Abstract
Oncological emergencies can occur at any time during the course of a malignancy and need to be recognized promptly to maximize successful outcomes. Emergencies are characterized as chemotherapy‐induced, paraneoplastic syndromes, or directly related to the neoplasm. Prompt identification with treatment of these emergencies can prolong survival and improve quality of life, even in the setting of terminal illness. This review aims to educate the reader on the pathophysiology, clinical presentation and treatment of some of these emergencies, and to review the current veterinary literature to help educate veterinarians in primary and tertiary facilities to know how to diagnose and treat these serious conditions.
Keywords: oncology, hypercalcaemia, tumour lysis syndrome, chemotherapy, cancer, paraneoplastic, canine, feline
Introduction
The care of oncology patients, when they develop acute complications either from their underlying neoplasm or secondary to therapy, presents a challenge for veterinary oncologists, general practitioners and emergency clinicians. An oncological emergency is defined as an acute condition that is caused by cancer (paraneoplastic syndrome) or its treatment requiring rapid intervention to avoid death or severe permanent damage (Cervantes & Chirivella 2004). In this review, we will discuss the pathophysiology, presentation, diagnosis and treatment of commonly encountered emergencies in oncology with a focus on chemotherapy‐induced emergencies, gastrointestinal emergencies and acute paraneoplastic illness.
Chemotherapy‐induced emergencies
Under the direction of a veterinary oncologist, chemotherapy administration is considered a relatively safe procedure with fewer than 25% of animals having adverse effects (AE) (Chun et al. 2001). A 2011 consensus document published by the Veterinary Cooperative Oncology Group (VCOG) provided practitioners with a grading scale for AE that can occur as well as the terminology to facilitate the accurate and consistent reporting of AE in patients receiving chemotherapy. Such standardization of terminology is essential for information transmission between clinicians and institutions (Yoon et al. 2007). These guidelines discuss a comprehensive range of AE and are divided into categories based on anatomy or pathophysiology with each given a grade of 1 through 5, from mild to death related AE. The reader is directed to the VCOG paper for further discussion of AE grading (VCOG 2011).
Neutropenia
Neutrophils are the most abundant white blood cell in cats and dogs and are produced following release of granulocyte colony stimulating factor (G‐CSF) which stimulates neutrophil production. Neutrophil transit time through the bone marrow is 6 days and the circulating half‐life is 4 to 8 h (Lucroy 2005). In dogs, about half of the neutrophils in circulation are in the circulating pool and half in the marginated pool. In cats, only a quarter of neutrophils are in the circulating pool (Schultze 2010). Classification of neutropenia in dogs and cats differ with a value of less than 2500 cells μL−1 in dogs and less than 2000 cells μL−1 in cats considered neutropenic (Brown & Rogers 2001). However, in the authors’ experience, it is not common to see clinical signs related to neutropenia unless the neutrophil count is below 1000 cells μL−1. Chemotherapy drugs are commonly associated with the development of neutropenia due to their myelosuppressive effects. In most instances of chemotherapy‐induced neutropenia, the patient is asymptomatic, so it may often go unnoticed or be found incidentally when a complete cell count (CBC) is rechecked prior to the next chemotherapy treatment. However, neutropenia can be accompanied by fever (temperature greater than 39.2°C), and clinical signs of infection may be present (Laforcade 2015). The true incidence of febrile neutropenia is not known but it is generally considered to be low. Risk factors for the development of neutropenia in dogs following chemotherapy administration include a lower body weight, dogs diagnosed with lymphosarcoma (LSA) and those who received doxorubicin (DOX) or vincristine (Brown & Rogers 2001; Sorenmo et al. 2010). With respect to cats developing neutropenia post chemotherapy administration, LSA was the most common diagnosis seen with both lomustine and vinca alkaloids associated with the development of neutropenia (Pierro et al. 2016).
Neutropenic patients may present to an emergency clinic with varying clinical signs, most commonly decreased appetite or lethargy after chemotherapy treatments. If these signs arise on or around the expected nadir of neutropenia of a recently‐administered chemotherapy drug (often 7–10 days after a chemotherapy treatment) then this is an indication that a bacterial infection may be present, especially if there is a concurrent fever (rectal temperature greater than 39.2°C). A CBC should be performed to confirm and determine the severity of the neutropenia. Patients with mild infections can often be treated on an outpatient basis, see Fig. 1.
Figure 1.
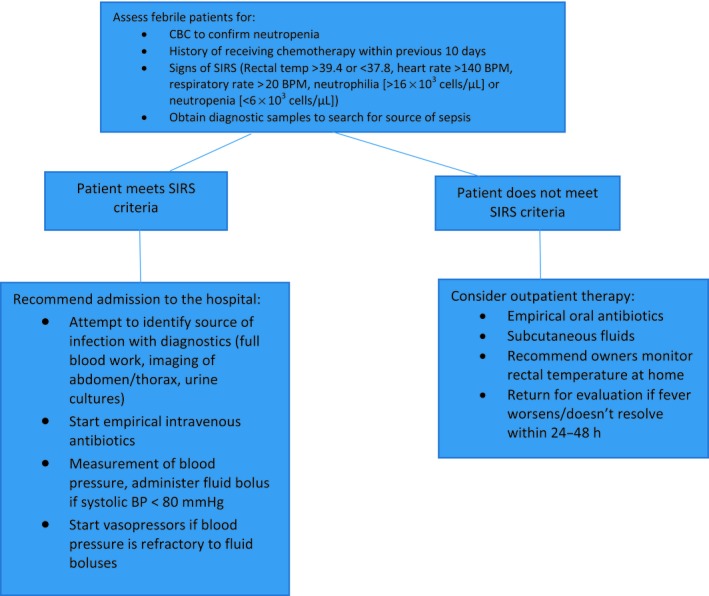
Management of febrile neutropenia.
Patients with more severe bacterial infections can present in systemic inflammatory response syndrome (SIRS) or in a state of sepsis. SIRS may resemble sepsis; however, it can occur in the absence of a documented infection (Brady & Otto 2001; Klainbart et al. 2017). For the definition of SIRS to be met veterinary patients must exhibit certain criteria, see Fig. 1, although such criteria are not considered 100% sensitive, however, the specificity can be quite high (Hauptman et al. 1997; Babyak & Sharp 2016). A recent study in a human emergency department also supported the use of SIRS criteria due to its high specificity and ease of use (Haydar et al. 2017). Sepsis is defined as a life‐threatening organ dysfunction caused by a dysregulated host response to infection (Singer et al. 2016). It has been noted in human literature that >50% of patients with febrile neutropenia or bacteraemia go on to develop sepsis (Penack et al. 2014). The incidence of sepsis from myelosuppressive chemotherapy in veterinary patients is relatively low and is likely multifactorial (Sorenmo et al. 2010). If left untreated, sepsis can be fatal.
The pathophysiology of sepsis remains incompletely understood but likely involves a combination of different cell types and a primary inflammatory stimulus under the influence of the innate immune system and T‐cells. The innate immune system responds to pathogens typically via receptors on the pathogen and a related ligand on an effector cell within the immune system. The major targets of these receptors are Pathogen‐associated molecular patterns (PAMPs) and Damage‐associated molecular patterns (DAMPs). The former responds to microbial pathogens while the latter respond to apoptotic cells. From a clinical standpoint, the end result of stimulation of either PAMPs or DAMPs is similar and once these receptors are stimulated, a cascade involving production of pro and anti‐inflammatory cytokines begins. Large production of proinflammatory cytokines is associated with the deleterious events observed in sepsis while an over‐production of anti‐inflammatory cytokines may lead to immunodeficiency and can also lead to a poor outcome (Pusterla et al. 2006; Briassoulis et al. 2010). Diagnostic measurement of cytokines may help physicians better characterize where in this pro or anti‐inflammatory cytokine spectrum individuals are falling however these are not widely available to most veterinarians at this time. Examples of such cytokines include TNF‐a which once released causes increased production of inducible nitric oxide synthase (iNOS) and cyclooxygenase‐2 (COX‐2), the former of which causes production of nitric oxide which can lead to hypotension. (Stylianou & Saklatvala 1998; Chaitanya et al. 2010). In contrast, COX‐2 will lead to significant inflammation. Other important cytokines produced include IL‐6 which triggers the acute phase response (APR) and is a potent pyrogen (Jawa et al. 2011). The APR is characterized by fever, activation of the coagulation and complement cascades and increased muscle and lipid metabolism (Cray et al. 2009). IL‐10 is the main anti‐inflammatory cytokine and acts to inhibit the release of other pro‐inflammatory cytokines and balance the pro and anti‐inflammatory processes occurring in sepsis. The end result of the release of such cytokines lead to multiple adverse effects across most of the body's organ systems and the clinical syndrome of sepsis.
For patients presenting septic, it is important to determine the source of infection where possible, in order to choose the most effective therapy. Diagnostic tests including imaging of the thorax and abdomen and/or blood and urine cultures can help identify the source of infection. Blood culture samples need to be obtained from different sites and should be obtained prior to starting antibiotics; however, this may not be feasible in all veterinary patients (Dellinger et al. 2012). Once necessary diagnostic samples have been taken, these patients should be started on broad spectrum antibiotics. Typically, empirical treatment using a beta lactam combined with a fluoroquinolone is pursued in small animal patients (Boothe 2015). Broad spectrum coverage to target both gram positive and negative microorganisms is recommended as gram negative organisms are the most commonly implicated infections in septic dogs and cats (Laforcade et al. 2003). Anaerobic coverage should also be considered for selected infections involving bone, deep or isolated areas and abscesses (Boothe 2015).
Sepsis remains a difficult syndrome to treat but improvements in the outcome of septic human patients have been accomplished with ‘bundle therapy,’ see Fig. 1. A bundle of therapy refers to therapies that, when instituted together, result in better outcomes than if each individual component were implemented alone (Cinel & Dellinger 2006). It is likely that a similar approach may improve outcomes in veterinary patients. One such bundle emphasizes the importance of measuring lactate within 6 hours of admission and reversing hyperlactataemia. Veterinary studies have found an improved prognosis with prompt lactate clearance (Bonczynski et al. 2003; Stevenson et al. 2007; Conti‐Patara et al. 2012). Other bundles recommend obtaining samples for culture or removal of the septic focus where possible and early administration of antibiotics, as previously discussed. The remaining bundles centre on the assessment and treatment of hypotension. Assessment of fluid responsiveness includes monitoring the animal's weight, vital parameters, blood pressure, mentation and urine output (UOP) via an indwelling urinary catheter. Finally, the guidelines also recommend the use of vasopressor agents in cases of refractory hypotension following fluid resuscitation. Noradrenaline is the first‐choice vasopressor in human medicine due to higher rates of adverse effects with adrenaline and dopamine (Puskarich 2012; De Backer et al. 2013, Vasu et al. 2012). Studies in veterinary medicine evaluating the superiority of various vasopressors are currently lacking. For clinicians managing septic patients, these can be quite labour‐intensive cases which typically require continuous monitoring and referral to speciality clinics is advisable to maximize success.
Myelosuppression and its secondary complications represent the major dose limiting toxicity of chemotherapy. Recombinant G‐CSF use in human oncology patients has been shown to redcue the severity and duration of neutropenia (Gabrilove et al. 1988; Morstyn et al. 1989; Trillet‐Lenoir et al. 1993). Clinical use of recombinant G‐CSF is limited in veterinary medicine due to its high cost in certain countries, the potential for auto‐antibody formation (Hammond et al. 1991), low incidence of febrile neutropenia, and lack of evidence that administration of G‐CSF will improve survival in cases of febrile neutropenia. Even severe neutropenia will generally resolve quickly secondary to production of endogenous G‐CSF (Britton et al. 2014), which is consistent with the authors’ experience. While there is currently not enough information to recommend the routine use of G‐CSF in veterinary patients, it should be noted that G‐CSF may be considered for patients in specialized circumstances where neutropenia may be severe and prolonged, such as those undergoing total body irradiation in combination with autologous peripheral blood progenitor cell transplants(, in cases of chemotherapy overdose and could be considered in difficult cases where the neutropenic chemotherapy patient is not responding to conventional therapy (Ogilvie et al. 1992; Escobar et al. 2012; Wells et al. 2014; Finlay et al. 2017).
Anaphylactic reactions to Antineoplastic agents
Several medications commonly used by oncologists can induce anaphylaxis, including l‐asparaginase (L‐asp), DOX and drugs in the taxane family (Chun et al. 2001; Poirier et al. 2004; Blake et al. 2016, Axiak et al. 2011; Kim et al. 2015; Silva et al. 2015). L‐asp is an enzyme derived from Escherichia coli, all formulations are immunogenic since they are foreign proteins (Rogers 1989). DOX can induce hypersensitivity via stimulating histamine release (Susaneck 1983; Phillips et al. 1998). The exact mechanism for hypersensitivity from taxane chemotherapy drugs (paclitaxel, docetaxel) is unknown, but, there are several theories including complement system activation induced by Cremophor (which is compounded with paclitaxel) and polysorbate 80 (which is compounded with docetaxel). Stimulation of the complement system then activates mast cells. Recent use of a water‐soluble veterinary formulation of paclitaxel (Paccal® Vet) without need for pre‐medication lends credence to this theory (Von Euler et al. 2013). Other theories include induced histamine release secondary to the drug's effects on basophils (Picard & Castells 2015).
Anaphylaxis can induce a range of acute clinical signs which can differ between species. In dogs dermal and gastrointestinal clinical signs predominate. The dermal signs are primarily secondary to the vasodilatory effects of histamine which causes erythema and pruritus. Many of the gastrointestinal signs occur secondary to hepatic congestion and portal hypertension which are also induced following histamine release leading to severe vomiting and diarrhoea (Shmuel & Cortes 2013). Gastrointestinal and respiratory tract signs are more typical in cats and they can present dyspneoic in severe cases (Shmuel & Cortes 2013; Kim et al. 2015; Murphy et al. 2016). Where anaphylaxis is suspected secondary to these drugs, administration should be stopped immediately. The animal should be treated with antihistamines, glucocorticoids and intravenous fluids to combat vasodilatory shock (Shmuel & Cortes 2013). However, the beneficial effects of glucocorticoids take several hours to occur and should never be considered the sole treatment for severe anaphylaxis (Shmuel & Cortes 2013). Furthermore, since histamine is just one of many vasodilatory substances released during anaphylaxis, anti‐histamines have limitations in animals with life‐threatening anaphylaxis. Intravenous fluids should be used judiciously in dyspnoeic animals; however, they should never be withheld as these patients are typically hypotensive either from hypovolaemic losses with vomiting and diarrhoea or have a relative hypovolaemia from vasodilatory shock. In severe cases where the blood pressure is refractory to the intravenous fluids and anti‐histamines, vasopressors are recommended. Adrenaline is considered the first line vasopressor for acute anaphylaxis by the World Allergy Association. There are no controlled trials in either veterinary or human medicine evaluating different vasopressors for anaphylaxis treatment. Such trials are considered potentially unethical because the preponderance of data in humans show that treatment with Adrenaline is critical for survival in many instances (Kemp et al. 2008; Shmuel & Cortes 2013). The beneficial effects of Adrenaline in cases of anaphylaxis stem from its effects on beta and alpha receptors. ß1 receptor agonism leads to positive inotropic effects increasing cardiac output. Stimulation of the ß1 receptor reduces release of inflammatory mediators by mast cells and has bronchodilatory effects (Shmuel & Cortes 2013). In addition, stimulation of alpha receptors by Adrenaline may help to alleviate laryngeal oedema and circumvent circulatory collapse via vasoconstriction (Estelle & Simons 2011). In dyspnoeic animals, supplemental oxygen should be provided. An inhaled bronchodilator can also be utilized to help relieve bronchospasm, but their use should not replace Adrenaline (Shmuel & Cortes 2013). After an anaphylactic event, the animal should not be administered the causative agent again. Although there is no way to completely prevent these reactions, some oncologists recommend the use of diphenhydramine and/or steroids as a premedication prior to the use of anti‐neoplastic agents that can induce anaphylaxis (Phillips et al. 1998; Silva et al. 2015).
Extravasation
Many chemotherapeutic agents can induce significant tissue injury if extravasation (EV) of the drug occurs during administration. Examples of such vesicants include commonly used chemotherapy agents such as DOX and the vinca alkaloids (Villalobos 2006). The exact mechanism behind extravasation injury is not completely understood, but it is thought to be due, at least in part, to tissue damage secondary to free radical formation or damage to DNA. Progression of the tissue damage and subsequent necrosis occurs following diffusion of drug/DNA complexes. Non‐DNA binding agents such as the vinca alkaloids are metabolized quickly, allowing normal healing to occur after the initial injury, while DNA binding agents (such as DOX) will remain in the tissues and cause more prolonged injury (Dorr 1990).
Tissue necrosis typically is noticeable 1‐10 days after injection depending on the drug used. Severity ranges from erythema to more serious open draining wounds. Immediate treatment (Table 1) includes applying cold packs in the case of DOX and warm packs with the vinca alkaloids (Dorr 1990; Pattison 2002; Villalobos 2006). In cases of DOX EV (Fig. 2), treatments include dimethyl sulphoxide (DMSO) and hyaluronidase (HU) (Villalobos 2006; Mouridsen et al. 2007). More recently, dexrazoxane use has shown benefit in dogs and this is now the treatment of choice for treatment of DOX extravasation (Mouridsen et al. 2007; Venable et al. 2012). Dexrazoxane acts as a metal ion chelator protecting against free radical toxicity induced by DOX‐iron complexes (Langer et al. 2000). Ideally, dexrazoxane should be administered within 3–6 h of a known or suspected DOX EV and then again at 24 and 48 h. Use of dexrazoxane can be limited by its cost and availability. For EV associated with vinca alkaloids, treatments include infiltration of HU which promotes the diffusion of a chemotherapeutic by temporarily decreasing glycosaminoglycans which control diffusion of materials in and out of cells (Dorr 1990; Raszka et al. 1990; Pattison 2002; Spugnini 2002; Villalobos 2006). HU has been used successfully in cases of EV in several dogs given DOX and vinca alkaloids (Spugnini 2002). Steps to reduce the risk of EV occurring following chemotherapy administration include not performing blood draws on peripheral veins to preserve them for intravenous catheter placement (Villalobos 2006). Intravenous catheters being used for administration of these agents should have been placed recently (ideally within 4 h) and the catheter should be checked rigorously for patency with saline before and after the chemotherapeutic agent is given (Villalobos 2006).
Table 1.
Treatment of extravasation
| Drug | Intervention | Advice |
|---|---|---|
| DOX | DMSO | Apply locally immediately of a known/suspected DOX EV |
| Dexrazoxane | Administer within 3–6 h of a known/suspected DOX EV | |
| Ice packs | Apply immediately of a known/suspected DOX EV | |
| Vinca Alkaloids | Hyaluronidase | Instil 1 mL hyaluronidase (150 U mL−1) for every 1 mL of extravasated drug through the existing catheter line |
| Hot packs | Apply immediately of a known/suspected vinca alkaloids EV |
Figure 2.
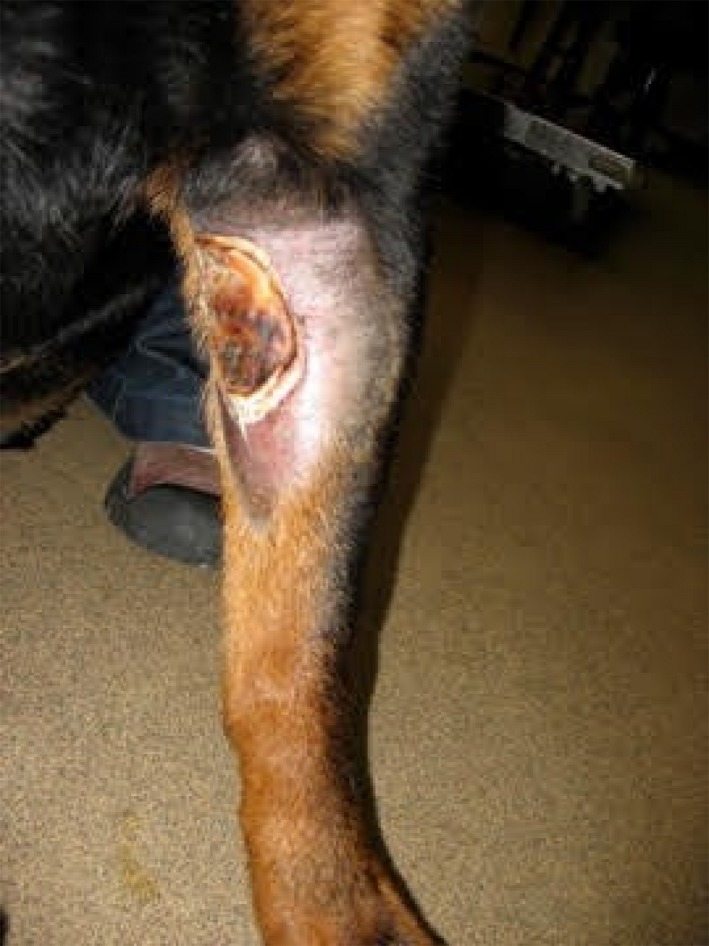
Extravasation secondary to doxorubicin in a dog.
Gastrointestinal toxicity
Although occurring less commonly in small animal patients compared to people, gastrointestinal toxicity (GIT) secondary to either the underlying cancer and/or chemotherapy can have a major impact on the patients’ quality of life. Risk factors for vomiting include administration of highly emetogenic chemotherapy, smaller patient size, repetitive administration of chemotherapy and a gastrointestinal comorbidity (Ogilvie et al. 1989; Moore et al. 1994; Vail 2009). There are two main causes of GIT in cancer patients: centrally mediated nausea and direct effects on the GI tract. The normal physiological pathway of vomiting is stimulated by several factors all of which converge on the vomiting centre within the medulla (Chapman 2015). This includes neural input via the vagal and sympathetic fibres secondary to gastrointestinal inflammation and/or over distension, anxiety/anticipation stimulating the cerebrum or excessive motion stimulating the vestibular centre. With respect to certain medications, including several chemotherapeutics, or toxins (e.g. uraemic, hepatic or endotoxins), these stimulate the chemoreceptor trigger zone (CTZ) which is a specialized region located within the fourth ventricle of the brain. This area is particularly sensitive to such substances because it lacks an intact blood brain barrier so emetic toxins and molecules can enter here relatively easily. Once stimulated, the receptors in the CTZ stimulate the vomiting centre in the brain. This area controls certain autonomic functions including swallowing, gastric sensation, baroreceptor function and pharyngeal sensation. Regardless of the exact stimulus, once the vomiting centre has been sufficiently stimulated, the process of vomiting begins (Chapman 2015). When caused by chemotherapy administration, centrally mediated nausea occurs acutely, either during the drug administration, or within the first few hours post‐treatment. This form of nausea is rarely an emergency and is generally controllable with injectable or oral antiemetics, which can be given prophylactically, prior to administration of a known emetogenic chemotherapeutic agent, or once signs of nausea are seen.
Decreased appetite, nausea, vomiting and diarrhoea commonly occur secondary to tumours that directly involve the GI tract or that cause external compression of the stomach or intestines. These signs occur secondary to GI obstruction, peritumoral inflammation, or affects GI motility and absorption. Ideally, treatment for this problem would be via effective treatment of the underlying cancer. This can be accomplished by surgical removal of the cancer or reducing tumour volume with other treatment modalities (e.g. chemotherapy, radiation therapy). Chemotherapy‐induced GIT can also occur secondary to the direct toxic effects of these drugs on the cells of the GI tract. Rapidly‐dividing multipotent stem cells in the intestinal crypts can be damaged by chemotherapy, resulting in depletion of enterocytes and villous blunting, which in turn leads to poor nutrient absorption (resulting in diarrhoea) and secretion of emetogenic factors (resulting in nausea and vomiting). These effects are often referred to as delayed GIT and generally occur 2–5 days after treatment, therefore if GI signs occur outside of this interval, it is important to consider other causes (unrelated to the chemotherapy) for these signs. The likelihood and severity of GIT post‐treatment varies with different chemotherapeutic agents.
Where vomiting is self‐limiting, animals can be fasted for several hours, although water should be provided. Once vomiting has ceased, a bland diet can be reintroduced (Vail 2009). Anti‐emetics can also be administered with the parenteral route preferred. Commonly used antiemetics include maropitant and ondansetron. For patients experiencing severe GI side effects, intravenous fluid therapy is recommended. A multimodal approach to antiemetic therapy may be required in these patients as their nausea can stem from both peripheral gastrointestinal tract dysfunction and stimulation at the central CTZ. For a full review of these anti‐emetics, the reader is directed elsewhere (Vail 2009; Whitehead et al. 2016).
Tumour lysis syndrome
Tumour Lysis Syndrome (TLS) occurs when large numbers of tumour cells release their contents (nucleic acids, cations and anions including potassium and phosphorus) into the bloodstream simultaneously. TLS can occur spontaneously or more commonly in response to therapy as a result of rapid tumour cell death following effective chemotherapy treatment. Nucleic acids are initially broken down and release purines, adenosine and guanosine. Both are then further metabolized into inosine and guanine, respectively. The result of this pathway is the formation of hypoxanthine which itself is further broken down into xanthine and finally uric acid via the enzyme xanthine oxidase (Rigas et al. 1956; Hochberg & Cairo 2008; Howard et al. 2011). Uric acid, in particular, can have deleterious effects on numerous organ system, especially the renal system. It has several negative effects on the kidneys including renal vasoconstriction, impaired autoregulation and crystal‐induced tissue damage (Ejaz et al. 2007; Shimada et al. 2009). Based on the few case reports in the veterinary literature, the clinical ramifications of such changes usually result in acute kidney injury (AKI) in dogs and cats (Calia et al. 1996; Mylonakis et al. 2007; Vickery & Thamm 2007). Interestingly, most breeds of dogs, except English Bulldogs and Dalmatians, oxidise uric acid into allantoin in the liver via uricase (Page et al. 1986). Thus, hyperuricaemia may not be as important in renal injury in canine cases of TLS.
Two classifications of TLS have been developed in people (Cairo & Bishop 2004). Laboratory TLS is defined as two or more of the following metabolic abnormalities occurring up to seven days after initiation of chemotherapy: hyperuricaemia, hyperkalaemia, hyperphosphataemia and hypocalcaemia. However, clinical TLS is accompanied by an increased creatinine, seizures, cardiac arrhythmias and/or death. Specific classifications for dogs and cats with TLS are lacking in veterinary literature.
Literature evaluating the clinical signs of TLS in small animal patients is limited to several published case reports. One such report described the development of TLS in a dog several hours after radiation therapy (RT) for LSA (Vickery & Thamm 2007). Following RT, the dog developed vomiting and diarrhoea and on physical examination was noted to be tachycardic, tachypnoeic with weak femoral pulses on palpation. A similar report in a cat with TLS illustrated the development of shock several hours following RT for mediastinal LSA (Calia et al. 1996).
In human medicine the severity of TLS likely depends on the type of cancer, the cancer burden and patient characteristics (Howard et al. 2011). Known patient characteristics which confer a higher risk in humans include pre‐existing chronic renal insufficiency, oliguria, dehydration, hypotension and acidic urine (Howard et al. 2011). Such characteristics are not reported in veterinary medicine. The exact prevalence of TLS in cats and dogs is unknown. In the authors’ experience, however, TLS may also be inaccurately blamed for severe post‐chemotherapy illness that is unrelated to tumour cell lysis. If characteristic electrolyte derangements are not seen, or if there has been minimal reduction in tumour volume, it is important to look for other causes for the presenting clinical signs.
Successful treatment of TLS depends on symptomatic management of the electrolyte disturbances and treatment of AKI, see Fig. 3. Current recommendations for management of AKI in humans include the discontinuation of nephrotoxic drugs, haemodynamic support with intravenous fluids, monitoring of UOP and, in cases of oliguria/anuria, vasoactive drugs (Keir & Kellum 2015). Once AKI is suspected, it is imperative that these patients are placed on a balanced intravenous fluid. An initial guide for prescribing intravenous fluids includes the following formula: percentage of dehydration x body weight in [kg] = fluid deficit in litres (Cavanagh et al. 2016). Even with cautious fluid administration, these patients are at risk of fluid overload which is typically defined as fluid accumulation more than 10% of baseline body weight (Cavanagh et al. 2016). Development of fluid overload and/or oliguria/anuria often indicates end‐stage AKI. Once this occurs, renal replacement therapy is considered gold standard for treatment; the prohibitive cost and availability affect its use in veterinary medicine. Vasodilatory medications have been studied for use in AKI as theoretically they can increase glomerular filtration rate (GFR) increasing UOP (Keir & Kellum 2015), see Table 2. When oliguria/anuria and/or fluid overload is suspected, the placement of an indwelling urinary catheter is recommended to measure UOP. Where the owner has declined referral for renal replacement therapy, it is recommended to trial the patient on a vasodilatory medication (see Table 2) in an attempt to increase UOP, although none of these medications have been proven superior. It is important to communicate to the owner that once oliguria/anuria occurs, without haemodialysis, there is a very low chance of conversion back to polyuria and the patient's prognosis at this point is grave.
Figure 3.
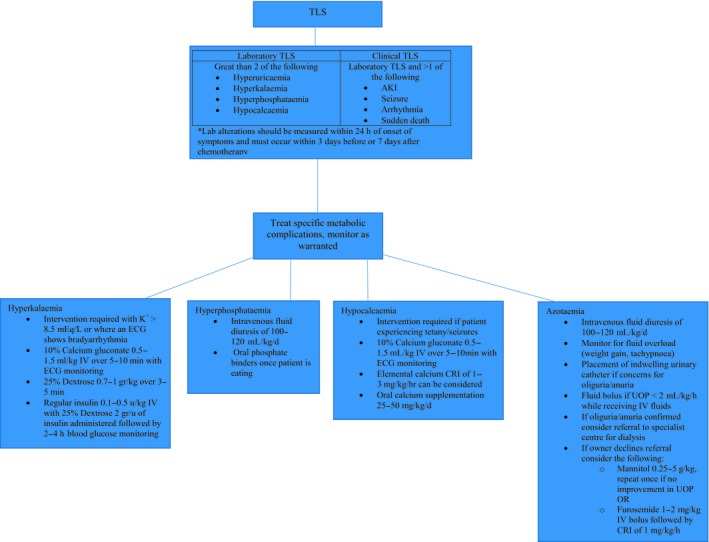
Management of tumor lysis syndrome.
Table 2.
Vasoactive drug options for use in AKI
| Vasoactive Drug | Drug Example | Dose | Comments | Adverse effects |
|---|---|---|---|---|
| Osmotic Diuretics | Mannitol | 0.25–0.5 gr kg−1, do not exceed 2 gr kg−1 | Dose can be repeated if no increased UOP noted within 30–60 min of administration | Volume overload, hyperosmolality |
| Thiazides | Hydrochlorothiazide | See comments | Generally these drugs are not potent enough to cause a significant increase in UOP | |
| Loop Diuretics | Furosemide | 1–2 mg kg−1 loading dose followed by infusion of 1 mg kg−1 h−1 for up to 6 h | If UOP does not increase within 6 h discontinue, if UOP increases reduce to 0.1 mg kg−1 h−1 to maintain urination | Dehydration, hypokalemia, metabolic alkalosis |
| Dopaminergic agonist | Dopamine | 2–5 mcg kg−1min−1 | The effectiveness of this drug has not been proven in human or veterinary medicine | Tachycardia, hypertension |
| DDopaminergic agonist | Fenoldopam |
0.8 mcg kg−1min−1 (dogs) 0.5 mcg kg−1 min−1 (cats) |
Studies in dogs/cats with acute kidney injury did not result in improved outcome | Hypotension |
Prevention of TLS is considered imperative in human medicine but due to the multifactorial nature of TLS pathogenesis, it is difficult to make specific recommendations about preventative strategies in veterinary medicine. It is likely more important that veterinarians recognize the clinical signs of TLS as successful treatment depends on prompt diagnosis and appropriate treatment (Fig. 2). The prognosis for veterinary patients remains poor although more recent case reports show success in managing these cases (Calia et al. 1996; Vickery & Thamm 2007).
Paraneoplastic syndromes
Hypercalcaemia of malignancy
Hypercalcaemia (HC) is a common electrolyte derangement managed by small animal emergency clinicians. There are several possible causes of HC which can be categorized into non‐pathologic, transient and pathological groups; pathological causes are most common. Non‐pathological HC stems from normal physiological growth in young animals while hypoadrenocorticism or hyperproteinaemia can cause transient elevations. In contrast, pathological causes include neoplasia, primary hyperparathyroidism and acute or chronic renal failure. In canine patients, neoplasia is the most common cause of HC. In cats it is the third most common cause behind idiopathic causes and renal disease (Savary et al. 2000; Scheck et al. 2011). This review will focus on the paraneoplastic causes of HC of which several mechanisms are believed to be responsible for this phenomenon.
Paraneoplastic causes of HC include: ectopic production of parathyroid hormone (PTH) or PTH‐related peptide (PTH‐rP), extensive and usually multifocal lytic bone tumours, tumour associated prostaglandins (PGE1 and PGE2), interleukin 1‐ β, transforming growth factor –β (TGF‐ß) and receptor activator of nuclear factor kappa‐ β ligand (RANKL) (Bergman 2013).
Excessive production of parathyroid hormone related protein (PTH‐rP) is one of the most important causes of HC in the setting of neoplasia. Parathyroid hormone (PTH) is produced by the chief cells in the parathyroid gland and is involved in minute to minute control of calcium homeostasis in the body (Scheck et al. 2011). PTH‐rP is structurally and functionally related to PTH and is produced both in the normal physiological conditions and by certain types of cancerous cells (Scheck et al. 2011). Both PTH and PTH‐rP act on the same receptors and their effects consistently overlap. PTH‐rP stimulates osteoclastic bone resorption, increased renal tubular calcium reabsorption and decreased renal tubular phosphate reabsorption (Scheck et al. 2011). Other tumour‐produced factors which can also lead to HC include interleukin (IL)‐1a, IL‐6, transforming growth factor (TGF) and TNF alpha, these may act synergistically with PTH‐rP (Weir et al. 1988). However, measurement of these cytokines is not commonly performed and plasma PTH‐rP is the only assay that is readily available (Stern et al. 1985; Sabatini et al. 1988; Johnson et al. 1989). Common cancers associated with this phenomenon include T‐cell LSA and apocrine gland anal sac adenocarcinomas (AGASACA) (Williams et al. 2003; Bennett et al. 2002; Weir et al. 1988). Concurrent HC has been associated with a poorer prognosis in both LSA and AGASACA (Weir et al. 1988; Bennett et al. 2002; Williams et al. 2003).
Osteolytic metastasis and consequent excessive calcium release from bone also results in HC and is most often seen with carcinomas of the prostate, mammary gland, liver and lung (Rosol 2000; Meuten 1984). The pathogenesis of increased bone resorption is likely due to secretion of cytokines which stimulate local bone resorption and indirect stimulation of bone resorption from local immune or bone cells (Powell et al. 1991, Meuten 1984). Cytokines which may be involved include PTH‐rP, TGF alpha and beta and prostaglandins. Primary bone tumours are rarely associated with HC in dogs and cats (Quigley & Leedale 1983; Jongeward 1985; Mauldin et al. 1988).
Haematological malignancies in the bone marrow such as multiple myeloma and LSA also results in cytokine induced HC (Matus et al. 1986). Evaluation of animals with LSA show that HC in dogs is most commonly associated with the T‐cell phenotype (Weir et al. 1988; Rosol et al. 1992; Fournel‐Fleury et al. 2002). Studies in dogs and cats with multiple myeloma show approximately 20% of those with multiple myeloma develop HC. In dogs with multiple myeloma, concurrent HC is a poor prognostic indicator (Patel et al. 2005). It is believed that HC occurs with multiple myeloma due to increased osteoclastic induced bone resorption secondary to cytokines, tumour necrosis factor‐β and IL‐6, secreted locally by the myeloma cells (Oyajobi & Mundy 2004; Oyajobi 2007).
HC has wide‐reaching effects on many organ systems leading to a variety of clinical signs including polydipsia, polyuria, anorexia and constipation (Scheck et al. 2011). HC results in both functional and structural changes to the kidneys, the latter of which may not be reversible even with treatment. HC leads to renal arterial vasoconstriction reducing GFR. Subsequent azotaemia can be worsened by acute tubular necrosis from the toxic effects of calcium on renal epithelium, nephrocalcinosis and tubulointerstitial inflammation (Scheck et al. 2011). HC also reduces the excitability of GI smooth muscle, while parietal cells release hydrogen ions as a response to HC (Scheck et al. 2011). Clinically important cardiac effects of HC are uncommon in dogs and cats, but arrhythmias can occur from calcium's effects on myocardial cells or secondary to cardiac tissue mineralization (Scheck et al. 2011).
In animals symptomatic for HC, the calcium level should be normalized quickly to avoid organ damage although long‐term control of HC requires definitive treatment of the underlying cause. The best way to correct HC is to effectively treat the underlying cancer, either by surgical removal of the tumour, or by reducing the tumour volume with medical therapy (e.g. chemotherapy for LSA). If reduction in tumour volume is not possible, then the several treatment options can be considered to help control the animal's HC and secondary symptoms. Correction of dehydration with intravenous fluids is of paramount importance with 0.9% NaCl preferred as this promotes calciuresis via increased GFR (Scheck et al. 2011). Loop diuretics can be utilized following rehydration; thiazide diuretics are contraindicated as they can worsen HC (Duarte et al. 1971). Steroids can also be utilized to reduce calcium via decreased bone and intestinal calcium resorption and increased renal calcium excretion (Peterson & Feinman 1982). Steroid administration can prevent diagnosis of LSA and must be used judiciously and only after diagnostic samples have been obtained. Calcitonin can have rapid effects, often within hours of administration, on calcium by reducing the activity and formation of osteoclasts, making it an attractive choice for the emergent treatment of HC (Dougherty et al. 1990; Scheck et al. 2011). Following administration, animals must be monitored for development of hypocalcaemia as its effects on reducing calcium are profound. Bisphosphonates are commonly used in veterinary medicine for chronic management of hypercalcaemia associated with osteolysis or in patients with HC and a gross tumour that is not responding to treatment (or that the owner has decided not to treat). They act by decreasing osteoclast activity. Calcium levels typically take 2–5 days to decrease and effects last for 4–6 weeks (Scheck et al. 2011). Possible adverse effects include oesophageal and gastrointestinal toxicity with oral administration and electrolyte abnormalities (Morden et al. 2015). There are reports of nephrotoxic effects from bisphosphonates, therefore ideally, they would only be used in a well‐hydrated patient, although no significant adverse effects were noted with its use in one cohort of dogs (Machado & Flombaum 1996; Fan et al. 2005). Rapid administration of IV pamidronate can increase its nephrotoxic potential so it is recommended that it be given at a rate of less than 200 mg h−1 (Adami & Zamberlan 1996). Caution should be taken with intravenous bisphosphonate administration as it can be a tissue irritant if EV of the drug occurs. It is important to educate the owner that while there are several treatment options for HC, without controlling the underlying cause it is difficult to maintain normocalcaemia.
Hypoglycaemia
Insulinoma is the most common malignancy associated with hypoglycaemia (HG); however, it has been reported with non‐islet cell tumours including hepatocellular carcinoma, hepatoma, LSA, leiomyosarcoma, adenocarcinoma and haemangiosarcoma among others (Patnaik et al. 1981; Cohen et al. 2003; Liptak et al. 2004; Battaglia et al. 2005). An insulinoma will produce excessive quantities of insulin leading to secondary HG as glucose is translocated into the cells. Theories postulated for how other malignancies can induce HG include secretion of insulin‐like substances, accelerated glucose utilization by the tumour and a failure of hepatic gluconeogenesis or glycogenolysis (Finora 2003). Animals experiencing HG often exhibit neurological clinical signs including weakness, disorientation, seizures or obtundation. In cases of insulinoma, a diagnosis of the HG can be ascertained via an insulin glucose ratio (Leifer et al. 1985). However, conditions other than insulinomas can yield increased ratios, and cases with confirmed insulinoma can still have normal insulin: glucose ratio making the diagnosis difficult (Thompson et al. 1995; Fernandez et al. 2009). In the case of extra‐pancreatic tumours, it can also be difficult to diagnose the exact cause of the HG. In the acute setting, HG patients are stabilized with a continuous infusion of dextrose. Medications, including glucocorticoids, glucagon and beta blockers, can also be used to promote normoglycaemia while more investigations and definitive treatments are pursued (Smith et al. 2000; Datte et al. 2016).
Neoplasia‐related emergencies
Haemorrhagic effusions
In some cases, animals may present acutely with clinical signs secondary to haemorrhagic effusion from the tumour. This review will focus on pericardial and peritoneal haemorrhagic effusions related to neoplasia. Less commonly, spontaneous haemorrhagic pleural effusion can also be associated with neoplasia; however, readers are directed elsewhere for information regarding this (Nakamura et al. 2008).
Pericardial effusions
Neoplasia is the most common cause of pericardial effusion in dogs, with the right atrium and heart base being the most common sites (Ware & Hopper 1999; Vicari et al. 2001; Ehrhart et al. 2002; Johnson et al. 2004). On histology, the most commonly identified neoplasm is haemangiosarcoma (HSA), but others have been reported, including chemodectomas, other neuroendocrine tumours, ectopic thyroid carcinoma, mesothelioma and LSA (Ware & Hopper 1999; MacDonald et al. 2009). Many studies of neoplastic PE in dogs are based on cardiac HSA due to its predominance. The most common presenting signs noted in dogs include muffled heart sounds, tachypnoea, weakness and exercise intolerance (Johnson et al. 2004). Many of these patients may exhibit abnormalities on an electrocardiograph (ECG) including electrical alternans and ventricular arrhythmias (Johnson et al. 2004). PE can also lead to the development of pericardial tamponade (PT), which if untreated is fatal. PT occurs when the intra‐pericardial pressure increases from its normal sub‐atmospheric pressure and becomes closer to the right atrial pressure, (Reed & Thomas 1984). Following PT, venous return, ventricular filling, stroke volume and cardiac output decrease. Initially, these patients compensate with increased heart rate and peripheral vascular resistance, but as left ventricular filling becomes more compromised cardiogenic shock ensues (Reed & Thomas 1984; Bouvy & Bjorling 1991). Patients presenting with PT exhibit weak peripheral pulses, jugular venous distention or a positive hepatojugular reflux, laboured breathing, ascites and vomiting (Ware 2015; Fahey et al. 2017). Pulsus paradoxus (PP) may also be found upon physical examination of these patients. On palpation of femoral pulses in patients with PP, weak pulses are noted during inspiration followed by a stronger pulse during expiration. Normally during inspiration, the right side of the heart fills and the interventricular septum (IVS) is shifted leftward to allow for the increased volume. However, in cases of PE, left ventricular (LV) filling has already been limited by the pressure created by the effusion on the heart wall. The combination of the normal physiological IVS shift during inspiration and the pathological reduction in LV filling from the PE result in reduced stroke volume during inspiration and a weaker femoral pulse on palpation. During expiration the thoracic pressure changes from negative to positive allowing improved LV filling and a palpably stronger femoral pulse. PP will not be present in all cases of PE and it should be noted that in human medicine it can also occur in cases of constructive pericarditis, pulmonary embolism, right ventricular infarct, severe hypovolaemia and circulatory shock, bilateral pleural effusion, tension pneumothorax, chronic obstructive pulmonary disease, extraneous cardiac compression and tracheal compression (Swami & Spodick 2003; Argulian & Messerli 2013). PP is best appreciated in dogs which are in lateral recumbency (Shaw & Rush 2007).
Echocardiography is a commonly used test for diagnosis of cardiac masses in dogs with PE and for PT. When performed by a board‐certified cardiologist this diagnostic test boasts a sensitivity between 82–99% to identify cardiac masses (MacDonald et al. 2009). Cardiac HSA are most commonly located within the right atrium and/or right auricle. Uncommonly, they can extend through the atrioventricular junction, septum, the ventricular wall and rarely into the heart base (MacDonald et al. 2009; Yamamoto et al. 2013). Ultrasound guided aspirates of a cardiac mass may be performed to ascertain a cytological diagnosis although because many of these tumours do not readily exfoliate, these samples may often be non‐diagnostic. In one study in six dogs with different causes of PE the procedure did not result in major complications (Pedro et al. 2016). The use of fluid cytology to obtain a diagnosis can also have mixed results depending on the underlying aetiology and is generally recommended to only submit when the effusion has a low haematocrit (Cagle et al. 2014). In such cases, where the effusion has a low haematocrit, it may be more likely to be due to a non‐neoplastic cause and clinicians should consider inflammatory and infectious disease differentials. A study evaluating the utility of fluid cytology to identify the cause of pericardial effusion noted that only 6 of 47 dogs had a cause identified (MacDonald et al. 2009). Of those 5/6 were due to infective pericarditis and 1/6 dog had lymphoma.
Primary treatment involves removal of the fluid via pericardiocentesis. Arrhythmias, particularly ventricular arrhythmias, are the most common complication and occur in approximately 10% of dogs within one hour of the procedure and in 15% within forty‐eight hours (Humm et al. 2009). Humm found in dogs that develop such events, 41% were euthanized or died within forty‐eight hours of diagnosis with the majority being suspected to have cardiac HSA (Humm et al. 2009). Unfortunately, in cases of HSA, PE likely recurs, and average survival time is consistently less than 30 days (Dunning et al. 1998; Ware & Hopper 1999; Johnson et al. 2004; MacDonald et al. 2009). While many treatments for cardiac HSA have been evaluated, survival times remain poor (Kerstetter et al. 1997; Jackson et al. 1999; Weisse et al. 2005; Ghaffari et al. 2014; Mullin et al. 2016). Reported survival times for pericardectomy alone was 52 days while mass resection alone or with chemotherapy resulted in a mean survival time of 46 and 164 days respectively (Kerstetter et al. 1997; Weisse et al. 2005). Prognosis can be better with other types of neoplasia depending on the tumour type and treatment pursued. Of the heart base tumours, mean survival after diagnosis of chemodectoma in dogs was over 200 days although the use of pericardiectomy can improve mean survival time further (Vicari et al. 2001; Ehrhart et al. 2002). Cardiac LSA can also result in PE. Survival with adjuvant chemotherapy was over 150 days in one study (MacGregor et al. 2005). Without chemotherapy, survival is similar to that of cardiac HSA (MacGregor et al. 2005).
Peritoneal effusions
Similar to PE, spontaneous haemorrhagic peritoneal effusion is a common syndrome noted in small animal practice. For dogs and cats presenting with haemorrhagic peritoneal effusion, malignancy is the most common cause, usually secondary to splenic ruptures (Mandell & Drobatz 1995; Pintar et al. 2003). HSA is the most common tumour associated with splenic neoplasia in dogs, accounting for 45–51% of splenic malignancies (Spangler & Culbertson 1992; Day et al. 1995; Spangler & Kass 1997; Hammond & Pesillo‐Crosby 2008). Dogs presenting with a non‐traumatic haemoperitoneum and a splenic mass have a 62‐70% chance of having HSA (Pintar et al. 2003; Hammond & Pesillo‐Crosby 2008; Aronsohn et al. 2009). In cats, causes of spontaneous haemoperitoneum are evenly distributed between neoplastic and non‐neoplastic causes with HSA being the most common neoplasm (Culp et al. 2010).
Presenting complaints of haemoperitoneum can be vague and include weakness, ataxia, abdominal distension, collapse and death (Pintar et al. 2003). There may also be a history of previous, milder episodes of weakness over the preceding few weeks. If weakness and a distended abdomen are seen in conjunction with pale mucous membranes at presentation, abdominal imaging (preferably ultrasound) should be performed immediately to look for free peritoneal fluid and intra‐abdominal masses, especially in the spleen. If effusion is present, then a sample can be obtained via abdominocentesis to determine if the fluid is haemorrhagic. Even if the peritoneal effusion is caused by a ruptured tumour, neoplastic cells are rarely seen on cytological evaluation of the fluid. Diagnostic tests should be performed to elucidate the cause of effusion, identify any haematological abnormalities which require intervention and identify metastasis prior to surgical intervention in cases of neoplasia. Blood abnormalities that may be present in cases with a haemoperitoneum consist of both regenerative and non‐regenerative anaemia, neutrophilia and thrombocytopenia (Scavelli et al. 1985 and Pintar et al. 2003). Thrombocytopenia occurs in 75–97% of cases of haemoperitoneum due to HSA (Hargis & Feldman 1991; Swardson et al. 1991; Pintar et al. 2003; Hammond & Pesillo‐Crosby 2008). Biochemical abnormalities are typically nonspecific (Pintar et al. 2003 and Culp et al. 2008). Coagulopathies are present in the majority of patients; disseminated intravascular coagulation (DIC) occurs in approximately 50% of dogs with HSA (Filppi et al. 1991; Maruyama et al. 2004 and Mischke et al. 2005)., Fine needle aspiration of any masses may be attempted, but can be non‐diagnostic, due to the vascular nature of many of these tumours and could cause further bleeding. Therefore, a preoperative diagnosis of the tumour may not be possible. Surgical resection is the best option for initial treatment of a bleeding mass, so it is reasonable to perform surgery to remove the mass and obtain a diagnosis through histopathological evaluation of the tumour tissue. If gross metastatic disease is present, however, surgery may not resolve the bleeding, so staging tests (3‐view thoracic radiographs, abdominal ultrasound and cardiac ultrasound) should be strongly advised prior to surgery. Thoracic radiographs are reported to have 78% sensitivity and 74% negative predictive value for detecting pulmonary metastasis of HSA when compared to post mortem findings (Holt et al. 1992). A non‐ruptured splenic mass is more likely to be benign (Cleveland & Casale 2016) but staging prior to surgery should still be advised.
Initial focus for the emergency clinician dealing with a haemoperitoneum is stabilization prior to surgery by re‐establishing the patient's effective circulating volume, maintaining their oxygen‐carrying capacity and arresting ongoing haemorrhage (Herold et al. 2008).
These patients are usually hypovolaemic secondary to the haemorrhage. Conventional fluid resuscitation (CR) can be used to replace these deficits and involves administration of an aliquot of fluid, which is approximately one‐quarter to one‐third of the estimated total blood volume (90 mL kg−1 in dogs and 60 mL kg−1 in cats), over 15–30 min. The patient's vital signs are reassessed following each aliquot and if still displaying signs of shock, the aliquot repeated. Evidence of stabilization on physical exam includes a normalization in blood pressure (90–160 mmHg), heart rate (80–140 BPM), respiratory rate (20‐40 BPM) and mentation (Rozanski & Rondeau 2002; Driessen & Brainard 2006; Hammond & Holm 2009). CR can lead to rapid redistribution of fluid to the interstitial space leading to oedema formation, hypothermia and may exacerbate bleeding by dislodging clots and diluting the circulating clotting factors. Another option specifically evaluated in canine cases of spontaneous haemoperitoneum is limited fluid volume resuscitation (LFVR) (Hammond & Holm 2009). This involves using the smallest possible volume of fluid to restore intravascular volume and minimize fluid extravasation into the interstitium. Typically, a colloid or hypertonic fluid is combined with isotonic crystalloids to achieve this goal using lower doses of fluids (5–10 mL kg−1) and re‐evaluating the patient's response. The blood pressure resuscitation endpoint with LFVR is lower than that of CR with values of 70 mmHg mean arterial pressure or 90 mmHg systolic arterial blood pressure being acceptable until definitive haemostasis is achieved. These values were derived as at a MAP of 60–70 mmHg, cerebral and renal blood flow are preserved (Hammond & Holm 2009). Invasive arterial blood pressure should be used to guide therapy with LVFR as non‐invasive measurements may overestimate blood pressures (Binns et al. 1995; Caulkett et al. 1998; Bosiack et al. 2010). Until definitive control of haemorrhage is achieved in these patients, small boluses of hypertonic saline and colloids are used to help resolve shock and perfuse the vital organs while the patient is being prepared for surgery. A prospective study comparing CR vs. LFVR for dogs with spontaneous haemoperitoneum showed that those receiving LFVR achieved stabilization faster than those receiving CR (Hammond et al. 2014). Disadvantages of LFVR include increased cost with colloids use, hypernatraemia due to hypertonic fluid and the need for rapid surgical control of haemorrhage once LVFR is employed to avoid organ dysfunction as the patient is being maintained at a lower blood pressure than compared to CR (Hammond et al. 2014).
A patient that is unresponsive to crystalloid or colloid fluid resuscitation, has evidence of severe haemorrhage or has elevated clotting times should be given a blood product (Herold et al. 2008; Culp & Silverstein 2015). Transfusion therapy is a treatment option that a clinician may employ in the pre‐operative, perioperative or post‐operative period as an effort to stabilize a case with a haemoperitoneum. Clinicians utilizing this therapy should aim to maintain the patient's haematocrit above 25% to stabilize signs of shock that are not responsive to fluid therapy and in the case of plasma transfusions to maintain clotting times within the normal range (Culp & Silverstein 2015). If blood products or a donor are not readily available, autologous blood transfusion (ABT) is another, albeit controversial, treatment option. The advantages disadvantages of ABT (Table 3) should be discussed with the owner. For routine transfusions, the blood product does not need to be administered warmed however if the clinician wants the product to be administered warmed, the blood can be warmed in a temperature‐controlled bowl (<39°C). Blood products should never be microwaved. Blood bags are connected to infusion sets which contain an in‐line micro‐filter (typically 170 micromilliliter pores). Sterility should be maintained at all times when connecting the bag to the fluid line. Ideally, the blood should be administered via gravitational flow however this may not be possible for smaller animals and infusion pump can be used in that case. The rate of transfusion will depend on the animal's cardiovascular status with the initial rate ideally being slow to observe any transfusion reactions. Transfusion of a single bag should be administered over 4 h to reduce the risk of bacterial contamination. The volume of blood to be transfused should be guided by goal directed therapy and the severity of anaemia, availability of blood products and the size of the patients. Several formulae have been described for dosing these products in dogs and cats. The two most accurate formulae in dogs include the following: (1) volume to be transfused (VT) = [(desired PCV – patient PCV/)donor blood PCV] x blood volume x body weight (kg) and (2) VT = required PCV% increased x 1.5 x body weight (kg) (Short et al. 2012). In studies in cats, the following formula used to predict the increase in PCV post transfusion was found to be most accurate: PCV% increase = VT (mL)/2 × body weight (kg) (Reed et al. 2014).
Table 3.
Autologous blood transfusion
| Advantages | Disadvantages |
|---|---|
| Decreased risk of transfusion reaction | Haemolysis |
| Decreased risk of inadvertent circulatory overload | Coagulopathy |
| Prevention of transfusion induced hypothermia | Microembolism of fat or air |
| Immediately available compatible blood | Potential spread of bacteraemia or sepsis |
| Potential spread of malignancy |
Conflicting reports exist in human literature as to the concern that ABT results in the dissemination of malignant cells (Burrows & Tartter 1982; Klimberg et al. 1986; Miller et al. 1991). Limited information is available in veterinary literature. Higgs et al. (2015) evaluated the outcome following ABT in 25 dogs secondary to thoracic or abdominal haemorrhage. In this study 32% of dogs needed an ABT as a result of a ruptured tumour, of those 75% had ruptured splenic masses. Seventeen of 25 dogs survived to discharge with 2/17 dying within 2 weeks of the ABT. Both dogs had a recurrence of haemoperitoneum (Higgs et al. 2015). Therefore if ABT is warranted it is important that the clinician discuss the benefits and risks associated so the owner may make an informed decision as to how to proceed.
The two methods for ABT include direct re‐infusion and cell salvage. As the latter requires specialized equipment and training it will not be discussed in further detail. Direct re‐infusion is the sterile collection of blood from the patient and re‐infusion into the same animal without processing and without the use of cell salvage device. While haemorrhage into a cavity is usually defibrinated within 45‐60 minutes, the addition of an anticoagulant is recommended with either method (Purvis 1995; Adamantos & Smith 2016).
Direct re‐infusion can be achieved either via a 2‐syringe technique (Robinson et al. 2016) or with a stopcock with or without the aid of underwater drainage set (Purvis 1995). In the 2‐syringe technique, blood is aspirated from the cavity into syringes containing acid citrate dextrose anticoagulant, transferred into a leur lock syringe attached to an infusion line and a 40‐micron blood filter and infused into the cephalic vein (Robinson et al. 2016). In the latter blood is aspirated from the cavity using an anticoagulant containing 60 mL syringe, stopcock and infusion line.
It is important the emergency clinician recognize patients with splenic masses and/or haemorrhagic peritoneal effusion may develop additional complications such as significant arrhythmias. Ventricular arrhythmias can be seen in both the perioperative and postoperative period and may be as a result of poor myocardial perfusion secondary to hypoxia, hypovolaemia, anaemia or a neurohormonal response associated splenic manipulation (Keyes & Rush 1994). Although arrhythmias can occur following splenectomies regardless of underlying splenic disease, it is much more likely associated with dogs who had ruptured splenic masses secondary to neoplasia (Marino et al. 1994). In one study dogs experiencing arrhythmias had a histopathological diagnosis of splenic HSA, haematoma and haemangioma in descending order (Keyes & Rush 1994). Ventricular arrhythmias resulting in haemodynamic instability may require treatment with an anti‐arrhythmogenic medication.
Part of the role of the emergency clinician in these cases also involves helping the owners make decisions regarding pursuing surgery for their pet. HSA is the most common splenic neoplasm in dogs and has a poor prognosis even with aggressive post‐operative therapy (Hammond & Pesillo‐Crosby 2008). Efforts have been made to help identify factors which may indicate a higher likelihood of HSA to help guide owners’ decisions in these difficult cases. Indicators of HSA in a 2016 study of dogs included preoperative anaemia, the presence of haemorrhagic ascites, thrombocytopenia and the need for a blood transfusion (Sherwood et al. 2016). The size or presence of focal as compared to those with multiple splenic masses may also have a bearing on prognosis with larger or more focal masses considered less likely to be malignant (Spangler & Kass 1997).
Prognosis following successful splenectomy depends on the underlying splenic disease. Long‐term survival for HSA remains poor; however, splenectomy can be curative with other tumours such as haemangioma and haematomas (Wendelburg et al. 2015). The median survival time for dogs with splenic HSA treated with splenectomy alone has been reported to be 86 days. In dogs that received chemotherapy (generally with doxorubicin‐containing protocols) post‐splenectomy, median survival times ranging from 125 days to 162 days have been reported. (Filppi et al. 1991; Ogilvie et al. 1996; Wood et al. 1998; Lana et al. 2007; Wendelburg et al. 2015; Matsuyama et al. 2017). In dogs, when haemoperitoneum is caused by some malignant tumours other than HSA (for example LSA and non‐angiomatous/non‐lymphomatous splenic sarcomas. In cases where there is not significant metastasis the prognosis is likely better and splenectomy alone can result in good survival, especially in cases of splenic marginal zone lymphomas (Gower et al. 2015; van Stee et al. 2015; Stefanello et al. 2011; Dervisis et al. 2017). Similarly, in cats, survival for those with neoplasia presenting with a haemoperitoneum was poor (Culp et al. 2010).
Pathological fractures with bone neoplasia
Appendicular osteosarcoma is the most common primary bone tumour in dogs (Brodey & Riser 1969). Less common primary bone tumours include chondrosarcoma, fibrosarcoma, HSA, histiocytic sarcoma and synovial cell sarcoma (Dernell et al. 2007). The metaphyseal region of long bones is the most common site for osteosarcoma in dogs with the distal radius and proximal humerus being the most common sites (Knecht & Priester 1978). Metastasis to bones, either from osteosarcoma, or from primary tumours in other organs, can also occur. The most frequent sites for bone metastases include the vertebrae, ribs, humerus and the femur with haematogenous spread more common than extension to the lymph nodes for animals with osteosarcoma (Hillers et al. 2005). When evaluating canine patients, several types of neoplasia can metastasize to bone including HSA, mammary gland carcinoma, urogenital carcinomas, squamous cell carcinomas and histiocytic sarcomas. These metastatic bone lesions are seen less frequently than primary bone tumours (Liu et al. 1977; Trost et al. 2014). When metastasis is seen in long bones, it mainly affects the proximal metaphysis, and vertebral metastases are usually seen in the bodies of the thoracic and lumbar vertebrae, although the spinous processes of the thoracic vertebrae are occasionally affected. (Trost et al. 2014). In contrast, in cats the most common secondary bone tumours identified included fibrosarcoma, squamous cell carcinoma, LSA, HSA, rhabdomyosarcoma and meningioma (Lui et al. 1974).
Common clinical signs related to bone neoplasms include lameness and localized limb swelling (Dernell et al. 2007). Lameness occurs secondary to periosteal inflammation, microfractures or less commonly pathological fractures (PF) secondary to the expanding tumour effects on the bones’ density (Dernell et al. 2007). The femur is most commonly associated with PF and the radius least likely (Rubin et al. 2015). Diagnosis of a bone tumour is usually obtained via a radiograph of the affected limb where an osteolytic or osteoblastic pattern is usually noted (Thrall 1998; Dernell et al. 2007). An osteolytic pattern is characterized by destruction of the bone with little or no defensive response. Contrastingly, an osteoblastic pattern is defined with new bone formation and an increased radiographic opacity of the affected bone (Kealy et al. 2010). Lytic tumours are more likely to experience PF compared with osteoblastic lesions (Rubin et al. 2015). Osteomyelitis, including fungal and bacterial infections, is the most likely differential diagnosis. A bone biopsy can be performed for histopathological diagnosis and cultures if warranted. Bone biopsy carries the risk of causing PF but in the authors’ experience this risk is low. Thoracic radiographs are also recommended although fewer than 15% of dogs affected by osteosarcoma will have clinically detectable metastasis at the time of initial diagnosis (Dernell et al. 2007).
The greatest density of afferent nociceptors are found along the periosteal surface and within the medullary cavity of the bone. Thus, PF in both human and veterinary patients are associated with intense, persistent pain (Ehrhart et al. 2013). Additional discomfort can be attributed to chemical mediators from non‐neoplastic stromal cells which stimulate nociceptors and osteoclastic bone resorption (Goblirsch et al. 2006, Clohisy & Mantyh 2003; [LM1]). For the emergency clinician, pain management and temporary stabilization of the fractured limb are the most important issues in these patients while amputation surgery is arranged. Patients with gross metastasis at the time of diagnosis and treated with amputation alone have a mean survival time of 133 days (Spodnick et al. 1992; Boerman et al. 2012; Ehrhart et al. 2013).
A multimodal approach to pain control provides synergistic analgesia while concurrently lowering the total dose of each analgesic resulting in fewer side effects (Jin & Chung 2001). In general, pain control in the hospitalized patient can be provided at regularly timed intervals or as a constant rate infusion (CRI). CRI allows one to titrate the dose allowing better control of acute pain in a hospital setting. Intermittent administration of a non‐steroidal anti‐inflammatory drug with an opioid could be considered. Additionally, an N‐methyl‐D‐aspartate antagonist such as amantadine or ketamine can be used to help alleviate central sensitization. Under sedation or anaesthesia, ultrasound guided nerve blocks of the sciatic, femoral, radial and brachial nerves can provide additional pain control, however, specialized equipment and experience may limit the availability of these options (Bhoi et al. 2012).
Once a PF has occurred, it generally will not heal well since the edges of the bone surrounding the fracture are diseased. Therefore, amputation of the affected limb is generally considered to be the treatment of choice, but it is best to look carefully for evidence of gross metastatic disease before deciding on whether to perform an amputation. If visible metastasis is present, especially is there is diffuse pulmonary metastatic disease or areas of bone metastasis, then the prognosis following amputation is very poor, and amputation may not improve quality of life for long, or at all, in which case, the owners may elect to forgo amputation. Stabilization of the fractured limb can be accomplished under heavy sedation or anaesthesia and allows the application of thick layers of padding and a splint in order to prevent worsening of soft tissue trauma, improve patient comfort and assist in weight bearing if ambulation is attempted. Other treatment options for PF, such as limb sparing surgery, chemotherapy and radiation are generally considered to be ineffective and are outside the scope of this review.
Conclusion
With an increase in the treatment options for cancer in small animal patients, veterinarians both in general practice and in the emergency setting can expect to see an increasing number of patients presenting with complications both from cancer itself and its treatment. Prompt recognition and appropriate therapy is required when managing these patients and management should include both a discussion with the owner about their goals and communication with a veterinary oncologist to maximize success. While few neoplasms can be fully cured in veterinary medicine, treatment of some of these oncological complications can be rewarding and prolong a good quality of life for the affected animals.
Source of funding
There was no external funding for this report.
Conflicts of interest
The authors report no conflicts of interest.
Ethics statement
The authors declare human ethics approval was not needed for this study
Contributions
KLT and LM conceived the review and with DH wrote the manuscript. All of the authors reviewed, revised and accepted the manuscript
Acknowledgement
The authors gratefully acknowledge the anonymous reviewers that assisted in the finalization of this manuscript
References
- Adamantos S. & Smith C. (2016) Alternative transfusion methods. Manual of Veterinary Transfusion Medicine and Blood Banking 21, 296–306. [Google Scholar]
- Adami S. & Zamberlan N. (1996) Adverse effects of bisphosphonates. Drug Safety 14, 158–170. [DOI] [PubMed] [Google Scholar]
- Argulian E. & Messerli F. (2013) Misconceptions and facts about pericardial effusion and tamponade. The American Journal of Medicine 126, 858–861. [DOI] [PubMed] [Google Scholar]
- Aronsohn M.G., Dubiel B., Roberts B. & Powers B.E. (2009) Prognosis for acute nontraumatic haemoperitoneum in the dog: a retrospective analysis of 60 cases (2003‐2006). Journal of the American Animal Hospital Association 45, 72–77. [DOI] [PubMed] [Google Scholar]
- Axiak S.M., Selting K.A., Decedue C.J., Henry C.J., Tate D., Howell J. et al. (2011) Phase I dose escalation safety study of nanoparticulatepaclitaxel (CTI 52010) in normal dogs. International Journal of Nanomedicine 6, 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak J.M. & Sharp C.R. (2016) Epidemiology of systemic inflammatory response syndrome and sepsis in cats hospitalized in a veterinary teaching hospital. Journalof the American Veterinary Medical Association 249, 65–71. [DOI] [PubMed] [Google Scholar]
- Battaglia L., Petterino C., Zappulli V. & Castagnaro M. (2005) Hypoglycaemia as a paraneoplastic syndrome associated with renal adenocarcinoma in a dog. Veterinary Research Communications 29, 671–675. [DOI] [PubMed] [Google Scholar]
- Bennett P.F., DeNicola D.B., Bonney P., Glickman N.W. & Knapp D.W. (2002) Canine anal sac adenocarcinomas: clinical presentation and response to therapy. Journal of Veterinary Internal Medicine 16, 100–104. [DOI] [PubMed] [Google Scholar]
- Bergman P.J. (2013) Paraneoplastic syndromes. Small Animal Clinical Oncology 5, 83–97. [Google Scholar]
- Bhoi S., Sinha T.P., Rodha M., Bhasin A., Ramchandani R. & Galwankar S. (2012) Feasibility and safety of ultrasound‐guided nerve block for management of limb injuries by emergency care physicians. Journal of Emergencies, Trauma, and Shock 5, 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns S.H., Sisson D.D., Buoscio D.A. & Schaeffer D.J. (1995) Doppler ultrasonographic, oscillometric sphygmomanometric, and photoplethysmographic techniques for noninvasive blood pressure measurement in anesthetized cats. Journal of Veterinary Internal Medicine 9, 405–414. [DOI] [PubMed] [Google Scholar]
- Blake M.K., Carr B.J. & Mauldin G.E. (2016) Hypersensitivity reactions associated with L‐asparaginase administration in 142 dogs and 68 cats with lymphoid malignancies: 2007‐2012. The Canadian Veterinary Journal 57, 176. [PMC free article] [PubMed] [Google Scholar]
- Boerman I., Selvarajah G.T., Nielen M., Kirpensteijn J. (2012) Prognostic factors in canine appendicular osteosarcoma‐ a meta‐analysis. BMC Veterinary Research, 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonczynski J.J., Ludwig L.L., Barton L.J., Loar A. & Peterson M.E. (2003) Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Veterinary Surgery 32, 161–166. [DOI] [PubMed] [Google Scholar]
- Boothe D.M. (2015) Antimicrobial use in the critical care patient. Small Animal Critical Care Medicine 2, 918–927. [Google Scholar]
- Bosiack A.P., Mann F.A., Dodam J.R., Wagner‐Mann C.C. & Branson K.R. (2010) Comparison ofultrasonic Doppler flow monitor, oscillometric, and direct arterial blood pressure measurements in ill dogs. Journal of Veterinary Emergency and Critical Care 20, 207–215. [DOI] [PubMed] [Google Scholar]
- Bouvy B.M., Bjorling D.E. (1991) Pericardial effusion in dogs and cats. I. Normal pericardium and causes and pathophysiology of pericardial effusion. The Compendium on continuing education for the practicing veterinarian (USA)
- Brady C.A. & Otto C.M. (2001) Systemic inflammatory response syndrome, sepsis, and multiple organ dysfunction. Veterinary Clinics: Small Animal Practice 31, 1147–1162. [DOI] [PubMed] [Google Scholar]
- Briassoulis G., Venkataraman S., Thompson A. (2010) Cytokines and metabolic patterns in pediatric patients with critical illness. Clinical and Developmental Immunology 2010, 354047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton B.M., Kelleher M.E., Gregor T.P. & Sorenmo K.U. (2014) Evaluation of factors associated with prolonged hospital stay and outcome of febrile neutropenic patients receiving chemotherapy: 70 cases (1997‐2010). Veterinary and Comparative Oncology 12, 266–276. [DOI] [PubMed] [Google Scholar]
- Brodey R.S. & Riser W.H. (1969) Canine osteosarcoma: a clinicopathological study of 194 cases. Clinical Orthopedics 62, 54–64. [PubMed] [Google Scholar]
- Brown M.R. & Rogers K.S. (2001) Neutropenia in dogs and cats: a retrospective study of 261 cases. Journal of the American Animal Hospital Association 37, 131–139. [DOI] [PubMed] [Google Scholar]
- Burrows L. & Tartter P. (1982) Effect of blood transfusions on colonic malignancy recurrence rate. Lancet 2, 662. [DOI] [PubMed] [Google Scholar]
- Cagle L.A., Epstein S.E., Owens S.D., Mellema M.S., Hopper K. & Burton A.G. (2014) Diagnostic yield of cytologic analysis of pericardial effusion in dogs. Journal of Veterinary Internal Medicine 28, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M.S. & Bishop M. (2004) Tumor lysis syndrome: new therapeutic strategies and classification. British Journal of Haematology 127, 3–11. [DOI] [PubMed] [Google Scholar]
- Calia C.M., Hohenhaus A.E., Fox P.R. & Meleo K.A. (1996) Acute tumorlysis syndrome in a cat with lymphoma. Journal of Veterinary Internal Medicine 10, 409–411. [DOI] [PubMed] [Google Scholar]
- Caulkett N.A., Cantwell S.L. & Houston D.M. (1998) A comparison of indirect blood pressure monitoring techniques in the anesthetized cat. Veterinary Surgery 27, 370–377. [DOI] [PubMed] [Google Scholar]
- Cavanagh A.A., Sullivan L.A. & Hansen B.D. (2016) Retrospective evaluation of fluid overload and relationship to outcome in critically ill dogs. Journal of Veterinary Emergency and Critical Care 26, 578–586. [DOI] [PubMed] [Google Scholar]
- Cervantes A. & Chirivella I. (2004) Oncological emergencies. Annals of Oncology 15, 299–306. [DOI] [PubMed] [Google Scholar]
- Chaitanya G.V., Franks S.E., Cromer W., Wells S.R., Bienkowska M., Jennings M.H. et al. (2010) Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphatic Research and Biology 8, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P.S. (2015) Regurgitation and vomiting. Small Animal Critical Care Medicine 2, 634–638. [Google Scholar]
- Chun R., Garrett L. & MacEwen E.G. (2001) Cancer chemotherapy. Small Animal Clinical Oncology 3, 92–118. [Google Scholar]
- Cinel I. & Dellinger R.P. (2006) Guidelines for severe infections: are they useful? Current Opinion in Critical Care 12, 483–488. [DOI] [PubMed] [Google Scholar]
- Cleveland M.J. & Casale S. (2016) Incidence of malignancy and outcomes for dogs undergoing splenectomy for incidentally detected non ruptured splenic nodules or masses: 105 cases (2009‐2013). Journal of the American Veterinary Medical Association 248, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Clohisy D.R. & Mantyh P.W. (2003) Bone cancer pain. Clinical Orthopaedics and Related Research 415, 279–288. [DOI] [PubMed] [Google Scholar]
- Cohen M., Post G.S. & Wright J.C. (2003) Gastrointestinal leiomyosarcoma in 14 dogs. Journal of Veterinary Internal Medicine 17, 107–110. [DOI] [PubMed] [Google Scholar]
- Conti‐Patara A., de Araújo Caldeira J., de Mattos‐Junior E., de Carvalho H.D.S., Reinoldes A., Pedron B.G. et al. (2012) Changes in tissue perfusion parameters in dogs with severe sepsis/septic shock in response to goal‐directed hemodynamic optimization at admission to ICU and the relation to outcome. Journal of Veterinary Emergency and Critical Care 22, 409–418. [DOI] [PubMed] [Google Scholar]
- Cray C., Zaias J. & Altman N.H. (2009) Acute phase response in animals: a review. Comparative Medicine 59, 517–526. [PMC free article] [PubMed] [Google Scholar]
- Culp W.T. & Silverstein D.C. (2015) Thoracic and Abdominal Trauma 138, 730. [Google Scholar]
- Culp W.T., Drobatz K.J., Glassman M.M. & Aronson L.R. (2008) Feline visceral hemangiosarcoma. Journal of Veterinary Internal Medicine 22, 148–152. [DOI] [PubMed] [Google Scholar]
- Culp W.T., Weisse C., Kellogg M.E., Gordon I.K., Clarke D.L., May L.R. & Drobatz K.J. (2010) Spontaneous hemoperitoneum in cats: 65 cases (1994‐2006). Journal of the American Veterinary Medical Association 236, 978–982. [DOI] [PubMed] [Google Scholar]
- Datte K., Guillaumin J., Barrett S., Monnig A. & Cooper E. (2016) Retrospective evaluation of the use of glucagon infusion as adjunctive therapy for hypoglycemia in dogs: 9 cases. Journal of Veterinary Emergency and Critical Care 26, 775–781. [DOI] [PubMed] [Google Scholar]
- Day M.J., Lucke V.M. & Pearson H. (1995) A review of pathological diagnoses made from 87 canine splenic biopsies. Journal of Small Animal Practice 36, 426–433. [DOI] [PubMed] [Google Scholar]
- De Backer D., Cortes D.O., Donadello K. & Vincent J.L. (2013) Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 5, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger R.P., Levy M.M., Rhodes A., Annane D., Gerlach H., Opal S.M. et al. (2012) Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock:2012. Critical Care Medicine 41, 580–637. [DOI] [PubMed] [Google Scholar]
- Dernell W.S., Ehrhart N.P., Straw R.C. & Vail D.M. (2007) Tumors of the skeletal system. Small Animal Clinical Oncology 3, 378–417. [Google Scholar]
- Dervisis N.G., Kiupel M., Qin Q. & Cesario L. (2017) Clinical prognostic factors in canine histiocytic sarcoma. Veterinary and Comparative Oncology 15, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Dorr R.T. (1990) Antidotes to vesicant chemotherapy extravasations. Blood Reviews 4, 41–60. [DOI] [PubMed] [Google Scholar]
- Dougherty S.A., Center S.A. & Dzanis D.A. (1990) Salmon calcitonin as adjunct treatment for vitamin D toxicosis in a dog. Journal of the American Veterinary Medical Association 196, 1269–1272. [PubMed] [Google Scholar]
- Driessen B. & Brainard B. (2006) Fluid therapy for the traumatized patient. Journal of Veterinary Emergency and Critical Care 16, 276–299. [Google Scholar]
- Duarte C.G., Winnacker J.L., Becker K.L. & Pace A. (1971) Thiazide‐induced hypercalcemia. New England Journal of Medicine 284, 828–830. [DOI] [PubMed] [Google Scholar]
- Dunning D., Monnet E., Orton E.C. & Salman M.D. (1998) Analysis of prognostic indicators for dogs with pericardial effusion: 46 cases (1985‐1996). Journal of the American Veterinary Medical Association 212, 1276–1280. [PubMed] [Google Scholar]
- Ehrhart N., Ehrhart E.J., Willis J., Sisson D., Constable P., Greenfield C. et al. (2002) Analysis of factors affecting survival in dogs with aortic body tumors. Veterinary Surgery 31, 44–48. [DOI] [PubMed] [Google Scholar]
- Ehrhart N.P., Ryan S.D. & Fan T.M. (2013) Tumors of the skeletal system. Small Animal Clinical Oncology 5, 463–503. [Google Scholar]
- Ejaz A.A., Mu W., Kang D.H., Roncal C., Sautin Y.Y., Henderson G. et al. (2007) Could uric acid have a role in acute renal failure? Clinical Journal of the American Society of Nephrology 2, 16–21. [DOI] [PubMed] [Google Scholar]
- Escobar C., Grindem C., Neel J.A. & Suter S.E. (2012) Hematologic changes after total body irradiation and autologous transplantation of hematopoietic peripheral blood progenitor cells in dogs with lymphoma. Veterinary Pathology 49, 341–343. [DOI] [PubMed] [Google Scholar]
- Estelle F. & Simons R. (2011) Anaphylaxis pathogenesis and treatment. Allergy 66, 31–34. [DOI] [PubMed] [Google Scholar]
- Fahey R., Rozanski E., Paul A. & Rush J.E. (2017) Prevalence of vomiting in dogs with pericardial effusion. Journal of Veterinary Emergency and Critical Care 27, 250–252. [DOI] [PubMed] [Google Scholar]
- Fan T.M., de Lorimier L.P., Charney S.C. & Hintermeister J.G. (2005) Evaluation of intravenous pamidronate administration in 33 cancer‐bearing dogs with primary or secondary bone involvement. Journal of Veterinary Internal Medicine 19, 74–80. [DOI] [PubMed] [Google Scholar]
- Fernandez N.J., Barton J. & Spotswood T. (2009) Hypoglycemia in a dog. Canadian Veterinary Journal 50, 423–426. [PMC free article] [PubMed] [Google Scholar]
- Filppi J., Hammer A.S., Couto C.G., Getzy D. & Shank K. (1991) Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. Journal of Veterinary Internal Medicine 5, 160–166. [DOI] [PubMed] [Google Scholar]
- Finlay J.R., Wyatt K. & North C. (2017) Recovery from cyclophosphamide overdose in a dog. Journal of the American Animal Hospital Association 53, 230–235. [DOI] [PubMed] [Google Scholar]
- Finora K. (2003) Common paraneoplastic syndromes. Clinical Techniquees in Small Animal Practice 18, 123–126. [DOI] [PubMed] [Google Scholar]
- Fournel‐Fleury C., Ponce F., Felman P., Blavier A., Bonnefont C., Chabanne L. et al. (2002) Canine T‐cell lymphomas: a morphological, immunological, and clinical study of 46 new cases. Veterinary Pathology 39, 92–109. [DOI] [PubMed] [Google Scholar]
- Gabrilove J.L., Jakubowski A., Scher H., Sternberg C., Wong G., Grous J. et al. (1988) Effect of granulocyte colony‐stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional‐cell carcinoma of the urothelium. New England Journal of Medicine 318, 1414–1422. [DOI] [PubMed] [Google Scholar]
- Ghaffari S., Pelio D.C., Lange A.J., Arndt J.W., Chretin J.D., Fiocchi S.C. et al. (2014) A retrospective evaluation of doxorubicin‐based chemotherapy for dogs with right atrial masses and pericardial effusion. Journal of Small Animal Practice 55, 254–257. [DOI] [PubMed] [Google Scholar]
- Goblirsch M., Zwolak P.P. & Clohisy D.R. (2006) Biology of bone cancer pain. Clinical Cancer Research 15, 6231–6235. [DOI] [PubMed] [Google Scholar]
- Gower K.L., Liptak J.M., Culp W.T., Bravo L., Powers B. & Withrow S.J. (2015) Splenic liposarcoma in dogs: 13 cases (2002–2012). Journal of the American Veterinary Medical Association 247, 1404–1407. [DOI] [PubMed] [Google Scholar]
- Hammond T.N., Holm J.L. (2009) Limited fluid volume resuscitation. Compendium: Continuing Education for Veterinarians 31, 309–321. [PubMed] [Google Scholar]
- Hammond T.N. & Pesillo‐Crosby S.A. (2008) Prevalence of hemangiosarcoma in anemic dogs with a splenic mass and hemoperitoneum requiring a transfusion: 71 cases (2003‐2005). Journal of the American Veterinary Medical Association 15, 553–558. [DOI] [PubMed] [Google Scholar]
- Hammond W.P., Csiba E., Canin A., Hockman H., Souza L.M., Layton J.E. & Dale D.C. (1991) Chronic neutropenia. A new canine model induced by human granulocyte colony‐stimulating factor. Journal of Clinical Investigation 87, 704–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond T.N., Holm J.L. & Sharp C.R. (2014) A pilot comparison of limited versus large fluid volume resuscitation in canine spontaneous haemoperitoneum. Journal of the American Animal Hospital Association 50, 159–166. [DOI] [PubMed] [Google Scholar]
- Hargis A.M. & Feldman B.F. (1991) Evaluation of haemostatic defects secondary to vascular tumours in dogs: 11 cases. Journal of the American Veterinary Medical Association 198, 891–894. [PubMed] [Google Scholar]
- Hauptman J.G., Walshaw R. & Olivier N.B. (1997) Evaluation of the sensitivity and specificity of diagnostic criteria for sepsis in dogs. Veterinary Surgery 26, 393–397. [DOI] [PubMed] [Google Scholar]
- Haydar S., Spanier M., Weems P., Wood S. & Strout T. (2017) Comparison of QSOFA score and SIRS criteria as screening mechanisms for emergency department sepsis. The American Journal of Emergency Medicine 35, 1730–1733. [DOI] [PubMed] [Google Scholar]
- Herold L.V., Devey J.J., Kirby R. & Rudloff E. (2008) Clinical evaluation and management of hemoperitoneum in dogs. Journal of Veterinary Emergency and Critical Care 18, 40–53. [Google Scholar]
- Higgs V.A., Rudloff E., Kirby R. & Linklater A.K.J. (2015) Autologous blood transfusion in dogs with thoracic or abdominal haemorrhage: 25 cases (2007‐2012). Journal of Veterinary Emergency and Critical Care 25, 731–738. [DOI] [PubMed] [Google Scholar]
- Hillers K.R., Dernell W.S., Lafferty M.H., Withrow S.J. & Lana S.E. (2005) Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986‐2003). Journal of the American Veterinary Medical Association 226, 1364–1367. [DOI] [PubMed] [Google Scholar]
- Hochberg J. & Cairo M.S. (2008) Rasburicase: future directions in tumor lysis management. Expert Opinion on Biological Therapy 8, 1595–1604. [DOI] [PubMed] [Google Scholar]
- Holt D., Van Winkle T., Schelling C. & Prymak C. (1992) Correlation between thoracic radiographs and postmortem findings in dogs with hemangiosarcoma: 77 cases (1984‐1989). Journal of the American Veterinary Medical Association 200, 1535–1539. [PubMed] [Google Scholar]
- Howard S.C., Jones D.P. & Pui C.H. (2011) The tumor lysis syndrome. New England Journal of Medicine 364, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm K.R., Keenaghan‐Clark E.A. & Boag A.K. (2009) Adverse events associated with pericardiocentesis in dogs: 85 cases (1999‐2006). Journal of Veterinary Emergency and Critical Care 19, 352–356. [DOI] [PubMed] [Google Scholar]
- Jackson J., Richter K.P. & Launer D.P. (1999) Thoracoscopic partial pericardiectomy in 13 dogs. Journal of Veterinary Internal Medicine 13, 529–533. [DOI] [PubMed] [Google Scholar]
- Jawa R.S., Anillo S., Huntoon K., Baumann H. & Kulaylat M. (2011) Interleukin‐6 in surgery, trauma, and critical care part II: clinical implications. Journal of Intensive Care Medicine 26, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin F. & Chung F. (2001) Multimodal analgesia for postoperative pain control. Journal of Clinical Anesthesia 13, 524–539. [DOI] [PubMed] [Google Scholar]
- Johnson R.A., Boyce B.F., Mundy G.R. & Roodman G.D. (1989) Tumors producing human tumor necrosis factor induce hypercalcemia and osteoclastic bone resorption in nude mice. Endocrinology 124, 1424–1427. [DOI] [PubMed] [Google Scholar]
- Johnson M.S., Martin M., Binns S. & Day M.J. (2004) A retrospective study of clinical findings, treatment and outcome in 143 dogs with pericardial effusion. Journal of Small Animal Practice 45, 546–552. [DOI] [PubMed] [Google Scholar]
- Jongeward S.J. (1985) Primary bone tumors. Veterinary Clinics of North America: Small Animal Practice 15, 609–641. [DOI] [PubMed] [Google Scholar]
- Kealy J.K., McAllister H. & Graham J.P. (2010) Diagnostic radiology and ultrasonography of the dog and cat. (pp 351–447). St Louis, MO: Elsevier Health Sciences. [Google Scholar]
- Keir I. & Kellum J.A. (2015) Acute kidney injury in severe sepsis: pathophysiology, diagnosis, and treatment recommendations. Journal of Veterinary Emergency and Critical Care 25, 200–209. [DOI] [PubMed] [Google Scholar]
- Kemp S.F., Lockey R.F. & Simons F.E.R. (2008) Epinephrine: the drug of choice for anaphylaxis–a statement of the World Allergy Organization. World Allergy Organization Journal 1, S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter K.K., Krahwinkel D.J., Millis D.L. & Hahn K. (1997) Pericardiectomy in dogs: 22 cases (1978‐1994). Journal of the American Veterinary Medical Association 211, 736–740. [PubMed] [Google Scholar]
- Keyes M. & Rush J. (1994) Ventriculararrhythmias in dogs with splenic masses. Journal of Veterinary Emergency and Critical Care 3, 33–38. [Google Scholar]
- Kim J., Doerr M. & Kitchell B.E. (2015) Exploration of paclitaxel (Taxol) as a treatment for malignant tumors in cats: a descriptive case series. Journal of Feline Medicine and Surgery 17, 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainbart S., Agi L., Bdolah‐Abram T., Kelmer E. & Aroch I. (2017) Clinical, laboratory, and hemostatic findings in cats with naturally occurring sepsis. Journal of the American Veterinary Medical Association 251, 1025–1034. [DOI] [PubMed] [Google Scholar]
- Klimberg J., Sirois R., Wajsman Z. & Baker J. (1986) Intraoperative autotransfusion in urologic oncology. Archives of Surgery 121, 1326–1329. [DOI] [PubMed] [Google Scholar]
- Knecht C.D. & Priester W.A. (1978) Musculoskeletal tumors in dogs. Journal of the American Veterinary Medical Association 172, 72–74. [PubMed] [Google Scholar]
- Laforcade A. (2015) Systemic inflammatory response syndrome. Small Animal Critical Care Medicine 2, 30–34. [Google Scholar]
- Laforcade A.M., Freeman L.M., Shaw S.P., Brooks M.B., Rozanski E.A. & Rush J.E. (2003) Hemostatic changes in dogs with naturally occurring sepsis. Journal of Veterinary Internal Medicine 17, 674–679. [DOI] [PubMed] [Google Scholar]
- Lana S., U'ren L., Plaza S., Elmslie R., Gustafson D., Morley P., Dow S. (2007) Continuous low‐dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. Journal of Veterinary Internal Medicine 21, 764–769. [DOI] [PubMed] [Google Scholar]
- Langer S.W., Sehested M. & Jensen P.B. (2000) Treatment of anthracycline extravasation with dexrazoxane. Clinical Cancer Research 6, 3680–3686. [PubMed] [Google Scholar]
- Leifer C.E., Peterson M.E., Matus R.E. & Patnaik A.K. (1985) Hypoglycemia associated with nonislet cell tumor in 13 dogs. Journal of the American Veterinary Medical Association 186, 53–55. [PubMed] [Google Scholar]
- Liptak J.M., Dernell W.S., Withrow S.J. (2004) Liver tumors in cats and dogs. Compendium: Continuing Education for Veterinarians 36, 50–57. [Google Scholar]
- Liu S.K., Dorfman H.D., Hurvitz A.I. & Patnaik A.K. (1977) Primary and secondary bone tumours in the dog. Journalof Small Animal Practice 18, 313–326. [DOI] [PubMed] [Google Scholar]
- Lucroy M.D. (2005) Managing febrile neutropenia in the chemotherapy patient. NAVC Proceedings, 651‐653.
- Lui S.K., Dorfman H.D., Patnaik A.K. (1974) Primary and secondary bone tumours in the cat. Journal of Small Animal Practice 15, 141–156. [DOI] [PubMed] [Google Scholar]
- MacDonald K.A., Cagney O. & Magne M.L. (2009) Echocardiographic and clinicopathologic characterization of pericardial effusion in dogs: 107 cases (1985‐2006). Journal of the American Veterinary Medical Association 235, 1456–1461. [DOI] [PubMed] [Google Scholar]
- MacGregor J.M., Faria M.L., Moore A.S., Tobias A.H., Brown D.J. & de Morais H.S. (2005) Cardiac lymphoma and pericardial effusion in dogs: 12 cases (1994–2004). Journal of the American Veterinary Medical Association 227, 1449–1453. [DOI] [PubMed] [Google Scholar]
- Machado C.E. & Flombaum C.D. (1996) Safety of pamidronate in patients with renal failure and hypercalcemia. Clinical Nephrology 45, 175–179. [PubMed] [Google Scholar]
- Mandell D.C. & Drobatz K. (1995) Feline Hemoperitoneum 16Cases (1986‐1993). Journal of Veterinary Emergency and Critical Care 5, 93–97. [Google Scholar]
- Marino D.J., Matthiesen D.T., Fox P.R., Lesser M.B. & Stamoulis M.E. (1994) Ventricular arrhythmias in dogs undergoing splenectomy: a prospective study. Veterinary Surgery 23, 101–106. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Miura T., Sakai M., Koie H., Yamaya Y., Shibuya H. et al. (2004) The incidence of disseminated intravascular coagulation in dogs with malignant tumor. Journal of Veterinary Medical Science 66, 573–575. [DOI] [PubMed] [Google Scholar]
- Matsuyama A., Poirier V.J., Mantovani F., Foster R.A. & Mutsaers A.J. (2017) Adjuvant doxorubicin with or without metronomic cyclophosphamide for canine splenic hemangiosarcoma. Journal of the American Animal Hospital Association 53, 304–312. [DOI] [PubMed] [Google Scholar]
- Matus R.E., Leifer C.E., MacEwen E.G. & Hurvitz A.I. (1986) Prognostic factors for multiple myeloma in the dog. Journal of the American Veterinary Medical Association 188, 1288–1292. [PubMed] [Google Scholar]
- Mauldin G.N., Matus R.E., Withrow S.J. & Patnaik A.K. (1988) Canine osteosarcoma. Journal of Veterinary Internal Medicine 2, 177–180. [DOI] [PubMed] [Google Scholar]
- Meuten D.J. (1984) Hypercalcemia. The Veterinary clinics of North America. Small animal practice 14, 891. [DOI] [PubMed] [Google Scholar]
- Miller G.V., Ramsden C.W. & Primrose J.N. (1991) Autologous transfusion: an alternative to transfusion with banked blood during surgery for cancer. British Journal of Surgery 78, 713–715. [DOI] [PubMed] [Google Scholar]
- Mischke R., Wohlsein P. & Schoon H.A. (2005) Detection of fibrin generation alterations in dogs with hemangiosarcoma using resonance thrombography. ThrombosisResearch 115, 229–238. [DOI] [PubMed] [Google Scholar]
- Moore A.S., Rand W.M., Berg J., L'heureux D.A., Dennis R.A. (1994) Evaluation of butorphanol and cyproheptadine for prevention of cisplatin‐induced vomiting in dogs. Journal of the American Veterinary Medical Association 205, 441–443. [PubMed] [Google Scholar]
- Morden N.E., Munson J.C., Smith J., Mackenzie T.A., Liu S.K. & Tosteson A.N. (2015) Oral bisphosphonates and upper gastrointestinal toxicity: a study of cancer and early signals of esophageal injury. Osteoporosis International 26, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morstyn G., Campbell L., Lieschke G., Layton J.E., Maher D., O'Connor M. et al. (1989) Treatment of chemotherapy‐induced neutropenia by subcutaneously administered granulocyte colony‐stimulating factor with optimization of dose and duration of therapy. Journal of Clinical Oncology 7, 1554–1562. [DOI] [PubMed] [Google Scholar]
- Mouridsen H.T., Langer S.W., Buter J., Eidtmann H., Rosti G., de Wit M. et al. (2007) Treatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studies. Annals of Oncology 18, 546–550. [DOI] [PubMed] [Google Scholar]
- Mullin C.M., Arkans M.A., Sammarco C.D., Vail D.M., Britton B.M., Vickery K.R. et al. (2016) Doxorubicin chemotherapy for presumptive cardiac hemangiosarcoma in dogs. Veterinary and Comparative Oncology 14, e171–83.. [DOI] [PubMed] [Google Scholar]
- Murphy L.A., Zuendt G.F., Nakamura R.K. & Gambardella P. (2016) Hypersensitivity reaction associated with subcutaneous glargine insulin therapy in a cat. Journal of Feline Medicine and Surgery Open Reports 2, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis M.E., Koutinas A.F., Papaioannou N. & Lekkas S. (2007) Acute tumour lysis syndrome in a dog with B‐Cell multicentric lymphoma. Australian Veterinary Journal 85, 206–208. [DOI] [PubMed] [Google Scholar]
- Nakamura R.K., Rozanski E.A. & Rush J.E. (2008) Non‐coagulopathic spontaneous hemothorax in dogs. Journal of Veterinary Emergency and Critical Care 18, 292–297. [Google Scholar]
- Ogilvie G.K., Moore A.S. & Curtis C.R. (1989) Evaluation of cisplatin‐induced emesis in dogs with malignant neoplasia: 115 cases (1984‐1987). Journal of the American Veterinary Medical Association 195, 1399–1403. [PubMed] [Google Scholar]
- Ogilvie G.K., Obradovich J.E., Cooper M.F., Walters L.M., Salman M.D. & Boone T.C. (1992) Use of recombinant canine granulocyte colony‐stimulating factor to decrease myelosuppression associated with the administration of mitoxantrone in the dog. Journal of Veterinary Internal Medicine 6, 44–47. [DOI] [PubMed] [Google Scholar]
- Ogilvie G.K., Powers B.E., Mallinckrodt C.H. & Withrow S.J. (1996) Surgery and doxorubicin in dogs with hemangiosarcoma. Journal of Veterinary Internal Medicine 14, 395–398. [DOI] [PubMed] [Google Scholar]
- Oyajobi B.O. (2007) Multiple myeloma/hypercalcemia. Arthritis Research & Therapy 9, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyajobi B.O. & Mundy G.R. (2004) Pathophysiology of myeloma bone disease. Multiple Myeloma and Related Disorders 2, 74–88. [Google Scholar]
- Page R.L., Leifer C.E. & Matus R.E. (1986) Uric acid and phosphorus excretion in dogs with lymphosarcoma. American Journal of Veterinary Research 47, 910–912. [PubMed] [Google Scholar]
- Patel R.T., Caceres A., French A.F. & McManus P.M. (2005) Multiple myeloma in 16 cats: a retrospective study. Veterinary Clinical Pathology 34, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A.K., Hurvitz A.I., Lieberman P.H. & Johnson G.F. (1981) Canine hepatocellular carcinoma. Veterinary Pathology 18, 427–438. [DOI] [PubMed] [Google Scholar]
- Pattison J. (2002) Managing cytotoxic extravasation. Nursing Times 98, 32–34. [PubMed] [Google Scholar]
- Pedro B., Linney C., Navarro‐Cubas X., Stephenson H., Dukes‐McEwan J., Gelzer A.R. & Kraus M.S. (2016) Cytological diagnosis of cardiac masses with ultrasound guided fine needle aspirates. Journal of Veterinary Cardiology 18, 47–56. [DOI] [PubMed] [Google Scholar]
- Penack O., Becker C., Buchheidt D., Christopeit M., Kiehl M., von Lilienfeld‐Toal M. et al. (2014) Management of sepsis in neutropenic patients: 2014 updated guidelines from the infectious diseases working party of the German society of hematology and medical oncology. Annals of Hematology 93, 1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.E. & Feinman J.M. (1982) Hypercalcemia associated with hypoadrenocorticism in sixteen dogs. Journal of American Veterinary Medical Association 181, 802–804. [PubMed] [Google Scholar]
- Phillips B.S., Kraegel S.A., Simonson E. & Madewell B.R. (1998) Acute reactions in dogs treated with doxorubicin: increased frequency with the use of generic formulation. Journal of Veterinary Internal Medicine 12, 171–172. [DOI] [PubMed] [Google Scholar]
- Picard M. & Castells M.C. (2015) Re‐visiting hypersensitivity reactions to Taxanes: a comprehensive review. Clinical Reviews in Allergy & Immunology 49, 177–191. [DOI] [PubMed] [Google Scholar]
- Pierro J., Krick E., Flory A., Regan R., DeRegis C., Boudreaux B. et al. (2016) Febrile neutropenia in cats treated with chemotherapy. Veterinary and Comparative Oncology 15, 550–556. [DOI] [PubMed] [Google Scholar]
- Pintar J., Breitschwerdt E.B., Hardie E.M. & Spaulding K.A. (2003) Acute nontraumatic hemoabdomen in the dog: a retrospective analysis of 39 cases (1987‐2001). Journal of the American Animal Hospital Association 39, 518–522. [DOI] [PubMed] [Google Scholar]
- Poirier V.J., Hershey A.E., Burgess K.E., Phillips B., Turek M.M., Forrest L.J. et al. (2004) Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. Journal of Veterinary Internal Medicine 18, 219–222. [DOI] [PubMed] [Google Scholar]
- Powell G.J., Southby J., Danks J.A., Stillwell R.G., Hayman J.A., Henderson M.A. et al. (1991) Localization of parathyroid hormone‐related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Research 51, 3059–3061. [PubMed] [Google Scholar]
- Purvis D. (1995) Autotransfusion in the emergency patient. Veterinary Clinics of North America: Small Animal Practice 25, 1291–1304. [DOI] [PubMed] [Google Scholar]
- Puskarich M.A. (2012) Emergency management ofsevere sepsis and septic shock. Current Opinion in Critical Care 18, 295–300. [DOI] [PubMed] [Google Scholar]
- Pusterla N., Magdesian K.G., Mapes S. & Leutenegger C.M. (2006) Expression of molecular markers in blood of neonatal foals with sepsis. American Journal of Veterinary Research 67, 1045–1049. [DOI] [PubMed] [Google Scholar]
- Quigley P.J. & Leedale A.H. (1983) Tumors involving bone in the domestic cat: a review of fifty‐eight cases. Veterinary Pathology 20, 670–686. [DOI] [PubMed] [Google Scholar]
- Raszka W.V., Keuser T.K., Smith F.R. & Bass J.W. (1990) The use of hyaluronidase in the treatment of intravenous extravasation injuries. Journal of Perinatology 10, 146–149. [PubMed] [Google Scholar]
- Reed J.R. & Thomas W.P. (1984) Hemodynamics of progressive pneumopericardium in the dog. American Journal of Veterinary Research 45, 301–307. [PubMed] [Google Scholar]
- Reed N., Espadas I., Lalor S.M. & Kisielewicz C. (2014) Assessment of five formulae to predict post‐transfusion packed cell volume in cats. Journal of Feline Medicine and Surgery 16, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas D.A., Duerst M.L., Jump M.E. & Osgood E.E. (1956) The nucleic acids and other phosphorus compounds of human leukemic leukocytes: relation to cell maturity. The Journal of Laboratory and Clinical Medicine 48, 356–378. [PubMed] [Google Scholar]
- Robinson D.A., Kiefer K., Bassett R. & Quandt J. (2016) Autotransfusion in dogs using a 2‐syinge technique. Journal of Veterinary Emergency and Critical Care 26, 766–774. [DOI] [PubMed] [Google Scholar]
- Rogers K.S. (1989) L‐asparaginase for treatment of lymphoid neoplasia in dogs. Journal of the American Veterinary Medical Association 194, 1626–1630. [PubMed] [Google Scholar]
- Rosol T.J. (2000) Pathogenesis of bone metastases: role of tumor‐related proteins. Journal of Bone and Mineral Research 15, 844–850. [DOI] [PubMed] [Google Scholar]
- Rosol T.J., Nagode L.A., Couto C.G., Hammer A.S., Chew D.J., Peterson J.L. et al. (1992) Parathyroid hormone (PTH)‐related protein, PTH, and 1, 25‐dihydroxyvitamin D in dogs with cancer‐associated hypercalcemia. Endocrinology 131, 1157–1164. [DOI] [PubMed] [Google Scholar]
- Rozanski E. & Rondeau M. (2002) Choosing fluids in traumatic hypovolemic shock: the role of crystalloids, colloids, and hypertonic saline. Journal of the American Animal Hospital Association 38, 499–501. [DOI] [PubMed] [Google Scholar]
- Rubin J.A., Suran J.N., Brown D.C. & Agnello K.A. (2015) Factors associated with pathological fractures in dogs with appendicular primary bone neoplasia: 84 cases (2007‐2013). Journal of the American Veterinary Medical Association 247, 917–923. [DOI] [PubMed] [Google Scholar]
- Sabatini M., Boyce B., Aufdemorte T., Bonewald L. & Mundy G.R. (1988) Infusions of recombinant human interleukins 1 alpha and 1 beta cause hypercalcemia in normal mice. Proceedings of the National Academy of Sciences 85, 5235–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary K., Price G.S. & Vaden S.L. (2000) Hypercalcemia in cats: a retrospective study of 71 cases (1991‐1997). Journal of Veterinary Internal Medicine 14, 184–189. [DOI] [PubMed] [Google Scholar]
- Scavelli T.D., Patnaik A.K., Mehlhaff C.J. & Hayes A.A. (1985) Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. Journal of the American Veterinary Medical Association 187, 817–819. [PubMed] [Google Scholar]
- Scheck P.A., Chew D.J., Nagodea L.A. & Rosol T.J. (2011) Disorders of calcium. Hypercalcemia and Hypocalcemia. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice 2, 120–195. [Google Scholar]
- Schultze A.E. (2010) Interpretation of canine leukocyte response. Schalm's Veterinary Hematology 6, 321–334. [Google Scholar]
- Shaw S.P., Rush J.E. (2007) Canine pericardial effusion: diagnosis, treatment, and prognosis. Compendium. [PubMed]
- Sherwood J.M., Haynes A.M., Klocke E., Higginbotham M.L., Thomson E.M., Weng H.Y. & Towle Millard H.A. (2016) Occurrence and clinicopathologic features of splenic neoplasia based on body weight: 325 dogs (2003‐2013). Journal of the American Animal Hospital Association 52, 220–226. [DOI] [PubMed] [Google Scholar]
- Shimada M., Johnson R.J., May W.S. Jr, Lingegowda V., Sood P., Nakagawa T. et al. (2009) A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrology Dialysis Transplantation 24, 2960–2964. [DOI] [PubMed] [Google Scholar]
- Shmuel D.L. & Cortes Y. (2013) Anaphylaxis in dogs and cats. Journal of Veterinary Emergency and Critical Care 23, 377–394. [DOI] [PubMed] [Google Scholar]
- Short J.L., Diehl S., Seshadri R. & Serrano S. (2012) Accuracy of formulas used to predict post‐transfusion packed cell volume rise in anemic dogs. Journal of Veterinary Emergency and Critical Care 22, 428–434. [DOI] [PubMed] [Google Scholar]
- Silva D.M., Franciosi A.I., Pezzini P.C. & Guérios S.D. (2015) Subcutaneous administration of paclitaxel in dogs with cancer: a preliminary study. The Canadian Veterinary Journal 56, 823. [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar‐Hari M., Annane D., Bauer M. et al. (2016) The third international consensus definitions for sepsis and septic shock (sepsis‐3). Journal of the American Medical Association 315, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.A., Fischer J.R. & Harkin K.R. (2000) Glucagon constant‐rate infusion: a novel strategy for the management of hyperinsulinemic‐hypoglycemic crisis in the dog. Journal of the American Animal Hospital Association 36, 27–32. [DOI] [PubMed] [Google Scholar]
- Sorenmo K.U., Harwood L.P., King L.G. & Drobatz K.J. (2010) Case‐control study to evaluate risk factors for the development of sepsis (neutropenia and fever) in dogs receiving chemotherapy. Journal of the American Veterinary Medical Association 236, 650–656. [DOI] [PubMed] [Google Scholar]
- Spangler W.L. & Culbertson M.R. (1992) Prevalence and type of splenic diseases in cats: 455 cases (1985‐1991). Journal of the American Veterinary Medical Association 201, 773–776. [PubMed] [Google Scholar]
- Spangler W.L. & Kass P.H. (1997) Pathologic factors affecting post splenectomy survival in dogs. Journal of Veterinary Internal Medicine 11, 166–171. [DOI] [PubMed] [Google Scholar]
- Spodnick G.J., Berg J., Rand W.M., Schelling S.H., Couto G., Harvey H.J. et al. (1992) Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978‐1988). Journal of the American Veterinary Medical Association 200, 995–999. [PubMed] [Google Scholar]
- Spugnini Enrico (2002) Use of hyaluronidase for the treatment of extravasation of chemotherapeutic agents in six dogs. Journal of the American Veterinary Medical Association 221, 1437–1440. [DOI] [PubMed] [Google Scholar]
- van Stee L.L., Boston S.E., Singh A., Romanelli G., Rubio‐Guzman A. & Scase T.J. (2015) Outcome and prognostic factors for canine splenic lymphoma treated by splenectomy (1995–2011). Veterinary Surgery 44, 976–982. [DOI] [PubMed] [Google Scholar]
- Stefanello D., Valenti P., Zini E., Comazzi S., Gelain M.E., Roccabianca P. et al. (2011) Splenic marginal zone lymphoma in 5 dogs (2001–2008). Journal of Veterinary Internal Medicine 25, 90–93. [DOI] [PubMed] [Google Scholar]
- Stern P.H., Krieger N.S., Nissenson R.A., Williams R.D., Winkler M.E., Derynck R. & Strewler G.J. (1985) Human transforming growth factor‐alpha stimulates bone resorption in vitro. Journal of Clinical Investigation 76, 2016–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson C.K., Kidney B.A., Duke T., Snead E.C., Mainar‐Jaime R.C. & Jackson M.L. (2007) Serial blood lactate concentrations in systemically ill dogs. Veterinary Clinical Pathology 36, 234–239. [DOI] [PubMed] [Google Scholar]
- Stylianou E. & Saklatvala J. (1998) Interleukin‐1. The International Journal of Biochemistry & Cell Biology 30, 1075–1079. [DOI] [PubMed] [Google Scholar]
- Susaneck S.J. (1983) Doxorubicin therapy in the dog. Journal of the American Veterinary Medical Association 182, 70–72. [PubMed] [Google Scholar]
- Swami A. & Spodick D.H. (2003) Pulsus paradoxus in cardiac tamponade: a pathophysiologic continuum. Clinical Cardiology 26, 215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swardson C., Hammer A.S., Couto C.G. & Getzy D. (1991) Hemostatic abnormalities in dogs with hemangiosarcoma. Journal of Veterinary Internal Medicine 5, 11–14. [DOI] [PubMed] [Google Scholar]
- Thompson J.C., Jones B.R. & Hickson P.C. (1995) The amended insulin to glucose ratio and diagnosis of insulinoma in dogs. New Zealand Veterinary Journal 43, 240–243. [DOI] [PubMed] [Google Scholar]
- Thrall D.E. (1998) Bone tumors versus bone infections In: Textbook of Veterinary Diagnostic Radiology (eds 6th edition Elsevier.), pp 161–168. WB Saunders: Philadelphia. [Google Scholar]
- Trillet‐Lenoir V., Green J., Manegold C., Von Pawel J., Gatzemeier U., Lebeau B. et al. (1993) Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. European Journal of Cancer 29, 319–324. [DOI] [PubMed] [Google Scholar]
- Trost M.E., Inkelmann M.A., Galiza G.J.N., Silva T.M. & Kommers G.D. (2014) Occurrence of tumours metastatic to bones and multicentric tumours with skeletal involvement in dogs. Journal of Comparative Pathology 150, 8–17. [DOI] [PubMed] [Google Scholar]
- Vail D.M. (2009) Supporting the veterinary cancer patient on chemotherapy: neutropenia and gastrointestinal toxicity. Topics in Companion Animal Medicine 24, 122–129. [DOI] [PubMed] [Google Scholar]
- Vasu T.S., Cavallazzi R., Hirani A., Kaplan G., Leiby B. & Marik P.E. (2012) Norepinephrine or dopamine for septic shock: systematic review of randomized clinical trials. Journal of Intensive Care Medicine 27, 172–178. [DOI] [PubMed] [Google Scholar]
- Venable R.O., Saba C.F., Endicott M.M. & Northrup N.C. (2012) Dexrazoxane treatment of doxorubicin extravasation injury in four dogs. Journal of the American Veterinary Medical Association 240, 304–307. [DOI] [PubMed] [Google Scholar]
- Veterinary Cooperative Oncology Group (2011) Veterinary co‐operative oncology group‐common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy indogs and cats v1. 0. Veterinary Comparative Oncology 14, 417–446. [DOI] [PubMed] [Google Scholar]
- Vicari E.D., Brown D.C., Holt D.E. & Brockman D.J. (2001) Survival times of and prognostic indicators for dogs with heart base masses: 25 cases (1986–1999). Journal of the American Veterinary Medical Association 219, 485–487. [DOI] [PubMed] [Google Scholar]
- Vickery K.R. & Thamm D.H. (2007) Successful treatment of acute tumor lysis syndrome in a dog with multicentric lymphoma. Journal of Veterinary Internal Medicine 21, 1401–1404. [DOI] [PubMed] [Google Scholar]
- Villalobos A. (2006) Dealing with chemotherapy extravasations: a new technique. Journal of the American Animal Hospital Association 42, 321–325. [DOI] [PubMed] [Google Scholar]
- Von Euler H., Rivera P., Nyman H. & Borgia O. (2013) A dose‐finding study with a novel water‐soluble formulation of paclitaxel for the treatment of malignant high‐grade solid tumours in dogs. Veterinary and Comparative Oncology 11, 243–255. [DOI] [PubMed] [Google Scholar]
- Ware W.A. (2015) Pericardial diseases. Small Animal Critical Care Medicine 45, 239–245. [Google Scholar]
- Ware W.A. & Hopper D.L. (1999) Cardiac tumors in dogs: 1982‐1995. Journal of Veterinary Internal Medicine 13, 95–103. [DOI] [PubMed] [Google Scholar]
- Weir E.C., Norrdin R.W., Matus R.E., Brooks M.B., Broadus A.E., Mitnick M. et al. (1988) Humoral hypercalcemia of malignancy in canine lymphosarcoma. Endocrinology 122, 602–608. [DOI] [PubMed] [Google Scholar]
- Weisse C., Soares N., Beal M.W., Steffey M.A., Drobatz K.J. & Henry C.J. (2005) Survival times in dogs with right atrial hemangiosarcoma treated by means of surgical resection with or without adjuvant chemotherapy: 23 cases (1986‐2000). Journalof the American Veterinary Medical Association 226, 575–579. [DOI] [PubMed] [Google Scholar]
- Wells J.E., Sabatino B.R. & Whittemore J.C. (2014) Cyclophosphamide intoxication because of pharmacy error in two dogs. Journal of the American Veterinary Medicine Association 245, 222–226. [DOI] [PubMed] [Google Scholar]
- Wendelburg K.M., Price L.L., Burgess K.E., Lyons J.A., Lew F.H. & Berg J. (2015) Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001‐2012). Journal of the American Veterinary Medical Association 247, 393–403. [DOI] [PubMed] [Google Scholar]
- Whitehead K., Cortes Y. & Eirmann L. (2016) Gastrointestinal dysmotility disorders in critically ill dogs and cats. Journal of Veterinary Emergency and Critical Care 26, 234–253. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Gliatto J.M., Dodge R.K., Johnson J.L., Gamblin R.M., Thamm D.H. et al. (2003) Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985‐1995). Journal of the American Veterinary Medical Association 15, 825–831. [DOI] [PubMed] [Google Scholar]
- Wood C.A., Moore A.S., Gliatto J.M., Ablin L.A., Berg R.J. & Rand W.M. (1998) Prognosis for dogs with state I or II splenic hemangiosarcoma treated by splenectomy alone: 32 cases (1991‐1993). Journal of the American Animal Hospital Association 34, 417–421. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Hoshi K., Hirakawa A., Chimura S., Kobayashi M. & Machida N. (2013) Epidemiological, clinical and pathological features of primary cardiac hemangiosarcoma in dogs: a review of 51 cases. Journal of Veterinary Medical Science 75, 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.Y., Yoon J.H., Min W.K., Lim W.K., Song J., Chae S.L. et al. (2007) Stanrdarization of terminology in laboratory medicine. Korean Journal of Laboratory Medicine 27, 151–155. [DOI] [PubMed] [Google Scholar]


