Abstract
The reduction of spermatozoa survival time is a major problem of canine chilled sperm for artificial insemination. The aim of the study was to improve the quality of canine chilled sperm during storage time. We therefore, evaluated the effects of eight treatments with different levels of soybean lecithin concentration (1, 3 and 5%) and egg yolk (20%) in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender on chilled canine sperm quality during 10 days of storage. The sperm motility was analysed by computer‐assisted sperm analysis (CASA), whereas plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential parameters were determined using a fluorescent staining combination of propidium iodide (PI), Hoechst 33342 (H342), fluorescein isothiocyanate‐conjugated Pisum sativum agglutinin (FITC‐PSA) and 5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethylbenzimidazolyl‐carbocyanine iodide (JC‐1) by confocal laser scanning microscope. The results showed that egg yolk was found to be better than soybean lecithin in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender for maintaining the quality of chilled canine sperm within 10 days of storage (P < 0.05). Although egg yolk in Tris‐citric‐fructose extender could maintain the motility better than other extenders, egg yolk in Tris‐citric‐fructose‐mineral salts extender was the highest in intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential (P < 0.05). In contrast, the sperm quality of soybean lecithin in Tris‐citric‐fructose‐mineral salts extender was lower than that of soybean lecithin in Tris‐citric‐fructose extender, and soybean lecithin 1% was greater than soybean lecithin 3% and 5% in plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential (P < 0.05). In conclusion, soybean lecithin cannot replace egg yolk in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extenders, and egg yolk in Tris‐citric‐fructose‐mineral salts extender is superior to other extenders in chilling canine sperm.
Keywords: canine, sperm, egg yolk, soybean, extender
Introduction
The oestrous cycle of bitches is different from the oestrous cycle of other domestic animals with a long inter‐oestrous interval of 6–7 months (Concannon 2011), while the oestrous cycle of sows, cows and mares is around 21 days (Frandson et al. 2009). In natural mating, however, the semen of 1 ejaculation only fertilises 1 bitch. Although the volume of canine semen is less than that of swine semen, canine sperm concentration is higher than swine sperm concentration with averages of 600 × 106 sperm mL−1 (Payan‐carreira et al. 2011) and 360 × 106 sperm mL−1 (Bajena et al. 2016) respectively. In addition, the uterus in bitches is similar in sows which have a long horn‐shaped uterus (Frandson et al. 2009). Thus, sperm dilution and artificial insemination (AI) are applicable in dogs.
To maintain the diluted sperm quality for AI techniques, sperm must be preserved by chilling or freezing (Thomassen & Farstad 2009). However, sperm cryopreservation involves specialised equipment and complicated processing than sperm chilling (Linde‐Forsberg 1991; Eilts 2005). In addition, the fertile capacity of chilled sperm is higher than that of frozen sperm (Linde‐Forsberg 1995). Hence, chilled sperm is more popular than frozen sperm in AI techniques.
The major limitation of chilled sperm is a reduced spermatozoa survival time. To improve the quality of chilled sperm during storage time, spermatozoa are diluted with an appropriate extender to provide energy, maintain pH and osmolality and protect the plasma membrane integrity, acrosome membrane integrity, mitochondrial membrane potential and DNA fragmentation against damage. In previous studies, the Tris‐citric‐fructose or glucose extender with 20% egg yolk was considered one of the most common extenders for chilled canine sperm that best maintains the quality of sperm during cooling storage (Rota et al. 1995; Ponglowhapan et al. 2004; Verstegen et al. 2005; Shahiduzzaman & Linde‐Forsberg 2007; Batista et al. 2012; Goericke‐Pesch et al. 2012; Rodenas et al. 2014). Recently, soybean lecithin used as an alternative to egg yolk in extender to avoid hygiene problems from bacterial contamination has obtained equal or superior results (Beccaglia et al. 2009a,2009b; Kmenta et al. 2011; Kasimanickam et al. 2012).
Furthermore, seminal plasma is a complex biological fluid containing ions (Na+, K+, Ca2+, Mg2+, Cl−), energy substrates (fructose, sorbitol, glycerylphosphorylcholine), and organic compounds (citric acid, amino acids, peptides, proteins, lipids, hormones, cytokines) (Wales & White 1965; Juyena & Stelletta 2012). It has crucial functions in sperm ejaculation and sperm survival in the female genital tract. The role of mineral ions is essential for maintaining osmotic balance, forming parts of principal enzymes relating to sperm metabolism and sperm function (Çevik et al. 2007; Juyena & Stelletta 2012; Smith et al. 2018). In previous studies, although canine seminal plasma has been found to be beneficial for chilled sperm plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential, it had detrimental effects on sperm motility (Rota et al. 1995; Treulen et al. 2012; Hori et al. 2017). The reduction in sperm motility is due to the decreased adenosine triphosphate (ATP) concentration in seminal plasma by acid and alkaline phosphatase activity (Günzel‐Apel & Ekrod 1991). Moreover, the centrifugation and removal of seminal plasma before diluting with extenders has been used, and no harmful effects on the function of chilled canine sperm have been found (Rota et al. 1995; Peña & Linde‐Forsberg 2000; Rijsselaere et al. 2002; Shahiduzzaman & Linde‐Forsberg 2007; Goericke‐Pesch et al. 2012). Thus, creating a new extender by adding mineral ions may increase sperm survival and improved chilled canine sperm quality without the addition of seminal plasma.
Therefore, the aim of the present study was to investigate the effects of egg yolk and soybean lecithin in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender on motility, plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential in chilled canine sperm during 10 days of storage.
Material and methods
Animals
A total of five healthy male dogs (American Bullies) aged 2–5 years were used. All dogs with proven fertility after natural mating were trained to ejaculate by digital manipulation before studying. The experiments were performed in accordance with the advice of the Institutional Animal Care and Use Committee of the Suranaree University of Technology, Nakhon Ratchasima, Thailand.
Semen collection and evaluation
Twenty ejaculates from five dogs were collected once per week by digital manipulation, and the three fractions were separated as described by Linde‐Forsberg (1991). The sperm‐rich fraction of ejaculate was deposited into prewarmed polypropylene‐calibrated tubes and placed in a water bath at 38°C. Immediately, each ejaculate was analysed to determine the semen volume, motility, concentration, viability and abnormal morphology before ejaculates of the five dogs were pooled. Only ejaculates with progressive motility >70%, sperm concentration >200 × 106 spermatozoa mL−1, sperm abnormal morphology <5% and sperm viability >90% were included in this study. The percentage of sperm progressive motility and sperm concentration were estimated using computer‐assisted sperm analysis (CASA). Sperm morphology and viability were determined using eosin‐nigrosin staining (Tamuli & Watson 1994).
Preparation of extenders
All chemicals used in this study were purchased from Sigma‐Aldrich (Singapore), and all solutions were prepared using sterile distilled water.
Extender was added to fresh semen prior to chilling and that eight semen extenders were compared and their compositions are displayed in Table 1.
Table 1.
The composition, pH and osmolality of the extenders
| Ingredients | Extenders | |||||||
|---|---|---|---|---|---|---|---|---|
| T‐EY | T‐SL1% | T‐SL3% | T‐SL5% | T‐M‐EY | T‐M‐SL1% | T‐M‐SL3% | T‐M‐SL5% | |
| Tris (mg) | 3025 | 3025 | 3025 | 3025 | 900 | 900 | 900 | 900 |
| Citric acid (mg) | 1700 | 1700 | 1700 | 1700 | 500 | 500 | 500 | 500 |
| Fructose (mg) | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 |
| NaCl (mg) | – | – | – | – | 450 | 450 | 450 | 450 |
| KHPO4 (mg) | – | – | – | – | 60 | 60 | 60 | 60 |
| KCl (mg) | – | – | – | – | 60 | 60 | 60 | 60 |
| CaHPO4 (mg) | – | – | – | – | 20 | 20 | 20 | 20 |
| MgCl2 (mg) | – | – | – | – | 10 | 10 | 10 | 10 |
| Egg yolk (mL) | 20 | – | – | – | 20 | – | – | – |
| Soybean lecithin (mg) | – | 1000 | 3000 | 5000 | – | 1000 | 3000 | 5000 |
| Gentamicin (mg) | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Distilled water (mL) | To 100 | To 100 | To 100 | To 100 | To 100 | To 100 | To 100 | To 100 |
| pH | 6.44 | 6.49 | 6.47 | 6.45 | 6.43 | 6.47 | 6.44 | 6.41 |
| Osmolality (mOsmol/kg) | 326 | 332 | 333 | 338 | 324 | 324 | 332 | 333 |
Tris‐citric‐fructose (T) buffer, pH 6.50; Osmolality, 328; Tris‐citric‐fructose‐mineral salts (T‐M) buffer, pH: 6.49; Osmolality, 325; EY, egg yolk; SL, soybean lecithin.
Therefore, there were eight extenders, including the Tris‐citric‐fructose‐egg yolk (T‐EY), Tris‐citric‐fructose‐soybean lecithin 1% (T‐SL1%), Tris‐citric‐fructose‐soybean lecithin 3% (T‐SL3%), Tris‐citric‐fructose‐soybean lecithin 5% (T‐SL5%), Tris‐citric‐fructose‐mineral salts‐egg yolk (T‐M‐EY), Tris‐citric‐fructose‐mineral salts‐soybean lecithin 1% (T‐M‐SL1%), Tris‐citric‐fructose‐mineral salts‐soybean lecithin 3% (T‐M‐SL3%) and Tris‐citric‐fructose‐mineral salts‐soybean lecithin 5% (T‐M‐SL5%). The composition of these extenders is shown in Table 1. Regarding the soybean lecithin extenders, the process of preparing the extenders was conducted with centrifuging and filtering as described by Axnér & Lagerson (2016).
Semen processing and experimental design
Pooled semen was divided into eight equal aliquots and placed in sterile tubes. The tubes were then centrifuged at 720g for 5 min, and the supernatants were discarded (Rijsselaere et al. 2002). Sperm pellets were resuspended in eight extenders to achieve the final sperm concentration of 100 × 106 spermatozoa mL−1 (Nizański et al. 2009; Batista et al. 2012). After that, equal volumes of 0.5 mL of every extended sperm were collected and put into 10 microcentrifuge tubes (1.5 mL). Then, they were placed in a plastic box containing water at 25°C. Next, extended sperm was cooled down gradually (0.3°C min−1) to 5°C for up to 1 h (Bouchard et al. 1990) and stored at 5°C during 10 days.
Sperm motility was analysed at 24‐h intervals over a period of 10 days. Plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential were evaluated every day in the first 4 days, and once every 2 days after that.
Sperm evaluation
Evaluation of sperm motility
The sperm motility was evaluated using computer‐assisted sperm analysis (CASA; Hamilton Thorne) Sperm Analyser (USA), version IVOS 14.0 (HTR‐IVOS 14.0). The technical settings are shown in supplementary material.
A volume of 5 μL of the chilled sperm samples was mounted in a 2X‐CEL counting chamber and was allowed to settle on the minitherm heating stage (38°C) before the analysis. For each sample, at least 200 spermatozoa from four randomly selected fields were evaluated. The percentage of total motility (TM%), the percentage of progressive motility (PM%), velocity average pathway (VAP), velocity straight line (VSL) and velocity curvilinear (VCL) parameters were recorded.
Evaluation of plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential
The plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential were evaluated by incubating spermatozoa with propidium iodide (PI), Hoechst 33342 (H342), fluorescein isothiocyanate‐conjugated Pisum Sativum Agglutinin (FITC‐PSA) and 5,5′,6,6′‐tetrachloro‐1,1′,3,3′‐tetraethylbenzimidazolyl‐carbocyanine iodide (JC‐1). A stock and work solution of PI, H342, FITC‐PSA and JC‐1 were prepared as described by Celeghini et al. (2007). A 100‐μL chilled sperm sample was put into a warmed microcentrifuge tube, and 10 μL of H342 (40 μg mL −1 in Dulbecco's phosphate‐buffered saline (DPBS)) was added. The mixture was incubated for 10 min at 38°C. After the incubation, 2 μL of PI (0.5 mg mL−1 in DPBS), 15 μL of JC‐1 (153 μmol L−1 JC‐1 in dimethyl sulfoxide (DMSO)) and 20 μL of FITC‐PSA (100 μg mL−1 in DPBS) were added to the sample. The sample was then incubated for 8 min at 38°C. To reduce the background fluorescence, unbound H342, PI, FITC‐PSA and JC‐1 were removed by adding 200 μL of DPBS, and spermatozoa were washed by centrifugation at 800g for 2 min (Chelucci et al. 2015). The supernatant was removed, and the pellet was resuspended in 100 μL of DPBS. After washing, an 8‐μL sample of stained spermatozoa was put on a slide and coverslipped. The slide was immediately examined by a confocal laser scanning microscope (CLSM; Nikon/Ni‐E, Japan). To evaluate the stained spermatozoa, at least 200 cells were identified in duplicate for each sample with a 60× objective lens. The spermatozoa with the intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential were PI‐ and FITC‐PSA‐negative, and H342‐ and JC‐1‐positive, while the spermatozoa with the damaged plasma membrane, damaged acrosome membrane and low mitochondrial membrane potential were PI‐ and FITC‐PSA‐positive, and H342 and JC‐1 negative (PI‐positive (+) = red‐stained nucleus; H342‐positive (+) = blue‐stained nucleus; FITC‐PSA positive (+) = yellow‐green acrosome region; JC‐1‐positive (+) = bright red‐orange in midpiece region; JC‐1 negative (−) = bright green in midpiece region). The staining standard of canine sperm in the fluorescent combination of H342, PI, FITC‐PSA and JC‐1 can be seen in Fig. 1.
Figure 1.
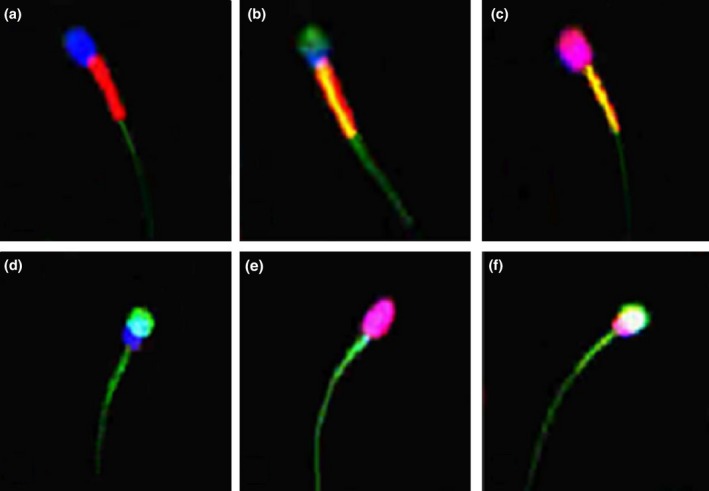
Canine spermatozoa stained with the fluorescent combination of H324, PI, FITC‐PSA and JC‐1 under a confocal laser scanning microscope (600 × magnification). (a) Intact plasma and acrosome membrane, and high mitochondrial membrane potential. (b) Intact plasma membrane, damaged acrosome membrane and high mitochondrial membrane potential. (c) Damaged plasma membrane, intact acrosome membrane and high mitochondrial membrane potential. (d) Intact plasma membrane, damaged acrosome membrane and low mitochondrial membrane potential. (e) Damaged plasma membrane, intact acrosome membrane and low mitochondrial membrane potential. (f) Damaged plasma and acrosome membrane, and low mitochondrial membrane potential.
Statistical analysis
Statistical analyses were performed with SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL, USA). All data are provided as mean ± standard deviation (SD). The Kolmogorov–Smirnov test was used for normality analysis of the parameters. Differences were examined by a two‐factor mixed analysis of variance (ANOVA) with interaction including time and extender as the main effects, followed by the post hoc analysis using Tukey test. When the results had a statistically significant interaction, the difference between groups at each level of each factor (time, extender) was determined. In this case, a modification of the repeated measurement command in the syntax was conducted by adding compare simple main effects for both time and extender factors. Pairwise comparisons were performed using a confidence interval adjustment by the Bonferroni method. A difference of P < 0.05 was considered significant.
Results
The composition of canine seminal fluid from American Bully dogs is shown in Table 2.
Table 2.
The composition of canine seminal fluid from American Bully dogs
| Components | Values (Mean ± SD) |
|---|---|
| pH | 6.45 ± 0.20 |
| Osmolality (mOsmol kg−1) | 324.50 ± 4.50 |
| Sodium (m‐equiv. L−1) | 155.00 ± 5.00 |
| Potassium (m‐equiv. L−1) | 13.20 ± 1.50 |
| Magnesium (m‐equiv. L−1) | 0.42 ± 0.01 |
| Calcium (m‐equiv. L−1) | 0.39 ± 0.01 |
| Phosphorus (mg%) | 7.15 ± 0.75 |
| Chloride (mg L−1) | 5466.50 ± 58.50 |
| Fructose (mg L−1) | 2.00 ± 0.10 |
| Lactic acid (mmol L−1) | 3.52 ± 0.48 |
| Citric acid (mmol L−1) | 3.63 ± 0.37 |
Sperm motility
The total motility (TM) and progressive motility (PM) of spermatozoa in the eight extenders are shown in Table 3. Overall, spermatozoa in T‐EY and T‐M‐EY extenders were high, and decreased gradually in TM (from 89.4 ± 1.9% to 65.2 ± 5.1% in T‐EY, and from 85.9 ± 3.8% to 13.0 ± 2.3% in T‐M‐EY) and in PM parameters (from 66.1 ± 3.3% to 32.2 ± 2.9% in T‐EY, and from 70.5 ± 4.8% to 3.6 ± 1.3% in T‐M‐EY) during the whole experimental period (10 days), while the percentage of TM and PM was reduced (P < 0.05) in T‐SL (from 92.2 ± 0.8% (day 1) to <5.2 ± 2.0% (day 10) in TM, from 82.0 ± 2.3% (day 1) to <2.4 ± 0.5 (day 9) in PM), in T‐M‐SL extenders (from 85.7 ± 3.2% (day 1) to <1.8 ± 0.5% (day 6) in TM, and from 61.1 ± 6.7% (day 1) to 0% (day 4) in PM). However, the sperm in T‐SL3% extender was the highest in TM during the first 5 days of storage (92.2 ± 0.8% (day 1) and 80.2 ± 2.3% (day 5)) and in PM during the first 3 days storage (82.0 ± 2.3% (day 1), and 60.9 ± 4.6% (day 3)) when compared to the rest extenders. Yet, there was no significant difference when it was compared to T‐EY, T‐SL1% and T‐M‐EY extenders (P > 0.05). In addition, from days 6 to 10, the percentage of TM and PM in T‐EY extender were the highest and had a significant difference when compared to that of other extenders (P < 0.05). Although T‐M‐EY extender was lower than T‐EY extender in TM and PM after day 6, it was still significantly higher than that in T‐SL and T‐M‐SL extenders (P < 0.05). Moreover, for T‐M‐SL extenders, TM and PM of the sperm decreased dramatically after day 3 and stopped rapidly on day 7 in TM as well as obtained a zero value on day 4 in the PM parameter.
Table 3.
Effects of egg yolk (EY) 20% and soybean lecithin (SL) at different concentrations (1%, 3% and 5%) in Tris‐citric‐fructose (T) or Tris‐citric‐fructose‐mineral salts (T‐M) extender on total motility (TM) and progressive motility (PM) parameters of chilled canine sperm during a storage period of 10 days at 5°C
| Parameters | Extenders | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TM (%) | T‐EY | 89.4 ± 1.9aA | 85.4 ± 1.7aAB | 83.4 ± 2.7aAB | 81.1 ± 2.6aB | 80.1 ± 3.4aB | 77.7 ± 4.5aB | 76.7 ± 4.9aB | 74.9 ± 4.7aBC | 69.5 ± 5.6aCD | 65.2 ± 5.1aD |
| T‐SL1% | 90.6 ± 2.5aA | 87.5 ± 0.8aAB | 84.9 ± 2.8aAB | 79.5 ± 3.3aB | 73.8 ± 1.0bC | 71.6 ± 1.3aC | 55.9 ± 4.5bcD | 15.0 ± 4.4dE | 12.1 ± 3.1cE | 2.1 ± 0.9cdF | |
| T‐SL3% | 92.2 ± 0.8aA | 89.3 ± 1.8aAB | 85.2 ± 2.2aAB | 82.8 ± 1.4aB | 80.2 ± 2.3aB | 74.1 ± 1.8aC | 54.8 ± 6.7cD | 26.1 ± 5.5cE | 15.2 ± 1.9cF | 5.2 ± 2.0cG | |
| T‐SL5% | 88.8 ± 3.5aA | 85.0 ± 5.2aA | 77.6 ± 4.7aB | 72.2 ± 4.3bB | 53.2 ± 4.1cC | 46.8 ± 4.9bD | 19.2 ± 3.1dE | 5.9 ± 1.3deF | 2.1 ± 0.9dF | 0.0 ± 0.0dF | |
| T‐M‐EY | 85.9 ± 3.8abA | 83.7 ± 2.5aAB | 81.6 ± 3.1aAB | 79.1 ± 2.7aB | 75.7 ± 1.8abBC | 71.8 ± 3.4aCD | 65.2 ± 5.1bD | 37.5 ± 8.0bE | 25.1 ± 5.4bF | 13.0 ± 2.3bG | |
| T‐M‐SL1% | 85.7 ± 3.2abA | 58.9 ± 8.7bB | 48.2 ± 6.5bC | 12.3 ± 2.4cD | 4.6 ± 1.1dE | 1.8 ± 0.5cE | 0.0 ± 0.0eE | 0.0 ± 0.0eE | 0.0 ± 0.0dE | 0.0 ± 0.0dE | |
| T‐M‐SL3% | 82.6 ± 4.0bA | 44.9 ± 7.5cB | 27.5 ± 4.2cC | 7.5 ± 1.4cdD | 2.8 ± 1.3dE | 1.1 ± 0.4cE | 0.0 ± 0.0eE | 0.0 ± 0.0eE | 0.0 ± 0.0dE | 0.0 ± 0.0dE | |
| T‐M‐SL5% | 80.2 ± 2.4bA | 37.8 ± 2.8cB | 23.5 ± 3.3cC | 2.4 ± 1.0dD | 1.1 ± 0.3dD | 0.0 ± 0.0cD | 0.0 ± 0.0eD | 0.0 ± 0.0eD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | |
| PM (%) | T‐EY | 66.1 ± 3.3cdeA | 61.8 ± 5.0bA | 58.5 ± 5.8aAB | 56.7 ± 6.5aAB | 52.0 ± 3.0aBC | 48.9 ± 4.6aCD | 46.7 ± 5.5aCD | 43.4 ± 6.3aD | 39.4 ± 4.0aE | 32.2 ± 2.9aF |
| T‐SL1% | 80.5 ± 2.2abA | 68.9 ± 1.1abB | 62.7 ± 1.2aB | 42.5 ± 4.9bC | 31.7 ± 5.9bD | 24.8 ± 6.1bcE | 11.5 ± 1.3cdF | 3.5 ± 1.5cG | 1.7 ± 0.3cG | 0.0 ± 0.0cG | |
| T‐SL3% | 82.0 ± 2.3aA | 73.9 ± 2.2aB | 60.9 ± 4.6aC | 44.8 ± 5.8bD | 36.9 ± 5.0bE | 27.6 ± 5.0bF | 16.6 ± 3.8cG | 4.4 ± 1.1cH | 2.4 ± 0.5cH | 0.0 ± 0.0cH | |
| T‐SL5% | 74.3 ± 1.6abcA | 65.3 ± 1.8bB | 45.5 ± 5.6bC | 38.6 ± 5.3bD | 19.5 ± 2.8cE | 13.4 ± 2.6cF | 4.3 ± 0.5deG | 1.7 ± 0.3cG | 0.0 ± 0.0cG | 0.0 ± 0.0cG | |
| T‐M‐EY | 70.5 ± 4.8bcdA | 67.3 ± 5.0abAB | 62.3 ± 4.1aABC | 59.5 ± 3.3aBC | 55.6 ± 7.5aC | 44.2 ± 9.1aD | 32.4 ± 4.9bE | 22.0 ± 3.3bF | 7.7 ± 3.6bG | 3.6 ± 1.3bH | |
| T‐M‐SL1% | 61.1 ± 6.7deA | 25.9 ± 3.0cB | 11.1 ± 3.0cC | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0eD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | |
| T‐M‐SL3% | 57.5 ± 2.6eA | 18.7 ± 2.5cdB | 11.6 ± 1.7cC | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0eD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | |
| T‐M‐SL5% | 58.2 ± 6.6eA | 12.6 ± 2.3 dB | 5.4 ± 2.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0dC | 0.0 ± 0.0dC | 0.0 ± 0.0eC | 0.0 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0cC |
Values are mean ± SD for four replicates, each being a pool of five ejaculates.
Lowercase superscript letters (a, b, c, d or e) in the same column indicates significant difference among extenders (P < 0.05) and uppercase superscript letters (A, B, C, D, E, F, G or H) in the same row indicates significant difference within extenders with different storage time (P < 0.05).
Another evolution that was observed in sperm motility characteristics was sperm velocity (VAP, VSL and VCL). Changes in the sperm velocity parameter during the storage period are given in Table 4. As TM and PM parameters, the sperm velocity parameters in T‐EY and T‐M‐EY extenders declined steadily and were highest among the extenders during 10 days storage. In particular, although T‐M‐EY extender was lower than T‐EY extender in VAP and VCL parameters, it was higher than that in the VSL parameter during the storage process without significant difference (P > 0.05). Furthermore, during the first 5 days, there was a slow change with no difference among T‐EY, T‐SL and T‐M‐EY extenders in these parameters. In contrast, the sperm velocity parameters in T‐M‐SL were only maintained for 3 days and decreased suddenly afterwards.
Table 4.
Effects of egg yolk (EY) 20% and soybean lecithin (SL) at different concentrations (1%, 3% and 5%) in Tris‐citric‐fructose (T) or Tris‐citric‐fructose‐mineral salts (T‐M) extender on average pathway velocity (VAP), straight line velocity (VSL) and curvilinear velocity (VCL) parameters of chilled canine sperm during a storage period of 10 days at 5°C
| Parameters | Extenders | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VAP (μm s−1) | T‐EY | 89.6 ± 0.5abA | 76.3 ± 5.2aB | 71.9 ± 3.5aB | 67.6 ± 6.5aB | 66.4 ± 7.3aB | 64.8 ± 7.6aB | 62.9 ± 7.8aB | 60.8 ± 7.5aBC | 59.1 ± 7.8aC | 55.6 ± 8.4aC |
| T‐SL1% | 93.5 ± 0.8aA | 76.1 ± 5.0aB | 67.8 ± 7.2aBC | 56.5 ± 6.5aC | 51.6 ± 4.9bD | 47.1 ± 6.4bE | 44.9 ± 4.8bE | 41.4 ± 8.0bE | 35.7 ± 6.9bcF | 0.0 ± 0.0cG | |
| T‐SL3% | 91.6 ± 1.6abA | 76.9 ± 2.9aB | 67.6 ± 8.7aBC | 56.3 ± 7.0aCD | 53.2 ± 7.8bD | 46.9 ± 4.7bE | 43.3 ± 1.9bE | 35.7 ± 1.6bcF | 32.7 ± 3.3cF | 0.0 ± 0.0cG | |
| T‐SL5% | 90.8 ± 4,4abA | 78.4 ± 5.8aA | 64.1 ± 6.7aB | 55.9 ± 5.9aB | 51.9 ± 5.7bB | 43.6 ± 2.9bC | 38.1 ± 7.5bD | 27.1 ± 2.3cE | 0.0 ± 0.0dF | 0.0 ± 0.0cF | |
| T‐M‐EY | 92.9 ± 4.0aA | 78.2 ± 4.3aAB | 71.6 ± 7.0aB | 64.9 ± 6.0aBC | 63.1 ± 6.0abC | 60.5 ± 6.1aC | 58.6 ± 5.9aCD | 53.3 ± 7.2abD | 46.8 ± 7.9bE | 43.1 ± 7.8bE | |
| T‐M‐SL1% | 82.3 ± 7.0abA | 59.7 ± 9.4bB | 38.6 ± 9.0bC | 0.0 ± 0.0bD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0cD | |
| T‐M‐SL3% | 79.6 ± 6.3bA | 47.2 ± 6.9bB | 37.1 ± 6.0bB | 0.0 ± 0.0bC | 0.0 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0dC | 0.0 ± 0.0dC | 0.0 ± 0.0cC | |
| T‐M‐SL5% | 79.7 ± 9.8bA | 55.0 ± 8.9bB | 35.6 ± 5.5bC | 0.0 ± 0.0bD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0cD | |
| VSL (μm s−1) | T‐EY | 77.0 ± 2.8abA | 62.1 ± 7.2abAB | 52.1 ± 4.0abBC | 47.4 ± 8.1abBCD | 45.0 ± 6.9abCD | 44.3 ± 6.9abCDE | 42.5 ± 6.4abCDE | 40.3 ± 4.4aCDE | 39.2 ± 4.0aDE | 36.3 ± 3.3aE |
| T‐SL1% | 74.0 ± 2.2abA | 59.4 ± 4.4abAB | 46.9 ± 5.7bBC | 41.4 ± 6.8bC | 34.8 ± 3.2bD | 33.8 ± 3.4bcD | 33.1 ± 2.7bcD | 29.7 ± 2.6bD | 23.0 ± 3.2bE | 0.0 ± 0.0cF | |
| T‐SL3% | 72.6 ± 5.8abA | 58.1 ± 6.3abAB | 47.2 ± 7.1bBC | 39.4 ± 4.7bC | 37.1 ± 5.9bC | 31.2 ± 4.2cD | 28.1 ± 2.3cE | 24.2 ± 2.0bcE | 22.4 ± 2.1bE | 0.0 ± 0.0cF | |
| T‐SL5% | 72.1 ± 8.5abA | 56.4 ± 6.2abB | 44.8 ± 6.4bBC | 36.6 ± 3.9bC | 34.5 ± 2.8bC | 29.1 ± 3.9cD | 25.9 ± 6.4cE | 15.6 ± 2.2cF | 0.0 ± 0.0cG | 0.0 ± 0.0cG | |
| T‐M‐EY | 86.1 ± 4.6aA | 69.1 ± 3.7aB | 63.2 ± 7.7aB | 55.5 ± 8.2aB | 53.5 ± 8.0aB | 49.2 ± 8.1aC | 47.6 ± 7.3aCD | 41.3 ± 1.0aD | 33.6 ± 4.1aE | 31.6 ± 4.0bE | |
| T‐M‐SL1% | 71.6 ± 8.4abA | 51.1 ± 7.6bB | 28.0 ± 4.4cC | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | |
| T‐M‐SL3% | 71.2 ± 6.2abA | 45.6 ± 7.9bcB | 29.3 ± 4.2cC | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0dD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | |
| T‐M‐SL5% | 65.8 ± 9.4bA | 32.6 ± 8.2cB | 25.5 ± 6.4cB | 0.0 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0dC | 0.0 ± 0.0dC | 0.0 ± 0.0dC | 0.0 ± 0.0cC | 0.0 ± 0.0cC | |
| VCL (μm s−1) | T‐EY | 148.0 ± 6.5bcA | 142.1 ± 7.4bcAB | 135.8 ± 8.6aABC | 127.2 ± 15.3aABCD | 124.8 ± 17.1aABCD | 121.8 ± 19.5aBCD | 119.3 ± 17.7aBCD | 115.7 ± 17.6aBCD | 112.7 ± 15.6aCD | 108.3 ± 14.4aD |
| T‐SL1% | 181.1 ± 8.8aA | 168.4 ± 10.9aAB | 144.9 ± 13.8aB | 116.1 ± 19.0aC | 104.2 ± 19.0aD | 98.6 ± 19.0aD | 87.1 ± 24.1abE | 58.6 ± 27.0bF | 46.6 ± 13.9cF | 0.0 ± 0.0cG | |
| T‐SL3% | 172.3 ± 1.6aA | 165.0 ± 7.6abAB | 143.6 ± 18.9aBC | 118.7 ± 17.6aC | 109.4 ± 24.6aD | 97.5 ± 15.0aE | 88.5 ± 6.3abE | 62.2 ± 8.6bF | 54.8 ± 73.6cF | 0.0 ± 0.0cG | |
| T‐SL5% | 164.3 ± 5.3abA | 158.7 ± 8.7abAB | 134.6 ± 17.5aBC | 108.7 ± 25.8aC | 97.2 ± 23.8aD | 89.4 ± 21.1aD | 75.4 ± 29.5bE | 42.2 ± 10.2bF | 0.0 ± 0.0dG | 0.0 ± 0.0cG | |
| T‐M‐EY | 133.5 ± 3.2cA | 129.3 ± 3.9cdAB | 123.1 ± 6.1aABC | 117.1 ± 2.8aABC | 112.9 ± 3.3aABC | 110.1 ± 4.0aABC | 106.0 ± 5.9abABCD | 101.7 ± 9.6aBCD | 90.6 ± 11.0bCD | 84.4 ± 12.7bD | |
| T‐M‐SL1% | 134.2 ± 8.7cA | 121.8 ± 7.5cA | 72.7 ± 19.0bB | 0.0 ± 0.0bC | 0.0 ± 0.0bC | 0.0 ± 0.0bC | 0.0 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0dC | 0.0 ± 0.0cC | |
| T‐M‐SL3% | 133.2 ± 7.2cA | 114.7 ± 16.8 dB | 78.9 ± 14.4bC | 0.0 ± 0.0bD | 0.0 ± 0.0bD | 0.0 ± 0.0bD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0cD | |
| T‐M‐SL5% | 131.9 ± 9.3cA | 87.9 ± 10.5eB | 59.4 ± 9.1bC | 0.0 ± 0.0bD | 0.0 ± 0.0bD | 0.0 ± 0.0bD | 0.0 ± 0.0cD | 0.0 ± 0.0cD | 0.0 ± 0.0dD | 0.0 ± 0.0cD |
Values are mean ± SD for four replicates, each being a pool of five ejaculates.
Lowercase superscript letters (a, b, c, d or e) in the same column indicates significant difference among extenders (P < 0.05) and uppercase superscript letters (A, B, C, D, E, F or G) in the same row indicates significant difference within extenders with different storage time (P < 0.05).
Plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential
The results of the plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential are presented in Table 5.
Table 5.
Percentage of intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential in chilled canine sperm diluted in Tris‐citric‐fructose (T) or Tris‐citric‐fructose‐mineral salts (T‐M) extender plus 20% egg yolk (EY) or soybean lecithin (SL) at different concentrations (1%, 3% and 5%) during a storage period of 10 days at 5 °C
| Parameters | Extenders | Day 1 | Day 2 | Day 3 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|---|---|
| Plasma membrane | T‐EY | 49.5 ± 4.3aA | 43.6 ± 7.0aAB | 38.6 ± 6.5abB | 34.0 ± 3.3aC | 30.2 ± 5.8aC | 26.7 ± 5.9aCD | 22.5 ± 8.7aD |
| T‐SL1% | 35.0 ± 3.7cA | 31.5 ± 2.8abA | 29.1 ± 1.8bcdA | 27.3 ± 2.7abAB | 20.8 ± 5.6abcB | 4.3 ± 2.3bC | 1.3 ± 0.4bC | |
| T‐SL3% | 36.0 ± 1.2bcA | 29.0 ± 3.3bB | 25.5 ± 2.7cdBC | 22.1 ± 2.3bcdC | 19.1 ± 3.7bcC | 6.3 ± 6.7bD | 0.7 ± 0.3bE | |
| T‐SL5% | 28.7 ± 2.8cA | 27.8 ± 2.8bA | 25.7 ± 3.7cdA | 16.8 ± 3.9cdB | 12.2 ± 1.7cB | 2.2 ± 1.0bC | 0.1 ± 0.0bC | |
| T‐M‐EY | 47.2 ± 7.5abA | 42.6 ± 5.3aA | 40.3 ± 4.5aA | 34.4 ± 5.7aB | 27.2 ± 3.2abBC | 20.4 ± 1.1aC | 14.9 ± 3.5aD | |
| T‐M‐SL1% | 39.4 ± 8.2abcA | 35.2 ± 8.0abAB | 29.9 ± 5.3abcB | 24.9 ± 5.0abcC | 15.5 ± 3.8cD | 1.8 ± 0.7bE | 0.3 ± 0.1bE | |
| T‐M‐SL3% | 35.3 ± 2.4cA | 29.6 ± 5.8bA | 23.7 ± 4.1cdB | 19.6 ± 2.3bcdBC | 15.2 ± 3.7cC | 1.8 ± 0.7bD | 0.1 ± 0.0bD | |
| T‐M‐SL5% | 28.2 ± 2.7cA | 22.8 ± 2.9bAB | 18.6 ± 5.2dBC | 15.1 ± 4.8dC | 13.3 ± 3.4cC | 1.5 ± 0.2bD | 0.1 ± 0.0bD | |
| Acrosome membrane | T‐EY | 47.7 ± 4.9bA | 42.0 ± 4.6abAB | 38.0 ± 4.0aB | 32.2 ± 5.3abC | 26.1 ± 4.0abD | 22.6 ± 1.6aD | 16.5 ± 4.2aE |
| T‐SL1% | 40.9 ± 6.3bA | 31.9 ± 3.8bcB | 28.2 ± 3.5bB | 25.7 ± 5.5bcB | 19.5 ± 4.0bC | 18.1 ± 3.9aC | 9.6 ± 4.7bcD | |
| T‐SL3% | 36.7 ± 3.2bcA | 25.2 ± 5.3cB | 20.6 ± 4.6bcBC | 17.0 ± 5.8cdC | 7.9 ± 2.2cD | 6.0 ± 1.7bD | 3.2 ± 1.7cD | |
| T‐SL5% | 25.1 ± 2.5cA | 19.1 ± 4.3cB | 14.1 ± 4.4cdBC | 11.0 ± 3.7dCD | 7.5 ± 3.0cDE | 5.7 ± 3.3bDE | 3.6 ± 2.5cE | |
| T‐M‐EY | 63.5 ± 7.2aA | 56.1 ± 11.1aB | 45.8 ± 5.7aC | 40.2 ± 5.7aD | 33.8 ± 4.1aE | 22.7 ± 3.9aF | 16.0 ± 1.6abG | |
| T‐M‐SL1% | 39.3 ± 8.9bA | 29.0 ± 7.4bcB | 16.4 ± 1.8cdC | 14.0 ± 3.3dCD | 9.9 ± 3.0cD | 3.5 ± 2.6bE | 2.0 ± 0.9cE | |
| T‐M‐SL3% | 25.1 ± 2.9cA | 21.0 ± 2.1cAB | 13.5 ± 3.4cdB | 8.8 ± 2.7dC | 5.1 ± 2.5cCD | 2.5 ± 1.6bCD | 1.6 ± 1.0cD | |
| T‐M‐SL5% | 24.5 ± 3.7cA | 16.8 ± 1.7cB | 8.8 ± 2.7dC | 6.4 ± 1.6dCD | 5.2 ± 1.6cCDE | 1.7 ± 0.5bDE | 0.8 ± 0.5cE | |
| Mitochondrial membrane potential | T‐EY | 62.5 ± 5.9aA | 54.2 ± 2.5abB | 49.1 ± 2.1aC | 42.3 ± 2.1aD | 39.4 ± 2.0aD | 36.0 ± 3.0aD | 21.3 ± 2.6aE |
| T‐SL1% | 58.0 ± 6.7abA | 44.5 ± 4.6bcB | 36.8 ± 3.2bC | 32.1 ± 4.3bD | 29.2 ± 3.5bD | 10.2 ± 2.2cE | 3.6 ± 2.6bF | |
| T‐SL3% | 41.4 ± 4.4cdA | 34.6 ± 3.1cdB | 28.9 ± 1.9cC | 24.1 ± 4.0bD | 19.2 ± 2.3cE | 5.6 ± 1.7cdF | 1.7 ± 0.8bG | |
| T‐SL5% | 33.7 ± 3.3deA | 25.2 ± 4.3deB | 17.0 ± 2.5deC | 10.3 ± 1.7cdD | 6.3 ± 1.5dDE | 2.1 ± 1.6dEF | 0.8 ± 0.7bF | |
| T‐M‐EY | 66.1 ± 3.8aA | 57.7 ± 5.3aB | 49.1 ± 3.6aC | 46.5 ± 3.8aCD | 43.4 ± 3.6aD | 29.9 ± 2.9bE | 17.9 ± 2.0aF | |
| T‐M‐SL1% | 47.2 ± 4.1bcA | 42.2 ± 3.9cA | 33.2 ± 2.8bcB | 28.0 ± 4.1bC | 20.0 ± 1.7cD | 5.6 ± 1.7cdE | 1.5 ± 0.3bF | |
| T‐M‐SL3% | 32.7 ± 4.1deA | 25.4 ± 5.2deB | 19.1 ± 2.9dC | 12.7 ± 1.4cD | 7.5 ± 2.8dE | 4.3 ± 3.6dEF | 0.7 ± 0.5bF | |
| T‐M‐SL5% | 25.7 ± 3.2eA | 17.3 ± 2.4eB | 11.0 ± 2.4eC | 4.3 ± 3.5dD | 2.1 ± 1.3dD | 1.0 ± 0.1dD | 0.1 ± 0.0bD |
Values are mean ± SD for four replicates, each being a pool of five ejaculates.
Lowercase superscript letters (a, b, c or d) in the same column indicates significant difference among extenders (P < 0.05) and uppercase superscript letters (A, B, C, D or E) in the same row indicates significant difference within extenders with different storage time (P < 0.05).
These parameters in all the extenders decreased gradually during the chilling storage. For plasma membrane integrity, the high values of intact plasma membrane were shown in both T‐EY (from 49.5 ± 4.3% to 22.5 ± 8.7%) and T‐M‐EY (from 47.2 ± 7.5% to 14.9 ± 3.5%) extenders during 10 days of storage. However, the percentage of intact plasma membrane in T‐SL1% and T‐M‐SL1% extenders was not significantly lower than that in T‐EY and T‐M‐EY extenders during the first 4 days (P > 0.05). In addition, there was a similar value of this parameter in T‐SL1% and T‐SL3% extenders during the whole storage period (P > 0.05). In contrast, T‐M‐EY extender had the highest value and was not significantly different from T‐EY extender in both the intact acrosome membrane (63.5 ± 7.2% vs. 47.7 ± 4.9% on day 1 and 22.7 ± 3.9% vs. 22.6 ± 1.6% on day 9 respectively) and high mitochondrial membrane potential parameters (66.1 ± 3.8% vs. 62.5 ± 5.9% on day 1, and 43.4 ± 3.6% vs. 39.4 ± 2.0% on day 6 respectively) (P < 0.05). Moreover, T‐SL1% was higher in the intact acrosome membrane and in high mitochondrial membrane potential values than T‐SL3%, but it was not significantly different (P > 0.05). Specifically, the proportion of these parameters in the extenders with high levels of soybean lecithin (T‐SL3%, T‐SL5%, T‐M‐SL3% and T‐M‐SL5%) reduced quickly and had a significant difference when compared to T‐EY and T‐M‐EY extenders during the whole storage period (P < 0.05).
The percentage of healthy sperm with the intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential are summarised in Table 6. In general, like the previous parameters, the proportion of healthy sperm achieved with the intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential in T‐M‐EY and T‐EY extenders were the highest when compared with that in other extenders during 10 days. However, during the first 6 days, the percentage of healthy sperm in T‐M‐EY (from 46.8 ± 7.9% to 20.8 ± 2.5%) was significantly higher than that in T‐EY extenders (from 42.2 ± 7.0% to 13.6 ± 3.2%) (P < 0.05), but it decreased suddenly after day 6, and obtained a similar value as in T‐EY extender on day 10 (5.4 ± 1.8% and 4.6 ± 0.8% respectively) (P > 0.05). Furthermore, although the healthy sperm in T‐SL1% extender was not significantly different from that in T‐M‐SL1% extender, it was significantly higher than that in the other extenders (T‐SL3%, T‐SL5%, T‐M‐SL3% and T‐M‐SL5%).
Table 6.
Percentage of healthy sperm with intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential in chilled canine sperm diluted in Tris‐citric‐fructose (T) or Tris‐citric‐fructose‐mineral salts (T‐M) extender plus 20% egg yolk (EY) or soybean lecithin (SL) at different concentrations (1%, 3% and 5%) during a storage period of 10 days at 5°C
| Extenders | Day 1 | Day 2 | Day 3 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|---|
| T‐EY | 42.2 ± 7.0aA | 31.9 ± 3.6bB | 27.4 ± 3.6bC | 22.2 ± 5.1bD | 13.6 ± 3.2bE | 11.4 ± 2.9aF | 5.4 ± 1.8aG |
| T‐SL1% | 25.9 ± 4.7bcA | 18.8 ± 1.2cB | 13.7 ± 2.2cC | 6.9 ± 1.5cD | 1.3 ± 0.5cE | 0.1 ± 0.0cE | 0.0 ± 0.0bE |
| T‐SL3% | 17.5 ± 2.5cdA | 8.0 ± 0.6deB | 4.9 ± 1.8dBC | 2.2 ± 0.4cdBC | 1.3 ± 0.5cC | 0.3 ± 0.1cC | 0.0 ± 0.0bC |
| T‐SL5% | 14.8 ± 1.8dA | 5.2 ± 1.7eB | 3.3 ± 2.1dBC | 2.2 ± 1.4cdCD | 0.8 ± 0.2cD | 0.1 ± 0.0cD | 0.0 ± 0.0bD |
| T‐M‐EY | 46.8 ± 7. 9aA | 39.0 ± 4.2aB | 36.2 ± 4.0aB | 29.1 ± 2.3aC | 20.8 ± 2.5aD | 6.9 ± 1.6bE | 4.6 ± 0.8aE |
| T‐M‐SL1% | 30.6 ± 2.0bA | 16.3 ± 0.8cB | 7.8 ± 1.6cdC | 6.8 ± 1.3cdC | 1.7 ± 1.0cD | 0.3 ± 0.1cD | 0.0 ± 0.0bD |
| T‐M‐SL3% | 20.2 ± 1.6bcdA | 13.7 ± 3.4cdB | 6.9 ± 1.5dC | 2.4 ± 0.8cdD | 0.45 ± 0.1cD | 0.0 ± 0.0cD | 0.0 ± 0.0bD |
| T‐M‐SL5% | 20.0 ± 1.7bcdA | 8.6 ± 2.1deB | 2.2 ± 1.0dC | 0.8 ± 0.3dC | 0.1 ± 0.0cC | 0.0 ± 0.0cC | 0.0 ± 0.0bC |
Values are mean ± SD for four replicates, each being a pool of five ejaculates.
Lowercase superscript letters (a, b, c, d or e) in the same column indicates significant difference among extenders (P < 0.05) and uppercase superscript letters (A, B, C, D, E, F or G) in the same row indicates significant difference within extenders with different storage time (P < 0.05).
Discussion
The study investigated the effects of egg yolk and soybean lecithin in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender on canine sperm quality. The obtained results clearly demonstrated that egg yolk is superior to soybean lecithin in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender when considering motility, plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential parameters during 10 days of chilling storage. The results of the current study are in agreement with the previous reports in dogs (Axnér & Lagerson 2016), rams (Ustuner et al. 2016), and bulls (Crespilho et al. 2012). They have confirmed that egg yolk extender was more efficient on sperm quality than soybean lecithin extender. This may be explained by the fact that both low‐density lipoproteins in egg yolk and soybean lecithin, known as a protective factor for sperm plasma membranes, are mainly composed of phospholipids. However, there is a difference in their mechanisms of action by their composition. Soybean lecithin is comprised almost entirely of phospholipids, whereas low‐density lipoprotein in egg yolk has both phospholipids and proteins. Proteins are necessary to keep phospholipid fractions in a solubilised form, and closely associated with the plasma membrane (Watson 1981). This leads to the interaction between low‐density lipoprotein in egg yolk and sperm plasma membrane (Belala et al. 2016). In addition, low‐density lipoprotein in egg yolk can decrease the binding of proteins in seminal plasma to sperm as well as reduce the phospholipid efflux from sperm membranes (Manjunath et al. 2002; Bergeron 2003). They can also prevent premature sperm capacitation and acrosome reaction (Witte et al. 2009). It is noteworthy that egg yolk is a conventional but effective extender for canine sperm preservation and cryopreservation (Silva et al. 2002; Ponglowhapan et al. 2004; Verstegen et al. 2005; Shahiduzzaman & Linde‐Forsberg 2007; Witte et al. 2009; Batista et al. 2012; Goericke‐Pesch et al. 2012; Treulen et al. 2012; Rodenas et al. 2014; Hori et al. 2017).
However, the results also indicated that although soybean lecithin extenders were lower than egg yolk extenders in almost all sperm quality parameters during 10 days of storage, they were similar to egg yolk extenders in the total motility and plasma membrane parameters during the first 6 days (in T‐SL1% and T‐SL3% extenders). Similar findings on chilled canine sperm were reported by Beccaglia et al. (2009a,2009b). They found that there was no significant difference between Tris‐citric‐fructose‐soybean lecithin (0.04%) and Tris‐citric‐fructose‐egg yolk (20%) extenders in motility, capacitation and zona pellucida binding during 4 days of chilling storage. In contrast to our results, Kmenta et al. (2011) reported that 0.8% lecithin extender was better than Tris‐citric‐fructose‐egg yolk (20%) extender in motility and viability of chilling canine sperm during 8 days when adding an enhancer (Tris buffer). In another study on chilled canine sperm, motility, plasma membrane integrity and mitochondrial membrane potential in soybean lecithin (0.4%) extender were found to be greater than those in 20% egg yolk extender during 10 days of storage (Kasimanickam et al. 2012). Furthermore, several investigations have proven that soybean lecithin could replace egg yolk in extender to protect sperm during cryopreservation in dogs (Beccaglia et al. 2009a,2009b; Dalmazzo et al. 2018), goats (Salmani et al. 2014; Chelucci et al. 2015; Yotov 2015), bulls (Aires et al. 2003; El‐sisy et al. 2016), rams (Masoudi et al. 2016), stallions (Nouri et al. 2013), rabbits (Nishijima et al. 2015) and fish (Yildiz et al. 2013). The difference between our results and previous studies may be due to the difference in soybean lecithin sources, the preparation for soybean lecithin extenders and the concentrations of soybean lecithin. de Paz et al. (2010) have found that various soybean lecithin sources have different compositions and effects on sperm quality. In addition, because soybean lecithin has amphiphilic characteristics, there are large lipid droplets in extender after diluting. Consequently, soybean lecithin is unsuitable for protecting sperm during storage. Thus, centrifuging and filtering are necessary to prepare soybean lecithin extenders as described earlier (de Paz et al. 2010; Vick et al. 2012; Axnér & Lagerson 2016). However, de Paz et al. (2010) have also reported that the centrifugation‐filtration process can reduce the quantity of phospholipids in extenders by 50%.
The study suggested that high concentrations of soybean lecithin have negative effects on sperm quality. Our results are in agreement with those reported by Forouzanfar et al. (2010) and Salmani et al. (2014). Their works have reported that the reduction of all sperm quality parameters at high levels of soybean lecithin may be due to the high viscosity of soybean lecithin in extenders. We also observed that there was more particle debris on 3% and 5% soybean lecithin extenders, as described by Forouzanfar et al. (2010). Moreover, Dalmazzo et al. (2018) have explained that high concentrations of soybean lecithin (phosphatidylcholine) can cause higher concentrations of exogenous phosphatidylcholine inside the mitochondria and lead to an imbalance between intracellular and extracellular as the result of reducing mitochondrial activity as well as motility.
The most important finding of our study is that T‐EY extender was inferior to T‐M‐EY extender in VSL parameter and in the percentage of healthy sperm with intact plasma membrane, intact acrosome membrane and high mitochondrial membrane potential during 10 days of storage. From a clinical point of view, VSL is most likely the most important parameter in the CASA system in which the average velocity of the sperm heads through a straight line connecting to the first point of the last track. In a previous study, the researchers demonstrated that the decline in VSL was highly correlated with the outcome of fertilisation in vitro in rat spermatozoa (Harry & Mehdi 1996). In addition, healthy sperm are defined as spermatozoa which have a good quality of plasma membrane, acrosome membrane and mitochondrial membrane potential. These spermatozoa also have a high survival potential in the female reproductive track as well as fertility ability (Grunewald et al. 2008). Moreover, although the proportion of healthy sperm in T‐M‐EY were higher than that in T‐EY during 6 days, it reduced suddenly after day 6 and gained a similar value with T‐EY extender on day 10. These factors may help to explain that T‐M‐EY extenders contain several mineral cations, such as Na+, K+, Mg2+, Ca2+, which are the main ions of seminal plasma, whose important functions are to maintain osmotic balance, form parts of principal enzymes relating to sperm metabolism and sperm function (Çevik et al. 2007; Juyena & Stelletta 2012; Smith et al. 2018). In particular, Mg2+ has a vital function in modulating the regulation of K+ (Na‐K pump) and Ca2+ (Owczarzy et al. 2008 ; Smith et al. 2018) as well as playing an essential role in enzymatic reactions involving anaerobic glycolysis and energy release from ATP for sperm activities (Wong et al. 2001; Asghari et al. 2016). Furthermore, Ca2+ ions also have an important role in intramitochondrial metabolism and energy production in cells (McCormack & Denton 1989). Mitochondria can import Ca2+ from cytosol into mitochondrial matrix via the mitochondrial uniporter (Walsh et al. 2009). When the concentration of free Ca2+ increases within the mitochondrial matrix, it activates several dehydrogenases and carriers. As a result, it increases, H+ extrusion, and ATP production as well as supports energy for cell activities (McCormack & Denton 1989; Hansford 1994; Santo‐Domingo & Demaurex 2010). Nevertheless, when the concentration of Ca2+ is overloaded, it can open the mitochondrial permeability transition pore (PTP) and deplete ATP. This leads to mitochondrial swelling, cytochrome C release and subsequently apoptosis (Demaurex & Distelhorst 2003; Giorgi et al. 2008). Therefore, sperm in T‐M‐EY extender have good quality in motility and mitochondrial membrane potential as well as plasma and acrosome membrane integrity during the former period of storage time and reduced quality in the last period of the storage time. Moreover, Baumgartner et al. (2009) and Voccoli et al. (2014) have shown that the apoptosis was not only the result of increased Ca2+ within the mitochondrial matrix, but also a powerful synergism of the combination between reactive oxygen species (ROS) production and mitochondrial Ca2+ overload. Thus, to optimise the effect of T‐M‐EY extender on sperm quality, the addition of antioxidant agents to this extender to reduce oxidative stress as well as apoptosis is necessary in the future.
In contrast, our results also indicated that T‐SL extender is more effective than T‐M‐SL extender in maintaining sperm quality parameters during storage. We have found that there is no synergy in the combination of soybean lecithin and Tris‐citric‐fructose‐mineral salts extender. The negative effects of T‐M‐SL extenders on sperm quality may be due to several nonorganic salts in these extenders, including NaCl, KCl, KHPO4, CaHPO4 and MgCl2. These nonorganic salts can induce a transition from spherical to long cylindrical micelles of soybean lecithin micelles by binding cations to the phosphate portion of lecithin headgroups (Lee et al. 2010; Markina et al. 2017). This results in an increase in the viscosity of soybean lecithin extenders as well as loss of cations and phospholipids after the centrifugation‐filtration processing.
Our results indicate that the healthy sperm are more correlated with intact sperm plasma membrane, intact acrosome membrane and high mitochondrial membrane potential than to sperm motility (see Table 3, 4). These results are similar to those reported by Volpe et al. (2009) in that the functional integrity of canine mitochondria is more strongly correlated to plasma membrane than to sperm motility. Nascimento et al. (2015) also demonstrated that there was no correlation between motility and mitochondrial membrane potential in canine sperm and suggested that when oxidative phosphorylation was inhibited, the energy from glycolysis in the sperm tail supported motility. Moreover, our results propose that the T‐M‐EY extender is more stable and suitable than the other extenders for protecting chilled canine sperm during 10 days of storage with a high motility and healthy sperm parameters, whereas T‐SL and T‐EY extenders are most productive in motility but less productive in healthy sperm parameters.
Conclusions
In conclusion, the results of our investigation revealed that egg yolk is greater than soybean lecithin in Tris‐citric‐fructose or Tris‐citric‐fructose‐mineral salts extender for chilling canine sperm. Egg yolk in Tris‐citric‐fructose‐mineral salts extender was superior to egg yolk in Tris‐citric‐fructose extender, whereas soybean lecithin in Tris‐citric‐fructose‐mineral salts extenders was inferior to Tris‐citric‐fructose‐soybean lecithin extenders in motility, plasma membrane integrity, acrosome membrane integrity and mitochondrial membrane potential. Further studies are necessary to study the addition of antioxidant into Tris‐citric‐fructose‐egg yolk or Tris‐citric‐fructose‐mineral salts‐egg yolk extender and evaluate more sperm quality parameters as DNA fragmentation and fertility ability.
Source of funding
This study was supported by an SUT‐PhD scholarship for ASEAN countries from Suranaree University of Technology in Thailand.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The Thai National Research Council's guidelines for the Care and Use of Laboratory Animals were followed.’
Contributions
Study design and manuscript preparation: VVN, PK. Laboratory work: VVN, SP, PK. Data analyses: VVN, PK. Manuscript review: SK, PK.
Supporting information
Table S1. The composition of canine seminal fluid from American Bully dogs and analysis methods
Acknowledgements
We express our sincere gratitude to the America Bully Dogs groups in Nakhon Ratchasima for their kind support.
References
- Aires V.A., Hinsch K., Mueller‐schloesser F., Bogner K. & Mueller‐schloesser S. (2003) In vitro and in vivo comparison of egg yolk‐based and soybean lecithin‐based extenders for cryopreservation of bovine semen. Theriogenology 60, 269–279. 10.1016/S0093-691X(02)01369-9. [DOI] [PubMed] [Google Scholar]
- Asghari A., Akbari G. & Galustanian G. (2016) Magnesium sulfate improves sperm characteristics against varicocele in rat. Crescent Journal of Medical and Biological Sciences 3, 55–59. [Google Scholar]
- Axnér E. & Lagerson E. (2016) Cryopreservation of dog semen in a tris extender with 1% or 2% soya bean lecithin as a replacement of egg yolk. Reproduction in Domestic Animals 51, 262–268. 10.1111/rda.12675. [DOI] [PubMed] [Google Scholar]
- Bajena M., Kondracki S., Iwanina M., Ysokińska W. & Adamiak A. (2016) Physical characteristics of ejaculates produced by insemination boars depending on the interval between successive ejaculate collections. Journal of Central European Agriculture 17, 260–271. 10.5513/JCEA01/17.2.1699. [DOI] [Google Scholar]
- Batista M., Santana M., Alamo D., González F., Niño T., Cabrera F. & Gracia A. (2012) Effects of incubation temperature and semen pooling on the viability of fresh, chilled and freeze‐thawed canine semen samples. Reproduction in Domestic Animals 47, 1049–1055. 10.1111/j.1439-0531.2012.02014.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner H.K., Gerasimenko J.V., Thorne C., Ferdek P., Pozzan T., Tepikin A.V. et al (2009) Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. Journal of Biological Chemistry 284, 20796–20803. 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccaglia M., Anastasi P., Chigioni S. & Luvoni G.C. (2009a) Tris‐lecithin extender supplemented with antioxidant catalase for chilling of canine semen. Reproduction in Domestic Animals 44, 345–349. 10.1111/j.1439-0531.2009.01410.x. [DOI] [PubMed] [Google Scholar]
- Beccaglia M., Anastasi P. & Luvoni G.C. (2009b) Freezing of canine semen in an animal‐free protein extender. Veterinary Research Communications 33, 77–80. 10.1007/s11259-009-9249-9. [DOI] [PubMed] [Google Scholar]
- Belala R., Delay J., Amirat L., Ropers M.‐H., Le Guillou J., Anton M. et al (2016) The benefits of liposomes for chilling canine sperm for 4 days at 4°C. Animal Reproduction Science 168, 100–109. 10.1016/j.anireprosci.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Bergeron A. (2003) Low‐density lipoprotein fraction from hen's egg yolk decreases the binding of the major proteins of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biology of Reproduction 70, 708–717. 10.1095/biolreprod.103.022996. [DOI] [PubMed] [Google Scholar]
- Bouchard G.F., Morris J.K., Sikes J.D. & Youngquist R.S. (1990) Effect of storage temperature, cooling rates and two different semen extender on canine spermatozoal motility. Theriogenology 34, 147–157. [DOI] [PubMed] [Google Scholar]
- Celeghini E.C.C., De Arruda R.P., De Andrade A.F.C., Nascimento J. & Raphael C.F. (2007) Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal and mitochondrial membranes. Reproduction in Domestic Animals 42, 479–488. 10.1111/j.1439-0531.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- Çevik M., Tuncer P.B., Taşdemir U. & Özgürtaş T. (2007) Comparison of spermatological characteristics and biochemical seminal plasma parameters of normozoospermic and oligoasthenozoospermic bulls of two breeds. Turkish Journal of Veterinary and Animal Sciences 31, 381–387. [Google Scholar]
- Chelucci S., Pasciu V., Succu S., Addis D., Leoni G.G., Manca M.E. et al (2015) Soybean lecithin‐based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology 83, 1064–1074. 10.1016/j.theriogenology.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Concannon P.W. (2011) Reproductive cycles of the domestic bitch. Animal Reproduction Science 124, 200–210. 10.1016/j.anireprosci.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Crespilho A.M., Sá Filho M.F., Dell'Aqua J.A., Nichi M., Monteiro G.A., Avanzi B.R. et al (2012) Comparison of in vitro and in vivo fertilizing potential of bovine semen frozen in egg yolk or new lecithin based extenders. Livestock Science 149, 1–6. 10.1016/j.livsci.2012.05.011. [DOI] [Google Scholar]
- Dalmazzo A., Losano J.D.A., Rocha C.C., Tsunoda R.H., Angrimani D.D.S.R., Mendes C.M. et al (2018) Effects of soy lecithin extender on dog sperm cryopreservation. Animal Biotechnology 29, 174–182. 10.1080/10495398.2017.1334662 [DOI] [PubMed] [Google Scholar]
- Demaurex N. & Distelhorst C. (2003) Cell biology: apoptosis ‐ the calcium connection. Science 300, 65–67. 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- Eilts B.E. (2005) Theoretical aspects of canine semen cryopreservation. Theriogenology 64, 692–697. 10.1016/j.theriogenology.2005.05.019. [DOI] [PubMed] [Google Scholar]
- El‐sisy G.A., El‐nattat W.S., El‐sheshtawy R.I. & El‐maaty A.M.A. (2016) Substitution of egg yolk with different concentrations of soybean lecithin in tris‐based extender during bulls’ semen preservability. Asian Pacific Journal of Reproduction 5, 514–518. 10.1016/j.apjr.2016.10.011. [DOI] [Google Scholar]
- Forouzanfar M., Sharafi M., Hosseini S.M., Ostadhosseini S., Hajian M., Hosseini L. et al (2010) In vitro comparison of egg yolk‐based and soybean lecithin‐based extenders for cryopreservation of ram semen. Theriogenology 73, 480–487. 10.1016/j.theriogenology.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Frandson R.D., Wilke W.L., Fails A.D. (2009) Anatomy and Physiology of Farm Animals, Seventh edn Wiley‐Blackwell: Ames, lowa 50014‐8300, USA: 10.1017/cbo9781107415324.004 [DOI] [Google Scholar]
- Giorgi C., Romagnoli A., Pinton P. & Rizzuto R. (2008) Ca2+ signaling, mitochondria and cell death. Current Molecular Medicine 8, 119–130. [DOI] [PubMed] [Google Scholar]
- Goericke‐Pesch S., Klaus D., Failing K. & Wehrend A. (2012) Longevity of chilled canine semen comparing different extenders. Animal Reproduction Science 135, 97–105. 10.1016/j.anireprosci.2012.08.032. [DOI] [PubMed] [Google Scholar]
- Grunewald S., Said T.M., Paasch U., Glander H.J. & Agarwal A. (2008) Relationship between sperm apoptosis signalling and oocyte penetration capacity. International Journal of Andrology 31, 325–330. 10.1111/j.1365-2605.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- Günzel‐Apel A.R. & Ekrod B. (1991) Influences of seminal plasma and extender on sperm motility, ATP‐concentration, and the activity of acid and alkaline phosphatases of beagle dog semen. Reproduction in Domestic Animals 26, 31–41. [Google Scholar]
- Hansford R.G. (1994) Physiological role of mitochondrial Ca2+ transport. Journal of Bioenergetics and Biomembranes 26, 495–508. [DOI] [PubMed] [Google Scholar]
- Harry D.M.M. & Mehdi A.A. (1996) Fertilizing capacity of rat spermatozoa is correlated with decline in straight‐line velocity measured by continuous computer‐aided sperm analysis: epididymal rat spermatozoa from the proximal cauda have a greater fertilizing capacity in vitro than those F. Journal of Andrology 17, 50–60. [PubMed] [Google Scholar]
- Hori T., Masuda T., Kobayashi M. & Kawakami E. (2017) Role of prostatic fluid in cooled canine epididymal sperm. Reproduction in Domestic Animals 52, 655–660. 10.1111/rda.12963. [DOI] [PubMed] [Google Scholar]
- Juyena N.S. & Stelletta C. (2012) Seminal plasma: an essential attribute to spermatozoa. Journal of Andrology 33, 536–551. 10.2164/jandrol.110.012583. [DOI] [PubMed] [Google Scholar]
- Kasimanickam V.R., Kasimanickam R.K., Memon M.A. & Rogers H.A. (2012) Effect of extenders on sperm mitochondrial membrane, plasma membrane and sperm kinetics during liquid storage of canine semen at 5°C. Animal Reproduction Science 136, 139–145. 10.1016/j.anireprosci.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Kmenta I., Strohmayer C., Müller‐schlösser F. & Schäfer‐somi S. (2011) Effects of a lecithin and catalase containing semen extender and a second dilution with different enhancing buffers on the quality of cold‐stored canine spermatozoa. Theriogenology 75, 1095–1103. 10.1016/j.theriogenology.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Lee H.Y., Diehn K.K., Ko S.W., Tung S.H. & Raghavan S.R. (2010) Can simple salts influence self‐assembly in oil? Multivalent cations as efficient gelators of lecithin organosols. Langmuir 26, 13831–13838. 10.1021/la1019108. [DOI] [PubMed] [Google Scholar]
- Linde‐Forsberg C. (1991) Achieving canine pregnancy by using frozen or chilled extended semen. The Veterinary Clinics of North America . Small Animal Practice 21, 467–485. 10.1016/s0195-5616(91)50054-1 [DOI] [PubMed] [Google Scholar]
- Linde‐Forsberg C. (1995) Artificial insemination with fresh, chilled extended, and frozen‐ thawed semen in the dog. Seminars in veterinary medicine and surgery (small animal) 10, 48–58. [PubMed] [Google Scholar]
- Manjunath P., Nauc V., Bergeron A. & Ménard M. (2002) Major proteins of bovine seminal plasma bind to the low‐density lipoprotein fraction of hen's egg yolk. Biology of Reproduction 67, 1250–1258. 10.1095/biolreprod.102.004358 [DOI] [PubMed] [Google Scholar]
- Markina A.A., Ivanov V.A., Komarov P.V., Khokhlov A.R. & Tung S.H. (2017) Self‐assembly of lecithin and bile salt in the presence of inorganic salt in water: mesoscale computer simulation. Journal of Physical Chemistry B 121, 7878–7888. 10.1021/acs.jpcb.7b04566. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., Zareh Shahneh A., Towhidi A., Kohram H., Esmaeili V. & Davachi N.D. (2016) Fertility and flow cytometry study of frozen‐thawed sperm in cryopreservation medium supplemented with soybean lecithin. Cryobiology 73, 69–72. 10.1016/j.cryobiol.2016.05.010. [DOI] [PubMed] [Google Scholar]
- McCormack J.G. & Denton R.M. (1989) The role of Ca2 + ions in the regulation of intramitochondrial metabolism and energy production in rat heart. Molecular and Cellular Biochemistry 89, 121–125. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2682206 [PubMed] [Google Scholar]
- Nascimento J.M., Shi L.Z., Chandsawangbhuwana C., Tam J., Durrant B., Botvinick E.L. & Berns M.W. (2015) Use of laser tweezers to analyze sperm motility and mitochondrial membrane potential. Journal of Biomedical Optics 13, 014002 10.1117/1.2839051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima K., Kitajima S., Koshimoto C., Morimoto M., Watanabe T., Fan J. & Matsuda Y. (2015) Motility and fertility of rabbit sperm cryopreserved using soybean lecithin as an alternative to egg yolk. Theriogenology 84, 1172–1175. 10.1016/j.theriogenology.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Nizański W., Klimowicz M., Partyka A., Savić M. & Dubiel A. (2009) Effects of the inclusion of equex STM into tris‐based extender on the motility of dog spermatozoa incubated at 5°C. Reproduction in Domestic Animals 44, 363–365. 10.1111/j.1439-0531.2009.01435.x. [DOI] [PubMed] [Google Scholar]
- Nouri H., Towhidi A., Zhandi M. & Sadeghi R. (2013) The effects of centrifuged egg yolk used with INRA plus soybean lecithin extender on semen quality to freeze miniature caspian horse semen. Journal of Equine Veterinary Science 33, 1050–1053. 10.1016/j.jevs.2013.03.184. [DOI] [Google Scholar]
- Owczarzy R., Moreira B.G., You Y., Behlke M.A. & Walder J.A. (2008) Supporting information to predicting stability of DNA duplexes in solutions containing magnesium and monovalent cations. Biochemistry 47, 5336–5353. 10.1021/bi702363u [DOI] [PubMed] [Google Scholar]
- Payan‐carreira R., Miranda S. & Ni W. (2011) Artificial Insemination in Dogs, Artificial Insemination in Farm Animals. InTechOpen, Open Access books. [Google Scholar]
- de Paz P., Esteso M.C., Alvarez M., Mata M., Chamorro C.A. & Anel L. (2010) Development of extender based on soybean lecithin for its application in liquid ram semen. Theriogenology 74, 663–671. 10.1016/j.theriogenology.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Peña A. & Linde‐Forsberg C. (2000) Effects of Equex, one‐ or two‐step dilution, and two freezing and thawing rates on post‐thaw survival of dog spermatozoa. Theriogenology 54, 859–875. [DOI] [PubMed] [Google Scholar]
- Ponglowhapan S., Essén‐Gustavsson B. & Linde Forsberg C. (2004) Influence of glucose and fructose in the extender during long‐term storage of chilled canine semen. Theriogenology 62, 1498–1517. 10.1016/j.theriogenology.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Rijsselaere T., Soom A.Van, Maes D. & Kruif A.De (2002) Effect of centrifugation on in vitro survival of fresh diluted canine spermatozoa. Theriogenology 57, 1669–1681. [DOI] [PubMed] [Google Scholar]
- Rodenas C., Parrilla I., Roca J., Martinez E.A. & Lucas X. (2014) Quality of chilled and cold‐stored (5°C) canine spermatozoa submitted to different rapid cooling rates. Theriogenology 82, 621–626. 10.1016/j.theriogenology.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Rota A., Strom B. & Linde‐Forsberg C. (1995) Effects of seminal plasma and three extenders on canine semen stored at 4°C. Theriogenology 44, 885–900. https://doi.org/0093-691x(95)00278-2 [DOI] [PubMed] [Google Scholar]
- Salmani H., Towhidi A., Zhandi M., Bahreini M. & Sharafi M. (2014) In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 68, 276–280. 10.1016/j.cryobiol.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Santo‐Domingo J. & Demaurex N. (2010) Calcium uptake mechanisms of mitochondria. Biochimica et Biophysica Acta ‐ Bioenergetics 1797, 907–912. 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Shahiduzzaman A.K.M. & Linde‐Forsberg C. (2007) Induced immotility during long‐term storage at +5 & #xB0;C does not prolong survival of dog spermatozoa. Theriogenology 68, 920–933. 10.1016/j.theriogenology.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Silva A.R., Cardoso R.D.C.S., Uchoa D.C. & da Silva L.M. (2002) Effect of tris‐buffer, egg yolk and glycerol on canine semen freezing. The Veterinary Journal 164, 244–246. 10.1053/tvjl.2002.0704 [DOI] [PubMed] [Google Scholar]
- Smith A.M.J., Bonato M., Dzama K., Malecki I.A. & Cloete S.W.P. (2018) Mineral profiling of ostrich (Struthio camelus) seminal plasma and its relationship with semen traits and collection day. Animal Reproduction Science 193, 98–106. 10.1016/j.anireprosci.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Tamuli M.K. & Watson P.F. (1994) Use of a simple stating technique to distinguish acrosomal changes in the live sperm sub‐population. Animal Reproduction Science 35, 247–254. [Google Scholar]
- Thomassen R. & Farstad W. (2009) Artificial insemination in canids: a useful tool in breeding and conservation. Theriogenology 71, 190–199. 10.1016/j.theriogenology.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Treulen F., Sánchez R. & Risopatrón J. (2012) Effects of seminal fluid fractions on plasma and acrosome membrane integrity and mitochondrial membrane potential determined by flow cytometry in chilled canine spermatozoa. Reproduction in Domestic Animals 47, 1043–1048. 10.1111/j.1439-0531.2012.02011.x. [DOI] [PubMed] [Google Scholar]
- Ustuner B., Alcay S., Toker M.B., Nur Z., Gokce E., Sonat F.A. et al (2016) Effect of rainbow trout (Oncorhynchus mykiss) seminal plasma on the post‐thaw quality of ram semen cryopreserved in a soybean lecithin‐based or egg yolk‐based extender. Animal Reproduction Science 164, 97–104. 10.1016/j.anireprosci.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Verstegen J.P., Onclin K. & Iguer‐Ouada M. (2005) Long‐term motility and fertility conservation of chilled canine semen using egg yolk added Tris‐glucose extender: in vitro and in vivo studies. Theriogenology 64, 720–733. 10.1016/j.theriogenology.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Vick M.M., Bateman H.L., Lambo C.A. & Swanson W.F. (2012) Improved cryopreservation of domestic cat sperm in a chemically defined medium. Theriogenology 78, 2120–2128. 10.1016/j.theriogenology.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Voccoli V., Tonazzini I., Signore G., Caleo M. & Cecchini M. (2014) Role of extracellular calcium and mitochondrial oxygen species in psychosine‐induced oligodendrocyte cell death. Cell Death and Disease 5, e1529 10.1038/cddis.2014.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe S., Leoci R., Aiudi G. & Lacalandra G.M. (2009) Relationship between motility and mitochondrial functional status in canine spermatozoa. Reproduction in Domestic Animals 44(SUPPL. 2), 275–278. 10.1111/j.1439-0531.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- Wales R.G. & White I.G. (1965) Some observations on the chemistry of dog semen. Journal of Reproduction and Fertility 9, 69–77. [DOI] [PubMed] [Google Scholar]
- Walsh C., Barrow S., Voronina S., Chvanov M., Petersen O.H. & Tepikin A. (2009) Modulation of calcium signalling by mitochondria. Biochimica et Biophysica Acta ‐ Bioenergetics 1787, 1374–1382. 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Watson P.F. (1981) The roles of lipid and protein in the protection of ram spermatozoa at 5 degrees C by egg‐yolk lipoprotein. Journal of Reproduction and Fertility 62, 483–492. 10.1530/jrf.0.0620483. [DOI] [PubMed] [Google Scholar]
- Witte T.S., Schäfer‐Somi S., Kuchar A., Möstl E., Iben C. & Aurich C. (2009) Effect of hen's egg yolk on capacitation and acrosome reaction of diluted canine spermatozoa. Animal Reproduction Science 110, 293–305. 10.1016/j.anireprosci.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Wong W.Y., Flik G., Groenen P.M.W., Swinkels D.W., Thomas C.M.G., Copius‐Peereboom J.H.J. et al (2001) The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reproductive Toxicology 15, 131–136. 10.1016/S0890-6238(01)00113-7. [DOI] [PubMed] [Google Scholar]
- Yildiz C., Bozkurt Y. & Yavas I. (2013) An evaluation of soybean lecithin as an alternative to avian egg yolk in the cryopreservation of fish sperm. Cryobiology 67, 91–94. 10.1016/j.cryobiol.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Yotov S. (2015) Effect of TFC‐based extenders with soybean lecithin and/or low concentration of glycerol on the quality of goat chilled‐stored semen. International Journal of Current Microbiology and Applied Sciences 4, 752–761. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The composition of canine seminal fluid from American Bully dogs and analysis methods


