Abstract
The network of Wingless/Int-1 (WNT)-induced signaling pathways includes β-catenin–dependent and –independent pathways. β-Catenin regulates T cell factor/lymphoid enhancer–binding factor (TCF/LEF)-mediated gene transcription, and in response to WNTs, β-catenin signaling is initiated through engagement of a Frizzled (FZD)/LDL receptor–related protein 5/6 (LRP5/6) receptor complex. FZDs are G protein–coupled receptors, but the question of whether heterotrimeric G proteins are involved in WNT/β-catenin signaling remains unanswered. Here, we investigate whether acute activation of WNT/β-catenin signaling by purified WNT-3A requires functional signaling through heterotrimeric G proteins. Using genome editing, we ablated expression of Gs/Golf/Gq/G11/G12/G13/Gz in HEK293 (ΔG7) cells, leaving the expression of pertussis toxin (PTX)-sensitive Gi/o proteins unchanged, to assess whether WNT-3A activates WNT/β-catenin signaling in WT and ΔG7 cells devoid of functional G protein signaling. We monitored WNT-3A–induced activation by detection of phosphorylation of LDL receptor–related protein 6 (LRP6), electrophoretic mobility shift of the phosphoprotein Dishevelled (DVL), β-catenin stabilization and dephosphorylation, and TCF-dependent transcription. We found that purified, recombinant WNT-3A efficiently induces WNT/β-catenin signaling in ΔG7 cells in both the absence and presence of Gi/o-blocking PTX. Furthermore, cells completely devoid of G protein expression, so called Gα-depleted HEK293 cells, maintain responsiveness to WNT-3A with regard to the hallmarks of WNT/β-catenin signaling. These findings corroborate the concept that heterotrimeric G proteins are not required for this FZD- and DVL-mediated signaling branch. Our observations agree with previous results arguing for FZD conformation–dependent functional selectivity between DVL and heterotrimeric G proteins. In conclusion, WNT/β-catenin signaling through FZDs does not require the involvement of heterotrimeric G proteins.
Keywords: G protein, G protein-coupled receptor (GPCR), WNT pathway, cell signaling, CRISPR/Cas, beta-catenin, conformational selection, Frizzled (FZD), WNT signaling, WNT-3A
Introduction
The Wingless/Int1 (WNT)3 family of secreted lipoglycoproteins initiates an elaborate network of signaling routes through interaction of diverse cell surface receptors, such as Frizzleds, LDL receptor–related protein 5/6, receptor tyrosine kinase–like orphan receptors ROR1/2, and the receptor tyrosine kinases RYK and PTK7 (1). Whereas the 10 mammalian isoforms of FZDs (FZD1–10) are seven transmembrane–spanning G protein–coupled receptors, LRP5/6, ROR1/2, RYK, and PTK7 are single transmembrane–spanning cell surface receptors for WNTs. The best-studied WNT pathway, the WNT/β-catenin pathway, is initiated through WNT interaction with FZD and LRP5/6 (Fig. 1) (1, 2). In the absence of WNTs, a constitutively active destruction complex leads to glycogen synthase kinase 3–mediated phosphorylation and proteasomal degradation of β-catenin, thereby maintaining low cytosolic β-catenin levels. WNT stimulation of the pathway leads to FZD/LRP5/6 signalosome formation, which includes phosphorylation of LRP5/6 and recruitment of the scaffold protein disheveled (DVL). Most importantly, formation of the WNT/FZD/LRP5/6/DVL complex at the plasma membrane results in the inactivation of the β-catenin destruction complex, manifesting in reduced glycogen synthase kinase 3 phosphorylation of β-catenin, increasing its cytosolic stabilization and nuclear translocation. In the nucleus, β-catenin acts as a transcriptional regulator for TCF/LEF transcription factors (2, 3). Because FZDs are heptahelical receptors, which function as G protein–coupled receptors (4), the question of the involvement of heterotrimeric G proteins in the WNT/β-catenin pathway was raised many years ago and has been a question of intense debate ever since (5, 6). On the one hand, experimental studies suggest that heterotrimeric G proteins participate in WNT-induced signal transduction to β-catenin–mediated transcriptional regulation (7–11) (for a recent review, see Ref. 4). On the other hand, an active FZD conformation that mediates G protein signaling is incapable of interacting with DVL and inducing DVL-dependent β-catenin–dependent or –independent WNT signaling (12). This conformational selection of pathway initiation indeed questions the involvement of heterotrimeric G proteins in WNT/β-catenin signaling. To answer the longstanding question about the potential role of heterotrimeric G proteins in the WNT-3A–induced β-catenin pathway, we here employ a recently developed cell system devoid of functional G protein signaling.
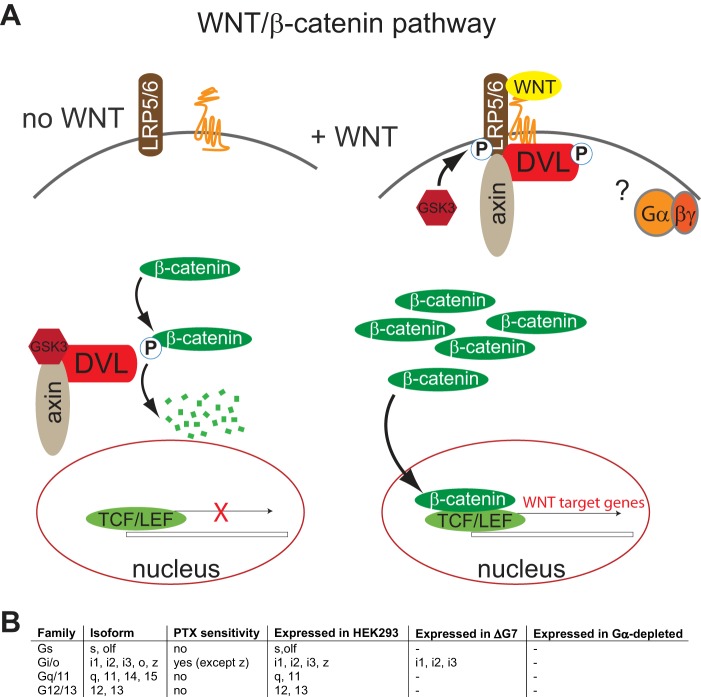
Figure 1.
WNT/β-catenin signaling and the potential role of heterotrimeric G proteins. A, the scheme presents an overview of the main players in the WNT/β-catenin pathway. In the subsequent experiments, hallmarks of this pathway serve as immunoblotting– and reporter assay–based measures of pathway activation, including P-LRP6, PS-DVL, β-catenin stabilization, β-catenin dephosphorylation, and TOPFlash activity, a luciferase-based transcriptional reporter assay for TCF/LEF activity. B, the table summarizes information about the family of heterotrimeric G proteins and their PTX sensitivity and expression in parental HEK293 cells, the ΔG7 HEK293 cells and the Gα-depleted cells, which underwent extensive genome editing to create a cell system without heterotrimeric G proteins functionally coupling to their receptors.
Results
ΔG7 HEK293 cells present a useful model to study signaling through heterotrimeric G proteins
HEK293 cells express a plethora of functional GPCRs, including FZDs, and a diverse set of heterotrimeric G proteins and modulators of G protein signaling (Fig. 1) (13). Whereas the possibilities to inhibit heterotrimeric G proteins pharmacologically are limited to PTX (selective for Gi/o proteins except for Gz) and recently discovered Gq/11 inhibitors (14, 15), genome editing has provided a valuable tool to assess GPCR coupling selectivity in HEK293 cells (16). Previously, ΔG6 cells, devoid of Gs/olf, Gq/11, and G12/13, were characterized in detail (17). These cells still express the Gi/o family of heterotrimeric G proteins, including the PTX-insensitive Gz. Here, we employ ΔG7 cells, which were directly derived from ΔG6 cells (17) and which are devoid of Gs/olf, Gq/11, G12/13, and Gz. Thus, in combination with PTX, ΔG7 cells provide a cellular “G protein–null” system devoid of functional heterotrimeric G proteins (Fig. 1).
WNT-3A induced hallmarks of activation of the WNT/β-catenin pathway independent of functional G protein signaling
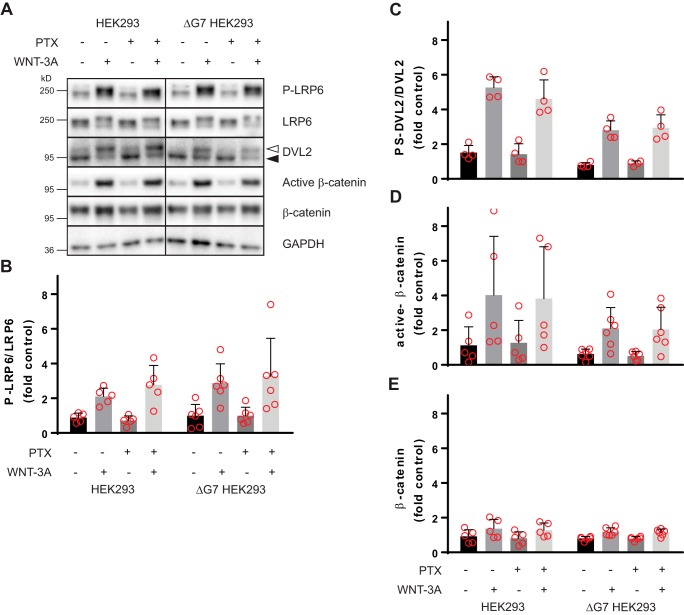
To address the longstanding question of whether heterotrimeric G proteins are required to mediate WNT-induced β-catenin signaling or not, we compared parental HEK293 cells with ΔG7 HEK293 cells in the absence and presence of Gi/o-blocking PTX treatment (100 ng/ml, overnight). Thereby, we created conditions that allow signaling through (i) all endogenously expressed heterotrimeric G proteins (parental HEK293 cells without PTX), (ii) all PTX-insensitive G proteins (parental HEK293 cells with PTX), (iii) all PTX-sensitive G proteins (ΔG7 HEK293 cells without PTX) and signaling (iv) in the absence of functional heterotrimeric G proteins (ΔG7 HEK293 cells with PTX). It is established that PTX-induced ADP-ribosylation of a conserved C-terminal cysteine in Gi/o proteins effectively uncouples Gi/o proteins from GPCRs (15). As shown previously for agonist-induced and Gi/o-coupled receptor–mediated G protein signaling, 50 ng/ml PTX are sufficient to completely block ligand-dependent dynamic mass redistribution, impedance, and cAMP responses in HEK293 cells (14). Therefore, 100 ng/ml PTX was chosen to sufficiently abrogate signaling through PTX-sensitive heterotrimeric G proteins to create “G protein–null” conditions in the ΔG7 cells. Stimulation with purified and recombinant WNT-3A, which activates WNT/β-catenin signaling in HEK293 and many other cells (7, 11, 18–20), was used to activate WNT/β-catenin signaling through endogenously expressed receptors. To monitor important hallmarks of WNT/β-catenin signaling in response to acute WNT-3A stimulation, we used immunoblotting-based detection of LRP6 phosphorylation (P-LRP6), the phosphorylation-dependent, electrophoretic mobility shift in DVL2 (PS-DVL), and stabilization and activation (dephosphorylation) of β-catenin (Fig. 2A). Stimulation with 300 ng/ml purified WNT-3A (2 h) induced distinct changes in P-LRP6, PS-DVL2, β-catenin levels, and β-catenin dephosphorylation in parental HEK293 cells. The experiments were performed in cells pretreated with the porcupine inhibitor C59 (10 nm; overnight) to reduce the autocrine activation of the pathway and to increase the response window upon stimulation with recombinant WNT-3A. Pathway activation after 2 h of WNT-3A exposure in parental HEK293 cells in the absence of PTX was indistinguishable from that in the presence of PTX. Furthermore, WNT-3A induced an identical WNT/β-catenin pathway activation profile in ΔG7 HEK293 cells irrespective of the presence or absence of PTX. Importantly, the amplitude and -fold increase of the WNT-3A–induced responses in the different conditions was similar irrespective of either PTX or the cell line used. Collectively, these findings argue that WNT-3A–induced WNT/β-catenin signaling in HEK293 cells does not require heterotrimeric G protein signaling.
Figure 2.
WNT-3A activates the WNT/β-catenin pathway in the absence of functional heterotrimeric G protein signaling. Parental HEK293 or ΔG7 HEK293 cells were stimulated with purified, recombinant WNT-3A (300 ng/ml, 2 h) in the absence and presence of PTX (100 ng/ml, overnight). Autocrine WNT signaling was reduced in all experiments by porcupine inhibition with C59 (10 nm, overnight). Pathway activation was visualized by detection of LRP6 phosphorylation (P-LRP6 over total LRP6), phosphorylated and shifted DVL2 (unshifted (filled triangle) and shifted (open triangle)), active β-catenin, and total β-catenin stabilization. GAPDH served as a visual loading control. A, a representative immunoblotting experiment from 4–6 independent experiments is shown. Probing with different antibodies was done after stripping on the same cell lysates. B–E, immunoblotting experiments were quantified by densitometry and normalized to a control sample (see “Experimental procedures”), and data were summarized in bar graphs. Error bars, S.D. Results were analyzed with one-way ANOVA and Tukey's multiple-comparison post hoc test. All WNT-3A–induced increases are statistically significant compared with unstimulated conditions (p < 0.05).
WNT-3A elicited TOPFlash transcriptional activity in the absence of functional signaling through heterotrimeric G proteins
TCF/LEF transcription factors mediate the regulation of WNT-induced target genes downstream of P-LRP6, PS-DVL2, and β-catenin. The TOPFlash luciferase reporter assay has been developed as a reliable, substantially amplified readout of WNT/β-catenin pathway activation (21). To investigate the role of heterotrimeric G proteins in the responsiveness of HEK293 cells to purified WNT-3A on the level of TCF/LEF transcription, we determined the concentration–response relationship between WNT-3A and TOPFlash signal (Fig. 3A). Whereas PTX pretreatment affected the WNT-3A–induced TOPFlash response in neither parental HEK293 nor ΔG7 HEK293 cells, there was a substantial difference in the amplitude of the TOPFlash response in ΔG7 HEK293 cells compared with parental HEK293 cells. First, these results further corroborate the concept that heterotrimeric G proteins are not required for WNT-3A–induced signal transduction to TCF/LEF-mediated transcription. Second, the larger amplitude of the WNT-3A–induced TOPFlash signal in ΔG7 HEK293 cells suggested that heterotrimeric G proteins negatively regulate WNT/β-catenin signaling. Based on the nature of the assay, where the TOPFlash (firefly luciferase) signal is normalized for transfection efficiency ratiometrically by the Renilla luciferase signal, we can exclude the possibility that the different amplitude originates purely from different transfectability of the two cell lines. Thus, we further explored the possibility that TOPFlash levels corresponding to parental HEK293 cells could be restored in ΔG7 HEK293 cells by retransfection of individual Gα subunits. We monitored TOPFlash levels in PTX-treated and WNT-3A–stimulated ΔG7 HEK293 cells transfected with either WT Gα or the constitutively active QL mutant of the Gα subunit of Gs, Gi1, Gq, or G12 (Fig. 3B). However, we did not observe a reduction of the TOPFlash signal under control conditions (pcDNA, PTX, and WNT-3A) with coexpression of any of the WT or constitutively active QL-Gα. Therefore, we conclude that differences in the signal amplitude do not simply originate in the genome editing with regard to G protein expression but could rather be consequences of additional compensation and signal rewiring (22).
Figure 3.
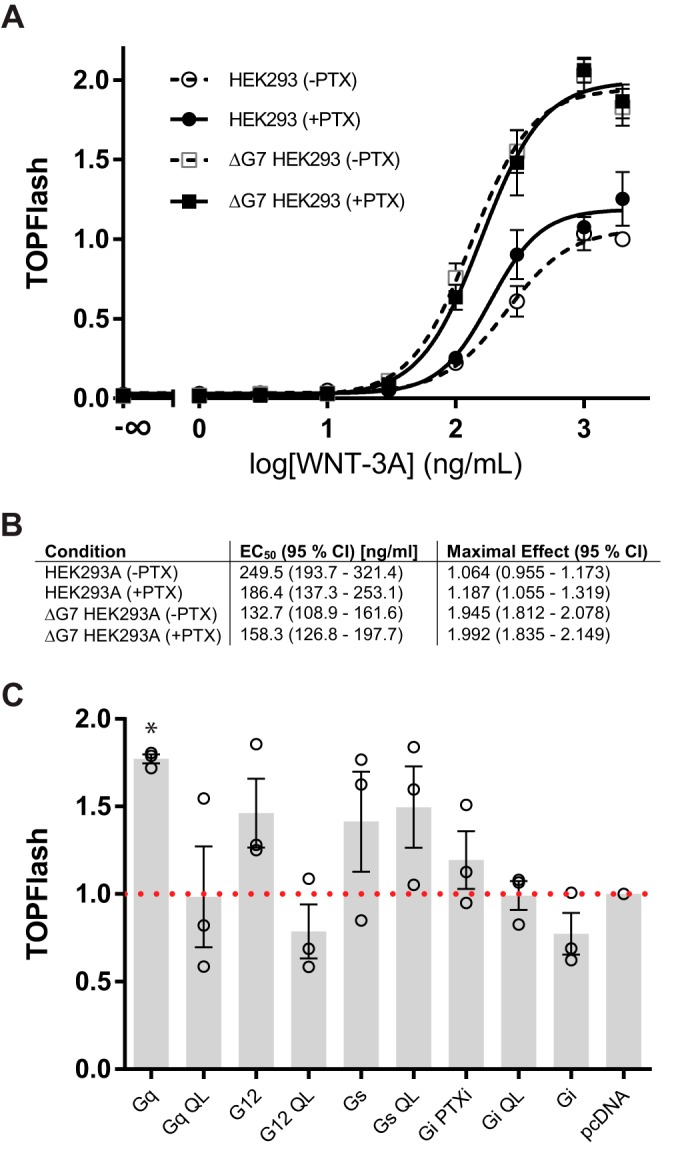
WNT-3A induces TCF/LEF transcriptional activity in the absence of functional heterotrimeric G protein signaling. A, HEK293 and ΔG7 HEK293 cells were transfected with M50 Super 8xTOPFlash and Renilla luciferase. After transfection, cells were serum-starved either in the absence or presence of PTX (100 ng/ml) for 4 h. Subsequently, cells were stimulated with increasing concentrations of purified, recombinant WNT-3A for 20 h. Luciferase data were normalized to the maximal response of parental HEK293 cells to 2000 ng/ml WNT-3A in the absence of PTX. Three independent experiments were done in duplicates. Data were fit to a four-parameter nonlinear regression using GraphPad Prism 6. Error bars, S.E. B, the table summarizes EC50 values and maximal effects of the TOPFlash signal evoked by WNT-3A under the different experimental conditions. C, TOPFlash activity was quantified in ΔG7 HEK293 cells, which were transfected with WT or constitutively active QL mutants of Gα proteins representing the four different families of G proteins (Gs, Gi1, Gq, and G12). Data are presented as the mean of four independent experiments performed in duplicates. Error bars, S.D. Data were normalized to pcDNA-transfected cells (red dotted line). Results were analyzed with one-way ANOVA and Tukey's multiple-comparison post hoc test. Significance levels are indicated as follows: *, p < 0.05, comparison with pcDNA.
WNT-3A maintains its ability to induce WNT/β-catenin signaling in Gα-depleted HEK293 cells
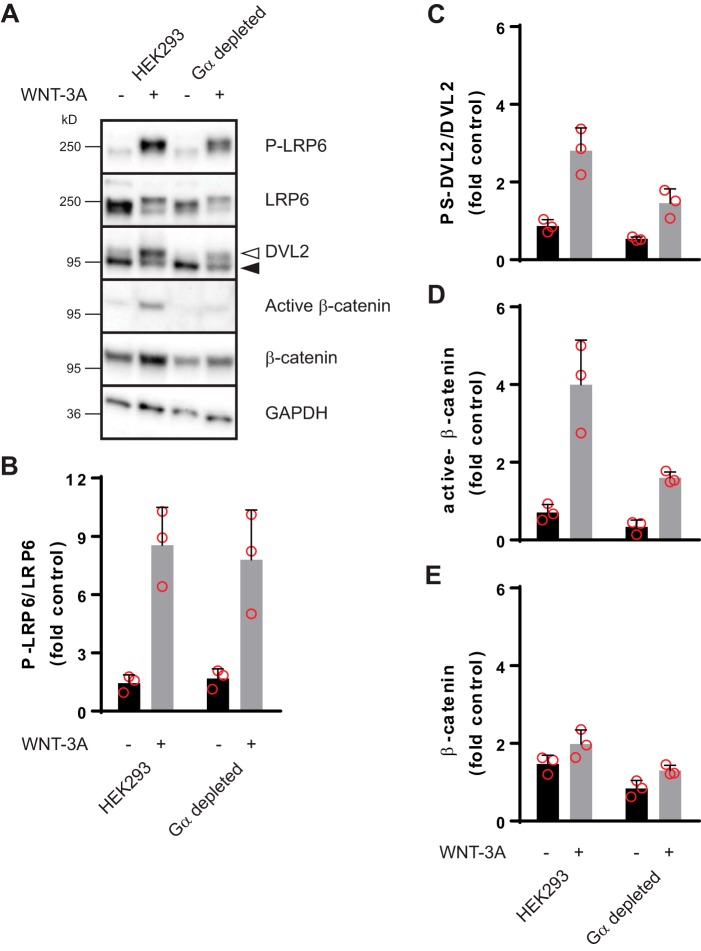
Whereas the ΔG7 cells offered the possibility to assess the role of Gi/o-independent and -dependent signaling in the WNT-3A–evoked WNT/β-catenin response, they come with the caveat that PTX-sensitive G proteins are still expressed although they are uncoupled from the GPCRs upon PTX treatment. Therefore, we complemented our experiments with a recently characterized, engineered cell line that does not express any functional heterotrimeric G proteins, the so-called Gα-depleted HEK293 cells (23). In accordance with the PTX-treated ΔG7 cells, WNT-3A maintained its ability to induce hallmarks of WNT/β-catenin signaling even in Gα-depleted HEK293 cells (Fig. 4). Despite the weaker overall levels of PS-DVL2, dephosphorylated (active) β-catenin, and total β-catenin in Gα-depleted HEK293 cells, the agonist-induced change over basal is similar in parental compared with Gα-depleted HEK293 cells. In line with the results from the ΔG7 cells, these data corroborate the conclusion that G proteins are not required for WNT-3A–induced WNT/β-catenin signaling.
Figure 4.
WNT-3A activates the WNT/β-catenin pathway in Gα-depleted HEK293 cells. Parental HEK293 or Gα-depleted HEK293 cells were stimulated with purified, recombinant WNT-3A (300 ng/ml, 2 h). Autocrine WNT signaling was reduced by porcupine inhibition with C59 (10 nm, overnight). Pathway activation was visualized by detection of LRP6 phosphorylation (P-LRP6 over total LRP6), phosphorylated and shifted DVL2 (unshifted (filled triangle) and shifted (open triangle)), active β-catenin, and total β-catenin stabilization. GAPDH served as a visual loading control. A, a representative immunoblotting experiment from three independent experiments. B–E, immunoblotting experiments were quantified by densitometry and normalized to a control sample (see “Experimental procedures”), and data were summarized in bar graphs. Error bars, S.D. Results were analyzed with unpaired Student's t test. All WNT-3A–induced increases are statistically significant compared with unstimulated conditions (p < 0.05).
Discussion
CRISPR/CAS9 genome-editing technology has provided valuable tools and cell systems to address the role of heterotrimeric G proteins in receptor-mediated cell responses (16). This methodological advance has now allowed us to conclude that heterotrimeric G proteins are not necessary to mediate major hallmarks of WNT/β-catenin signaling and TCF/LEF transcriptional activity in response to recombinant and purified WNT-3A. This stands in contrast to several reports that have used antisense technology and genetic and pharmacological means to implicate a direct, functional role of heterotrimeric G proteins in WNT/β-catenin signaling in different biological systems (7–9, 11, 24, 25).
Importantly, the present data support an emerging concept of functional selectivity downstream of WNT-stimulated FZDs clearly distinguishing between FZD–DVL and FZD–G protein routes. Whereas we have previously shown that WNT-3A leads to parallel induction of WNT/β-catenin and WNT/G protein/extracellular signal–regulated kinase 1/2 (ERK1/2) signaling in primary microglia and microglia-like cell lines, we were at that time not able to clarify whether signaling is routed through one or several FZD isoforms to achieve pathway selectivity (11, 26). Recently, however, we have discovered a conserved, molecular switch in Class F receptors that defines downstream functional selectivity through conformational selection of FZD–G protein over FZD–DVL interaction (12). This model is in good agreement with the current data set, underlining that G proteins are not necessary for the DVL-dependent WNT/β-catenin pathway.
Nevertheless, the substantial body of information about a role of heterotrimeric G protein signaling in the WNT/β-catenin pathway should be carefully re-assessed with regard to a potential modulatory function of G proteins toward WNT/β-catenin signaling. At first sight, the comparison of the concentration–response to WNT-3A in the TOPFlash readout using parental HEK293 and ΔG7 HEK293 cells suggests that the amplitude of the signal depends directly on functional expression of heterotrimeric G proteins. This simplistic explanation, however, did not withstand the control experiment of reintroducing WT or constitutive active G proteins into ΔG7 HEK293 cells. Reexpression of G proteins did not diminish the WNT-3A–induced TOPFlash response toward the levels observed in parental HEK293 cells. Furthermore, the complexity of the phenomenon is underlined by the lack of a difference between the parental and the ΔG7 HEK293 cells in the responses measured after 2 h of WNT stimulation compared with the TOPFlash responses assessed 20 h post-stimulation. Long-term stimulation allows more time for activation of compensatory mechanisms upon WNT stimulation, and basal TOPFlash levels in both cell types are similar. In addition, removal of G protein expression in the ΔG7 HEK293 cells could affect cross-talking signaling pathways, which then in turn could affect ligand efficacy in WNT/β-catenin signaling. For example, the loss of G proteins in ΔG7 HEK293 cells might sensitize the system to FZD–DVL interaction, allowing a larger WNT-induced input toward TCF. This could originate in the larger availability of FZDs for DVL interaction due to the absence of G proteins engaging in inactive state assembly with FZDs (27–29). Furthermore, it is possible that certain combinations of FZD and heterotrimeric G proteins could lead to a direct, FZD-mediated, and G protein-dependent activation of the WNT/β-catenin pathway, similar to what is known for other GPCRs (30, 31). At this point, we remain without a mechanistic explanation for the different amplitudes of the WNT-3A–induced TOPFlash signal in parental and ΔG7 HEK293 cells. On the other hand, we postulate a complex modulatory function of G proteins toward the WNT/β-catenin pathway, which will require further investigations.
In summary, we use genome editing in combination with PTX to create a cell system devoid of functional signaling through heterotrimeric G proteins. Under “G protein–null” conditions, WNT-3A was still able to induce WNT/β-catenin signaling, underlining that heterotrimeric G proteins are not necessary for this signaling branch downstream of FZDs. Our findings suggest a still poorly defined modulatory role for heterotrimeric G proteins toward WNT/β-catenin signaling and are in agreement with the recently proposed mechanism of conformational selection of pathway selectivity at the level of FZD (12).
Experimental procedures
Cell culture, transfection, and treatments
HEK293A, ΔG7 HEK293A, and Gα-depleted HEK239A cells were cultured in Dulbecco's modified Eagle's medium (HyClone SH30081) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Thermo Fisher Scientific, catalog nos. 10270106 and 15140) in a humified incubator at 37 °C and 5% CO2. All cell culture plastics were from Sarstedt unless stated otherwise. Transfection was done with Lipofectamine 2000 (Thermo Fisher Scientific, catalog no. 11668).
Generation of ΔG7 HEK293A cells by CRISPR/Cas9 system
ΔG7 HEK293A cells were derived during a process of generating fully G protein–KO cells (23). Briefly, the GNAZ gene in previously established HEK293 cells devoid of three Gα families (the Gαs, the Gαq, and the Gα12 families) was mutated by a CRISPR/Cas9 system using a GNAZ sgRNA–targeting sequence (5′-GATGCGGGTCAGCGAGTCGATGG-3′; including a NGG PAM sequence) (17). Introduction of a 5-bp deletion (frameshift mutation) into the GNAZ gene was verified by direct sequencing as described elsewhere (23).
Immunoblotting
HEK293 and ΔG7 HEK293 cells were seeded into 24-well plates at 5 × 105 cells/well, or HEK293 and Gα-depleted HEK239 cells were seeded into PDL-coated 24-well plates. 4 h after seeding, medium was changed to starvation medium with 2-[4-(2-methylpyridin-4-yl)phenyl]-N-[4-(pyridin-3-yl)phenyl]acetamide (C59; 10 nm (32, 33)) and with or without PTX (100 ng/ml) overnight. The next day, cells were stimulated for 2 h with recombinant and purified WNT-3A (300 ng/ml). Subsequently, cells were lysed in 2× Laemmli buffer and sonicated. Samples were analyzed by SDS-PAGE on 7.5% Mini-PROTEAN TGX precast polyacrylamide gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes using a Trans-Blot Turbo system (Bio-Rad). Membranes were blocked for 1 h in 5% milk in TBS-T and incubated overnight at 4 °C with the primary antibody in blocking buffer as follows: rabbit anti-P-LRP6 (1:500; Cell Signaling Technology, catalog no. 2568), rabbit anti-LRP6 (1:1000; Cell Signaling Technology, catalog no. 3395), rabbit anti-DVL2 (1:1000; Cell Signaling Technology, catalog no. 3216), mouse anti-active-β-catenin (1:500; Millipore, catalog no. 05-665), mouse anti-β-catenin (1:2000; BD Biosciences, catalog no. 610154), rabbit anti-GAPDH (1:8000; Cell Signaling Technology, catalog no. 2118). Proteins were detected with a horseradish peroxidase–conjugated secondary antibody (goat anti-rabbit or goat anti-mouse; 1:7500; Thermo Fisher Scientific, catalog nos. 31460 and 31430) and Clarity Western ECL Blotting Substrate (Bio-Rad). Densitometry was done using ImageJ 1.52h. For each membrane, the same lysate from unstimulated HEK293 cells was prepared and run in parallel with the experimental samples. The control lysate was used for normalization of each individual antibody.
TCF/LEF luciferase reporter assay (TOPFlash)
Cells were cultured in 48-well plates to 80% confluence and transiently transfected with 50 ng of M50 Super 8xTOPFlash (Addgene, catalog no. 12456) plasmid, 25 ng of pRL-TK Luc (Promega E2241) plasmid, and 175 ng of pcDNA 3.1+. 4 h after transfection, medium was changed to 180 μl of starvation medium (no fetal bovine serum) with or without PTX (111 ng/μl). After 4 h, WNT-3A was added to a final volume of 200 μl/well. 20 h after stimulation, cells were analyzed with the Dual-Luciferase reporter assay system (Promega E1910) according to the manufacturer's instructions in a white 96-well plate with solid flat bottom (Greiner Bio-One) with the following modifications. Cells were lysed in 50 μl of Passive Lysis Buffer, and 25 μl of LARII and Stop & Glo reagent was used for each well. The analysis was done on a CLARIOstar microplate reader (BMG) reading 580 ± 40 nm for firefly luciferase and 480 ± 40 nm for Renilla luciferase.
Statistical analysis
Statistical analysis was done with GraphPad Prism 6. Densitometry data were analyzed with one-way ANOVA and Tukey's multiple-comparison post hoc or unpaired Student's t test. Significance levels were determined as p < 0.05.
Author contributions
C.-F. B. and G. S. conceptualization. C.-F. B. performed all experiments, data analysis, and preparation of figures. A. I. provided validated parental and ΔG7 and Gα-depleted HEK293 cells. C. F. B. and G. S. wrote the paper. G. S. supervision, resources, and funding acquisition.
Acknowledgments
We thank Anna Krook (Department of Physiology and Pharmacology, Karolinska Institutet) for providing access to the CLARIOstar plate reader.
This work was supported by a grant from the Karolinska Institutet, Swedish Research Council Grant 2017-04676, Swedish Cancer Society Grant CAN2017/561, Novo Nordisk Foundation Grant NNF17OC0026940, Stiftelsen Olle Engkvist Byggmästare Grant 2016/193, and the Emil and Wera Cornells Stiftelse.
- WNT
- Wingless/Int-1 family of proteins
- ΔG7 HEK293 cells
- HEK293 cells devoid of Gs/olf, Gq/11, G12/13, and Gz
- DVL
- Dishevelled
- FZD
- Frizzled
- GPCR
- G protein–coupled receptor
- LRP5/6
- LDL receptor-related protein 5/6
- P-LRP6
- phosphorylated LRP6
- PTX
- pertussis toxin
- TCF/LEF
- T cell factor/lymphoid enhancer–binding factor
- ANOVA
- analysis of variance
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- P-LRP6
- LRP6 phosphorylation
- PS-DVL
- phosphorylation-dependent, electrophoretic mobility shift in DVL2.
References
- 1. Driehuis E., and Clevers H. (2017) WNT signalling events near the cell membrane and their pharmacological targeting for the treatment of cancer. Br. J. Pharmacol. 174, 4547–4563 10.1111/bph.13758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeBruine Z. J., Xu H. E., and Melcher K. (2017) Assembly and architecture of the Wnt/β-catenin signalosome at the membrane. Br. J. Pharmacol. 174, 4564–4574 10.1111/bph.14048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angers S., and Moon R. T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 10.1038/nrm2717 [DOI] [PubMed] [Google Scholar]
- 4. Schulte G., and Wright S. C. (2018) Frizzleds as GPCRs: more conventional than we thought! Trends Pharmacol. Sci. 39, 828–842 10.1016/j.tips.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 5. Malbon C. C. (2011) Wnt signalling: the case of the “missing” G-protein. Biochem. J. 433, e3–e5; Correction (2011) Biochem. J. 434, 575–575 10.1042/BJ20102111 [DOI] [PubMed] [Google Scholar]
- 6. Schulte G., and Bryja V. (2007) The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol. Sci. 28, 518–525 10.1016/j.tips.2007.09.001 [DOI] [PubMed] [Google Scholar]
- 7. Liu X., Rubin J. S., and Kimmel A. R. (2005) Rapid, Wnt-induced changes in GSK3β associations that regulate β-catenin stabilization are mediated by Gα proteins. Curr. Biol. 15, 1989–1997 10.1016/j.cub.2005.10.050 [DOI] [PubMed] [Google Scholar]
- 8. Liu T., DeCostanzo A. J., Liu X., Wang H., Hallagan S., Moon R. T., and Malbon C. C. (2001) G protein signaling from activated rat frizzled-1 to the β-catenin-Lef-Tcf pathway. Science 292, 1718–1722 10.1126/science.1060100 [DOI] [PubMed] [Google Scholar]
- 9. Katanaev V. L., Ponzielli R., Sémériva M., and Tomlinson A. (2005) Trimeric G protein-dependent frizzled signaling in Drosophila. Cell 120, 111–122 10.1016/j.cell.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 10. Koval A., Ahmed K., and Katanaev V. L. (2016) Inhibition of Wnt signalling and breast tumour growth by the multi-purpose drug suramin through suppression of heterotrimeric G proteins and Wnt endocytosis. Biochem. J. 473, 371–381 10.1042/BJ20150913 [DOI] [PubMed] [Google Scholar]
- 11. Halleskog C., and Schulte G. (2013) Pertussis toxin-sensitive heterotrimeric Gαi/o proteins mediate WNT/β-catenin and WNT/ERK1/2 signaling in mouse primary microglia stimulated with purified WNT-3A. Cell. Signal. 25, 822–828 10.1016/j.cellsig.2012.12.006 [DOI] [PubMed] [Google Scholar]
- 12. Wright S. C., Kozielewicz P., Kowalski-Jahn M., Petersen J., Bowin C. F., Slodkowicz G., Marti-Solano M., Rodríguez D., Hot B., Okashah N., Strakova K., Valnohova J., Babu M. M., Lambert N. A., Carlsson J., and Schulte G. (2019) A conserved molecular switch in Class F receptors regulates receptor activation and pathway selection. Nat. Commun. 10, 667 10.1038/s41467-019-08630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atwood B. K., Lopez J., Wager-Miller J., Mackie K., and Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12, 14 10.1186/1471-2164-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrage R., Schmitz A. L., Gaffal E., Annala S., Kehraus S., Wenzel D., Büllesbach K. M., Bald T., Inoue A., Shinjo Y., Galandrin S., Shridhar N., Hesse M., Grundmann M., Merten N., et al. (2015) The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156 10.1038/ncomms10156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sunyer T., Monastirsky B., Codina J., and Birnbaumer L. (1989) Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol. Endocrinol. 3, 1115–1124 10.1210/mend-3-7-1115 [DOI] [PubMed] [Google Scholar]
- 16. Milligan G., and Inoue A. (2018) Genome editing provides new insights into receptor-controlled signalling pathways. Trends Pharmacol. Sci. 39, 481–493 10.1016/j.tips.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 17. Grundmann M., Merten N., Malfacini D., Inoue A., Preis P., Simon K., Rüttiger N., Ziegler N., Benkel T., Schmitt N. K., Ishida S., Müller I., Reher R., Kawakami K., Inoue A., et al. (2018) Lack of β-arrestin signaling in the absence of active G proteins. Nat. Commun. 9, 341 10.1038/s41467-017-02661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bryja V., Schulte G., and Arenas E. (2007) Wnt-3a utilizes a novel low dose and rapid pathway that does not require casein kinase 1-mediated phosphorylation of Dvl to activate β-catenin. Cell. Signal. 19, 610–616 10.1016/j.cellsig.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 19. Bryja V., Gradl D., Schambony A., Arenas E., and Schulte G. (2007) β-Arrestin is a necessary component of Wnt/β-catenin signaling in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 104, 6690–6695 10.1073/pnas.0611356104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Willert K., Brown J. D., Danenberg E., Duncan A. W., Weissman I. L., Reya T., Yates J. R. 3rd, and Nusse R. (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 10.1038/nature01611 [DOI] [PubMed] [Google Scholar]
- 21. Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., and Clevers H. (1997) Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275, 1784–1787 10.1126/science.275.5307.1784 [DOI] [PubMed] [Google Scholar]
- 22. Luttrell L. M., Wang J., Plouffe B., Smith J. S., Yamani L., Kaur S., Jean-Charles P. Y., Gauthier C., Lee M. H., Pani B., Kim J., Ahn S., Rajagopal S., Reiter E., Bouvier M., et al. (2018) Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci. Signal. 11, eaat7650 10.1126/scisignal.aat7650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hisano Y., Kono M., Cartier A., Engelbrecht E., Kano K., Kawakami K., Xiong Y., Piao W., Galvani S., Yanagida K., Kuo A., Ono Y., Ishida S., Aoki J., Proia R. L., et al. (2019) Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J. Exp. Med. jem.20181895 10.1084/jem.20181895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feigin M. E., and Malbon C. C. (2007) RGS19 regulates Wnt-β-catenin signaling through inactivation of Gαo. J. Cell Sci. 120, 3404–3414 10.1242/jcs.011254 [DOI] [PubMed] [Google Scholar]
- 25. Wu C., Zeng Q., Blumer K. J., and Muslin A. J. (2000) RGS proteins inhibit Xwnt-8 signaling in Xenopus embryonic development. Development 127, 2773–2784 [DOI] [PubMed] [Google Scholar]
- 26. Kilander M. B. C., Halleskog C., and Schulte G. (2011) Purified WNTs differentially activate β-catenin-dependent and -independent pathways in mouse microglia-like cells. Acta Physiol. (Oxf.) 203, 363–372 10.1111/j.1748-1716.2011.02324.x [DOI] [PubMed] [Google Scholar]
- 27. Hot B., Valnohova J., Arthofer E., Simon K., Shin J., Uhlén M., Kostenis E., Mulder J., and Schulte G. (2017) FZD10-Gα13 signalling axis points to a role of FZD10 in CNS angiogenesis. Cell. Signal. 32, 93–103 10.1016/j.cellsig.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 28. Arthofer E., Hot B., Petersen J., Strakova K., Jäger S., Grundmann M., Kostenis E., Gutkind J. S., and Schulte G. (2016) WNT stimulation dissociates a frizzled 4 inactive-state complex with Gα12/13. Mol. Pharmacol. 90, 447–459 10.1124/mol.116.104919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kilander M. B., Petersen J., Andressen K. W., Ganji R. S., Levy F. O., Schuster J., Dahl N., Bryja V., and Schulte G. (2014) Disheveled regulates precoupling of heterotrimeric G proteins to Frizzled 6. FASEB J. 28, 2293–2305 10.1096/fj.13-246363 [DOI] [PubMed] [Google Scholar]
- 30. Nag J. K., Rudina T., Maoz M., Grisaru-Granovsky S., Uziely B., and Bar-Shavit R. (2018) Cancer driver G-protein coupled receptor (GPCR) induced β-catenin nuclear localization: the transcriptional junction. Cancer Metastasis Rev. 37, 147–157 10.1007/s10555-017-9711-z [DOI] [PubMed] [Google Scholar]
- 31. Fujino H., and Regan J. W. (2001) FP prostanoid receptor activation of a T-cell factor/β-catenin signaling pathway. J. Biol. Chem. 276, 12489–12492 10.1074/jbc.C100039200 [DOI] [PubMed] [Google Scholar]
- 32. Proffitt K. D., Madan B., Ke Z., Pendharkar V., Ding L., Lee M. A., Hannoush R. N., and Virshup D. M. (2013) Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 73, 502–507 10.1158/0008-5472.CAN-12-2258 [DOI] [PubMed] [Google Scholar]
- 33. Valnohova J., Kowalski-Jahn M., Sunahara R. K., and Schulte G. (2018) Functional dissection of the N-terminal extracellular domains of Frizzled 6 reveals their roles for receptor localization and Dishevelled recruitment. J. Biol. Chem. 293, 17875–17887 10.1074/jbc.RA118.004763 [DOI] [PMC free article] [PubMed] [Google Scholar]