Abstract
Triple-negative breast cancer (TNBC) is known to have a poor prognosis and limited treatment options, namely chemotherapy. Different molecular studies have recently classified TNBC into different subtypes opening the door to potential new-targeted treatment options. In this review, we discuss the current standard of care in the treatment of TNBC in the neoadjuvant, adjuvant and metastatic settings. In addition, we summarize the ongoing phase III clinical trials evaluating different associations between the 3 pillars of anticancer treatment: chemotherapy, targeted therapy and immunotherapy.
Keywords: breast cancer, triple-negative, immunotherapy, PARP inhibitors
Introduction
Breast cancer continues to be the second cause of death in women worldwide.1 Triple-negative breast cancer (TNBC) is defined by the lack of expression of estrogen (ER), progesterone (PR) and HER2 receptors. TNBC represents approximately 10–15% of all diagnosed breast cancers.2 The pattern of metastatic spread in TNBC is different from the other breast cancer subtypes with a higher likelihood of brain and lung involvement and less frequent bone lesions; in addition, this is the tumor subtype with the poorest prognosis between all breast cancer subtypes.3
In the current era, more in-depth studies have divided TNBC into different subtypes, according to their molecular characteristics. By analyzing gene-expression profile of TNBC, Lehman et al showed the existence of 6 different subtypes: basal-like 1 and 2, immunomodulatory, mesenchymal, mesenchymal stem-like and luminal androgen receptors.4 In a more recent study, the same authors re-classified these tumors into 4 groups: basal-like 1, basal-like 2, mesenchymal and luminal androgen receptor.5 Another classification for TNBC was suggested by Burstein et al describing four subtypes: luminal androgen receptor, mesenchymal, basal-like immune-suppressed and basal-like immune-activated.6 In the same study, the basal-like immune-activated subtype showed to be associated with good prognosis, which is compatible with the results of other studies showing better outcomes for TNBC having lymphocytic infiltration.7,8
TNBC is more often associated with hereditary conditions as compared to other breast cancer subtypes. For instance, among newly diagnosed breast cancer patients, <10% have BRCA1 or BRCA2 mutated genes but this percentage is higher among patients with TNBC with around 35% of BRCA1 and 8% of BRCA2 mutations in this population. Among BRCA1 mutation carriers, more than one-third have TNBC.9 TNBC diagnosed in women at the age of 60 years or less is considered a criterion to test for BRCA mutations.9 Tumors missing the germline mutations in BRCA1/2 but keeping the same characteristics are classified as “BRCAness.”10
In this review, we discuss the standard of care in the treatment of TNBC in the neoadjuvant, adjuvant and metastatic settings. In addition, we summarize the ongoing phase III clinical trials evaluating different associations between the 3 pillars of anticancer treatments: chemotherapy, targeted therapy and immunotherapy.
Different subtypes of TNBC
Basal-like 1 and 2 subtypes
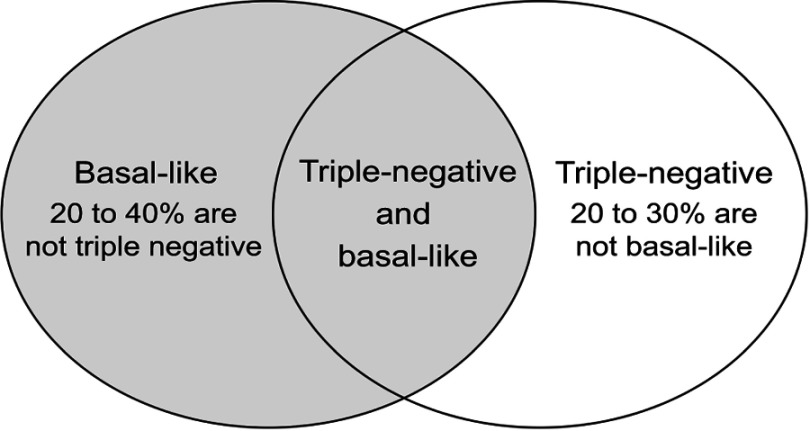
It is estimated that 75% of TNBC belong to the basal-like subtypes, and TNBC forms the largest part of the basal-like subtypes11 (Figure 1).
Figure 1.
Basal-like subtype and triple-negative breast cancer.
Basal-like 1 is associated with an elevated DNA damage response and Ki67 levels.4 Burstein et al showed that basal-like immune-suppressed subtypes of TNBC have downregulation of B cell, T cell and natural killer in both cytokines and immune pathways, which results in worse prognosis for these subtypes.6 Mostly, all cell lines harboring mutations in BRCA1 and BRCA2 have correlation with the gene patterns of the basal-like subtype.12
Luminal androgen receptor subtype
The luminal androgen receptor subtype contains pathways that regulate steroid synthesis, porphyrin metabolism and androgen/estrogen metabolism.6 In this subtype, the androgen receptor is heavily expressed, with an expression 10-fold greater than the other subtypes.4
Mesenchymal and mesenchymal stem-like subtypes
In addition to the mesenchymal subtypes having pathways included in the motility and cell differentiation, the mesenchymal stem-like subtype is characterized by having components interfering with the EGFR, calcium signaling, G-protein receptors.11
Immunomodulatory subtype
In the classification of Burstein et al,6 immunomodulatory subtype is considered as another type of basal-like subtype, ie, the basal-like immune-activated subtype. It has a favorable prognosis. It is characterized by an upregulation in genes responsible for T-cell, B-cell and natural killer, by having a high expression of STAT genes.
Standard of care in TNBC
The standard of care in patients with TNBC defined by the guidelines of the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO) is reported in this section.
Neoadjuvant treatment
The addition of carboplatin in the neoadjuvant setting showed to increase the rate of pathological complete response in TNBC from 37.0% to 52.1% (OR 1.96, 95% CI 1.46–2.62). Consequently, it can be considered a possible option in patients with TNBC at the cost of more frequent hematological toxicities.13
For patients with TNBC treated in the neoadjuvant setting but with residual disease post-chemotherapy at the time of surgery, the CREATE-X trial demonstrated improved outcomes when administering capecitabine for six to eight cycles as adjuvant treatment. Disease-free survival rate at 5 years was improved with capecitabine by around 14% (69.8% vs 56.1%; HR 0.58; 95% CI 0.39–0.87) and overall survival (OS) at 5 years was improved by around 8% (78.8% vs 70.3%; HR 0.52; 95% CI 0.30–0.90).14 The post-neoadjuvant setting has gained great attention after the publication of the CREATE-X trial and several studies are currently investigating new treatment options for patients with residual disease at the time of surgery.15
Adjuvant treatment
The vast majority of TNBC benefit from adjuvant chemotherapy with the possible exception of some low-risk histologic subtypes (secretory juvenile, apocrine, or adenoid cystic carcinomas). When adjuvant chemotherapy is indicated, anthracycline- and taxane-based regimens are considered the optimal strategy.16
Dose-dense chemotherapy is of special interest in these aggressive tumors. In fact, efficacy may be enhanced when increasing the intensity of treatment by giving individual drugs sequentially at full dose rather than in lower-dose concurrently, or by shortening the intervals between cycles. This was evaluated in an individual patient-level meta-analysis of trials comparing 2-weekly versus standard 3-weekly schedules and of trials comparing sequential versus concurrent administration of anthracycline and taxane chemotherapy. Data were provided for 26 trials including 37,298 patients, most aged younger than 70 years. It showed that fewer breast cancer recurrences were seen with dose-intense than with standard-schedule chemotherapy (10-year recurrence 28.0% vs 31.4%; RR 0.86; 95% CI 0.82–0.89). Similarly, 10-year breast cancer mortality was reduced (18.9% vs 21.3%; RR 0.87, 95% CI 0.83–0.92), as was all-cause mortality (22.1% vs 24.8%; RR 0.87, 95% CI 0.83–0.91).17
The role of platinum agents in the early setting is currently being evaluated. The US trial EA1131 is an ongoing randomized phase III post-operative trial comparing single-agent platinum-based chemotherapy to capecitabine in patients with residual TNBC with residual disease after standard neoadjuvant chemotherapy. The primary objective consists in comparing the invasive disease-free survival.18
Treatment of advanced disease
Chemotherapy
In patients with advanced TNBC treated with an anthracycline with or without a taxane in the neoadjuvant or adjuvant setting, carboplatin demonstrated comparable efficacy and a more favorable toxicity profile than docetaxel.19 In the subgroup of patients with germline BRCA1/2-mutated breast cancer, carboplatin showed to double the objective response rate as compared to docetaxel (68% vs 33%, P=0.01).19 This suggests the importance of characterizing the BRCA1/2 mutation status of patients with advanced disease to also help informing on the choices of the best first-line chemotherapy approach.
Poly ADP-ribose polymerase (PARP) inhibitors
Olaparib FDA- and EMA-approved targeted therapy
In metastatic patients harboring a germline BRCA mutation, olaparib has shown important activity in both TNBC and luminal-like disease.20–22 The OlympiAD study was designed to compare the use of olaparib versus standard single-agent chemotherapy (capecitabine, eribulin, or vinorelbine in 21-day cycles) in BRCA-mutated breast cancer patients. Among the 302 patients that underwent randomization, 205 received olaparib and 97 received standard chemotherapy. Response rate was 59.9% in patients receiving olaparib and 28.8% in patients receiving standard chemotherapy. The rate of adverse events was higher (50.6%) in the chemotherapy group versus the olaparib group (36.6%). Median progression-free survival (PFS) was 7.0 months with olaparib and 4.2 months with chemotherapy (HR 0.58; 95% CI 0.43–0.80). However, no significant difference was observed in OS that was 19.3 months with olaparib and 17.1 months with standard therapy (HR 0.90; 95% CI 0.66–1.23).23,24
Talazoparib FDA-approved targeted therapy
With a similar design as the OlympiAD study, the EMBRACA trial showed important activity for talazoparib in the treatment of metastatic breast cancer patients harboring a germline BRCA mutation including women with TNBC.25 This was a randomized open-label phase III study that included 431 patients divided into two groups: 287 patients received talazoparib and 144 received standard chemotherapy (capecitabine, eribulin, gemcitabine and vinorelbine). A significantly longer median PFS, the primary outcome of the study, was observed with the group receiving talazoparib (8.6 months vs 5.6 months; HR 0.54; 95% CI 0.41–0.71). The objective response rate was also higher in the talazoparib group than in the chemotherapy group (62.6% vs 27.2%; OR, 5.0; 95% CI, 2.9–8.8). Median OS was 22.3 months (95% CI 18.1–26.2) in the talazoparib group and 19.5 months (95% CI 16.3–22.4) in the chemotherapy group, with no significant difference (HR 0.76; 95% CI 0.55–1.06).
Respectively, hematologic grades 3–4 adverse events (primarily anemia) and nonhematologic grade 3 adverse events occurred in 55% and 32% of the patients who received talazoparib and each in 38% of the patients who received standard therapy.
In this trial, quality of life in the two treatment arms was assessed. In the patient-reported outcomes analysis, a significant overall improvement was seen in the global health status/quality of life with the use of talazoparib as compared to chemotherapy.26
Immunotherapy
Atezolizumab has shown safety and good clinical activity in TNBC.27 Chemotherapy, taxanes in particular, may enhance tumor antigens release by activating toll-like receptors and promoting dendritic cell activity.28 Based on this rationale, a phase III trial randomized patients with metastatic TNBC to first-line atezolizumab plus nab-paclitaxel and placebo plus nab-paclitaxel.29 This study had two primary end points: PFS and OS. A total of 451 patients were included in each treatment group. A better PFS was obtained in the atezolizumab plus nab-paclitaxel group (7.2 months vs 5.5 months; HR 0.80; 95% CI 0.69–0.92). OS with atezolizumab plus nab-paclitaxel was 21.3 months as compared to 17.6 months in the chemotherapy alone group (HR 0.84; 95% CI 0.69–1.02). A predefined subgroup analysis showed a greater benefit with the addition of immunotherapy among patients having PD-L1 positive tumors: median PFS was 7.5 months versus 5.0 months (HR 0.62; 95% CI 0.49–0.78) and median OS was 25.0 months versus 15.5 months (HR 0.62; 95% CI 0.45–0.86) favoring the group receiving atezolizumab plus nab-paclitaxel. Recently, the combination of atezolizumab plus nab-paclitaxel has been approved by FDA as first-line therapy in patients with PD-L1 positive TNBC.30
The results of several ongoing trials are awaited to further investigate the role of immunotherapy for the treatment of patients with TNBC in all disease settings (Table 1).
Table 1.
Ongoing phase III clinical trials with immunotherapy in patients with triple-negative breast cancer
| ClinicalTrials.gov identifier | Number of patients | Management options | Class of treatment | Study arms | Primary outcome |
|---|---|---|---|---|---|
| NCT02954874 | 1000 | Adjuvant | Immunotherapy | Arm I: no treatments Arm II: pembrolizumab |
Invasive disease-free survival |
| NCT03498716 | 2300 | Adjuvant | Immunotherapy + chemotherapy | Atezolizumab + adjuvant Antracycline/Taxane versus Chemotherapy alone | Invasive disease-free survival |
| NCT03197935 | 204 | Neoadjuvant | Immunotherapy + chemotherapy | Atezolizumab and chemotherapy versus placebo and chemotherapy | Percentage of participants with pCR and percentage of participant with pCR in sub-population with PD-L1 positive |
| NCT03281954 | 1520 | Neoadjuvant and adjuvant | Immunotherapy + chemotherapy | Neoadjuvant chemotherapy + atezolizumab versus placebo and adjuvant atezolizumab versus placebo | Pathologic complete response in the breast and lymph nodes and event-free survival |
| NCT03036488 | 1175 | Neoadjuvant and adjuvant | Immunotherapy + chemotherapy | Pembrolizumab plus chemotherapy versus placebo + chemotherapy in neoadjuvant setting and pembrolizumab versus placebo in adjuvant setting | pCR and event-free survival |
| NCT03125902 | 540 | Metastatic | Immunotherapy + chemotherapy | Atezolizumab and paclitaxel versus placebo and paclitaxel | Progression-free survival |
| NCT03371017 | 350 | Metastatic | Immunotherapy + chemotherapy | Atezolizumab versus placebo | Overall survival |
Abbreviations: pCR, pathologic complete response; PD-L1, programmed death-ligand 1.
Promising agents in TNBC
PARP inhibitors beyond olaparib/talazoparib and the metastatic setting
Several other PARP inhibitors beyond olaparib and talazoparib are currently under investigation for the treatment of patients with BRCA-mutated breast cancer.31 Veliparib has been investigated in breast cancer patients with metastatic disease in combination with chemotherapy. A significant anti-tumor effect was shown with the combination of veliparib plus temozolomide.32 In the BrighTNess phase III randomized trial, the addition of veliparib to carboplatin and standard neoadjuvant chemotherapy did not show any advantage related to pathologic complete response compared to carboplatin and standard chemotherapy.33
To investigate the activity of PARP inhibitors in TNBC patients, both in the adjuvant and the post-neoadjuvant settings, the phase III OLYMPIA trial (ClinicalTrials.gov identifier: NCT02032823) is currently randomizing early HER2-negative breast cancer patients harboring BRCA germline mutations to 1 year of olaparib or placebo after surgery and standard chemotherapy.15
Androgen receptor inhibitors
In a study by Gucalp et al,34 242 patients with TNBC were tested for androgen receptors, and 12% of them were found positive. This phase II study used single-agent bicalutamide showing a 6-month clinical benefit rate of 19% (95% CI 7–39%).
Enzalutamide was also studied in a subset of TNBC tumors with expression of androgen receptors.35 In the overall population, the study showed a clinical benefit rate at 16 weeks of 25% (95% CI 17–33%). In a biomarker exploratory analysis, patients having positive androgen-driven gene signatures showed greater clinical benefit rate (39% vs 11%).
Trials are ongoing to assess enzalutamide in this setting. As an example, the NCT02750358 trial is designed to determine the feasibility of adjuvant enzalutamide for the treatment of patients with TNBC.
Antibody–drug conjugates
Sacituzumab govitecan is an antibody–drug conjugate in which SN-38 (an active metabolite of the topoisomerase I inhibitor, irinotecan) is coupled to a monoclonal antibody targeting anti-trophoblast cell-surface antigen 2 (Trop-2).36 Trop-2, stimulates cancer-cell growth and is detected in breast cancer cells, including TNBC.36 This molecule allows the delivery of the drug to the tumors both intracellularly and in the tumor microenvironment. The safety and efficacy of this treatment were evaluated in a phase I/II trial in patients with TNBC that have received a median of 3 previous therapies.36 Overall response rate was 33.3% and clinical benefit rate was 45.4%. Median PFS was 5.5 months and median OS was 13.0 months.37
A randomized phase III trial (ASCENT, NCT02574455) is currently comparing sacituzumab govitecan to standard chemotherapy in the treatment of patients with metastatic TNBC with prior exposure to taxane.
Additional therapeutic agents
TNBC is considered having a high prevalence of PI3K/AKT pathway activation.38 The LOTUS trial was a randomized phase II trial that investigated the addition of ipatasertib (an orally administered, ATP-competitive, selective AKT inhibitor) to paclitaxel as first-line treatment.39 Patients were randomized to ipatasertib and paclitaxel vs paclitaxel and placebo. Median PFS was 6.2 months in the group receiving ipatasertib vs 4.9 months in the group receiving paclitaxel and placebo (HR 0.60; 95% CI 0.37–0.98). Further investigations are ongoing in the NCT03337724 phase III trial evaluating the efficacity of ipatasertib with paclitaxel versus paclitaxel and placebo in 450 participants.
AZD5363 is a highly selective, oral, small molecule AKT inhibitor, that is being investigated in addition to paclitaxel as first-line therapy for TNBC. Results presented at ASCO 2018 showed that the combination resulted in a significantly longer PFS with a median PFS of 5.9 compared to 4.2 months, and longer OS with a median OS of 19.1 vs 12.6 months (HR 0.64; 95% CI 0.40–1.01). AZD5363 warrants further investigation for the treatment of TNBC.40
Conclusion
TNBC is a heterogeneous disease characterized by many subtypes that differ in natural history and may be candidates to different treatment options. Besides standard anthracycline- and taxane-based chemotherapy, recent studies have better elucidated the potential role of platinum agents in both the neoadjuvant and metastatic settings. In patients with germline BRCA mutations, PARP inhibitors have proved to be effective treatment options in the metastatic setting and are currently being explored in the early setting. Immunotherapy also proved to be effective and has recently become a standard of care in metastatic breast cancer.
Several new promising treatment options are under active evaluation in many clinical trials. Among them, the most promising strategies include androgen receptor-inhibitors, antibody–drug conjugate (eg, sacituzumab govitecan) and AKT inhibitors.
After many years without breakthroughs in the field of TNBC and with chemotherapy remaining the only treatment option in this setting, several promising agents are becoming available or are in late stage of clinical development giving hope for a more personalized therapy also in patients with this breast cancer subtype.
Acknowledgment
Matteo Lambertini acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium) during the writing of this article.
Disclosure
Matteo Lambertini served as a consultant for Teva and received honoraria from Theramex and Takeda outside the submitted work. All remaining authors declared no conflicts of interest in this work.
References
- 1.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43–46. doi: 10.7314/apjcp.2016.17.4.2119 [DOI] [PubMed] [Google Scholar]
- 2.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9 [DOI] [PubMed] [Google Scholar]
- 3.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11(6):e0157368. doi: 10.1371/journal.pone.0157368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–1698. doi: 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast 2017;35:142–150. doi: 10.1016/j.breast.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. doi: 10.1200/JCO.18.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peshkin BN, Alabek ML, Isaacs C. BRCA1/2 mutations and triple negative breast cancers. Breast Dis. 2010;32(1–2). doi: 10.3233/BD-2010-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–819. doi: 10.1038/nrc1457 [DOI] [PubMed] [Google Scholar]
- 11.Hubalek M, Czech T, Müller H. Biological subtypes of triple-negative breast cancer. Breast Care. 2017;12(1):8–14. doi: 10.1159/000455820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefansson OA, Jonasson JG, Johannsson OT, et al. Genomic profiling of breast tumours in relation to BRCA abnormalities and phenotypes. Breast Cancer Res. 2009;11(4):R47. doi: 10.1186/bcr2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497–1508. doi: 10.1093/annonc/mdy127 [DOI] [PubMed] [Google Scholar]
- 14.Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147–2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 15.Caparica R, Lambertini M, Pondé N, Fumagalli D, de Azambuja E, Piccart M. Post-neoadjuvant treatment and the management of residual disease in breast cancer: state of the art and perspectives. Ther Adv Med Oncol. 2019;11:1758835919827714. doi: 10.1177/1758835919827714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393(10179):1440–1452. doi: 10.1016/S0140-6736(18)33137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platinum based chemotherapy or capecitabine in treating patients with residual triple-negative basal-like breast cancer following neoadjuvant chemotherapy – full text view – ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT02445391. Accessed March23, 2019.
- 19.Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24(5):628. doi: 10.1038/s41591-018-0009-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6 [DOI] [PubMed] [Google Scholar]
- 22.Poggio F, Bruzzone M, Ceppi M, et al. Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open. 2018;3(4):e000361. doi: 10.1136/esmoopen-2018-000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450 [DOI] [PubMed] [Google Scholar]
- 24.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. doi: 10.1093/annonc/mdz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ettl J, Quek RGW, Lee K-H, et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: patient-reported outcomes from the EMBRACA phase III trial. Ann Oncol. 2018;29(9):1939–1947. doi: 10.1093/annonc/mdy257 [DOI] [PubMed] [Google Scholar]
- 27.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a Phase 1 study. JAMA Oncol. 201. 9;5(1):74–82. doi: 10.1001/jamaoncol.2018.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi: 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 30.Esteva FJ, Hubbard-Lucey VM, Tang J, Pusztai L. Immunotherapy and targeted therapy combinations in metastatic breast cancer. Lancet Oncol. 2019;20(3):e175–e186. doi: 10.1016/S1470-2045(19)30026-9 [DOI] [PubMed] [Google Scholar]
- 31.Livraghi L, Garber JE. PARP inhibitors in the management of breast cancer: current data and future prospects. BMC Med. 2015;13(1):188. doi: 10.1186/s12916-015-0425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palma JP, Wang Y-C, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15(23):7277–7290. doi: 10.1158/1078-0432.CCR-09-1245 [DOI] [PubMed] [Google Scholar]
- 33.Loibl S, O’Shaughnessy J, Untch M, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. doi: 10.1016/S1470-2045(18)30111-6 [DOI] [PubMed] [Google Scholar]
- 34.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884–890. doi: 10.1200/JCO.2016.71.3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardia A, Mayer IA, Diamond JR, et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan(IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2017;35(19):2141–2148. doi: 10.1200/JCO.2016.70.8297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. doi: 10.1056/NEJMoa1814213 [DOI] [PubMed] [Google Scholar]
- 38.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–3815. doi: 10.1200/JCO.2014.59.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360–1372. doi: 10.1016/S1470-2045(17)30450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid P, Abraham J, Chan S, et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J Clin Oncol. 2018;36(15_suppl):1007. doi: 10.1200/JCO.2018.36.15_suppl.100729432078 [DOI] [Google Scholar]