Abstract
Peste‐des‐petits‐ruminants (PPR) and Goat pox (GTP) are two devastating and economically important transboundary animal diseases of small ruminants in Africa and Asia that have been difficult to control. This study however, investigated an outbreak of PPR and GTP in a mixed flock of indigenous sheep and goats in Kanam, North Central Nigeria. A total of nine sera and seven tissues (lungs, spleen, scab and skin) samples were collected and analysed in the laboratory using competitive enzyme linked immunosorbent assay (cELISA) for PPR antibodies and polymerase chain reaction (PCR) for detection of PPR virus (PPRV) and GTP virus (GTPV). Gene fragments of the nucleoprotein of PPRV and the G‐protein‐coupled chemokine receptor (GPCR) of GTPV were amplified and sequenced to confirm the presence of the causative viruses. Serologically, antibodies to PPRV were detected in all (9/9) sera collected. GTPV and PPRV was detected in corresponding samples (42.8% n = 3/7) of the scab/skin samples collected by both PCR and RT‐PCR technique. The phylogenetic analysis of PPRV revealed that the virus belongs to lineage IV and clustered with viruses from Gabon and Cameroon. Similarly, the GTPV also clustered with other sequences from Burkina Faso and Yemen. The positive cELISA, RT‐PCR and PCR results from samples collected from the same animals confirmed co‐infection of PPR and GTP in this mixed flock of sheep and goats. This is the first report of concurrent infection of PPR and GTP in mixed flock of sheep and goats in Nigeria. Our findings underscore the need for farmers to vaccinate their flock to control spread and economic losses as result of these diseases.
Keywords: co‐infection, peste‐des‐petits‐ruminants, goat pox, mixed flock, Nigeria
Introduction
Peste‐des‐petits‐ruminants (PPR) also known as goat plague is a severe economically, high contagious and transboundary viral disease of sheep and goats (Luka et al. 2011). PPR virus (PPRV) the causative agent of PPR is a single stranded non‐segmented RNA virus classified under the genus Morbillivirus within the family Paramyxoviridae (Kumar et al. 2014). PPR is characterised by pyrexia, diarrhoea, respiratory distress and muco‐purulent oculo‐nasal discharge with matting of the eyelids (Luka et al. 2011). Similarly, Goat pox (GTP) is also a disease of sheep and goats characterised by pyrexia, generalised skin and internal pox lesions and lymphadenopathy (Rao & Bandyopadhyay 2000). The GTP virus (GTPV) is an enveloped, double‐stranded DNA virus that belongs to the genus Capripoxvirus and family Poxviridae (Tulman et al. 2002). PPR and GTP are both endemic in the same regions of the World which includes: Middle East, Asia and Africa (Boshra et al. 2015). The two diseases are highly contagious among small ruminants causing huge economic losses due to their high morbidity and mortality rates (Boshra et al. 2015). Mixed natural infections involving PPRV and GTPV or PPRV and contagious ecthyma (Orf) has been reported in Asia (Malik et al. 2011), but mixed infection of PPR and GTP in Nigeria has not been investigated. Our study investigated an outbreak of co‐infection of PPR and GTP in a mixed flock of indigenous sheep and goats in Kanam, Local Government Area, Plateau State, Nigeria.
Case report
Case history
In October 2016, a suspected outbreak of PPR and GTP was reported at Tuntung, Plateau state, Nigeria which is located at latitude 9°5′–12°6′N and longitude 8°3′–11°6′E. Tuntung is an agrarian community with most households rearing livestock as a source of income. A livestock owner reported high morbidity and mortality in his flock of indigenous sheep and goats with an estimated flock size of 500 Yankasa breed of sheep and 250 Kano brown breed of goats. The animals were kept under extensive husbandry system without feed supplementation and history of vaccination against PPR and GTP. Twenty (n = 20) sheep and 60 goats that were showing clinical signs suggestive of PPR and GTP were examined.
Sample collection and post mortem examination
Samples were collected from sheep and goats showing clinical signs suggestive to be PPR or GTP, sera and skin scab samples were collected from sheep (2) and goats (5). Post mortem (PM) examination was carried out on a dead goat reported during the field investigation and tissues samples (lungs, spleen and skin) were collected. The samples were transported to the National Veterinary Institute Vom, Nigeria for laboratory examination.
Laboratory investigations
All sera collected were analysed for specific antibodies to PPRV using competitive enzyme linked immuo‐sorbent assay (cELISA) test kit obtained from IDvet (IDvet®, Grabels, France). The test was performed according to the manufacturer's instructions. DNA and RNA were extracted from homogenates of skin, scab and lungs using QIAamp® DNA and QIAamp® Viral Mini Kits (Qiagen, Hilden, Germany) following the manufacturer's instruction. Lyophilized freeze‐dried GTP and PPR vaccines (National Veterinary Research Institute, Vom) were included as positive controls. Detection of GTPV was carried out by targeting the RPO30 gene of the virus (Lamien et al. 2011; Le Goff et al. 2009). Similarly, the nucleoprotein gene of PPRV was used for the detection of the virus (Couacy‐Hymann et al. 2002). The polymerase chain reaction (PCR) products were sequenced by Inqaba Biotech®, (South Africa). Sequence analysis and phylogenetic relationships were determined for both using MEGA 7 version 7.1 (Kumar et al. 2016) with 1000 bootstrap replicates.
Results
The clinical signs observed during the field investigation in the mixed flock of sheep and goats were fever (40.5–41°C), muco‐purulent oculo‐nasal discharges (with 56.7% of the goats and 19% of sheep affected), coughing, respiratory dyspnoea, scab and vesicles around the mouth. Other clinical signs observed include mastitis and pox lesions on the mammary gland (in 1.2% of the goats only), generalised pox lesions (in 57.03% of the goats and 29.2% of the sheep) (Fig. 1a–d). The estimated morbidity rates were 85.1% in goats and 55.2% in sheep. While mortality rates were 49% (245/500) in the flock of sheep and 64.4% (161/250) in the flock of goats. The age range of the goats was <1 month–5 years, while the ages of the sheep was 2 months–5 years. The PM examination revealed nodular skin lesions, pox lesions on the lungs and gastro intestinal tract with fatty degeneration were observed. The cELISA result revealed that all (9/9) sera samples collected were positive for PPRV antibodies. The sera samples were collected from sheep (2) and goats (7). Of the seven tissue/scab samples (one skin, four scab, one spleen and one lung samples) analysed for GTP, 42.8% (3/7) of the samples collected detected GTPV by PCR (Fig. 2b). The RT‐PCR results revealed that PPRV was detected in all the samples (2/2) analysed, which were the lungs and spleen samples collected from one dead goat. Furthermore, the results also revealed that both PPR and GTP were confirmed by cELISA and PCR in sheep (2) and goats (3). Likewise in one goat sample, PPR was confirmed by cELISA and RT‐PCR, while the GTP was confirmed by PCR in the same. The phylogenetic analysis of the circulating PPRV revealed that it belongs to lineage IV where the sequence obtained clustered with other virus sequences from Nigeria, Morrocco, China and Cameroon (Fig. 3). Phylogenetic tree of GTPV Kanam, Nigeria revealed similarities with GTP viruses from Burkina Faso and Yemen as presented in Fig. 4.
Figure 1.
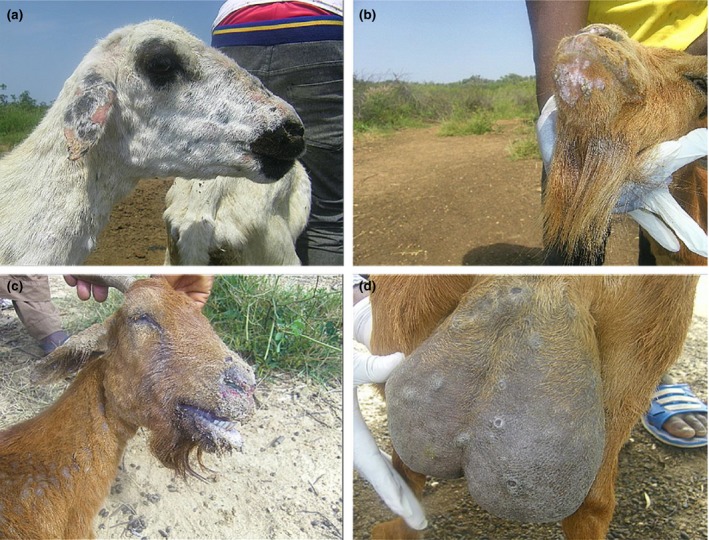
(a) A sheep showing nodular skin lesion of goat pox in the flock. (b) Goat pox lesions in a goat. (c) Clinical sign of PPR in one of the goat with muco‐purulent oculo‐nasal discharges. (d) Mastitis and pox lesions in another goat in the mixed flock. PPR, peste‐des‐petits‐ruminants.
Figure 2.
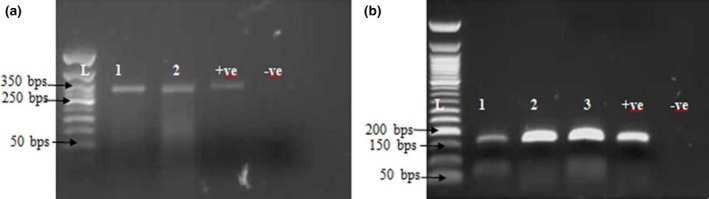
(a) Photograph of the RT‐PCR product of the detection of PPRV, L is the 50 bps ladder while Lanes 1–2 are the samples with +ve and –ve as the positive and negative controls respectively. The PCR product was amplified at 350 bps. (b) The PCR product of Goat pox virus, L is the 50 bps ladder, Lane 1–3 are the field samples, +ve is the positive control which the vaccine virus, while −ve is the negative control. The positive samples are amplified at 172 bps.
Figure 3.
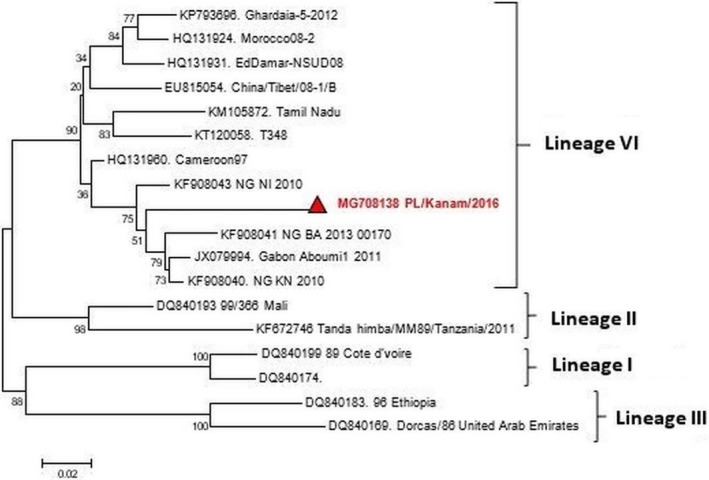
Phylogenetic analysis of the peste‐des‐petits‐ruminants virus based on nucleoprotein gene of the virus.
Figure 4.
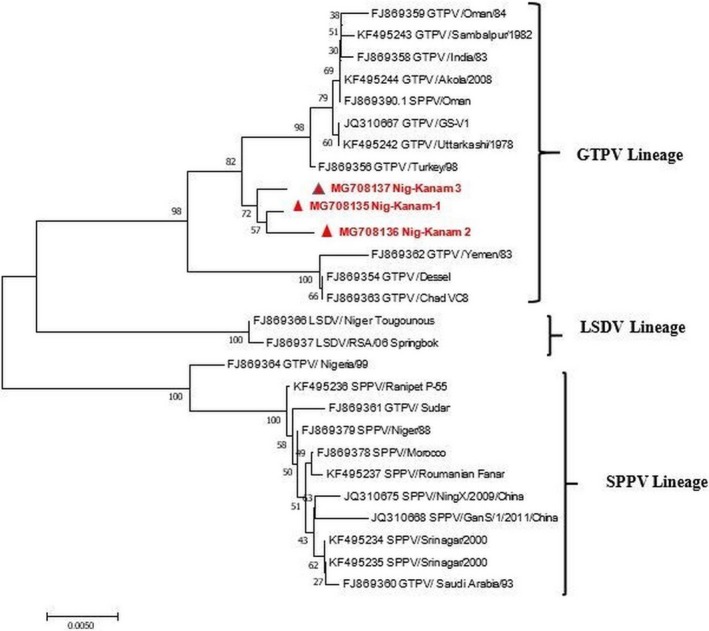
Phylogenetic analysis of the Goat pox virus based on the G‐protein‐coupled chemokine receptor (GPCR) gene of the virus.
Discussion
Peste‐des‐petit ruminants and GTP are transboundary small ruminants' diseases of socio‐economic relevance to farmers in sub Saharan Africa. Our study reported a case of co‐infection of PPR and GTP in a mixed flock of indigenous sheep and goats in Tutung, Plateau state, Nigeria. The mixed infections were confirmed by the detection of antibodies and viral antigen of PPR and viral antigen only of GTP by PCR respectively. GTPV was confirmed by amplifying the RPO30 gene with an expected band size of 172 bps confirmed that we were dealing with GTPV and not sheep pox virus which is amplified at 150 bps (Lamien et al. 2011). Interestingly, given the fact that it was a mixed flock, both sheep and goats were affected. The clinical signs of generalised pox lesions, muco‐purulent oculo‐nasal discharges, high morbidity and mortality observed in the flock of sheep and goats are characteristic of GTP and PPR (Babiuk et al. 2008; Boshra et al. 2015). Based on the history obtained from the livestock owner, the animals were never vaccinated against either PPR or GTP, therefore, the antibodies/antigen of PPRV and GTPV detected were from field infection and not as result of vaccination. The high mortality rate observed in the sheep (49%) and goats (64.4%) recorded in the mixed flock may be attributed to exacerbating effect of either PPR or GTP which are reported to individually cause severe mortalities in endemic areas (Luka et al. 2011; Malik et al. 2011). High mortalities of 74.6% and 84% have been reported by Malik et al. (2011) and Karim et al. (2016) respectively in co‐infections of PPR and GTP in India among flocks of goats. Mixed infections are a common occurrence in livestock production with severe economic consequences compared to singular infections (Malik et al. 2011). Additionally, previous reports of co‐infections of PPR and GTP were in only goats not in mixed flock of sheep and goats, although Ramakrishnan et al. (2016) reported GTP in a mixed flock of Sheep and goats in India. The phylogenetic analysis revealed that the PPRV obtained clustered with viruses in lineage IV similar to those from Cameroon and Gabon. However, our finding is worrisome, because of the increasing prevalence of lineage IV in Nigeria as earlier reported by Woma et al. (2016). However, the recent security challenges in the north eastern parts of the Nigeria and also free movement of livestock into country may be responsible for the spread and endemicity of newer strains or lineages of transboundary diseases like PPR. This study is also the first report of the characterisation of GTPV from field outbreak in Nigeria. In conclusion, this is the first report of co‐infection of PPRV and GTPV in a mixed flock of sheep and goats in Nigeria. Due to the severe socio‐economic consequences of PPR and GTP and the availability of vaccines against these diseases, farmers are encouraged to vaccinate their flocks to minimise losses.
Source of funding
The research study was funded by the authors.
Conflicts of interest
The authors of this article hereby declare that there exists no potential conflict of interest.
Ethical statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and no ethical approval was required for this case report.
Contributions
AJA, YD and PO designed the study. AJA, YD carried out the field sampling. PL, NC, JAA, TYW, ARJ, MBB, OA carried out laboratory analysis. AJA, PL and OBA drafted the manuscript and every author read and approved the manuscript.
Acknowledgement
The authors acknowledge the National Veterinary Research Institute where the laboratory investigation was done. We also acknowledge Dr. N. Kujul and Gambo Pazam that first reported the outbreak.
References
- Babiuk S., Bowden T.R., Boyle D.B., Wallace D.B. & Kitching R.P. (2008) Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transboundary and Emerging Disease 55, 263–272. [DOI] [PubMed] [Google Scholar]
- Boshra H., Truong T., Babiuk S. & Hemida M.G. (2015) Seroprevalence of sheep and goat pox, peste des petits ruminants and Rift Valley fever in Saudi Arabia. PLoS One 10, e0140328 10.1371/journal.pone.0140328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couacy‐Hymann E., Roger F., Hurard C., Guillou J.P., Libeau G. & Diallo A. (2002) Rapid and sensitive detection of peste des petits ruminants virus by a polymerase chain reaction assay. Journal of Virological Methods 100, 17–25. [DOI] [PubMed] [Google Scholar]
- Karim A., Bhattacharjee U., Puro K., Shakuntala I., Sanjukta R., Das S. et al (2016) Detection of Peste des petits ruminants virus and goatpox virus from an outbreak in goats with high mortality in Meghalaya state, India. Veterinary World 9, 1025–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Maherchandani S., Kashyap S.K., Singh S.V., Sharma S., Chaubey K.K. & Ly H. (2014) Peste des petits ruminants virus infection of small ruminants: a comprehensive review. Viruses 6, 2287–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura T. (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamien C.E., Le Goff C., Silber R., Wallace D.B., Gulyaz V., Tuppurainen E. et al (2011) Use of the capripoxvirus homologue of vaccinia virus 30 kDa RNA polymerase subunit (RPO30) gene as a novel diagnostic and genotyping target: development of a classical PCR method to differentiate goat poxvirus from sheep poxvirus. Veterinary Microbiology 149, 30–39. [DOI] [PubMed] [Google Scholar]
- Le Goff C., Lamien C.E., Fakh E., Chadeyras A., Aba‐Adulugba E., Libeau G. et al (2009) Capripoxvirus G‐protein‐coupledchemokine receptor: a host‐range gene suitable for virus animal origin discrimination. Journal of General Virology 90, 1967–1977. [DOI] [PubMed] [Google Scholar]
- Luka P.D., Erume J., Mwiine F.N., Ayebazibwe C. & Shamaki D. (2011) Molecular characterization and phylogenetic study of peste des petits ruminants viruses from North central States of Nigeria. BMC Veterinary Research 7, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Y.S., Singh D., Chandrashekar K.M., Shukla S., Sharma K., Vaid N. & Chakravarti S. (2011) Occurrence of dual infection of Peste des petits ruminants and goatpox in indigenous goats of central India. Transboundary and Emerging Disease 58, 268–273. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan M.A., Santhamani R. & Pandey A.B. (2016) Capripox outbreak in a mixed flock of sheep and goats in India. Transboundary and Emerging Diseases 64, 27–30. 10.1111/tbed.12604 [DOI] [PubMed] [Google Scholar]
- Rao T.V.S. & Bandyopadhyay S.K. (2000) A comprehensive review of goat pox and sheep pox and their diagnosis. Animal Health Research Reviews 1, 127–136. [DOI] [PubMed] [Google Scholar]
- Tulman E.R., Afonso C.L., Lu Z., Zsak L., Sur J.‐H., Sandybaev N.T.U. et al (2002) The genomes of sheeppox and goatpox viruses. Journal of Virology 76, 6054–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woma T.Y., Qasim A.M., Sabi A.A., Abraham M.N., Olaiya O.D., Bailey D. et al (2016) Co‐circulation of peste‐des‐pe tits‐ruminants virus Asian lineage IV with lineage II in Nigeria. Transboundary and Emerging Disease 63, 235–242. 10.1111/tbed.12387 [DOI] [PubMed] [Google Scholar]


