Mortality rates among critically ill patients with AKI requiring kidney replacement therapy (KRT) remain unacceptably high (1). Various therapeutic strategies have been investigated to improve outcomes in this high-risk patient population, including alterations to the intensity of KRT (2), alterations in KRT modality (3), and alternations to various components of the KRT circuitry (4). Unfortunately, the vast majority of randomized controlled trials (RCTs) conducted to date in this space have been negative.
The optimal timing of KRT initiation represents an increasing focus among investigators studying critically ill patients with AKI. The underlying premise is straightforward: earlier initiation of KRT could result in meaningful health benefits via both “classical” and “nonclassical” effects of KRT. Classic effects include regulation of volume and acid-base status, maintenance of electrolyte homeostasis, and clearance of uremic toxins. Nonclassic effects include clearance of other harmful substances from the circulation, such as proinflammatory cytokines (e.g., IL-6) (5). On the other hand, earlier initiation of KRT could also result in harm because it will inevitably expose some patients to KRT who will never need it. Premature KRT can lead to catheter-related blood stream infections, bleeding, procedure-related hypotension, and excessive clearance of small molecules that could have unintended and relatively understudied adverse effects (6). Thus, striking the right balance in the timing of KRT initiation is key.
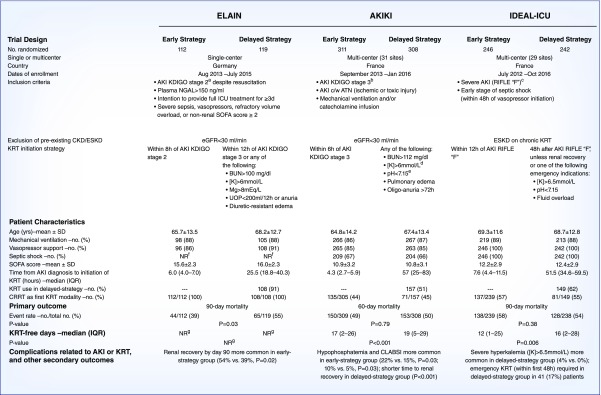
Two RCTs, both published in mid-2016, investigated whether an early KRT initiation strategy improves mortality among critically ill patients with AKI (Figure 1). The Early versus Late Initiation of Renal Replacement Therapy in Critically Ill Patients with AKI (ELAIN) trial (7) was a single-center trial comparing early versus delayed KRT initiation among 231 critically ill patients. Patients were included if they had moderate AKI (Kidney Disease Improving Global Outcomes [KDIGO] stage 2) in addition to at least one of the following: severe sepsis, shock requiring vasopressors, refractory volume overload, or a nonkidney Sequential Organ Failure Assessment score ≥2. Most of the patients were postsurgical, with cardiac surgery accounting for approximately half of the overall population. Patients were randomly assigned to initiate KRT within 8 hours of development of stage 2 AKI (early initiation group), or under either of the following scenarios: within 12 hours of development of stage 3 AKI, or immediately for any urgent indication (delayed initiation group). Patients assigned to the early compared with delayed initiation group had lower 90-day mortality (39% versus 55%; P=0.03).
Figure 1.
Timing of KRT in AKI: placing the IDEAL-ICU trial in context. aDefined as a two-fold increase in SCr or UOP<0.5 ml/kg per hour for ≥12 hours. bDefined as a three-fold increase in SCr, an increase in SCr to ≥4 mg/dl, UOP<0.3 ml/kg per hour for ≥24 hours, or anuria for ≥12 hours. cDefined as a three-fold increase in SCr, an acute increase in SCr ≥0.5 to a value ≥4 mg/dl, UOP<0.3 ml/kg per hour for ≥24 hours, or anuria for ≥12 hours. dOr [K]>5.5 mmol/L despite medical treatment. eIn the context of either pure metabolic acidosis or mixed acidosis. fThe prevalence of septic shock on enrollment was not reported in ELAIN, but only approximately 32% of patients had severe sepsis. gNot reported, but the median duration of KRT was significantly lower in the early-strategy group (9 days [IQR, 4–44 days]) compared with the delayed-strategy group (25 days [IQR, 7 to ≥90 days]; P=0.04). ATN, acute tubular necrosis; CLABSI, central line-associated bloodstream infection; CRRT, continuous RRT; c/w, consistent with; IQR, interquartile range; K, potassium concentration; Mg, magnesium; NGAL, neutrophil gelatinase-associated lipocalin; NR, not reported; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; UOP, urine output.
The Artificial Kidney Initiation in Kidney Injury (AKIKI) trial (8) was a multicenter trial comparing early versus delayed KRT initiation among 619 critically ill patients. Patients were included if they had severe AKI (KDIGO stage 3) consistent with ischemic or toxic acute tubular necrosis and had the need for either mechanical ventilation or vasopressor support. Approximately two thirds of the patients had septic shock on enrollment. Patients were randomly assigned to initiate KRT within 6 hours of development of stage 3 AKI (early initiation group) or immediately for urgent indications (delayed initiation group). Patients assigned to the early compared with delayed initiation group had similar 60-day mortality (49% versus 50%; P=0.79).
The ELAIN and AKIKI trials lead to opposite conclusions. Given the conflicting findings from these two RCTs, results from the recently published Initiation of Dialysis Early versus Late in the Intensive Care Unit (IDEAL-ICU) trial (9) are a welcome addition (Figure 1). IDEAL-ICU was a multicenter trial that sought to enroll 864 critically ill patients in a trial of early versus delayed KRT initiation, which would have made it the largest trial to date to investigate this question. However, the trial only enrolled 488 patients, as it was stopped early for futility. Inclusion criteria for the IDEAL-ICU trial were similar to AKIKI: patients were required to have severe AKI (Risk, Injury, Failure, Loss, and End-Stage Kidney Disease [RIFLE] class F, essentially identical to KDIGO stage 3). Additionally, all patients in IDEAL-ICU were required to have early-stage septic shock (within 48 hours of initiation of vasopressors). Patients were randomly assigned to initiate KRT within 12 hours of development of RIFLE class F AKI (early initiation group) or under either of the following scenarios: after 48 hours of development of RIFLE class F AKI, assuming AKI had not yet recovered, or immediately for any urgent indication for KRT (delayed initiation group). Patients assigned to the early compared with delayed initiation group had similar 90-day mortality (58% versus 54%; P=0.38). A notable limitation is that the median net fluid balance was similar between the early versus delayed groups for the first several days; thus, IDEAL-ICU does not specifically address whether early fluid removal is beneficial in AKI.
What can we learn from these three trials? Specifically, why was ELAIN positive whereas AKIKI and IDEAL-ICU were negative? ELAIN differed from the other two trials in several important ways. First, ELAIN was single-center and had the smallest sample size; thus, its findings have a high index of fragility. Another key difference is that the early initiation group in ELAIN was considerably earlier than the early initiation groups of the other two trials (within 8 hours of AKI stage 2 in ELAIN versus within 6 and 12 hours of AKI stage 3 in AKIKI and IDEAL-ICU, respectively). Additionally, ELAIN included a much higher proportion of surgical patients and a much smaller proportion of patients with severe sepsis or septic shock compared with AKIKI and IDEAL-ICU (in the latter, all patients were required to have septic shock). In ELAIN, refractory fluid overload, including pulmonary edema, was one of four acute severity-of-illness characteristics that allowed entry into the study; in contrast, pulmonary edema was an exclusion criterion in AKIKI. Nonetheless, in ELAIN there was no difference between groups in daily fluid balance during the first 3 days after randomization; thus, early, aggressive ultrafiltration is unlikely to account for the observed benefit of early KRT in ELAIN. In ELAIN, all patients initiated KRT using a continuous KRT modality; in AKIKI and IDEAL-ICU, continuous KRT was used as the initial therapy in only approximately 50% of patients. Patients enrolled in ELAIN had a higher mean Sequential Organ Failure Assessment score compared with the other two trials (Figure 1). As a result of these conflicting findings, larger, more definitive trials are needed. Such trials are ongoing.
The Standard versus Accelerated Initiation of RRT in AKI (STARRT-AKI trial; Clinicaltrials.gov identifier: NCT02568722) is an ambitious and extremely important trial in nephrology that will hopefully shed light on the decades-old question over when to start KRT. STARRT-AKI will test accelerated versus standard initiation of KRT in a pragmatic, multinational, multicenter RCT of 2866 participants, which exceeds by two-fold the total enrollment in ELAIN, AKIKI, and IDEAL-ICU combined. STARRT-AKI is unique in another respect: in addition to formal inclusion and exclusion criteria on the basis of laboratory and clinical parameters, the study team must confirm with the critical care or nephrology attending caring for the patient that clinical equipoise exists, meaning that the patient is neither urgently in need of nor definitely inappropriate for KRT initiation. Participants in STARRT-AKI can have relatively early AKI, like the ELAIN trial, and will include individuals with sepsis as well as postoperative AKI. The AKIKI2 trial (Clinicaltrials.gov identifier: NCT03396757) plans to enroll 810 participants into a “delayed” versus “even more delayed” RCT. In the delayed arm, KRT will be initiated as with the delayed strategy in the original AKIKI trial, whereas the even more delayed arm will commence KRT only because of potentially life-threatening indications, such as severe hyperkalemia, acidemia, pulmonary edema, or BUN>140 mg/dl.
As we await the results of STARRT-AKI and AKIKI2, in our opinion clinicians should consider the delayed strategy as standard of care in most critically ill patients with severe AKI. Additionally, until definitive evidence of benefit for an early initiation strategy is provided, an individualized approach seems warranted. Such an approach would incorporate data on the AKI inciting event, the serum creatinine (and overall) trajectory, the likelihood of renal recovery, the degree of volume overload, and many other clinical factors that are difficult to summarize in a standardized protocol. Other RCTs to improve KRT delivery and outcomes in AKI, such as testing fluid balance goals or use of novel technologies, are also greatly needed, and may represent a promising area of investigation, particularly if early versus late initiation trials continue to fail to provide answers.
In conclusion, it is exciting to see clinicians and investigators committed to developing a solid evidence base for one of the most subjective decisions in all of nephrology. The real “IDEAL” trial, of course, would be one identifying a drug or treatment that prevents clinicians from having to make the decision to dialyze in the first place: by preventing AKI.
Disclosures
Dr. Leaf is supported by grants from BioPorto Diagnostics outside the submitted work. Dr. Waikar reports receiving a grant from Allena and has received consulting income from Allena, Cerus, CVS, GSK, Harvard Clinical Research Institute, Janssen, Mass Medical International, Strataca, Takeda, Venbio, and Wolters Kluwer. Dr. Waikar has also provided expert witness consultation for litigation related to Granuflo, Omniscan, statins, cisplatin nephrotoxicity, and mercury exposure.
Funding
Dr. Leaf is supported by National Institute of Diabetes and Digestive and Kidney Diseases (K23DK106448) and an American Society of Nephrology Foundation for Kidney Research Carl W. Gottschalk research scholar grant.
Acknowledgments
The content of this article does not reflect the views or opinions of the American Society of Nephrology (ASN) or the CJASN. Responsibility for the information and views expressed therein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bagshaw SM, Uchino S, Kellum JA, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Bellomo R; Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators: Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care 28: 1011–1018, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P; VA/NIH Acute Renal Failure Trial Network: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF; Hemodiafe Study Group: Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: A multicentre randomised trial. Lancet 368: 379–385, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Mao Z, Kang H, Hu J, Zhou F: Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: A meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care 20: 144, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahaba AA, Elawady GA, Rehak PH, List WF: Procalcitonin and proinflammatory cytokine clearance during continuous venovenous haemofiltration in septic patients. Anaesth Intensive Care 30: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Brugnara C, Betensky RA, Waikar SS: Reductions in red blood cell 2,3-diphosphoglycerate concentration during continuous renal replacement therapy. Clin J Am Soc Nephrol 10: 74–79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315: 2190–2199, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D; AKIKI Study Group: Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 375: 122–133, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, Lebert C, Bohé J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network: Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 379: 1431–1442, 2018 [DOI] [PubMed] [Google Scholar]