Abstract
There is a resurgence in clinical adoption of home hemodialysis globally driven by several demonstrated clinical and economic advantages. Yet, the overall adoption of home hemodialysis remains under-represented in most countries. The practicality of managing ESKD with home hemodialysis is a common concern among practicing nephrologists in the United States. The primary objective of this invited feature is to deliver a practical guide to managing ESKD with home hemodialysis. We have included common clinical scenarios, clinical and infrastructure management problems, and approaches to the day-to-day management of patients undergoing home hemodialysis.
Keywords: management; end-stage kidney disease; Hemodialysis, Home; Kidney Failure, Chronic; kidney transplantation
Introduction
Frequent home hemodialysis (HD) has been associated with several clinical benefits documented by observational and randomized, controlled trials, including BP, extracellular volume, and phosphate control and lessened medication burden (1,2). Frequent home HD was associated with a reduction in left ventricular hypertrophy and stabilization of left ventricular ejection fraction (3,4). Observational studies have demonstrated an association between intensive home HD and lower risk for death (5). From a patient experience perspective, patients on home HD have reported higher quality of life and independence (6). Taken together, home HD is considered an economically dominant kidney replacement modality to manage ESKD (7,8). However, practical considerations in the utilization of home HD remain a common concern for most practicing United States nephrologists (9). This review will explore these practical issues and serve to demonstrate an approach to managing ESKD with home HD.
Patient Selection and Training
Patient enrolment and training are the first critical steps in managing ESKD with home HD. Table 1 summarizes reasons to prescribe home HD. Table 2 explores characteristics of patients who are potential home HD candidates. Although registry data have shown that patients on home HD are generally younger with fewer comorbidities, several studies have focused on the potential use of home HD as salvage therapy (10,11). Although about 30% of patients on home HD perform treatment without a partner, for those who do have a partner, the use of caregiver- or skilled worker–assisted home HD also broadened the general applicability of home HD as a dialysis modality to a larger pool of patients (12,13). These studies emphasized the need to improve system factors to enable patients who are intellectually and physically capable of performing self-care and home HD. Schatell (14) identified several patient barriers and highlighted possible solutions that could be enacted in a multidisciplinary manner, which are summarized in Table 3. A salient example includes patients who are frail due to medical issues or immobility, for whom rehabilitation should be attempted first before home HD training. Indeed, in some centers, patients with higher medical complexity have realized benefits from home HD due to the longer duration and increased frequency of treatment in the home setting (15). Timelines for training differ but range between 3 and 8 weeks, with training sessions done at a specified home HD unit with trained and motivated specialized nurse educators. Patients are selected from a variety of sources, including predialysis CKD clinics, transplant clinics, and in-center HD and peritoneal dialysis clinics. The prevalence of each group will differ by center, but education and planning should be offered to all groups. There is also an increasing number of electronic tools (e.g., “My life, My Dialysis, My Choice” [http://www.mydialysischoice.org]) that aim to prioritize and match patients’ preference and values with their dialysis modality choice. The motivation to learn and the willingness to train for home HD remain the most important unmeasured characteristic for patient selection.
Table 1.
Proposed practical reasons to prescribe home HD
| Eliminating long interdialytic interval |
| Reducing excessive interdialytic weight gain and intradialytic hypotension while lowering ultrafiltration rate (especially with long dialysis) |
| Controlling BP and phosphate control with reduced medication |
| Preventing myocardial stunning, stabilizing LV function, and decreasing LV mass |
| Improving quality of life, chance of successful pregnancy, and lower death risk |
HD, hemodialysis; LV, left ventricular.
Table 2.
Potential candidates for home hemodialysis
| Motivated patients with ESKD who are capable of self-care |
| Patients who are motivated to work or continue schooling |
| Suitable patients who require home to home transition (from peritoneal dialysis to home hemodialysis) |
| Patients with ESKD and the following clinical conditions |
| (1) Obstructive sleep apnea |
| (2) Difficult to control hypertension |
| (3) Intradialytic hemodynamic compromise (e.g., hypotension or cramps) |
| (4) Refractory volume overload (associated with decompensated right heart failure or uncontrolled ascites) |
| (5) Prolonged recovery time and/or inadequate uremia control (e.g., uremic cardiomyopathy) |
| Women with ESKD who are planning to conceive |
Table 3.
Suggested strategies to enhance patient suitability for home hemodialysis
| Factor | Intervention |
|---|---|
| Poor personal hygiene | Hygiene education and dialysis partner training |
| Frail/nonambulatory | Physiotherapy, occupational therapy, and dialysis care partner training |
| Illiterate | Multimedia training |
| Hearing impaired | Vibration/light alarms |
Studies have also focused on various aspects of patient training. The use of the Visual, Aural, Read/write, and Kinesthetic sensory learning style assessment (the VARK tool) has classified patient learning style preferences and observed an association between visual learners and reduced hazards for adverse events (such as bacteremia) (16). Although most home HD centers have designed a training curriculum (averaging between 3 and 8 weeks), the verification of actual acquisition of knowledge and competency is not always easily demonstrated. Efforts using standardized examinations and high-fidelity simulation have also tried to test the validity of knowledge transmission and have been linked to a reduced need for home visits after graduation to home HD.
Home preparation is an important logistical step in patient training, and it involves relevant plumbing and electrical changes to accommodate the dialysis machine and water treatment system. This step is usually undertaken during the training process to prepare the patient for transition to the home environment. Water testing is included in patient training curricula in some centers, with emphasis on technical support and backup for any patient issues.
A home visit generally addresses the practicality of a patient performing treatment ranging from hygienic issues and technique audit/coaching to assessing the home environment for logistic and supply storage issues. As nursing educators teach and interact with patients, the relationship fostered is also inherently one of psychosocial support for patients, and at times, a home visit can serve that purpose as well. There are no specific standardized criteria or recommended frequencies of home visits, with some centers prioritizing initial home visits after training and others having continuing visits on an annual or semiannual basis.
Access and Needling Considerations
Vascular access is vital to the success of all HD modalities. Given the complexity of home HD training, some patients may initiate with a permanent cuffed central venous catheter (CVC). Recently, Rivara et al. (17) have shown that, in patients who started home HD with a CVC, >50% had switched to a permanent arteriovenous (AV) access after 1 year. From a pragmatic perspective, it is likely more important to capitalize on the patient’s motivation to pursue home HD. Ideally, with appropriate planning and predialysis follow-up, AV access (both grafts and fistulas) should be sought; however, at times, because of failure of maturation/difficulty with needling by experienced cannulators, CVC is used to start home HD training. In select patients, such as those in whom an appropriate AV access would not be possible (elderly with poor access, patients who are vasculopathic, and those with decompensated congestive heart failure), CVC may be the most suitable option and would not preclude such patients from home HD.
Cannulation is taught to patients or primary caregivers by highly trained clinical staff. Instruction checklists, videos, and demonstrations are used for teaching purposes of cannulation. There is, predictably, concern over the risk of infection with repeated use of AV access in the home setting (18). Because this presents a safety issue, concerted effort is placed on hygiene, appropriate use of antimicrobial topical treatment (e.g., Mupirocin), and cannulation technique in education for patients on home HD. Two techniques are taught: button hole and rope ladder technique. Use of rope ladder technique is associated with lower infection risk, but it is balanced against perceptions by some of patient comfort, increased risk of infiltration, increased hematomas, and needling attempts (19). For patients with AV fistulas of adequate length and superficial depth, those with poor vision or tremor, and those with AV grafts, rope ladder is preferred. For patients with short, tortuous, or aneurysmal AV fistulas, button hole may be considered. Caution is particularly noted for button hole for patients with a history of access infections or prostheses, including mechanical heart valves (20). Equally important, it must be noted that button hole cannulation should not be used in patients with AV graft.
In some centers, patients performing home HD nocturnally are encouraged to use intravenous needles with cannula (such as Supercath Clampcath Needles or Nipro Biohole Cath) due to the risk of infiltration with sharp needles. Major issues with needling and strategies for resolving them are summarized in Table 4.
Table 4.
Challenges with needling and potential solutions
| Needling Issue | Possible Intervention |
|---|---|
| Painful needling | Warm compresses and topical anesthetic |
| Fear of needles | Stepwise approach of observing needling in other patients, themselves, and holding needles |
| Difficulty cannulating | Review of technique in home hemodialysis unit, auditing technique, and examining access/button hole sites if used |
Regardless of technique, auditing patients’ cannulation procedure along with their adherence to aseptic and infection precautions may aid in decreasing infection rates. Examples of such audits and checklists have been used by Rousseau-Gagnon et al. (21). Equally important, patients should be trained to recognize signs and symptoms of infection by the home HD staff to expedite diagnosis and treatment of access-related infections appropriately. In patients using button hole, superficial cellulitis with no fever should be treated with oral antibiotics for a 2-week course, with relocation of the button hole site. Deeper abscesses and bacteremia are treated for a longer course (4–6 weeks with intravenous antibiotics), with surgical intervention as needed (22). If patients recognize any signs of infection, they are coached to contact their home HD unit to arrange follow-up, ensure appropriate infectious workup, and obtain parenteral antibiotics with dialysis.
Risk factors for noninfectious access complications have been examined previously. History of kidney transplantation, use of AV grafts, and vintage of access were significant predictors of access dysfunction. Interventions and surveillance are not standardized, but some centers monitor AV access annually and as needed intermittently with AV access flow measurement. Ultimately, each center should decide on how best to monitor access surveillance, because evidence on optimal monitoring in home HD is lacking.
Dialysis Machines
Intuitively, a dialysis machine that is easier to operate is ideal for home HD, but ultimately, any available HD machine can be used to provide home HD (23). Size of machine as well as water and dialysate delivery are features that may differentiate between platforms. From a home environment perspective, dialysis machine size and footprint are important features in determining its suitability. Other features, such as reliability, lower sound level, simplicity, and accessibility, are desirable properties for a home machine. Conventional technologies allow nontraditional/nonmunicipal water to be used (i.e., wells and septic fields). Water purification systems are otherwise installed in the home by technicians. Table 5 summarizes different categories of available machines, with salient features for each platform. In North America, the Food and Drug Administration has approved Fresenius and NxStage systems for home HD use, with NxStage being approved for both solo short daily and nocturnal use.
Table 5.
Types of hemodialysis machines used at home
| Features | |
|---|---|
| Single-pass systems | Most commonly used system globally, with onscreen instructions and fail-safe alarms |
| Bigger, more complex, requiring maintenance, cleaning of components within the circuit | |
| Plumbing and electric modification may be needed | |
| Low-flow systems | Proprietary dialysis fluid bags, travel friendly but need to travel or ship fluid, much like peritoneal dialysis |
| Option for online dialysate generator, not portable | |
| Machine portable, no wet circuit, single cartridge that patient engages as a singular step, with all pathways working automatically | |
| Maximum dialysate 60 L and singular cartridge dialyzer option, limits individualized prescription |
It is noteworthy to comment that other technologies are being developed for home HD. Sorbent dialysis, which had been previously used in the 1970s and 1980s, is currently being redesigned for the home environment (24). This system uses a sorbent column to adsorb uremic retention solutes. Ion exchangers are used to provide the appropriate biochemistry to the patient. A catalytic converter may be used to regenerate the appropriate cartridge system(s). Other systems may use a traditional single-pass system but with integrated or portable water treatment. Finally, hemodiafiltration systems combining diffusive and convective principles with the use of ultrapure water are being explored for use in Europe and Australia (23).
Treatment Schedule, Dialysis Prescription, and Clinical Considerations
Home HD prescriptions have a high degree of variability globally. Outside of the United States, patients on home HD use both traditional single-pass system machines designed for conventional HD and low-flow devices. In the United States, there is an overall higher prevalence of low-flow system machines (25).
It is important to note that home HD prescriptions should strive for optimal dialysis delivery rather than pursuing adequacy for patients with ESKD (26). Optimal dialysis, as the main driver of the home HD prescription, should encompass factors, including solute balance, quality of life, symptom control, BP and volume control, cardiac structural and functional status, nutrition and mineral balance, and overall survival.
Simplistically, one can modify home HD prescription by changing dialysis frequency, duration, or both. More frequent home HD (five to six sessions per week) has been demonstrated to correlate with (1) BP control with less vasoactive medications requirement, (2) regression of left ventricular hypertrophy, and (3) phosphate control with more liberalization of diet (27–31). Of note, pregnant patients have a unique set of dialysis prescription requirements. The advent of the nocturnal HD regimen (five to six sessions per week for 6–8 hours per session) has been associated with higher live birth rate, gestational age, and birth weight compared with standard HD parameters (32,33). Their requirements are summarized in Table 6. Alternate HD (four sessions per week) done either nocturnally or during the day has also increased in popularity. Logistically, alternate day 4-day diurnal therapy and long alternate HD mitigate the “long” interdialytic break and provide a mechanism to deliver moderate ultrafiltration rate. However, the availability of evidence in support of this mode of home HD is relatively scarce and warrants further attention. Short daily home HD using standard blood and variable dialysis flows is presently the most common home HD modality in the United States. Indeed, there is an emerging body of observational literature in support of this modality, including cardiovascular health, hospitalization, and overall survival (34–37).
Table 6.
Home hemodialysis parameters
| Modality | Treatments per Week | Sessional Duration, h | Blood Flow Rate, ml/min | Dialysate Flow Rate, ml/min | Calcium, mmol/L | Phosphate Additive |
|---|---|---|---|---|---|---|
| Conventional | 3 | 3–5 | 300–400 | 500–800 | 1.25 | None |
| Alternate nightly | 3.5 | 6–8 | 250–350 | 300–500 | 1.25 | Rare |
| Short daily | 5–6 | 2–3.5 | 350–400 | 500–800 | 1.25 | None |
| Nocturnal | 4–6 | 6–8 | 250–350 | 300 | 1.5–1.75 | 20%–30% |
| Low-flow dialysate, short daily | 5–6 | 2.5–4 | 300–400 | 90–300 | 1.5 | None |
| Low-flow dialysate, nocturnal | 3.5–6 | 6–8 | 300–350 | 83–166 | 1.5 | None |
Recently, the focus on goal-directed dialysis has gained more attention. To this aim, one may want to define the patient’s priority while undertaking home HD. For instance, if patients are pursuing a palliative approach, it is plausible that an incremental approach may be more than appropriate. Taken together, the spectrum of home HD prescription offers the flexibility and plausibility to titrate the dose to the targeted clinical outcome.
Patient Safety Issues
Although home HD offers flexibility in dialysis dosing and schedules, there is an innate tradeoff between dialysis provision, quality, and safety. Indeed, Tong et al. (38,39) identified several apprehensions relating to independently performing complex medical treatments at home and the possibility of managing life-threatening complications. Consequently, a thriving home HD program should foster a culture of safety and quality to all stakeholders. Several fundamental principles have been proposed (40,41).
(1) The home environment should be conducive to patient safety at all times. This includes ensuring that the patient can access all lines and monitors quickly, with wetness detectors placed under the dialysis machine and commercially available blood leakage alarms.
(2) From a dialysis prescription perspective, safeguards should be set to limit total daily fluid removal. Appropriate access alarm ranges should be set to alert potential access malfunctions.
(3) Clinical and technical assistance should be accessible to alleviate potential patient anxieties, especially in the initial phase of therapy. This may include an on-call nurse/technologist who can address any immediate concerns during the dialysis session.
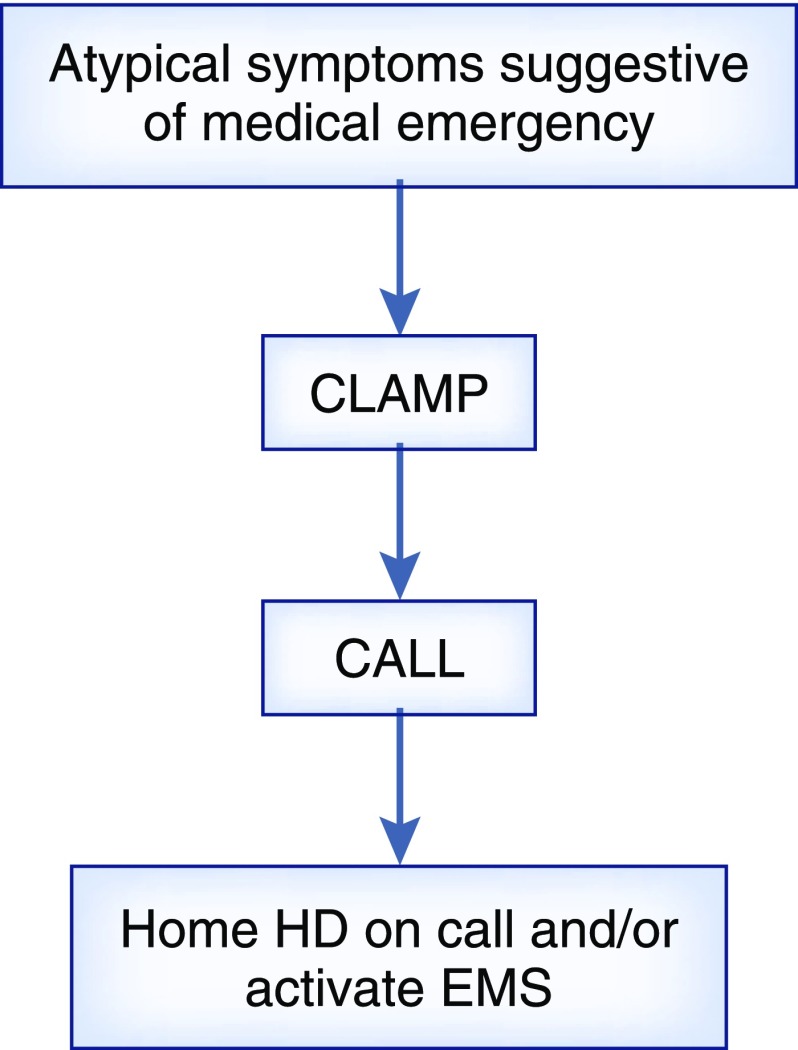
(4) A simplified approach to managing complications should be taught, such as the “clamp and call” mechanism as outlined in Figure 1. This is a strategy that may be used for intradialytic complications, such as blood loss.
(5) All home HD units should also monitor appropriate quality metrics, such as
(a) prevalent patient count,
(b) training success rate,
(c) technique survival rate,
(d) hospitalization (cardiovascular and infectious) rate,
(e) access-related infection,
(f) patient attrition rate (and reason), and
(g) noninfectious access complications.
Adverse events in home HD have been reported (42–44). In most patients, serious adverse events are rare, which is likely a function of training and reinforcement of best practices with patients. Invariably, most adverse events are driven by human error, and they are often related to vascular access (cannulation, connection, and disconnection). This highlights the need for constant support, retraining, auditing of techniques, and creating a “blameless” environment where patients are encouraged to report all events.
Figure 1.
In the work by Pauly et al. (41), the clamp and call approach is used to manage complications experienced in home hemodialysis (HD). Atypical symptoms include chest pressure, palpitations, neurologic changes, and presyncope. EMS, emergency medical services.
The provision of respite care and dialysis is often suggested to alleviate caregiver burden. To date, there is a paucity of data to document the actual effect of caregiver assistance. Similarly, psychologic support through social work and psychiatry involvement is used in some programs to address mental health issues. Presently, there is no standard approach for social support for patients on home HD. Given the high attrition rate of home HD, strategies to enhance technique survival are urgently warranted. Proper resources at the unit level are necessary, and future progress in training, technology, telehealth, and transitional care is desired to grow all home therapies.
Finally, as technology continues to evolve, the use of remote monitoring of patients on home HD may also become a potential pathway to offer an enhanced safety environment. Growth in telehealth may be associated with reduction of burden, decreasing training time and improving retention (45). However, the logistics, cost, and balance between patient intrusion and safety will require further investigation (46–48).
Infrastructure and Perspective
We have tried to outline several important practical considerations in the day-to-day management of ESKD with home HD. The most important component of any home dialysis management is, in fact, the team infrastructure. Prior published literature has already substantiated the value of a high-performing home dialysis center with adequate critical mass of patients and highly trained staffs (10). Indeed, technique survival is higher with larger home dialysis centers (in both home HD and peritoneal dialysis). Appropriate staffing ratios for nursing and allied health have been previously published (49). Most notably, we have also highlighted several clinical domains starting from patient selection and enrollment to the practice culture of quality and safety.
Moving forward, innovative strategies to enhance clinical delivery and follow-up of patients on home HD will broaden the generalizability of home HD to a wider patient population.
Disclosures
Dr. Chan reports grants from the Medtronic Investigator-Initiated Grant Program and other funding from Baxter and NxStage outside the submitted work. Dr. Ibrahim has nothing to disclose.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Marshall MR, Hawley CM, Kerr PG, Polkinghorne KR, Marshall RJ, Agar JW, McDonald SP: Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis 58: 782–793, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Bakris GL, Burkart JM, Weinhandl ED, McCullough PA, Kraus MA: Intensive hemodialysis, blood pressure, and antihypertensive medication use. Am J Kidney Dis 68[5S1]: S15–S23, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Trinh E, Chan CT: Intensive home hemodialysis results in regression of left ventricular hypertrophy and better clinical outcomes. Am J Nephrol 44: 300–307, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Chan C, Floras JS, Miller JA, Pierratos A: Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 17: 1518–1521, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Nesrallah GE, Lindsay RM, Cuerden MS, Garg AX, Port F, Austin PC, Moist LM, Pierratos A, Chan CT, Zimmerman D, Lockridge RS, Couchoud C, Chazot C, Ofsthun N, Levin A, Copland M, Courtney M, Steele A, McFarlane PA, Geary DF, Pauly RP, Komenda P, Suri RS: Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol 23: 696–705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO: Hemodialysis and peritoneal dialysis: Patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol 1: 1191–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 7.McFarlane PA, Bayoumi AM, Pierratos A, Redelmeier DA: The quality of life and cost utility of home nocturnal and conventional in-center hemodialysis. Kidney Int 64: 1004–1011, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Klarenbach S, Tonelli M, Pauly R, Walsh M, Culleton B, So H, Hemmelgarn B, Manns B: Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. J Am Soc Nephrol 25: 587–594, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karopadi AN, Mason G, Rettore E, Ronco C: The role of economies of scale in the cost of dialysis across the world: A macroeconomic perspective. Nephrol Dial Transplant 29: 885–892, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Moran J, Kraus M: Starting a home hemodialysis program. Semin Dial 20: 35–39, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Wong JH, Pierratos A, Oreopoulos DG, Mohammad R, Benjamin-Wong F, Chan CT: The use of nocturnal home hemodialysis as salvage therapy for patients experiencing peritoneal dialysis failure. Perit Dial Int 27: 669–674, 2007 [PubMed] [Google Scholar]
- 12.Tennankore KK, Kim SJ, Chan CT: The feasibility of caregiver-assisted home nocturnal hemodialysis. Nephron Clin Pract 122: 17–23, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Pierratos A, Tremblay M, Kandasamy G, Woodward G, Blake P, Graham J, Hebert M, Harvey R: Personal Support Worker (PSW)-supported home hemodialysis: A paradigm shift. Hemodial Int 21: 173–179, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Schatell D: MATCH-D: A roadmap to home dialysis therapy. Nephrol News Issues 21: 41, 43–44, 2007 [PubMed] [Google Scholar]
- 15.Rioux JP, Faratro R, Chan CT: Nocturnal home hemodialysis: Implementation, quality assurance and future challenges. Minerva Urol Nefrol 62: 103–110, 2010 [PubMed] [Google Scholar]
- 16.Auguste BL, Al-Muhaiteeb A, Chan CT: The effect of learning styles on adverse events in home hemodialysis patients. Clin J Am Soc Nephrol 13: 782–783, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivara MB, Soohoo M, Streja E, Molnar MZ, Rhee CM, Cheung AK, Katz R, Arah OA, Nissenson AR, Himmelfarb J, Kalantar-Zadeh K, Mehrotra R: Association of vascular access type with mortality, hospitalization, and transfer to in-center hemodialysis in patients undergoing home hemodialysis. Clin J Am Soc Nephrol 11: 298–307, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suri RS, Li L, Nesrallah GE: The risk of hospitalization and modality failure with home dialysis. Kidney Int 88: 360–368, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR: A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol 7: 1632–1638, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesrallah GE, Mustafa RA, MacRae J, Pauly RP, Perkins DN, Gangji A, Rioux JP, Steele A, Suri RS, Chan CT, Copland M, Komenda P, McFarlane PA, Pierratos A, Lindsay R, Zimmerman DL: Canadian Society of Nephrology guidelines for the management of patients with ESRD treated with intensive hemodialysis. Am J Kidney Dis 62: 187–198, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Rousseau-Gagnon M, Faratro R, D’Gama C, Fung S, Wong E, Chan CT: The use of vascular access audit and infections in home hemodialysis. Hemodial Int 20: 298–305, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Ball LK: The buttonhole technique: Strategies to reduce infections. Nephrol Nurs J 37: 473–477, 2010 [PubMed] [Google Scholar]
- 23.Marshall M, Chan C: Implementing Hemodialysis in the Home: A Practical Manual, International Society for Hemodialysis, 2016. John Wiley & Sons, Inc., Hoboken, NJ [Google Scholar]
- 24.Agar JW: Review: Understanding sorbent dialysis systems. Nephrology (Carlton) 15: 406–411, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Lockridge RS Jr, Pipkin M: Short and long nightly hemodialysis in the United States. Hemodial Int 12[Suppl 1]: S48–S50, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Twardowski ZJ: We should strive for optimal hemodialysis: A criticism of the hemodialysis adequacy concept. Hemodial Int 7: 5–16, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Jun M, Jardine MJ, Gray N, Masterson R, Kerr PG, Agar JW, Hawley CM, van Eps C, Cass A, Gallagher M, Perkovic V: Outcomes of extended-hours hemodialysis performed predominantly at home. Am J Kidney Dis 61: 247–253, 2013 [DOI] [PubMed] [Google Scholar]
- 28.van Eps CL, Jeffriess L, Haluska B, Hawley CM, Coombes J, Matsumoto A, Jeffries JK, Johnson DW, Campbell SB, Isbel NM, Mudge DW, Marwick T: Cardiac and vascular structure and function parameters do not improve with alternate nightly home hemodialysis: An interventional cohort study. BMC Nephrol 12: 51, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eps CL, Jeffries JK, Anderson JA, Bergin PT, Johnson DW, Campbell SB, Carpenter SM, Isbel NM, Mudge DW, Hawley CM: Mineral metabolism, bone histomorphometry and vascular calcification in alternate night nocturnal haemodialysis. Nephrology (Carlton) 12: 224–233, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Van Eps CL, Jeffries JK, Johnson DW, Campbell SB, Isbel NM, Mudge DW, Hawley CM: Quality of life and alternate nightly nocturnal home hemodialysis. Hemodial Int 14: 29–38, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS; FHN Trial Group: In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig KL, Podymow T, Pauly RP: Intensifying renal replacement therapy during pregnancy: The role for nocturnal home hemodialysis. Int Urol Nephrol 42: 137–139, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Hladunewich MA, Hou S, Odutayo A, Cornelis T, Pierratos A, Goldstein M, Tennankore K, Keunen J, Hui D, Chan CT: Intensive hemodialysis associates with improved pregnancy outcomes: A Canadian and United States cohort comparison. J Am Soc Nephrol 25: 1103–1109, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, Daugirdas JT: Daily hemodialysis: A systematic review. Clin J Am Soc Nephrol 1: 33–42, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Culleton BF, Asola MR: The impact of short daily and nocturnal hemodialysis on quality of life, cardiovascular risk and survival. J Nephrol 24: 405–415, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, Kendrick CA, James SH, Miller BW, Schulman G, Salusky IB, Kliger AS; Frequent Hemodialysis Network (FHN) Trial Group: Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol 23: 727–738, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, Chertow GM, Ornt DB, Raimann JG, Larive B, Kliger AS; FHN Trial Group: Effect of frequent hemodialysis on residual kidney function. Kidney Int 83: 949–958, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong A, Palmer S, Manns B, Craig JC, Ruospo M, Gargano L, Johnson DW, Hegbrant J, Olsson M, Fishbane S, Strippoli GF: The beliefs and expectations of patients and caregivers about home haemodialysis: An interview study. BMJ Open 3: e002148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong A, Palmer S, Manns B, Craig JC, Ruospo M, Gargano L, Johnson DW, Hegbrant J, Olsson M, Fishbane S, Strippoli GF: Clinician beliefs and attitudes about home haemodialysis: A multinational interview study. BMJ Open 2: e002146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley CM, Jeffries J, Nearhos J, Van Eps C: Complications of home hemodialysis. Hemodial Int 12[Suppl 1]: S21–S25, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Pauly RP, Eastwood DO, Marshall MR: Patient safety in home hemodialysis: Quality assurance and serious adverse events in the home setting. Hemodial Int 19[Suppl 1]: S59–S70, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Wong B, Zimmerman D, Reintjes F, Courtney M, Klarenbach S, Dowling G, Pauly RP: Procedure-related serious adverse events among home hemodialysis patients: A quality assurance perspective. Am J Kidney Dis 63: 251–258, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Allcock K, Jagannathan B, Hood CJ, Marshall MR: Exsanguination of a home hemodialysis patient as a result of misconnected blood-lines during the wash back procedure: A case report. BMC Nephrol 13: 28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murlidharan P, Chan CT, Bargman JM: Catastrophic hypercalcemia as a technical complication in home hemodialysis. NDT Plus 4: 251–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinhandl ED, Collins AJ: Relative risk of home hemodialysis attrition in patients using a telehealth platform. Hemodial Int 22: 318–327, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Marshall MR, Pierratos A, Pauly RP: Delivering home hemodialysis: Is there still a role for real-time treatment monitoring? Semin Dial 28: 176–179, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Cafazzo JA, Leonard K, Easty AC, Rossos PG, Chan CT: Patient perceptions of remote monitoring for nocturnal home hemodialysis. Hemodial Int 14: 471–477, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Hoy C: Remote monitoring in nocturnal home hemodialysis 2003. Hemodial Int 8: 144–150, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Schachter ME, Tennankore KK, Chan CT: Determinants of training and technique failure in home hemodialysis. Hemodial Int 17: 421–426, 2013 [DOI] [PubMed] [Google Scholar]