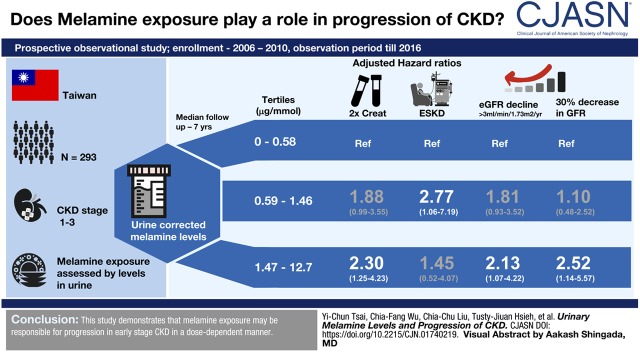
Visual Abstract
Keywords: Melamine; renal function decline; chronic kidney disease; glomerular filtration rate; melamine; creatinine; Cross-Sectional Studies; Prospective Studies; Public Health; Kidney Function Tests; Renal Insufficiency, Chronic; kidney; Triazines; Neoplasms
Abstract
Background and objectives
CKD is a global public health problem. Some cross-sectional studies have associated environmental melamine exposure with kidney diseases, but evidence is limited.
Design, setting, participants, & measurements
We conducted this prospective cohort study to enroll patients with eGFR≥30 ml/min per 1.73 m2 in 2006–2010. Urinary corrected melamine levels (ratio of urinary melamine to urinary creatinine) were measured by liquid chromatography/tandem mass spectrometry at enrollment. Kidney outcomes included doubling of serum creatinine levels, eGFR decline >3 ml/min per 1.73 m2 per year, and 30% decline in eGFR in the first 2 years. Subjects were followed until targeted kidney outcomes, cancer, death, last contact, or the end of observation in December 2016.
Results
In a total of 293 subjects, the median urinary corrected melamine level was 0.97 (interquartile range, 0.43–2.08) μg/mmol. Over a median follow-up period of 7.0 years, serum creatinine levels doubled in 80 subjects (27%). Subjects in the highest tertile of urinary melamine level 12.70 μg/mmol) had a 2.30 (95% confidence interval, 1.25 to 4.23; P<0.01) hazard risk for doubling of serum creatinine compared with those in the lowest tertile (0.02–0.58 μg/mmol). Similar significant dose-response results were found in eGFR decline >3 ml/min per 1.73 m2 per year and 30% decline in eGFR in the first 2 years.
Conclusions
Urinary melamine level is significantly associated with kidney function deterioration in patients with early-stage CKD.
Introduction
CKD is a worldwide public health threat. Taiwan has the highest incidence of CKD worldwide, with about one tenth of its population diagnosed with CKD (1). Because of its silent symptoms and signs, CKD is difficult to detect clinically before irreversible changes occur.
The well known traditional risk factors for CKD include aging, hypertension, and diabetes. However, since the 2008 melamine-tainted milk contamination in China, this emerging environmental hazard has gradually attracted clinical attention, because it has been linked with rapid progression of CKD (2,3). In that incident, melamine was added to milk or animal feed to increase protein content. It was worrisome, because exposure to excess concentrations of melamine increases the risk of calcium urolithiasis and kidney injury in children (3,4).
Even after measures were taken in response to the 2008 melamine incident, this substance remains in use in manufacturing and is in products, such as some cooking utensils, plates, plastic products, and packaging for food products around the world (https://www.fda.gov/food/resourcesforyou/consumers/ucm199525.htm). Our previous studies have found that daily use of melamine tableware is the main source of melamine exposure in humans in Taiwan (5), and environmental low-dose melamine exposure has been associated with the risk of kidney stones (6). Melamine tableware manufacturing workers with higher urinary melamine levels resulting from exposure to ambient melamine had increased concentrations of urinary N-acetyl β-d-glucosaminidase and β2-microglobulin, both surrogate markers for kidney tubular injury (7). In addition, one in vitro study suggested that low-dose melamine-induced kidney tubular cell damage may be caused by oxidative stress and inflammation (8), a major cause of poor kidney progression.
On the basis of the above findings, we hypothesized that environmental exposure to melamine may play a pathophysiologic role in kidney injury and affect the progression of kidney function in the general population, especially vulnerable populations, such as those with early-stage CKD. We conducted a prospective cohort study of patients with early-stage CKD to examine the association between baseline urinary melamine levels and adverse kidney outcomes represented by doubling of serum creatinine levels and rapid decline in kidney function, including eGFR decline >3 ml/min per 1.73 m2 per year during the follow-up period and 30% decline in eGFR in the first 2 years.
Materials and Methods
Study Subjects
This prospective study enrolled subjects from a previously established cohort of patients with CKD in the Interdisciplinary CKD Care Program conducted at Kaohsiung Medical University Hospital (KMUH), a tertiary hospital in southern Taiwan. This integrative care program was implemented by the KMUH’s Division of Nephrology for the comprehensive and continuous care of patients with CKD. Patients with CKD are recruited into this program when they have a urinary protein-to-creatinine ratio (UPCR) ≥0.15 g/g or eGFR<60 ml/min per 1.73 m2 for >3 months (9). The eGFR was calculated using the four-variable equation of the Modification of Diet in Renal Disease study (eGFR=186× serum creatinine−1.154× age−0.203×0.742 [if a woman]) (10). CKD was staged according to Kidney Disease Outcomes Quality Initiative definitions on the basis of eGFR (milliliters per minute per 1.73 m2; stage 1, ≥90 with UPCR≥0.15 g/g; stage 2, 60–89 with UPCR≥0.15 g/g; stage 3a, 45–59; stage 3b, 30–45).
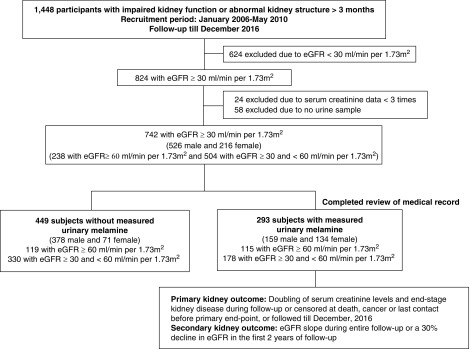
Between January 2006 and May 2010, 1448 patients with CKD participated in this integrative care program (Figure 1). After excluding 624 subjects with baseline eGFR <30 ml/min per 1.73 m2, 24 subjects with fewer than three serum creatinine measurements, and 58 subjects without baseline urine samples, 742 subjects, including 238 with eGFR≥60 ml/min per 1.73 m2 and 504 with eGFR≥30 and <60 ml/min per 1.73 m2, were left. Because of the greater proportion of men to women in 742 subjects, we chose the ratio of men to women to be 1:1 and then matched their ages in the two sex groups for additional completed review of medical record (Supplemental Table 1). Finally, we analyzed 293 subjects with urinary melamine. All subjects were followed to the occurrence of targeted kidney outcomes, cancer, death, last contact, or the end of observation in December 2016. The protocol for this study was approved by the Institutional Review Board of the KMUH. Informed consent was obtained in written form from all study subjects, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Figure 1.
The flow chart of study design and subjects enrollment.
Data Collection
We collected demographic characteristics, a history of current or ever cigarette smoking and alcohol drinking defined as positive smoking and drinking history, and clinical data from interviews and medical records of subjects at enrollment. Body mass index was calculated as body weight divided by squared body height. Subjects were classified as having hypertension on the basis of history or use of antihypertensive drugs. Diabetes was defined by medical history and blood glucose values using American Diabetes Association criteria, oral hypoglycemia agent use, or insulin use. Heart disease was defined as a history of acute or chronic ischemic heart disease, myocardial infarction, or heart failure. Information regarding participant use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers before and after enrollment was obtained from medical records.
Subjects were asked to fast for at least 12 hours before blood and one-spot urine samples were collected the next morning for biochemistry studies. Serum creatinine was measured according to the compensated Jaffé (kinetic alkaline picrate) method using the same autoanalyzer (Roche/Integra 400; Roche Diagnostics) and a calibrator that could be used in isotope dilution mass spectrometry (11). Protein in urine was expressed as UPCR. Hematuria was defined as occult blood in urine ≥1+ using dipstick analysis.
Urinary Melamine Measurement
Urinary melamine was measured using isotopic liquid chromatography/tandem mass spectrometry (API4000Q; Applied Biosystems/MDS SCIEX) at enrollment. We have demonstrated that melamine levels in one-spot overnight urine samples can represent the previous 24-hour total urinary melamine excretions in both adults and children (6,12). The details of the methods used to measure urinary melamine have been described previously (6,12,13). Urinary melamine levels were expressed as nanograms per milliliter or micrograms per millimole creatinine without and with the correction of urinary creatinine (urinary corrected melamine levels), respectively. Laboratory personnel did not have knowledge of the study subjects’ information or clinical data during measurement of urinary melamine and creatinine levels (Supplemental Material).
Kidney Outcomes
Subjects were contacted at outpatient clinics at 3-month intervals to assess clinical status and measure eGFR. The primary kidney outcomes were defined as doubling of serum creatinine levels compared with baseline and progression to ESKD with initial dialysis, whereas the secondary kidney outcome was rapid decline in kidney function, including eGFR decline >3 ml/min per 1.73 m2 per year (eGFR slope) and 30% decline in eGFR within the first 2 years. The eGFR slope was defined as annual change in eGFR during follow-up period, and it was measured by the regression coefficient between eGFR and time in units of milliliters per minute per 1.73 m2 per year on the basis of all eGFR measurements obtained from enrollment to the end of observation period. We excluded eGFR measurements during hospitalization or AKI events. Rapid eGFR decline was defined as the lowest quartile (eGFR decline >3 ml/min per 1.73 m2 per year, an integer near the cutoff point between the lowest two quartiles of the eGFR slope) (14). Percentage change in eGFR was calculated as (last eGFR − first eGFR)/first eGFR) ×100% in the first 2-year follow-up (15). As outlined in Kidney Disease Improving Global Outcomes (16), ≥30% change in eGFR was considered as an indicator of poor outcomes in morbidity and mortality.
Statistical Analyses
Baseline characteristics of all subjects were stratified by tertiles of urinary corrected melamine levels, with cutoffs set at 0.58 and 1.46 μg/mmol. Multiple imputation-expectation maximization was used to handle missing data. The differences between continuous variables across groups were tested by one-way ANOVA or the Kruskal–Wallis H test, whereas the difference between categorical variables was tested by the chi-squared test. Spearman correlations were used to examine the relationship between urinary corrected melamine levels and continuous clinical variables. Skewed distribution of continuous variables was log10 transformed to approximate normal distribution before additional analyses.
Kaplan–Meier survival analysis was used to analyze the association of urinary corrected melamine levels with the risk of doubling of serum creatinine levels. Cox proportional hazards modeling was used to examine the relationship between urinary corrected melamine levels and the risk of doubling of serum creatinine levels and progression to ESKD. Each subject accumulated person-time starting from the enrollment to doubling of creatinine levels, ESKD, or the end of this study. Logistic regression models were used to evaluate the relationship between urinary corrected melamine levels and rapid decrease in kidney function: either eGFR decline >3 ml/min per 1.73 m2 per year or 30% change in eGFR in the first 2 years. The covariates in the models included age, sex, diabetes, hypertension, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker usage, body mass index, baseline eGFR, and UPCR cut at 1 g/g on the basis of well known risk factors of CKD progression. We stratified subjects by diabetes mellitus and eGFR of 60 or 45 ml/min per 1.73 m2 to further examine the synergistic interaction between continuous urinary corrected melamine levels and diabetes mellitus group (yes versus no) or eGFR level in kidney outcomes. In the sensitivity analysis, urinary creatinine level was also adjusted to minimize the influence of low urinary creatinine secretion. Also, we used a new method that incorporates both covariate-adjusted standardization and creatinine as a covariate in the model to control measurement error bias caused by urinary diluteness (17). All statistical operations were conducted using SPSS 18.0 version and SAS 9.3. All P values were two sided, and significance was set at <0.05.
Results
Characteristics of Entire Cohort
Of 293 subjects with urinary melamine data (115 with eGFR ≥60 ml/min per 1.73 m2 and 178 with eGFR≥30 and <60 ml/min per 1.73 m2), the mean age was 57±14 years old, and 54% were men (Table 1). Overall median eGFR was 52 ml/min per 1.73 m2, and the median urinary corrected melamine level was 0.97 (0.43–2.08) μg/mmol creatinine. Urinary corrected melamine levels were significantly and negatively correlated with baseline eGFR, serum albumin, and hemoglobin, whereas they were significantly and positively correlated with baseline UPCR and serum uric acid (Supplemental Table 2).
Table 1.
Baseline characteristics of a cohort of people with CKD from a tertiary hospital in southern Taiwan
| Characteristics | Entire Cohort | Urinary Corrected Melamine Levelsa | ||
|---|---|---|---|---|
| Tertile 1, 0–0.58 μg/mmol | Tertile 2, 0.59–1.46 μg/mmol | Tertile 3, 1.47–12.70 μg/mmol | ||
| N | 293 | 98 | 97 | 98 |
| Demographics | ||||
| Age, yr | 57±14 | 53±15 | 59±13 | 58±14 |
| Sex, men | 159 (54) | 59 (60) | 56 (58) | 44 (45) |
| Smoke, current/past | 64 (22) | 25 (26) | 18 (19) | 21 (21) |
| Alcohol, current/past | 33 (11) | 8 (8) | 17 (18) | 8 (8) |
| Hypertension | 247 (84) | 80 (82) | 82 (85) | 85 (87) |
| Diabetes mellitus | 111 (38) | 33 (34) | 35 (36) | 43 (44) |
| Cardiovascular disease | 46 (16) | 10 (10) | 13 (13) | 23 (24) |
| Hyperlipidemia | 136 (46) | 45 (46) | 46 (47) | 45 (46) |
| Kidney stone | 10 (3) | 2 (2) | 5 (5) | 3 (3) |
| CKD cause | ||||
| Chronic GN | 132 (45) | 49 (50) | 45 (46) | 38 (39) |
| Diabetic kidney disease | 90 (31) | 28 (29) | 29 (30) | 33 (34) |
| eGFR≥60 ml/min per 1.73 m2 | 115 (39) | 54 (55) | 38 (39) | 23 (23) |
| eGFR≥30 and <60 ml/min per 1.73 m2 | 178 (61) | 44 (45) | 59 (61) | 75 (77) |
| ACEI/ARB usage | 197 (67) | 65 (66) | 61 (63) | 71 (72) |
| BMI, kg/m2 | 25.8±4.1 | 25.7±4.2 | 25.8±4.3 | 25.7±4.0 |
| Laboratory data | ||||
| BUN, mg/dl | 20 (15–25) | 18 (12–22) | 20 (16–25) | 22 (17–29) |
| Creatinine, mg/dl | 1.4±0.5 | 1.2±0.5 | 1.4±0.4 | 1.5±0.5 |
| eGFR, ml/min per 1.73 m2 | 59±29 | 71±35 | 56±20 | 51±24 |
| Uric acid, mg/dl | 7.1±1.7 | 6.7±1.8 | 7.2±1.6 | 7.2±1.7 |
| Glycated hemoglobin, % | 5.9 (5.5–6.9) | 5.9 (5.5–6.6) | 6.1 (5.6–6.8) | 6.1 (5.5–7.2) |
| Hemoglobin, g/dl | 13.0±2.0 | 13.7±2.0 | 12.9±2.1 | 13.0±1.9 |
| Albumin, g/dlb | 4.1±0.5 | 4.2±0.5 | 4.1±0.5 | 4.1±0.5 |
| Phosphate, mg/dl | 3.7 (3.3–4.0) | 3.6 (3.3–3.9) | 3.7 (3.2–4.0) | 3.7 (3.4–4.1) |
| Calcium, mg/dl | 9.1±0.6 | 9.1±0.5 | 9.1±0.6 | 9.2±0.5 |
| Cholesterol, mg/dl | 200±58 | 197±42 | 202±71 | 202±59 |
| Triglyceride, mg/dl | 126 (87–185) | 134 (88–186) | 127 (89–183) | 119 (82–185) |
| hsCRP, mg/L | 1.6 (0.6–4.3) | 1.5 (0.6–4.1) | 1.6 (0.5–4.5) | 1.6 (0.7–4.5) |
| Urinary corrected melamine levels, μg/mmol | 0.97 (0.43–2.08) | 0.32 (0.19–0.43) | 0.97 (0.74–1.18) | 2.79 (2.06–4.60) |
| Occult blood in urine ≥1+ | 146 (50) | 51 (53) | 41 (42) | 54 (56) |
| UPCR, g/g | 0.5 (0.2–1.3) | 0.4 (0.2–1.0) | 0.5 (0.2–1.3) | 0.6 (0.2–1.8) |
Continuous variables are expressed as both mean ± SD and median (interquartile range), and categorical variables are expressed in number (percentage). Missing data numbers: calcium (34), phosphate (38), cholesterol (3), triglyceride (3), glycated hemoglobin (23), high sensitivity C-reactive protein (hsCRP) (36), urine protein-to-creatinine ratio (UPCR) (13). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index.
Three tertiles of urinary corrected melamine levels (micrograms per millimole) were 0.02–0.58, 0.59–1.46, and 1.47–12.7, with cutoffs of at 0.58 and 1.46 μg/mmol.
Urinary Corrected Melamine, Doubling of Serum Creatinine Levels, and ESKD
Over a median follow-up period of 7.0 (4.3–8.2) years, 80 (27%) subjects reached doubling of serum creatinine levels, and 34 subjects (12%) had ESKD. Two subjects developed cancer, and six subjects died (from cardiovascular diseases [n=2], sepsis [n=2], or respiratory failure [n=2]) before they had doubling of serum creatinine levels; their data were censored (Table 2). There was no significant difference in the proportions of the censored subjects across the tertiles of urinary corrected melamine levels (Supplemental Table 3).
Table 2.
Kidney outcomes of study subjects in total and tertiles by baseline urinary corrected melamine levels
| Kidney Outcomes | Entire Cohort | Urinary Corrected Melamine Levelsa | ||
|---|---|---|---|---|
| Tertile 1, 0–0.58 μg/mmol | Tertile 1, 0–0.58 μg/mmol | Tertile 1, 0–0.58 μg/mmol | ||
| N | 293 | 98 | 98 | 98 |
| Follow-up time, yr | 6.3±2.6 | 7.0±2.5 | 6.1±2.3 | 5.8±2.8 |
| No. of serum creatinine measurements | 22 (13–32) | 23 (13–31) | 20 (13–28) | 23 (12–37) |
| Primary kidney outcome | ||||
| Doubling of serum creatinine levels | 80 (27) | 18 (18) | 25 (26) | 37 (38) |
| ESKD | 34 (12) | 7 (7) | 15 (16) | 12 (12) |
| Secondary kidney outcome | ||||
| eGFR slope, ml/min per 1.73 m2 per year | −1.8 (−4.2 to −0.3) | −1.4 (−3.2 to −0.2) | −1.8 (−5.0 to 0.2) | −2.6 (−5.3 to −0.9) |
| eGFR decline >3 ml/min per 1.73 m2 per year | 110 (38) | 28 (29) | 39 (40) | 44 (45) |
| 30% decline of eGFR in the first 2 yr | 62 (21) | 14 (14) | 17 (18) | 31 (32) |
Continuous variables are expressed as both mean ± SD and median (interquartile range), and categorical variables are expressed in number (percentage). Conversion factor for units for eGFR is milliliters per minute per 1.73 m2 to milliliters per second per 1.73 m2.
Three tertiles of urinary corrected melamine levels (micrograms per millimole) were 0.02–0.58, 0.59–1.46, and 1.47–12.7, with cutoffs of at 0.58 and 1.46 μg/mmol.
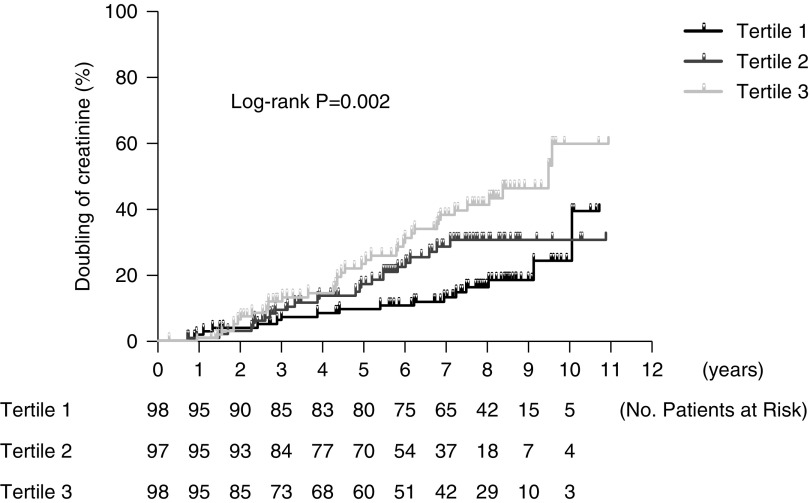
There was a stepwise increase in the proportion of those having doubling of serum creatinine levels from the lowest tertile of urinary corrected melamine levels to the highest (P<0.01) (Table 2). Kaplan–Meier survival curve showed the higher the tertile, the greater the proportion of subjects having doubling of serum creatinine levels (P=0.002) (Figure 2). Compared with the lowest tertile, the highest tertile had 2.30 (95% confidence interval, 1.25 to 4.23) risk of doubling of serum creatinine levels in the multivariable Cox regression model (Table 3). However, there was no significant correlation between urinary melamine and ESKD in subjects with eGFR≥30 ml/min per 1.73 m2.
Figure 2.
A stepwise increase in the risk of doubling of serum creatinine levels from tertile 1 to tertile 3 of urinary corrected melamine levels.
Table 3.
Relationship of urinary corrected melamine levels with doubling of serum creatinine levels and ESKD
| Urinary Corrected Melamine Levels | Event N (%)/Total Person-Yr | Crude HR (95% CI) | Adjusted HR (95% Cl)a |
|---|---|---|---|
| Doubling of serum creatinine levels | |||
| Tertile 1b | 18 (18)/686 | Reference | Reference |
| Tertile 2 | 25 (26)/590 | 1.70 (0.92 to 3.12) | 1.88 (0.99 to 3.55) |
| Tertile 3 | 37 (38)/568 | 2.62 (1.49 to 4.60) | 2.30 (1.25 to 4.23) |
| ESKD | |||
| Tertile 1b | 7 (7)/705 | Reference | Reference |
| Tertile 2 | 15 (16)/637 | 2.59 (1.05 to 6.37) | 2.77 (1.06 to 7.19) |
| Tertile 3 | 12 (12)/638 | 2.01 (0.79 to 5.10) | 1.45 (0.52 to 4.07) |
HR, hazard ratio; 95% CI, 95% confidence interval.
Adjusting for age, sex, body mass index, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker usage, diabetes mellitus, hypertension, eGFR, and urinary protein-to-creatinine ratio cut at 1 g/g.
Three tertiles of urinary corrected melamine levels (micrograms per millimole) were 0.02–0.58, 0.59–1.46, and 1.47–12.7, with cutoffs of at 0.58 and 1.46 μg/mmol.
Urinary Corrected Melamine and Rapid Decrease in Kidney Function
The median eGFR slope of all subjects was −1.8 ml/min per 1.73 m2 per year (Table 2). During the follow-up period, 110 (38%) subjects had decline of eGFR>3 ml/min per 1.73 m2 per year, and 62 (21%) reached 30% decline in eGFR within the first 2 years. Subjects in the highest tertile had higher risk of eGFR decline >3 ml/min per 1.73 m2 per year and 30% decline in eGFR within the first 2 years compared with those in the lowest tertile (Table 4).
Table 4.
Relationship of urinary corrected melamine levels with rapid decrease in kidney function
| Urinary Corrected Melamine Levels | Event N (%) | Crude OR (95% Cl) | Adjusted OR (95% Cl)a |
|---|---|---|---|
| eGFR decline >3 ml/min per 1.73 m2 per year | |||
| Tertile 1b | 28 (29) | Reference | Reference |
| Tertile 2 | 40 (41) | 1.75 (0.97 to 3.19) | 1.81 (0.93 to 3.52) |
| Tertile 3 | 43 (44) | 1.96 (1.08 to 3.54) | 2.13 (1.07 to 4.22) |
| 30% decrease of eGFR in the first 2 yr | |||
| Tertile 1b | 14 (14) | Reference | Reference |
| Tertile 2 | 17 (18) | 1.28 (0.59 to 2.76) | 1.10 (0.48 to 2.52) |
| Tertile 3 | 31 (32) | 2.78 (1.37 to 5.64) | 2.52 (1.14 to 5.57) |
OR, odds ratio; 95% CI, 95% confidence interval.
Adjusting for age, sex, body mass index, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker usage, diabetes mellitus, hypertension, eGFR, and urinary protein-to-creatinine ratio cut at 1 g/g.
Three tertiles of urinary corrected melamine levels (micrograms per millimole) were 0.02–0.58, 0.59–1.46, and 1.47–12.7, with cutoffs of at 0.58 and 1.46 μg/mmol.
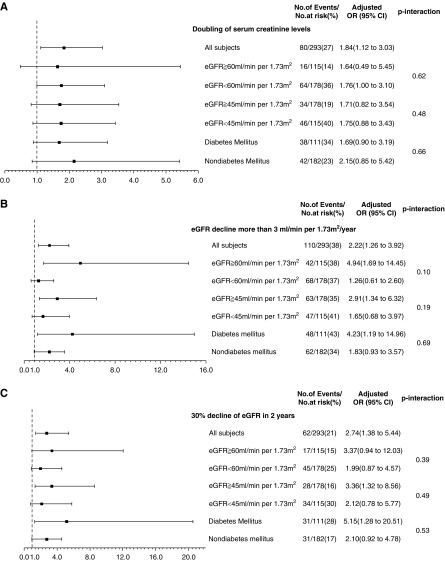
We performed subgroup analysis of study subjects stratified by eGFR and diabetes mellitus for the investigation of the synergistic interaction of continuous urinary corrected melamine with diabetes mellitus and eGFR in kidney outcomes (Figure 3). Subjects with baseline eGFR ≥45 ml/min per 1.73 m2 were found to have positive correlations between urinary corrected melamine levels and rapid decline in kidney function. The association of urinary corrected melamine with eGFR decline >3 ml/min per 1.73 m2 per year and 30% decline in eGFR within the first 2 years is shown in subjects with diabetes mellitus. However, we did not find a significant synergistic interactive effect of urinary melamine levels and diabetes mellitus or eGFR on kidney function (P value interaction >0.05).
Figure 3.
Subjects with baseline eGFR ≥45 ml/min per 1.73 m2 or diabetes mellitus have positive correlations between urinary corrected melamine levels and rapid decrease in kidney function. Ratios were adjusted for age, sex, body mass index, diabetes mellitus, hypertension, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers use, baseline eGFR, and urinary protein-to-creatinine ratio cut at 1 g/g. 95% CI, 95% confidence interval.
Sensitivity Analyses
We excluded the subjects with doubling of creatinine levels within 2 years to diminish the effect of reverse causality, and the association of urinary corrected melamine levels with adverse kidney outcomes remained consistent (Supplemental Table 4).
To minimize the influence of low urinary creatinine secretion on the relationship between urinary uncorrected melamine levels and kidney outcomes, we treated urinary creatinine levels as an independent variable. Also, we used a new method of covariate-adjusted standardization plus creatinine adjustment to minimize the effect of urine dilution on urinary marker measurement (17). Consistent results were found in both analyses (Supplemental Tables 5 and 6).
Discussion
This study demonstrates that baseline urinary corrected melamine levels are positively and significantly associated with doubling of serum creatinine levels and rapid decline in kidney function in patients with CKD stages 1–3, indicating that patients with early-stage CKD and high urinary melamine level have higher risk for poor kidney progression. To the best of our knowledge, this is the first study to find an association between environmental melamine exposure and adverse kidney outcomes in a clinical setting.
The etiology of development and progression of early-stage CKD is multifactorial (18). In addition to several demographic characteristics, such as age, sex, race, and family history, there are other well known risk factors for CKD, including diabetes, hypertension, obesity, and genetics (18,19). Environmental factors, particularly daily contact of chemicals (such as melamine), have been less studied and underappreciated for their possible link with kidney disease (20,21). Exposure to environmental low-dose melamine has been linked to risk of kidney stones in adults (6,22,23). Our previous studies have found a positive association between urinary melamine level and kidney tubular injury in both a high-exposure group of melamine tableware manufacturing workers and a susceptible group of adults with kidney stones (7,12). This study adds additional evidence of the association of urinary melamine with rapid kidney function progression in the early-stage CKD population, even after adjusting for well known risk factors. On the basis of these findings, environmental melamine exposure may have the potential to be a contributing factor to onset or progression of CKD.
In additional subgroup analyses, we found a positive association between urinary melamine and rapid decline of kidney function in patients with eGFR≥45 ml/min per 1.73 m2 but not in those with eGFR≥30 and <45 ml/min per 1.73 m2. A possible explanation may be that the influence of baseline kidney function has a greater effect on kidney progression than melamine exposure in late-stage CKD. Additionally, a significant correlation is seen in patients with diabetes and CKD, but it is not seen in patients with CKD who are not diabetic. Our findings suppose that melamine exposure is independently associated with poor kidney outcomes in the CKD population, especially the early-stage CKD and diabetic CKD populations.
We further compared urinary melamine levels in different populations and found that urinary melamine levels measured in patients with CKD are higher than those in healthy controls, lower than those in patients with calcium stones (6), and significantly lower than those in melamine workers (7) (Supplemental Table 7). Although patients with calcium stones and melamine workers have higher urinary melamine levels, only the change in the markers of kidney tubular injury (but normal kidney function) is shown. However, in early-stage CKD, relative low melamine levels may cause rapid decline in kidney function, meaning that patients with CKD have lower threshold of the concentration of melamine exposure in consequent kidney injury. Our study demonstrated that urinary melamine levels have a correlation with the deterioration of CKD progression. These findings remind clinical physicians of recognizing melamine exposure in daily life, such as heating high acidic foods to extreme temperatures (160°F) using the plastic or heating foods/drinks on melamine-based dinnerware in microwave ovens, and the potential risk of melamine in rapid decline in kidney function. Furthermore, this study provides clinical application in evaluating consequent kidney outcomes in patients of early-stage CKD.
This study has some limitations. First, we did not record the source exposure of melamine in daily life. Because only one baseline measurement of urinary melamine level was available, which is probably not representative of long-term and cumulative environmental melamine exposure, this may cause random misclassification of exposure and underestimate the results. Additional study is necessary to investigate the effect of longitudinal melamine excretion on CKD progression. Additionally, the relative small numbers of study subjects and ESKD events may not be enough to detect a significant association between urinary melamine and ESKD.
In conclusion, baseline urinary corrected melamine levels have significant association with rapid kidney progression in patients with early-stage CKD. This study analyzes the hazard of melamine exposure in consequent kidney outcomes and provides future applications in CKD progression.
Disclosures
Dr. Chen, Dr. Chiu, Dr. Hsieh, Dr. Hwang, Dr. Lin, Dr. Liu, Dr. Tsai, Dr. C-F Wu, and Dr. M-T Wu have nothing to disclose.
Funding
This work was supported by grants from the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan; Taiwan’s Ministry of Science and Technology grants MOST 107-2632-B-037-002 and MOST 106-2632-B-037-001; Taiwan’s National Health Research Institutes grant NHRI-EX106-10209PI; and Kaohsiung Medical University grants KMU-DK105007 and KMU-DK106007.
None of supporters had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01740219/-/DCSupplemental.
Supplemental Material. Urinary melamine measurement.
Supplemental Table 1. The clinical characteristics of subjects with CKD and eGFR≥30 ml/min per 1.73 m2 with or without baseline urinary melamine levels.
Supplemental Table 2. The Spearman correlation between urinary corrected melamine levels and some clinical data related to kidney outcomes in study subjects.
Supplemental Table 3. Distribution of cancer or other causes of death during follow-up by tertiles of urinary corrected melamine levels.
Supplemental Table 4. Relationship of urinary corrected melamine levels with different kidney outcomes in subjects entering doubling of creatinine levels after 2 years of enrollment.
Supplemental Table 5. Relationship of urinary uncorrected melamine levels and different kidney outcomes adjusting for other covariates plus urinary creatinine levels.
Supplemental Table 6. Relationship of urinary corrected melamine levels with different kidney outcomes using the method of covariate-adjusted standardization plus creatinine adjustment.
Supplemental Table 7. Comparison of demographic characteristics and laboratory data between patients with calcium urolithiasis, one cohort from the occupational setting with melamine tableware manufacturing workers (melamine workers), and healthy controls in our earlier study.
References
- 1.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Guan N, Fan Q, Ding J, Zhao Y, Lu J, Ai Y, Xu G, Zhu S, Yao C, Jiang L, Miao J, Zhang H, Zhao D, Liu X, Yao Y: Melamine-contaminated powdered formula and urolithiasis in young children. N Engl J Med 360: 1067–1074, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bhalla V, Grimm PC, Chertow GM, Pao AC: Melamine nephrotoxicity: An emerging epidemic in an era of globalization. Kidney Int 75: 774–779, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Dalal RP, Goldfarb DS: Melamine-related kidney stones and renal toxicity. Nat Rev Nephrol 7: 267–274, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Wu CF, Hsieh TJ, Chen BH, Liu CC, Wu MT: A crossover study of noodle soup consumption in melamine bowls and total melamine excretion in urine. JAMA Intern Med 173: 317–319, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Liu CC, Wu CF, Chen BH, Huang SP, Goggins W, Lee HH, Chou YH, Wu WJ, Huang CH, Shiea J, Lee CH, Wu KY, Wu MT: Low exposure to melamine increases the risk of urolithiasis in adults. Kidney Int 80: 746–752, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Wu CF, Peng CY, Liu CC, Lin WY, Pan CH, Cheng CM, Hsieh HM, Hsieh TJ, Chen BH, Wu MT: Ambient melamine exposure and urinary biomarkers of early renal injury. J Am Soc Nephrol 26: 2821–2829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh TJ, Hsieh PC, Tsai YH, Wu CF, Liu CC, Lin MY, Wu MT: Melamine induces human renal proximal tubular cell injury via transforming growth factor-β and oxidative stress. Toxicol Sci 130: 17–32, 2012 [DOI] [PubMed] [Google Scholar]
- 9.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Vickery S, Stevens PE, Dalton RN, van Lente F, Lamb EJ: Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol Dial Transplant 21: 2439–2445, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Lin YT, Tsai MT, Chen YL, Cheng CM, Hung CC, Wu CF, Liu CC, Hsieh TJ, Shiea J, Chen BH, Wu MT: Can melamine levels in 1-spot overnight urine specimens predict the total previous 24-hour melamine excretion level in school children? Clin Chim Acta 420: 128–133, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Liu CC, Hsieh TJ, Wu CF, Tsai YC, Huang SP, Lee YC, Huang TY, Shen JT, Chou YH, Huang CN, Wu WJ, Wu MT: Urinary melamine excretion and increased markers of renal tubular injury in patients with calcium urolithiasis: A cross-sectional study. Environ Pollut 231: 1284–1290, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS: Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 17.O’Brien KM, Upson K, Cook NR, Weinberg CR: Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124: 220–227, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glassock RJ, Warnock DG, Delanaye P: The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat Rev Nephrol 13: 104–114, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Kazancioğlu R: Risk factors for chronic kidney disease: An update. Kidney Int Suppl (2011) 3: 368–371, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS: Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis 17: 254–264, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Weaver VM, Fadrowski JJ, Jaar BG: Global dimensions of chronic kidney disease of unknown etiology (CKDu): A modern era environmental and/or occupational nephropathy? BMC Nephrol 16: 145, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu CF, Liu CC, Chen BH, Huang SP, Lee HH, Chou YH, Wu WJ, Wu MT: Urinary melamine and adult urolithiasis in Taiwan. Clin Chim Acta 411: 184–189, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Liu CC, Wu CF, Wu MT: Reappraisal of melamine exposure and adult calcium urolithiasis. Kidney Int 82: 361–362, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.