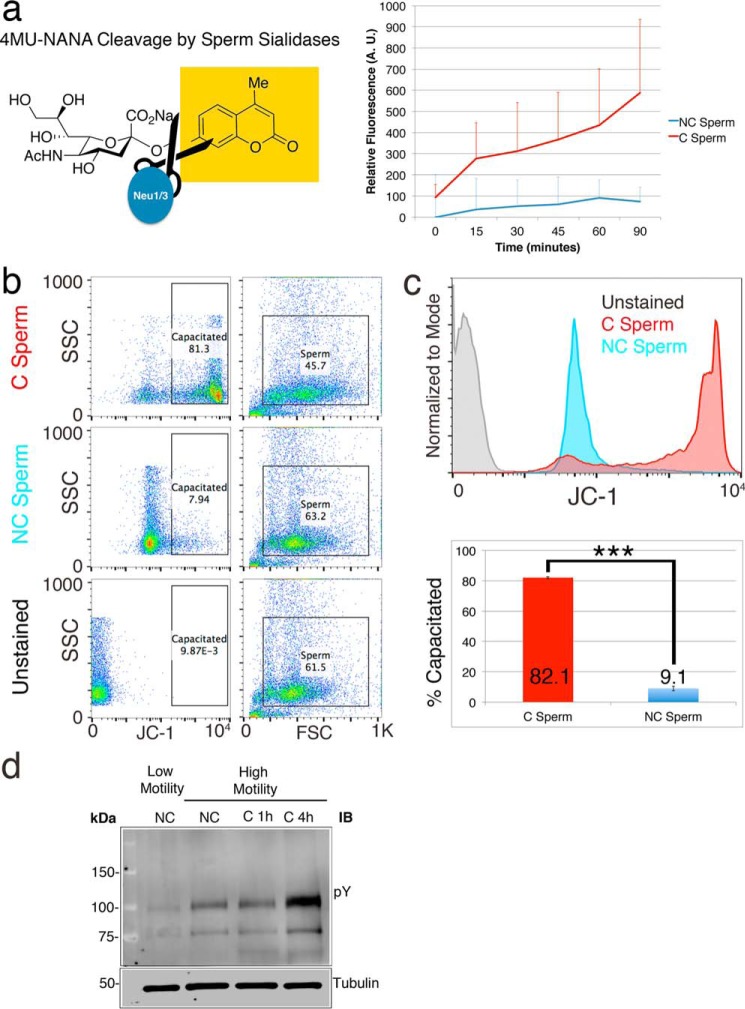
We measured the temporal dynamics of sialic acid loss in capacitating sperm using a reporter molecule 4-MU-NANA. Cleavage of the sialic acid analog NANA results in the emission of fluorescence from the separated 4-MU molecule. Sperm were incubated together with 4MU-NANA in either noncapacitating or capacitating buffers, and the resulting changes in fluorescence were measured via plate reader. Sperm show an increase in fluorescence immediately upon exposure to capacitating conditions, indicative of sialidase activity (Fig. 2a). Noncapacitated sperm do not show any increase in fluorescence. The percentage of capacitated sperm following in vitro capacitation was measured via flow cytometry by probing for an increase in mitochondrial activity using the mitochondrial membrane potential probe JC-1 (29). The majority of C sperm showed a robust increase in JC-1 compared with NC sperm (Fig. 2, b and c) and staining of Western blots with antiphosphotyrosine antibody confirms capacitation (Fig. 2d).
Figure 2.
Confirmation of sperm capacitation and sialic acid shedding. Sperm co-incubated with the fluorogenic sialic acid analog 4-MU-NANA were exposed to a noncapacitating or capacitating buffer. Only sperm in the capacitating buffer show an immediate increase in fluorescence (a), indicative of rapidly activated sperm sialidases. Sperm capacitation was confirmed by using the fluorescent molecule JC-1 to probe for increased mitochondrial activity. The majority of sperm incubated under capacitating conditions show an increase in JC-1 staining (b and c), compared with sperm in noncapacitating conditions (∼81 versus ∼10%, respectively). Staining with anti-phosphotyrosine antibody on Western blotting shows an increase of phosphorylation between low motility (LM) and high motility (HM) human sperm and with increased duration (1 versus 4 h) of in vitro exposure to capacitating conditions (d).
Capacitated sperm do not increase neutrophil activation in vitro
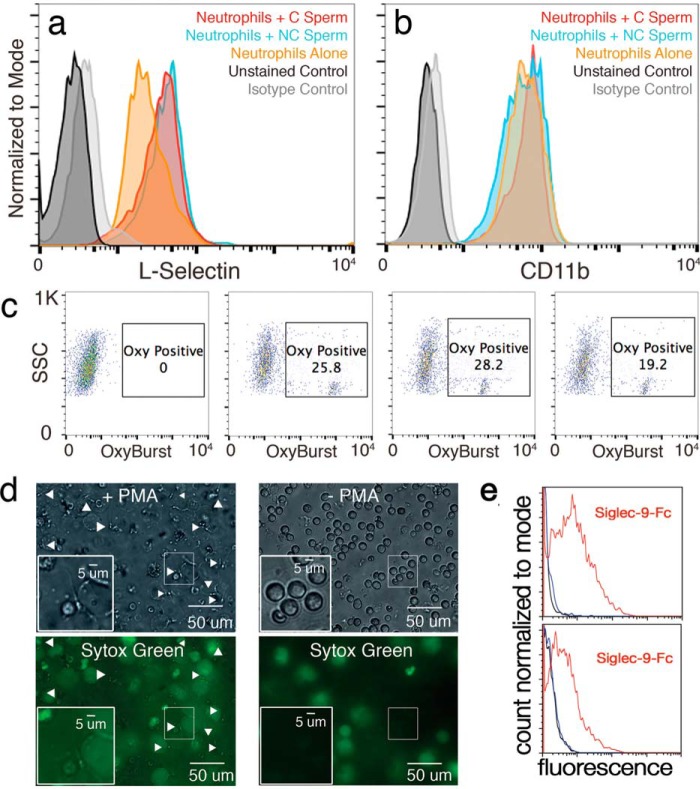
We are using in vitro capacitation as a biologically relevant way of shedding sialic acids from sperm to test the effect of reduced sialylation on interactions with leukocytes. We investigated the differential impact of sperm with more (NC sperm) or less sialic acid (C sperm) exposure on neutrophil activation in vitro using four different measures: L-selectin shedding, expression of CD11b, reactive oxygen species (ROS) production, and NET formation. First, activation of human neutrophils was determined by shedding of the quiescence marker L-selectin after exposure to C and NC human sperm. The amount of L-selectin present on neutrophils after incubation in vitro was probed using L-selectin antibodies and analyzed via flow cytometry. In one trial, exposure to both C and NC sperm preserved L-selectin as compared with untreated neutrophil controls (Fig. 3a), but this effect was not consistent (Fig. S1). We next determined the reactivity state of neutrophils exposed to C and NC sperm by assessing levels of the activation marker CD11b. Anti-CD11b reactivity was measured via flow cytometry. C sperm did not generate activation of neutrophils in vitro, except for a small subpopulation in 1 of 3 experiments (Fig. S2). In 1 of 3 replicates, C sperm generated the weakest activation response in neutrophils as compared with that of NC sperm and untreated neutrophils (Fig. 3b). Neutrophils can perform their microbicidal function via the creation of ROS following endocytosis of foreign microbes (30). Thus, we investigated the generation of reactive oxygen species in neutrophils after exposure to C and NC sperm as an alternate measure of neutrophil activation. ROS production was probed using OxyBURST and measured via flow cytometry. C sperm did not generate a strong increase in ROS production by neutrophils (19.2% OxyBURST positive) compared with that of NC sperm (28.2%) and untreated neutrophils (25.8%, Fig. 3c). Neutrophils can also immobilize and destroy pathogenic threats via the creation of neutrophil extracellular traps (NETs) (31). Neutrophils triggered by phorbol 12-myristate 13-acetate (PMA) displayed a robust increase in NET formation that immobilized both NC and C sperm (Fig. 3d). Neutrophils that were not exposed to PMA and briefly exposed to NC or C sperm remained membrane intact and did not capture NC or C sperm. We confirmed engagement of Siglec-9 by sperm from two different donors by flow cytometry using recombinant Siglec-9-Fc chimera protein (Fig. 3e).
Figure 3.
Impact of sperm on neutrophil activation. Flow cytometry analysis of the expression of several neutrophil reactivity markers (a–c). Neutrophils co-incubated with NC or C sperm were stained with antibodies targeting several markers of neutrophil activation and measured using flow cytometry. Co-incubation of isolated human leukocytes with NC or C sperm show no additional shedding of the neutrophil quiescence marker L-selection when compared with leukocytes alone (a). Isolated human neutrophils co-incubated with C or NC sperm show similar CD11b expression compared with isolated leukocytes alone (b). Isolated human neutrophils co-incubated with C sperm show a nonsignificant decrease in the percentage of OxyBURST-positive cells (positivity indicates an increase in intracellular reactive oxygen species) compared with isolated neutrophils alone or neutrophils co-incubated with NC sperm (c). PMA-induced isolated human neutrophils trigger NET formation and capture live NC and C sperm (d). Captured sperm are indicated by white arrowheads. e, sperm from two different donors stained with Siglec-Fc chimeric recombinant protein: black is control, blue is Siglec-5::FC, red is Siglec-9::FC.
The human endometrium expresses Siglecs and downstream signaling proteins
Although our data clearly demonstrate that reduced levels of sperm sialic acids do not activate neutrophils in vitro, we decided to investigate the expression of Siglecs in the uterus.
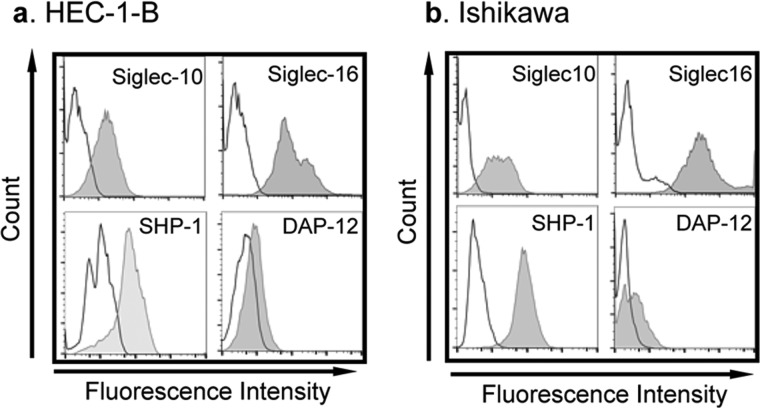
As heavily sialylated NC sperm directly interact with the endometrium, we hypothesized that the endometrium may express Siglecs. Therefore, we probed for the expression of various Siglecs in human pre-menopausal uterine samples and found that Siglec-10 was expressed primarily by the epithelium (n = 7) (Fig. 4). We also screened two human endometrial cell lines, Ishikawa and HEC-1B, for cell-surface Siglec expression by flow cytometry analysis (Table 1, Fig. 5). Ishikawa and HEC-1B cell lines have been used extensively to investigate hormonal responses of various genes or in the context of infection (32–35). These analyses revealed HEC-1B (Fig. 5a, Fig. S3) and Ishikawa (Fig. 5b, Fig. S4) express two inhibitory Siglecs, Siglec-10 and Siglec-11, as well as activating Siglecs (Siglec-16). Importantly, both SHP-1, the downstream signaling molecule of immune-inhibitory Siglec-10 and Siglec-11, and DAP12, the downstream signaling molecule of immune-activating Siglec-16, are expressed in the two endometrial cell lines (Fig. 5).
Figure 4.
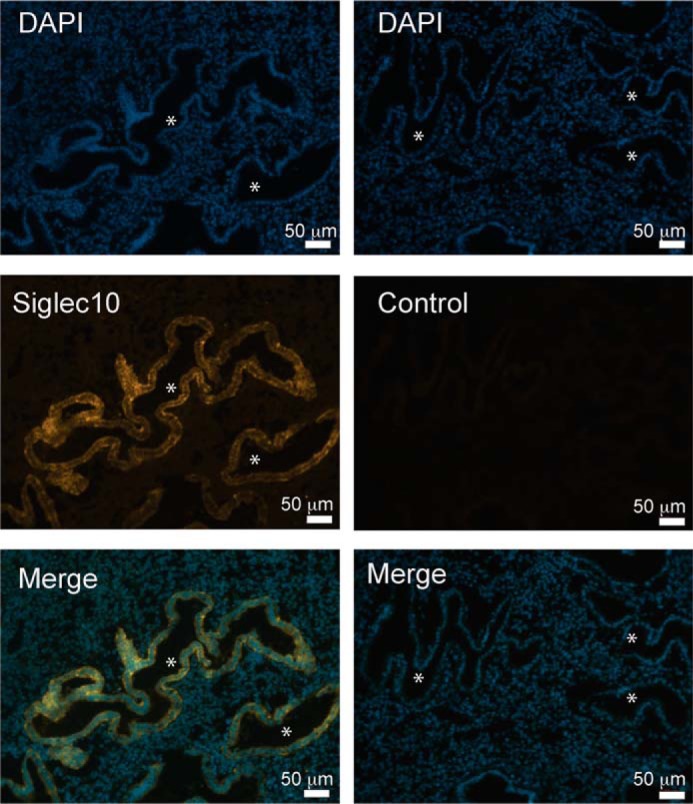
Siglec-10 is expressed in the human endometrium. Immunofluorescence staining of Siglec-10 (orange) and all nuclei (4′,6-diamidino-2-phenylindole, DAPI). Siglec-10 expression is observed exclusively in the endometrium and not in the underlying stroma. Asterisks indicate the uterine lumen. Images are representative for a sample of n = 7.
Table 1.
Siglec expression in two human endometrial cells lines
| Cell line |
||
|---|---|---|
| HEC-1-B | Ishikawa | |
| Siglec-3 | Absent | Absent |
| Siglec-5/14 | Absent | Absent |
| Siglec-7 | Absent | Absent |
| Siglec-9 | Absent | Absent |
| Siglec-10 | High | High |
| Siglec-11 | Low | Low |
| Siglec-16 | High | High |
Figure 5.
Siglecs and their downstream signaling proteins of Siglecs are present in endometrial cancer cell lines. Flow cytometry analysis of the expression of select Siglec and their signaling molecules in endometrial cell lines. Both HEC-1B (a) and Ishikawa (b) cell lines express Siglec-10 and Siglec-16. Both cell lines also express SHP-1 and DAP12, the downstream signaling molecules of Siglec-10 and Siglec-16, respectively.
Human sperm bind Siglec-10 in a sialic acid-dependent manner
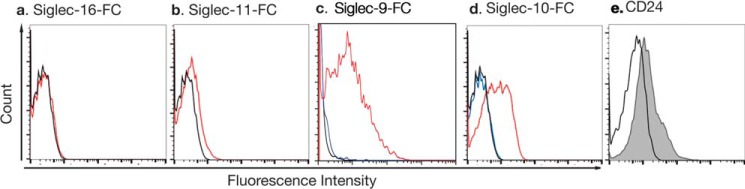
To investigate the possible role of Siglecs in the leukocytic reaction, we assessed the binding of recombinant Siglec-Fc fusion proteins expressed by the endometrium to noncapacitated human sperm. Siglec-Fc fusion proteins contain the V-set and one or more underlying C-set of a given Siglecs in-frame to the human IgG Fc region. We tested the binding of Siglec-10, -11, and -16-Fc fusion proteins to sperm. Sperm did not bind Siglec-11 or Siglec-16-Fc proteins (Fig. 6, a and b). However, sperm robustly bound Siglec-10-Fc fusion proteins (Fig. S5). In addition, this binding was sialic acid dependent as no binding was observed using a Siglec-10 mutant protein with a R120K mutation, which abrogates its sialic acid binding ability by virtue of lacking the critical arginine residue in the outermost V-set domain responsible for sialic acid binding (Fig. 6c). Furthermore, we found by flow cytometry (FACS) analysis using an anti-CD24 antibody, that a subset (∼25%) of human sperm expresses the glycoprotein CD24, a known ligand for Siglec-10 (Fig. 6d).
Figure 6.
Human spermatozoa carry sialylated ligands for Siglec-10. Flow cytometry analysis indicates that human sperm do not bind Siglec-11-Fc (a) and Siglec-16-Fc (b) (black line is control and red is the Siglec-Fc fusion protein). Human sperm do bind Siglec-9 (c) and Siglec-10-Fc (d) in a sialic acid-dependent manner (black is control, red is WT Siglec-10-Fc, blue is Siglec-10(R120K)-Fc). A subset of sperm also express CD24 (e), a known sialylated ligand of Siglec-10.
Mouse endometrium also expresses Siglecs
We initially focused on Siglec-G, the mouse homologue of Siglec-10 that was expressed in the human post-mortem endometrium and human endometrial cell lines. However, both IHC and FACS analyses revealed an absence of Siglec-G expression in the mouse endometrium. We then screened the expression of other Siglecs in mouse uterus by qPCR (Fig. 7a). We found that Siglec-3 was highly expressed in the mouse uterus and confirmed the presence of Siglec-3 protein by IHC analysis, specifically in the uterine epithelium (Fig. 7b).
Figure 7.
Siglec-3 is expressed in the mouse uterine epithelium. qPCR analysis (a) of the whole mouse uteri indicates that Siglec-3 expression is higher than Siglec-E and -F. Immunofluorescence staining for Siglec-3 (b) indicates Siglec-3 expression is enriched in the epithelium and epithelium glands as compared with the underlying stroma (asterisks indicate the uterine lumen). c, quantification of Siglec-3 antibody staining.
Discussion
As sialic acids and Siglecs have well-established roles in modulation of immune responses, we hypothesized that sperm sialic acids could interact with various inhibitory Siglecs expressed by neutrophils to inhibit their activation during the leukocytic reaction. By using in vitro capacitation as a biologically relevant way of shedding sialic acids from sperm, we sought to determine whether capacitated sperm, with reduced levels of sialic acid, activated neutrophils more than noncapacitated sperm. Surprisingly, we determined that co-incubation of neutrophils with capacitated sperm did not result in additional neutrophil reactivity than when neutrophils were co-incubated with NC sperm. Moreover, co-incubation of neutrophils with C sperm resulted in similar levels of neutrophil reactivity when measured via CD11b levels, reactive oxygen species production, and in the shedding of L-selectin (36). Furthermore, neutrophils are capable of capturing both NC and C sperm via the formation of NETs in vitro. These data indicate that high levels of sperm-associated sialic acids do not reduce neutrophil activation in vitro.
Although sperm capacitated in vitro shed sialic acid, some residual sialic acid is retained on the sperm surface (19). This residual sialic acid may be sufficient to engage inhibitory Siglecs on neutrophils, explaining the lack of differences in neutrophil activation as compared with NC sperm. In addition, the higher mortality of sperm kept under noncapacitating conditions in vitro may confound the interpretation of the data. Although efforts are made to keep the number of live sperm cells equal between conditions, NC sperm have a higher rate of cell death than C sperm, as evidenced by a higher proportion of propidium iodide-positive cells (Fig. S6). The high rate of death in NC sperm likely results in the presentation of danger-associated molecular patterns (DAMPs) that have been shown to contribute to the overall reactivity of nearby immune cells (37). Controlling for a higher level of dead or moribund sperm in the noncapacitated condition is challenging because viability-enhancing components such as albumin also function as key capacitation factors in vitro. Although future experiments might address neutrophil reactivity to increasing proportions of dead sperm, the current data must be interpreted with the caveat that the observed neutrophil reactivity toward NC and C sperm is confounded by a roughly 20% difference in sperm viability between the two conditions. In addition, others have previously suggested that a primary purpose of the leukocytic reaction is to remove dead and/or dying sperm (38). As the NC sperm samples contain a higher percentage of such cells, the resultant DAMPs may generate a danger signal that overpowers the reduction of immunoreactivity imparted by a sperm sialic acid-inhibitory Siglec interactions.
Aside from immune cells, sperm also come into contact with the endometrium during the leukocytic reaction. We sought to investigate if sperm could be interacting with the endometrium in a sialic acid-dependent manner. Unlike for neutrophils, the expression of Siglecs in the uterus has not previously been reported. We have now documented the expression of Siglecs-10, -11, and -16 in human endometrial cell lines for the first time. Siglecs-10 and -11 are inhibitory receptors and Siglec-16 is an activating receptor that is known to be polymorphic in humans with a common loss-of-function (pseudogenized) variant (44). Endometrial cells also express SHP1 and DAP12, the respective downstream signaling components of inhibitory and activating Siglecs. Preliminary bioinformatic analysis (using oPOSSUM) indicates that estrogen response elements are in the genomic sequences flanking Siglec-11 and Siglec-16. Transcription-binding sites for one of more transcription factors (AP-1, SP-1, NF-κB, and CREB1) known to act in the tethering pathway for estrogen receptors have been identified in the genomic sequences flanking all three Siglecs by bioinformatics analysis. The presence of these transcription factor-binding sites indicates that Siglecs expressed by endometrial cells may be transcriptionally up-regulated by estrogen exposures. Future experiments where the expression of endometrial Siglecs before and after exogenous estrogen treatment should be very informative in this regard.
We have shown Siglec-10 expression in pre-menopausal human endometrium and have established that highly sialylyated noncapacitated human sperm bind Siglec-10 in a sialic acid-dependent manner. We also report that a subset of sperm bears CD24, a sialic acid containing glycoprotein that is an established activator of Siglec-10 signaling (39). Although a recent paper has identified the presence of Siglec-10 in first trimester placental cells (40), the majority of work on Siglec-10, and its mouse homologue Siglec-G, concerns its role in the immune system. Generally, Siglec-10/G is considered an inhibitor of immune responses in various cellular contexts. For example, mouse Siglec-G functions in B1a cells to inhibit their proliferation and induce their tolerance to self-antigens (41). Consequently, genetic ablation of Siglec-G (in select genetic backgrounds) results in an age-dependent autoimmunity phenotype and increases the severity of collagen-induced arthritis in an autoimmune prone mouse model (42). CD24 is a ligand for Siglec-G and Siglec-10 in mouse and human, respectively. In vivo, Siglec-G and CD24 are required to inhibit the production of inflammatory cytokines in an acetaminophen-induced liver necrosis model and graft versus host disease (39). Our results indicate that sialic acid-containing ligands, including those carried by CD24, on human sperm may interact with endometrial expressed Siglec-10 and with neutrophil Siglec-9 during the leukocytic reaction (Fig. 8). In vivo, this interaction could inhibit the release of cytokines by the endometrium, decrease the recruitment and activation of leukocytes to the uterine lumen during the leukocytic reaction, and result in increased sperm survival. Future experiments exposing live sperm to cultured female tract cell lines should be very informative in this regard.
Figure 8.
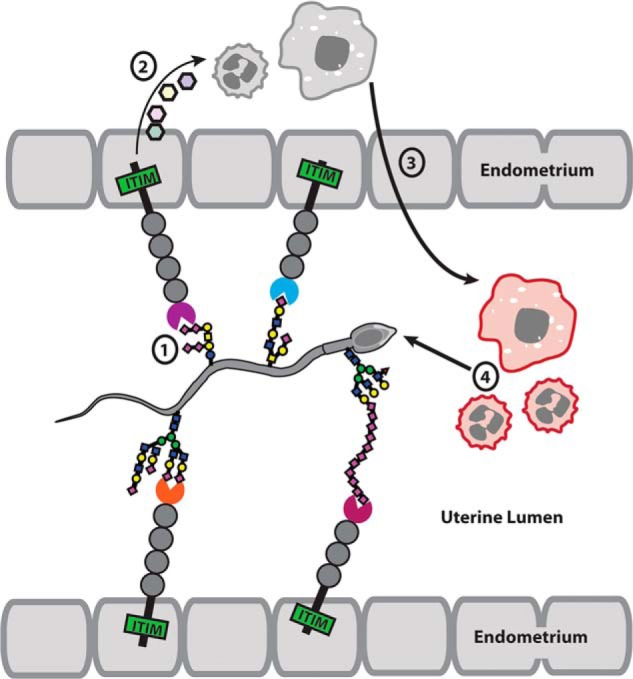
Sperm sialic acid and endometrial Siglecs may interact to modulate the female immune response to sperm. Both human and the mouse endometrium express inhibitory Siglecs (Siglec-10 and Siglec-3, respectively). When bound by sialic acid on sperm, the expressed inhibitory Siglecs may inhibit the immune response of the endometrium by inhibiting the release of cytokines, complement or other pro-inflammatory proteins via SHP-1 signaling. Sperm may further directly interact with neutrophils and macrophages and inhibit their immune response.
Although Siglec-11 and Siglec-16 were expressed by the endometrial cell lines, the Siglec-11-Fc and Siglec-16-Fc proteins did not bind to human sperm in vitro. Although Siglecs are defined as sialic acid-binding lectins, the precise specificities for sialylated glycan patterns of each Siglec remain poorly defined. In addition, Siglecs aggregate on the cell surface and such aggregation strongly influences their binding to cis or trans ligands (43). The use of soluble Siglec-Fc proteins as molecular probes does not fully mimic these cell-surface mechanisms. It is possible that sperm do carry ligands for Siglec-11 and Siglec-16, but that using the Siglec-Fc fusion proteins does not allow to reconstitute the in vivo cell-surface mechanisms. As Siglecs-11 and -16 are paired receptors, and their precise specificity for glycan patterns is poorly defined (a problem for Siglecs in general), they could possibly also bind molecular patterns of pathogens, rather than SAMPs, explaining their lack of binding to sperm in our assay.
To generate a model for investigating the role of Siglec-10 in the leukocytic reaction, we sought to determine the expression of Siglec-G, the mouse homologue of Siglec-10, in the mouse uterus. We were surprised to find that Siglec-G was not expressed in the mouse uterus. We did, however, determine that another inhibitory Siglec, Siglec-3, is expressed in the mouse uterine epithelium and may be under hormonal control. Although no reproductive problems have been reported within Siglec-3 knockout mice, it would be very interesting to determine whether these female mutants show increased infiltration of leukocytes into the uterine lumen after mating.
In conclusion, we provide definite evidence for the presence of Siglec innate immune receptors in the female reproductive tract and their capacity to recognize sialylated glycans on sperm in humans and mice. Our results suggest that sperm engage female innate immune receptors en route to fertilization. The female immune system may spare some allogeneic sperm cells, in part because sperm are coated with highly sialylated glycan patterns that is shared between sperm and local female cells. Altogether, our results suggest that the immune paradox of mammalian insemination may in part be resolved via the sensing of shared, species-specific glycan patterns on sperm by innate immune-modulating receptors expressed along the endometrium. Further insights into the precise mechanism of these interactions will require in vitro experiments with human sperm and endometrial cell lines to measure alterations in endometrial cell signaling cascades and/or cytokine expression.
Experimental procedures
In vitro interactions between human sperm and activated neutrophils
Human sperm were collected from volunteers via masturbation under UCSD human subjects protocol number 16027, liquefied at room temperature for 30 min, and washed with TYH media before being exposed to NC TYH media or capacitating conditions (C) TYH plus 5 mg/ml of BSA and 15 mm NaHCO3 for 4 h at 37 °C and 5% CO2, as described previously (45). Blood was collected in heparin-coated tubes from human donors using UCSD human subjects protocol number 170921X. This study abides by the Declaration of Helsinki for all human sample collection. Red blood cells were removed from whole blood using an Easysep magnetic separation kit (Stemcell Technologies) with anti-glycophorin A-conjugated magnetic beads. The remaining plasma and leukocytes were co-incubated for 30 min with NC sperm, C sperm, or sperm-free capacitation media (C buffer alone), fixed in 10% PFA on ice for 20 min, and stained with antibody staining: FITC mouse anti-CD62L IgG1 (Biolegend, 304802) or mouse IgG1, κ-isotype control (BD Biosciences, 349041), FITC rat anti-CD11b (Tonbo Biosciences, 70-0112-OWL-A11503) or rat IgG2b, κ-isotype control (BD Biosciences, 554688). Phagosomal ROS production was analyzed using Fc-OxyBURST Green Assay Reagent (ThermoFisher, F2902) for 15 min and then fixed. The stained sperm and leukocytes were submitted to flow cytometry on a BD FACSCalibur. The population was gated based on forward and side scatter to analyze a minimum of 10,000 neutrophils for each experiment. Data were analyzed using FlowJo. NET formation was visualized using the live cell-impermeable DNA stain SYTOX Green (ThermoFisher, S7020).
Sialidase activity and confirmation of capacitation
Swim-up sperm were incubated with 100 pm 4-MU-NANA (Sigma, M8639) and exposed to an equal volume of either NC or C buffer. Control samples contained the NC or C buffer alone. Samples were read on a Molecular Devices SpectraMAX M3 plate reader using excitation and emission values of 365 and 450 nm, respectively. Confirmation of sperm capacitation was performed after 1 h of capacitation with the MitoProbe JC-1 Assay Kit for Flow Cytometry (Molecular Probes, MP34142) following the manufacturer's recommendations. Tyrosine phosphorylation was confirmed by staining with anti-phosphotyrosine mouse monoclonal at dilution of 1:10,000 (Cell Signaling, 9411).
Endometrial cell line maintenance and flow cytometry experiments
Ishikawa cells and HEC-1B cells were purchased from Sigma or ATTC, respectively. Both cell lines were maintained in RPMI with phenol red and supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamate. For the flow cytometry based screen for Siglec expression, cells were fixed for 10 min in 10% PFA. 106 cells were submitted to antibody staining for each experiment. The following primary antibodies were used: APC mouse anti-human Siglec-3 IgG1 (BD, 551378), PE mouse anti-human CD170 (Biolegend, catalog number 352004), APC mouse anti-human Siglec-7 IgG1 (R&D Systems, FAB1138), mouse anti-human Siglec-9 (R&D Systems, AF1139), APC mouse anti-human Siglec-10 (Biolegend, 347606), mouse antihuman Siglec-11 (R&D Systems, MAB3258), mouse anti-human Siglec-11 (R&D Systems, MAB3258), rabbit anti-human SHP-1 (Abcam, 131537), and goat anti-human DAP12 (Novus Biologicals, NBP1–52376). All unconjugated Siglec antibodies were stained with Alexa Fluor 647 goat anti-mouse IgG. The SHP-1 antibody was stained with Alexa Fluor 488 goat anti-rabbit IgG. The DAP12 antibody was stained with Alexa Fluor 647 donkey anti-goat IgG. Siglec staining was determined by flow cytometry on a BD FACSCalibur. A minimum of 10,000 events were counted for each experiment. Data were analyzed using FlowJo.
Siglec-Fc fusion protein purification
Purification of human Siglecs was performed as previously described (46). Briefly, 18 μg of each human Siglec-Fc plasmid were transfected into 2 × 107 HEK293A cells. Transfected cells were grown in 50% Dulbecco's modified Eagle's medium, 50% RPMI supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, and 1% Nutridoma for 4 days. Secreted Siglec-Fc proteins were purified from conditioned media using protein A-Sepharose beads and treated with Arthrobacter ureafaciens sialidase (AUS) to remove any possible interaction with sialic acid containing secreted proteins. All Siglec-Fc encoding plasmids were kindly provided by Dr. Ajit Varki.
Human sperm isolation and binding to Siglec-Fc by flow cytometry analysis
Human ejaculate was donated by volunteers recruited under UCSD IRB protocol number 160274. To remove seminal proteins and any other contaminates, donated ejaculate was washed three times with 10 times volume of PBS and filtered through glass wool. The isolated sperm were then fixed in 2% PFA for 10 min at room temperature. 106 sperm cells in suspension were incubated with a Siglec-Fc for 30 min followed by a 30-min incubation with R-phycoerythrin AffiniPure F(ab′)2 fragment goat anti-human IgG, Fcγ fragment specific (Jackson ImmunoResearch, 109-116-170). For CD24 staining, 106 sperm cells were incubated with Alexa Fluor 647 mouse anti-human CD24 (Biolegend, 311109). Binding of the Siglec-Fc and CD24 expression was determined by flow cytometry on a BD FACSCalibur. A minimum of 10,000 events were counted per experiment. Data were analyzed using FlowJo.
Mouse estrus tracking and uterine qPCR
C57/B6 mice were kept under UCSD IACUC protocol number S16223. The estrus cycle was tracked by vaginal cytology for a minimum of 2 weeks before mice were sacrificed. Briefly, the vagina of mice was washed with ∼100 μl of PBS and the isolated cells were analysis under a dissecting microscope. The stage in the estrus cycle was determined by the proportion of cornified epithelial cells, nucleated epithelial cells, and leukocytes as described in Caligioni (47). 3 to 4-month-old female in proestrus or estrus were humanely euthanized. Harvested uterine horns were homogenized. Total mRNA was isolated using the Qiagen RNA mini kit, which contains on column DNase. All isolated RNA was submitted to UV spectroscopy and samples had A260/A280 readings between 1.8 and 2.0. For all samples 2 μg of RNA were converted to cDNA using the Bio-Rad iScript kit. qPCR was performed using gene-specific primers, the Power SYBR Green master mix (Invitrogen, number 4367659), and the Bio-Rad CFX96 Real-time system. Sequence for Siglec-3 primers (ID: 10946590a1), Siglec-E (ID: 13626036a1), and Siglec-F (ID: 28864163a1) used were taken from Primerbank (48). qPCR was performed on two biological replicates in triplicate. Ct values were normalized to the Ct values for TBP. TBP was used as a reference gene as its Ct values were more similar to that the Siglecs.
Immunofluorescence staining of human and mouse uterus
Frozen samples of pre-menopausal human uterine biopsies were kindly provided by Dr. Nissi Varki. Mouse uterine horns at pro-estrus and estrus were harvested from 3- to 4-month-old mice. Both human and mouse tissues were flash frozen in optimal cutting temperature (OCT®) and sectioned into 7-μm slices. Human endometrial samples were stained with goat anti-human Siglec-10 (Santa Cruz, sc-240882) or mouse anti-human Siglec-11 (R&D Systems, MAB3258) and visualized with Cy3 bovine anti-goat IgG (Jackson ImmunoResearch, 705-165147) or Cy3 donkey anti-mouse IgG (Jackson ImmunoResearch, 715-165-150), respectively. Mouse uteri were stained wsith rabbit anti-mouse Sigelc-3 (Santa Cruz, sc-28810) and visualized with Alexa Fluor 488 goat anti-rabbit IgG (A11008). All images were taken using Zeiss AXIO Observer D1 Inverted Disc Fluorescence Microscope. For image quantification, exposure time was the same and the fluorescence intensity for a given antibody stain was quantified using ImageJ. Five images from four different mice were used for quantification.
Author contributions
E. T., H. S. R., and P. G. conceptualization; E. T., H. S. R., R. W., and P. G. data curation; E. T., H. S. R., and P. G. formal analysis; E. T. and H. S. R. validation; E. T., H. S. R., R. W., and P. G. investigation; E. T., H. S. R., R. W., and P. G. methodology; E. T., H. S. R., R. W., and P. G. writing-original draft; E. T., H. S. R., and P. G. project administration; E. T., H. S. R., and P. G. writing-review and editing; P. G. resources; P. G. supervision; P. G. funding acquisition.
Supplementary Material
Acknowledgments
We thank Ajit Varki for the Siglec-Fc chimera plasmids, Nissi Varki for the frozen human samples, Anel Lizcano and Ross Corriden for guidance with neutrophil isolation, and Stevan Springer for critical feedback. We gratefully acknowledge the University of California, San Diego, Neuroscience Microscopy Facility supported by Grant P30 NS047101.
This work was supported by National Institutes of Health NIGMS Grant GM1R01GM095882, the Mizutani Foundation for Glycosciences, the G. Harold and Leila Y. Mathers Foundation, and a University of California, San Diego, Institutional Research and Academic Career Development fellowship and National Institutes of Health NIGMS Grant K12GM068524 (to E. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S6.
- SAMP
- self-associated molecular pattern
- DAMP
- danger-associated molecular pattern
- C
- capacitated
- IHC
- immunohistochemistry
- ITIM
- immunoreceptor tyrosine-based inhibition motif
- ITAM
- immunoreceptor tyrosine-based activation motif
- JC-1
- 5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- NC
- uncapacitated
- Siglec
- sialic acid-binding immunoglobulin-like lectin
- TBP
- TATA-binding protein
- 4MU-NANA
- 4-methylumbelliferyl αDN-acetylneuraminic acid
- ROS
- reactive oxygen species
- NET
- neutrophil extracellular trap
- PMA
- phorbol 12-myristate 13-acetate
- qPCR
- quantitative PCR
- SHP-1
- Src homology region 2 domain-containing phosphatase-1.
References
- 1. Tecle E., and Gagneux P. (2015) Sugar-coated sperm: unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 82, 635–650 10.1002/mrd.22500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandtzaeg P. (1997) Mucosal immunity in the female genital tract. J. Reprod. Immunol. 36, 23–50 10.1016/S0165-0378(97)00061-2 [DOI] [PubMed] [Google Scholar]
- 3. Pandya I. J., and Cohen J. (1985) The leukocytic reaction of the human uterine cervix to spermatozoa. Fertil. Steril. 43, 417–421 10.1016/S0015-0282(16)48442-6 [DOI] [PubMed] [Google Scholar]
- 4. Thompson L. A., Barratt C. L., Bolton A. E., and Cooke I. D. (1992) The leukocytic reaction of the human uterine cervix. Am. J. Reprod. Immunol. 28, 85–89 10.1111/j.1600-0897.1992.tb00765.x [DOI] [PubMed] [Google Scholar]
- 5. Katila T. (2012) Post-mating inflammatory responses of the uterus. Reprod. Domest. Anim. 47, Suppl. 5, 31–41 10.1111/j.1439-0531.2012.02120.x [DOI] [PubMed] [Google Scholar]
- 6. Vilés K., Rabanal R., Rodríguez-Prado M., and Miró J. (2013) Influence of seminal plasma on leucocyte migration and amount of COX-2 protein in the jenny endometrium after insemination with frozen-thawed semen. Anim. Reprod. Sci. 143, 57–63 10.1016/j.anireprosci.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 7. Zandieh Z., Ashrafi M., Jameie B., Amanpour S., Mosaffa N., Salman Yazdi R., Pacey A., and Aflatoonian R. (2015) Evaluation of immunological interaction between spermatozoa and fallopian tube epithelial cells. Andrologia 47, 1120–1130 10.1111/and.12391 [DOI] [PubMed] [Google Scholar]
- 8. Varki A. (2011) Since there are PAMPs and DAMPs, there must be SAMPs? glycan “self-associated molecular patterns” dampen innate immunity, but pathogens can mimic them. Glycobiology 21, 1121–1124 10.1093/glycob/cwr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Springer S. A., and Gagneux P. (2013) Glycan evolution in response to collaboration, conflict, and constraint. J. Biol. Chem. 288, 6904–6911 10.1074/jbc.R112.424523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varki A, Schnaar RL, Schauer R. (2015) Sialic acids and other nonulosonic acids in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds) pp. 179–191, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 11. Crocker P. R., Paulson J. C., and Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- 12. Varki A., and Gagneux P. (2012) Multifarious roles of sialic acids in immunity. Ann. N.Y. Acad. Sci. 1253, 16–36 10.1111/j.1749-6632.2012.06517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blaum B. S., Hannan J. P., Herbert A. P., Kavanagh D., Uhrín D., and Stehle T. (2015) Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat. Chem. Biol. 11, 77–82 10.1038/nchembio.1696 [DOI] [PubMed] [Google Scholar]
- 14. Varki A., and Angata T. (2006) Siglecs: the major subfamily of I-type lectins. Glycobiology 16, 1R–27R 10.1093/glycob/cwj008 [DOI] [PubMed] [Google Scholar]
- 15. Crocker P. R., and Redelinghuys P. (2008) Siglecs as positive and negative regulators of the immune system. Biochem. Soc. Trans. 36, 1467–1471 10.1042/BST0361467 [DOI] [PubMed] [Google Scholar]
- 16. Wira C. R., Patel M. V., Ghosh M., Mukura L., and Fahey J. V. (2011) Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am. J. Reprod. Immunol. 65, 196–211 10.1111/j.1600-0897.2011.00970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tollner T. L., Bevins C. L., and Cherr G. N. (2012) Multifunctional glycoprotein DEFB126: a curious story of defensin-clad spermatozoa. Nat. Rev. Urol. 9, 365–375 10.1038/nrurol.2012.109 [DOI] [PubMed] [Google Scholar]
- 18. Srivastava P. N., and Abou-Issa H. (1977) Purification and properties of rabbit spermatozoal acrosomal neuraminidase. Biochem. J. 161, 193–200 10.1042/bj1610193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ma F., Wu D., Deng L., Secrest P., Zhao J., Varki N., Lindheim S., and Gagneux P. (2012) Sialidases on mammalian sperm mediate deciduous sialylation during capacitation. J. Biol. Chem. 287, 38073–38079 10.1074/jbc.M112.380584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huo L. J., Ma X. H., and Yang Z. M. (2002) Assessment of sperm viability, mitochondrial activity, capacitation and acrosome intactness in extended boar semen during long-term storage. Theriogenology 58, 1349–1360 10.1016/S0093-691X(02)00953-6 [DOI] [PubMed] [Google Scholar]
- 21. Okabe M. (2015) Mechanisms of fertilization elucidated by gene-manipulated animals. Asian J. Androl. 17, 646–652 10.4103/1008-682X.153299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee S. K., Kim C. J., Kim D. J., and Kang J. H. (2015) Immune cells in the female reproductive tract. Immune Netw. 15, 16–26 10.4110/in.2015.15.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suarez S. S. (2016) Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185–194 10.1007/s00441-015-2244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ardighieri L., Lonardi S., Moratto D., Facchetti F., Shih IeM., Vermi W., and Kurman R. J. (2014) Characterization of the immune cell repertoire in the normal fallopian tube. Int. J. Gynecol Pathol. 33, 581–591 10.1097/PGP.0000000000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma F., Deng L., Secrest P., Shi L., Zhao J., and Gagneux P. (2016) A mouse model for dietary xenosialitis: antibodies to xenoglycan can reduce fertility. J. Biol. Chem. 291, 18222–18231 10.1074/jbc.M116.739169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cornish A. L., Freeman S., Forbes G., Ni J., Zhang M., Cepeda M., Gentz R., Augustus M., Carter K. C., and Crocker P. R. (1998) Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood 92, 2123–2132 [PubMed] [Google Scholar]
- 27. Ali S. R., Fong J. J., Carlin A. F., Busch T. D., Linden R., Angata T., Areschoug T., Parast M., Varki N., Murray J., Nizet V., and Varki A. (2014) Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 211, 1231–1242 10.1084/jem.20131853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nasu K., and Narahara H. (2010) Pattern recognition via the Toll-like receptor system in the human female genital tract. Mediators Inflamm. 2010, 976024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson M. J., Chapman S. J., Videan E. N., Evans E., Fritz J., Stoinski T. S., Dixson A. F., and Gagneux P. (2007) Functional evidence for differences in sperm competition in humans and chimpanzees. Am. J. Phys. Anthropol. 134, 274–280 10.1002/ajpa.20674 [DOI] [PubMed] [Google Scholar]
- 30. Lamb F. S., Hook J. S., Hilkin B. M., Huber J. N., Volk A. P., and Moreland J. G. (2012) Endotoxin priming of neutrophils requires endocytosis and NADPH oxidase-dependent endosomal reactive oxygen species. J. Biol. Chem. 287, 12395–12404 10.1074/jbc.M111.306530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., and Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 32. Tamm-Rosenstein K., Simm J., Suhorutshenko M., Salumets A., and Metsis M. (2013) Changes in the transcriptome of the human endometrial Ishikawa cancer cell line induced by estrogen, progesterone, tamoxifen, and mifepristone (RU486) as detected by RNA-sequencing. PLoS ONE 8, e68907 10.1371/journal.pone.0068907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang Q., Su Q., Wang G., Bi F., and Sa R. (2012) Effect of AT1R knockdown on Ishikawa cell proliferation induced by estrogen. Arch. Gynecol. Obstet. 286, 481–487 10.1007/s00404-012-2305-7 [DOI] [PubMed] [Google Scholar]
- 34. Boehme K., Simon S., and Mueller S. O. (2009) Gene expression profiling in Ishikawa cells: a fingerprint for estrogen active compounds. Toxicol. Appl. Pharmacol. 236, 85–96 10.1016/j.taap.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 35. Guseva N. V., Dessus-Babus S. C., Whittimore J. D., Moore C. G., and Wyrick P. B. (2005) Characterization of estrogen-responsive epithelial cell lines and their infectivity by genital Chlamydia trachomatis. Microbes Infect. 7, 1469–1481 10.1016/j.micinf.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 36. Lizcano A., Secundino I., Döhrmann S., Corriden R., Rohena C., Diaz S., Ghosh P., Deng L., Nizet V., and Varki A. (2017) Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood 129, 3100–3110 10.1182/blood-2016-11-751636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miller Y. I., Choi S. H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C. J., Que X., Montano E., Shaw P. X., Tsimikas S., Binder C. J., and Witztum J. L. (2011) Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 108, 235–248 10.1161/CIRCRESAHA.110.223875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aitken R. J., Baker M. A., and Nixon B. (2015) Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress. Asian J. Androl. 17, 633–639 10.4103/1008-682X.153850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen G. Y., Tang J., Zheng P., and Liu Y. (2009) CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323, 1722–1725 10.1126/science.1168988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sammar M., Siwetz M., Meiri H., Fleming V., Altevogt P., and Huppertz B. (2017) Expression of CD24 and Siglec-10 in first trimester placenta: implications for immune tolerance at the fetal-maternal interface. Histochem. Cell Biol. 147, 565–574 10.1007/s00418-016-1531-7 [DOI] [PubMed] [Google Scholar]
- 41. Nitschke L. (2015) Siglec-G is a B-1 cell inhibitory receptor and also controls B cell tolerance. Ann. N.Y. Acad. Sci. 1362, 117–121 10.1111/nyas.12826 [DOI] [PubMed] [Google Scholar]
- 42. Bökers S., Urbat A., Daniel C., Amann K., Smith K. G., Espéli M., and Nitschke L. (2014) Siglec-G deficiency leads to more severe collagen-induced arthritis and earlier onset of lupus-like symptoms in MRL/lpr mice. J. Immunol. 192, 2994–3002 10.4049/jimmunol.1303367 [DOI] [PubMed] [Google Scholar]
- 43. Razi N., and Varki A. (1999) Cryptic sialic acid binding lectins on human blood leukocytes can be unmasked by sialidase treatment or cellular activation. Glycobiology 9, 1225–1234 10.1093/glycob/9.11.1225 [DOI] [PubMed] [Google Scholar]
- 44. Wang X., Mitra N., Cruz P., Deng L., NISC, Comparative Sequencing Program, Varki N, Angata T., Green E. D., Mullikin J., Hayakawa T., and Varki A. (2012) Evolution of siglec-11 and siglec-16 genes in hominins. Mol. Biol. Evol. 29, 2073–2086 10.1093/molbev/mss077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Munné S., and Estop A. M. (1993) Chromosome analysis of human spermatozoa stored in vitro. Hum. Reprod. 8, 581–586 10.1093/oxfordjournals.humrep.a138100 [DOI] [PubMed] [Google Scholar]
- 46. Angata T., and Varki A. (2000) Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs: evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 275, 22127–22135 10.1074/jbc.M002775200 [DOI] [PubMed] [Google Scholar]
- 47. Caligioni C. S. (2009) Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4, 10.1002/0471142301.nsa04is48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spandidos A., Wang X., Wang H., and Seed B. (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucl. Acids Res. 38, D792–D799 10.1093/nar/gkp1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.