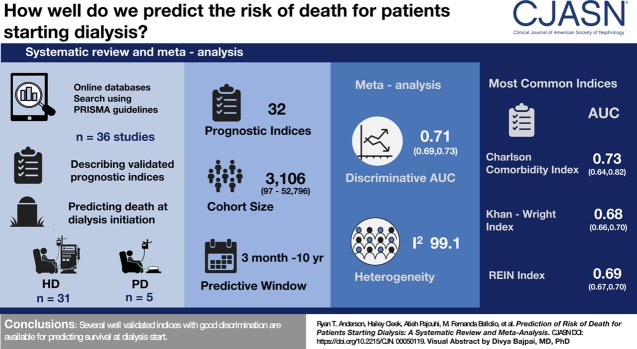
Visual Abstract
Keywords: dialysis, mortality, mortality risk, Prognosis, peritoneal dialysis, Humans, Nephrologists, Renal Dialysis, Prognosis, Patient Selection, Area Under Curve, Cohort Studies, Acute Kidney Injury, Prevalence, Risk, Bias, Comorbidity
Abstract
Background and objectives
Dialysis is a preference-sensitive decision where prognosis may play an important role. Although patients desire risk prediction, nephrologists are wary of sharing this information. We reviewed the performance of prognostic indices for patients starting dialysis to facilitate bedside translation.
Design, setting, participants, & measurements
Systematic review and meta-analysis following the PRISMA guidelines. We searched Ovid MEDLINE, Ovid Embase, Ovid Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus for eligible studies of patients starting dialysis published from inception to December 31, 2018. Selection Criteria: Articles describing validated prognostic indices predicting mortality at the start of dialysis. We excluded studies limited to prevalent dialysis patients, AKI and studies excluding mortality in the first 1–3 months. Two reviewers independently screened abstracts, performed full text assessment of inclusion criteria and extracted: study design, setting, population demographics, index performance and risk of bias. Pre-planned random effects meta-analysis was performed stratified by index and predictive window to reduce heterogeneity.
Results
Of 12,132 articles screened and 214 reviewed in full text, 36 studies were included describing 32 prognostic indices. Predictive windows ranged from 3 months to 10 years, cohort sizes from 46 to 52,796. Meta-analysis showed discrimination area under the curve (AUC) of 0.71 (95% confidence interval, 0.69 to 073) with high heterogeneity (I2=99.12). Meta-analysis by index showed highest AUC for The Obi, Ivory, and Charlson comorbidity index (CCI)=0.74, also CCI was the most commonly used (ten studies). Other commonly used indices were Kahn-Wright index (eight studies, AUC 0.68), Hemmelgarn modification of the CCI (six studies, AUC 0.66) and REIN index (five studies, AUC 0.69). Of the indices, ten have been validated externally, 16 internally and nine were pre-existing validated indices. Limitations include heterogeneity and exclusion of large cohort studies in prevalent patients.
Conclusions
Several well validated indices with good discrimination are available for predicting survival at dialysis start.
Introduction
Dialysis is a lifesaving therapy for patients with ESKD, but its utility is increasingly questioned for frail and multimorbid patients (1,2), who experience rapid functional decline (3) and high symptom burden (4). Qualitative studies and surveys report suffering and decisional regret (5,6), yet patients are reluctant to discontinue dialysis treatments (7). Ideally, clinicians counseling patients with ESKD would present patients with all treatment options and likely outcomes before dialysis initiation (8) and use shared decision making focused on patients’ goals and preferences (8).
Prognostic information is an essential component of shared decision making (9), and dialysis patients desire this information (5,10). Nephrologists feel unprepared for these discussions (10,11) and avoid giving prognostic information even when asked (10). Consequently, attempts to help patients choose a treatment usually do not include prognostic information (12,13). As a result, patients may assent to dialysis without understanding the risks and benefits, a failure of both shared decision making and respect for patient autonomy (7,14). Both existing guidelines and the American Society of Nephrology Choosing Wisely campaign emphasize that individualized prognostic information should be included in the decision to initiate dialysis (15,16).
The objective of our study was to perform a systematic review and meta-analysis to identify validated prognostic indices that predict mortality for patients with ESKD starting dialysis. At this crucial decision point, there may be value in identifying patients at higher mortality risk for goals of care discussion and patients with better prognosis for kidney transplantation. A scoping review was done as part of the Kidney Disease Improving Global Outcomes (KDIGO) work on kidney palliative care but, to our knowledge, there has been no systematic review of the availability and performance of prognostic indices applied at dialysis start (17).
Materials and Methods
Protocol
We developed a protocol before the review was initiated, and incorporated feedback from content experts. We adhered to the recommendations made in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (18), except for registration of our protocol.
Eligibility Criteria
We included original research articles and observational studies that described validated prognostic indices for patients with incident ESKD, with the outcomes of patient death and at least 3 months of prognostication.
We excluded studies not specific to CKD or ESKD, studies that were limited to patients with AKI in hospital or intensive care unit, studies limited to prevalent dialysis patients, studies that excluded early death (usually first 3 months), and studies where an index was just used as a variable in a regression model. Letters, editorials, narrative reviews, commentaries, and case reports were excluded but used as sources for references.
Data Sources and Searches
A medical librarian (P.J.E.) searched Ovid MEDLINE, Ovid Embase, Ovid Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus from inception to December 31, 2018. No language restrictions were applied to the search strategy, which used controlled vocabulary and was supplemented with keywords (Supplemental Appendix 1). We also screened reference lists of included articles and included the list of prognostic articles identified by the KDIGO expert review (17).
Study Selection
All titles and abstracts were independently screened by two college and medical students (R.T.A., H.C., A.M., and A.M.M). Each full text was distributed to two coauthors (R.T.A., H.C., B.T., A.H., R.M.G.C., M.A.F., and M.E.W.) for independent determination of inclusion and data abstraction with at least one physician in each pair. The senior author (B.T.) adjudicated for disagreement. Articles in other languages were reviewed by at least one physician at our institution fluent in the language. Abstract authors were noted and monitored intermittently for subsequent publication. Reviewers were not masked to the authors, journals, or results of studies. At this step, the unweighted Cohen κ statistic was used for quantifying the chance-corrected agreement between reviewers.
Data Extraction
Pertinent data were extracted and assessed for quality with the use of standardized predefined forms (Supplemental Appendices 2 and 3), including study design, study setting and population, demographics of the development and validation cohorts.
Metrics of Model Performance
The primary outcome of interest for meta-analysis was discrimination as measured by the c-statistic or area under receiver operating characteristics curve (AUC). Data on calibration was also collected positive predictive values (PPVs), negative predictive values, calibration plots, Hosmer–Lemeshow goodness-of-fit test results, and chi-squared test results. Discrimination refers to the ability to distinguish between persons with a particular characteristic and those without. Calibration refers to the accuracy of a model’s predictions (19,20).
Quality Assessment, Risk of Bias, and Clinical Usefulness
Quality and risk of bias in each study were assessed with the checklist developed by Hayden et al. (21). Clinical usefulness was defined as a combination of utility and usability as described by Tangri et al. (22).
Missing Data
If studies were missing data or the outcomes were unclear, we contacted the authors by email. We received and incorporated an answer from one (23). Missing confidence intervals for the reported c-statistic were calculated where possible and otherwise imputed on the basis of similar studies, as per the Cochrane handbook (24).
Data Synthesis and Statistical Analyses
Weighted averages were calculated for demographic information. Clinical heterogeneity was assessed by determining whether the characteristics of participants, interventions, comparison group, and outcome measures were similar across studies. When data for more than three cohorts was available random effects meta-analysis and Forrest plots were conducted with pooled estimates and associated 95% confidence intervals. Meta-analysis was performed using OpenMeta Analyst software for meta‐analyses following a random‐effects model as described by DerSimonian‐Laird, to account for clinical and methodologic heterogeneity between studies (25). To quantify the degree of statistical heterogeneity, I2 was used.
A preplanned subgroup analysis by prediction index and length of predictive window was performed. We also performed a subgroup analysis by whether the data were from development, internal validation or external validation cohorts. We performed the following additional sensitivity analyses, excluding studies that were not externally validated and excluding the three smallest studies (n<100) and the two outliers in terms of length of prediction (8 and 10 years).
Results
Summary Measures
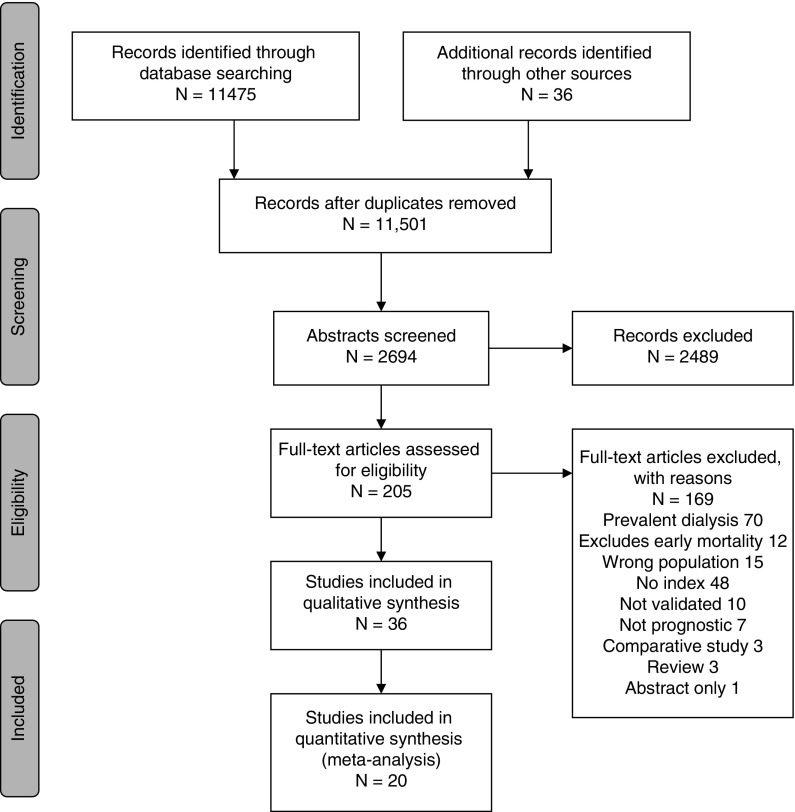
After screening 12,132 titles, we retrieved 214 references for full-text review. We selected 36 articles describing 32 unique indices predicting mortality for patients with incident ESKD (Figure 1). For inclusion of the articles reviewed in full text, the κ score was 0.86. Table 1 shows the included articles, indices used, and performance metrics. Supplemental Table 1 shows a list of articles excluded after full text review.
Figure 1.
Flow diagram of the literature search and selection.
Table 1.
Included studies showing cohort size and characteristics, indices developed, and validated predictive window and performance
| Study, Year | Index | Length | Discrimination | Calibration | Validation Type | Source of Cohort | Cohorta | Mortality | |
|---|---|---|---|---|---|---|---|---|---|
| Number (Female) | Age (SD) | ||||||||
| Barrett et al., 1997 (55) | Foley | 6 mo | NR | NR | N/A | Canadian academic medical centers HD (62%) and PD patients | 822 (40.9%) | 58.3 (15.4) | 13.7% |
| Beddhu et al., 2002 (26) | CCI (Beddhu) | 2 yr | NR | NR | Internal | US academic medical center PD patients | 97 (58%) | 53 (14) | 21.6% |
| Chandna et al., 1999 (61) | Kahn-Wright | 1 yr | 0.70 (NR) | NR | N/A | UK dialysis unit HD (NR) and PD patients | 292 (34%) | 61.3 (15.8) | 44% |
| Cheung et al., 2014 (23) | nCI-Liu | 6 mo | 0.62/0.65b | NR | N/A | USRDS HD patients | 44,109 (46%) | 77.2 (6.5) | 23.3% |
| REIN | 0.63/0.66b | ||||||||
| HEC | 0.65/0.68b | ||||||||
| Cho et al., 2017 (27) | CCI | Avg f/u 47.1 mo | 0.82 | NR | Internal | Korean health insurance database PD patients | 7606 (43.6%)a | 54.5 (13.8) | 39.5% |
| mCCI-IPD | 0.82 | 664 (40.1%) | 48.4 (14.1) | 12.3% | |||||
| mCCI-IHD | 0.82 | 24,738 (40.5%) | 57.9 (NR) | ||||||
| Couchoud et al., 2009 (33) | REIN | 6 mo | 0.70 | Observed versus predicted H-L P=93 | Internal and external | French ERSD all-comers Renal Epidemiology and Information Network HD (NR) and PD patients | 2500 (39.6%)a | 80.9 (4.1) | 19% |
| REIN | 3 mo | 0.74 | 1642 (40.5%) | 81.1 (4.1) | |||||
| REIN | 12 mo | 0.68 | |||||||
| Couchoud et al., 2015 (37) | Updated REIN | 3 mo | 0.76 | NR | Internal | French dialysis centers REIN HD (NR) and PD patients | 12,500 (39.6%)a | NR | 10.5% |
| 3 mo | 0.75 | 11,848 (NR) | NR | ||||||
| 3 mo | 0.79 | 2490 (NR) | NR | ||||||
| Davies et al., 2002 (34) | Davies | 2 yr | NR | NR | External | UK dialysis center Stoke PD study PD patients | 303 (NR) | 58.8 (NR) | 35% |
| Kahn-Wright | 5 yr | ||||||||
| Di Iorio et al., 2004 (28) | CCI (Di Iorio) | 15 mo | NR | NR | Internal | Italian dialysis centers HD patients | 515 (38.6%) | 63.2 (15.4) | 14.6% |
| Doi et al., 2015 (43) | Doi | 1 yr | 0.83 | Observed versus predicted | Internal | Japanese dialysis centers HD patients | 688 (33.4%) | 69 (NR) | 9% |
| Fernandez Lucas et al., 2007 (38) | ACPI | 3 yr | 0.75 | NR | External | Spanish dialysis center HD (80%) and PD patients | 304 (37%) | 64 (15) | 31% |
| CCI | 0.76 | ||||||||
| CCI-H | 0.71 | ||||||||
| Floege et al., 2015 (45) | AROii | 3 mo | 0.71 | Observed versus predicted 1-yr R2= 0.94-yr R2= 0.98 | Internal | Development: AROii study population Validation: DOPPS III study population HD patients | 9277 (40.2%)a | 64.4 (14.7) | 17.6% |
| AROii | 1 yr | Range 0.75–0.76 | 4247 (NR) | NR | |||||
| AROii | 2 yr | Range 0.73–0.75 | |||||||
| Kahn-Wright | 2 yr | 0.66 | |||||||
| nCI-Liu | 2 yr | 0.60 | |||||||
| Foley et al., 1994 (39) | Foley | 6 mo | 0.90 | Observed versus predicted histogram | External | Canadian hospital HD patients | 325a(35%) | 55 (NR) | 22.4% |
| Fried et al., 2001 (35) | CCI | 3 yr | NR | NR | External existing index | US dialysis centers PD patients | 268 (35.1%) | 56.0 (14.6) | 20.5% |
| Fried et al., 2003 (36) | CCI | N/A | NR | NR | External existing indices | US dialysis centers PD patients | 415 (37.8%) | 55.9 (14.8) | NR |
| Davies | |||||||||
| SF-36 MCS | |||||||||
| Karnofsky scale | |||||||||
| Geddes et al., 2006 (44) | RBM | 1 yr | NR | Observed versus expected bar graph | Internal | European dialysis centers ERA-EDTA Registry HD (86%) and PD patients | 1139a (42.3%) | 63.4 (13.5) | 12.8% |
| CCI-H | 5 yr | 1171 (39.8%) | 60.4 (15.5) | 27.3% | |||||
| Gomez et al., 2015 (62) | CCI | 6.5 yr | 0.61 | NR | Internal and external existing indices | Canadian academic medical center HD (78%) and PD patients | 771 (38%) | 62.6 (15.1) | 40.3% |
| CCI-H | 0.62 | ||||||||
| Hemmelgarn et al., 2003 (29) | CCI-H | 2 yr | 0.73 | NR | Internal and external modification of a validated scale subsequently validated in several studies | Canadian dialysis centers HD (77%) and HD patients | 237 (36.7%)a | 62.5 (15.2) | 23.6% |
| CCI | 0.72 | ||||||||
| Inaguma et al., 2017 (46) | Barthel | NR | NR | NR | External existing Index | Japanese dialysis centers Aichi Cohort Study of the Prognosis in Patients Newly Initiated into Dialysis cohort HD patients | 1496 (32.3%) | 67.4 (13.0) | 17.5% |
| Ivory et al., 2017 (63) | Ivory | 6 mo | 0.75 | H-L P=21.9 calibration curve | Internal and in separate cohort | Australian and New Zealand dialysis centers Australia and New Zealand Dialysis and Transplant Registry; modality not reported | 23,658 (40.1%)a | 60.6 (14.9) | 6.1% |
| Ivory | 0.71 | 32,664 (NR) | |||||||
| Ivory | 0.76 | 5518 (NR) | |||||||
| REIN | 0.70 | H-L P=15.6 | 5518 (NR) | ||||||
| Wagner | 0.69 | H-L P=6.1 | 5518 (NR) | ||||||
| Wagner | 0.73 | H-L P=29.5 | 32,664(NR) | ||||||
| Kan et al., 2013 (42) | nCI-Liu | 10 yr | 0.91 | NR | Internal and external | Taiwan National Health Insurance Research Database HD (96%) and HD patients | 21,043 (55.6%) | NR | 55.3% |
| CCI | 0.90 | ||||||||
| Khan et al., 1993 (31) | Kahn-Wright | 2 yr | NR | NR | External modification of a validated scale subsequently validated in several studies | UK dialysis centers HD (NR) and PD patients | 375 (42.9%) | 53.9 (18) | 35% |
| Khan et al., 1998 (64) | Kahn-Wright | 2 yr | NR | NR | External existing index | UK dialysis centers HD (NR) and PD patients | 1407 (40%) | 55.6 (15.7) | NR |
| Lee et al., 2017 (65) | Multidimensional frailty core | Avg f/u 18 mo | NR | NR | External existing index | Korean dialysis center HD patients | 46 (37%) | 71.5 (NR) | |
| Lopez Revuelta et al., 2004 (66) | SF-36 PCS | 2 yr | NR | NR | External existing indices | Spanish hospitals HD (80%) and PD patients | 318 (39.6%) | 60.2 (14.6) | 25% |
| SF– 36 MCS | |||||||||
| Karnofsky scale | |||||||||
| Ma et al., 2018 (67) | CCI | 3 yr | NR | NR | External existing indices | Chinese dialysis unit PD patients | 461 (47%) | 57.7 (13.7) | 49.9% |
| CCI-H | |||||||||
| nCI-Liu | |||||||||
| Marinovich et al., 2010 (68) | New index | 1 yr | 0.74 | NR | New index not validated, external validation existing indices | Argentinian dialysis centers National Registry of the Instituto Centro Unico Cordinator de Ablación e Implante de Organos HD patients | 5360 (33.8%) | 58.9 (15.5) | 20.4% |
| CCI | 0.70 | ||||||||
| ACPI | 0.68 | ||||||||
| Kahn-Wright | 0.67 | ||||||||
| CCI-H | 0.63 | ||||||||
| Mauri et al., 2008 (40) | RMRC | 1 yr | 0.78 | Observed versus predicted H-L P=49 | Internal and external | Spanish dialysis centers Catalan Renal Registry HD patients | 3445 (37.8%)a | 64.6 (14.4) | 16.5% |
| 2283 (NR) | |||||||||
| Nicolucci et al., 1992 (69) | ICED | 1,2 and 5 yr | 0.594 | External existing index | Italian dialysis center HD (61%) and PD patients | 255 (43.5%) | 54 (NR)c | 16%, 27% and 49% at 1, 2 and 5y | |
| Obi et al., 2018 (49) | Obi low GFR | 3,6,9 and 12 mo | 0.71 | Observed versus predicted | Internal and in separate cohorts | Development: US Veterans Affairs dialysis cohort; Validation: US health system; Data from USRDS HD (94%) and PD patients | 15,142 (2%)a | 69.4 (11.1) | 20.0% |
| 7463 (1.9%) | 68 (11) | ||||||||
| Obi high GFR | 0.66 | 8888 (1%)a | 72 (10) | 39.8% | |||||
| 4385 (1%) | 72 (10) | ||||||||
| Obi low GFR men only | 0.77 | 1872 (0%) | 64 (13) | ||||||
| Obi low GFR women only | 0.74 | 1491 (100%) | 65 (15) | ||||||
| Obi high GFR men only | 0.71 | 570 (0%) | 66 (13) | ||||||
| Obi high GFR women only | 0.67 | 351 (100%) | 65 (14) | ||||||
| Otero-López et al., 2012 (70) | RMRC score | 1 yr | 0.59 | Observed versus predicted | External existing indices | Spanish hospital HD (89%) and PD patients | 63 (40%) | 80.4 (3.9) | 20.6% |
| REIN | 6 mo | 0. 68 | NR | 27% | |||||
| Peeters et al., 2016 (30) | Abbreviated REIN | 3 mo | 0.74 | NR | Internal modified existing index | Belgian dialysis centers Neederlandstalige Vereniging voor Nefrologie HD (87%) and PD patients | 2679 (43.3%) | 67.6 (14.3) | 19.6% |
| 6 mo | 0.74 | ||||||||
| 12 mo | 0.74 | ||||||||
| Postorino et al., 2007 (50) | NYHA | Avg f/u 41 mo | 0.74 | NR | Existing indices | Italian dialysis centers Calabrian Registry of Uremia, Dialysis, and Transplantation HD (82%) and PD patients | 1322 (41.6%) | 60.8 (NR) | 41.7% |
| ESRD-SI (RDSS) | 0.70 | ||||||||
| Kahn-Wright | 0.69 | ||||||||
| Thamer et al., 2015 (47) | Thamer, Simple | 6 mo | 0.72 | NR | Internal | USRDS HD (96%) and PD patients | 52,796 (46.2%)a | 76.9 (6.5) | 20.3% |
| Comprehensive | |||||||||
| REIN | 0.68 | 16,645 (46.9%) | 76.8 (NR) | ||||||
| Wagner | 0.73 | ||||||||
| Van Manen et al., 2002 (71) | New Index | 2 yr | 0.72 | NR | Internal | Dutch dialysis centers HD (61%) and PD patients | 616 (39%) | 59.3 (15.1) | 25% |
| Kahn-Wright | 0.73 | ||||||||
| Davis | 0.74 | 589 (40%) | 59.1 (15.8) | ||||||
| CCI | 0.72 | ||||||||
| Wick et al., 2017 (48) | Wick | 6 mo | NR | H-L P=2 | Internal | Canadian dialysis centers Northern and Southern Alberta Renal Program registries HD (85%) and PD patients | 2199 (39.2%) | 75.2 (6.5) | 17.1% |
NR, not reported; N/A, Not applicable; HD, Hemodialysis; PD, peritoneal dialysis; CCI, Charlson comorbidity index; nCI, new comorbidity index; USRDS, US Renal Data System; REIN, Renal Epidemiology and Information Network; HEC, hospice eligibility criteria; Avg f/u, Average follow up; mCCI-IPD, Modified Charlson comorbidity index - Intermittent Peritoneal Dialysis; mCCI-IHD, Modified Charlson comorbidity index - Intermittent Hemodialysis; H-L, Hosmer–Lemeshow; ACPI, age-comorbidity prognostic index; CCI-H, Charlson comorbidity index Hemmelgarn modification; AROii, Analyzing data, Recognizing excellence, and Optimizing outcomes; DOPPS, Dialysis Outcomes and Practice Patterns Study; SF-36, Short Form 36; MCS, mental component score; RBM, Rule-Based Model; ERA-EDTA, European Renal Association - European Dialysis and Transplant Association; PCS, physical component score; RMRC, Registre de Malalts Renals de Catalunya; ICED, Index of Coexisting Disease; NYHA, New York Heart Association; ESRD-SI, End-Stage Renal Disease Severity Index; RDSS, Renal Disease Severity Score.
Training/development cohort.
Adjusted for age.
Median age.
Synthesis of Results
The 36 studies included a total of 299,373 patients. Sixteen papers developed 18 new indices or modified an existing index in 140,108 patients with an average cohort size of 3.106 (range 97–52,796) and a weighted average age of 69.7 years. Of the 18 new indices, 11 had internal validation and nine compared the performance of their new index to existing ones (Table 1). Five studies modified existing indices to better fit their population or available data (26–30). Thirteen studies were solely validation studies existing indices, often more than one (Table 1). Predicted survival time ranged from 3 months to 10 years. The most commonly used indices were the Charlson comorbidity index (CCI) (ten studies) and its modifications (eight studies), the Renal Epidemiology and Information Network (REIN) score (five studies) and modifications (two studies), and the Kahn-Wright Index (eight studies) (31–33). Five studies were limited to patients on peritoneal dialysis (26,27,34–36). Of the 32 indices reported, nine were preexisting indices from other disease states (Barthel, CCI, Hospice criteria, Karnofsky, New York Heart Association Heart failure score, Frailty score, Short Form 36 physical and mental component scores), nine have been validated externally (age-comorbidity prognostic index, CCI Hemmelgarn, Davies, Foley, Kahn-Wright Index, new comorbidity index (NCI)-Liu, REIN Renalts, Registre Malalts Renals de Catalunya (RMRC), Wagner) (29,31,32,34,37–42), 13 only have internal validation (CCI-Beddhu, CCI-Di Iorio, Doi, mCCI-IPD, mCCI-IHD, REIN updated, Analyzing Data, Recognizing Excellence, and Optimizing Outcomes (AROii), REIN abbreviated, Rule Based Model, Thamer Simple and Comprehensive, Van Manen, Wick), and one was validated in a separate population cohort in the original development study (Ivory). Only five indices in four papers had a c-statistic >0.8, indicating excellent discrimination (27,39,42,43) and most indices had c-statistics between 0.70 and 0.79, suggesting good discrimination; other studies had worse discrimination or did not report it. Eleven studies reported calibration (most by reporting a Hosmer–Lemeshow P value >0.05), but two studies chose a cutoff and calculated sensitivity and specificity of 63%–84% or PPV of 80%–93% for a certain cutoff and age group (23,44), and one study published a nomogram to guide the choice of a clinically meaningful cutoff (45).
Meta-Analysis
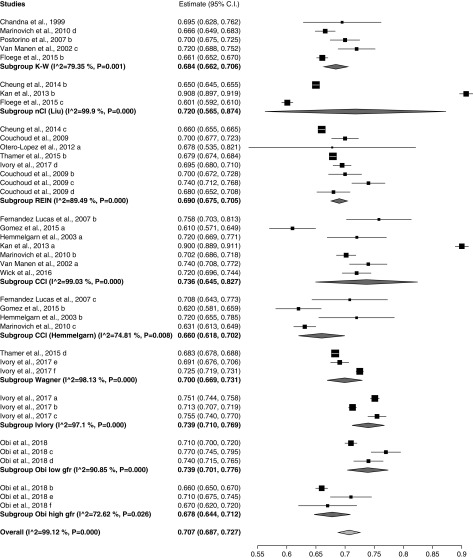
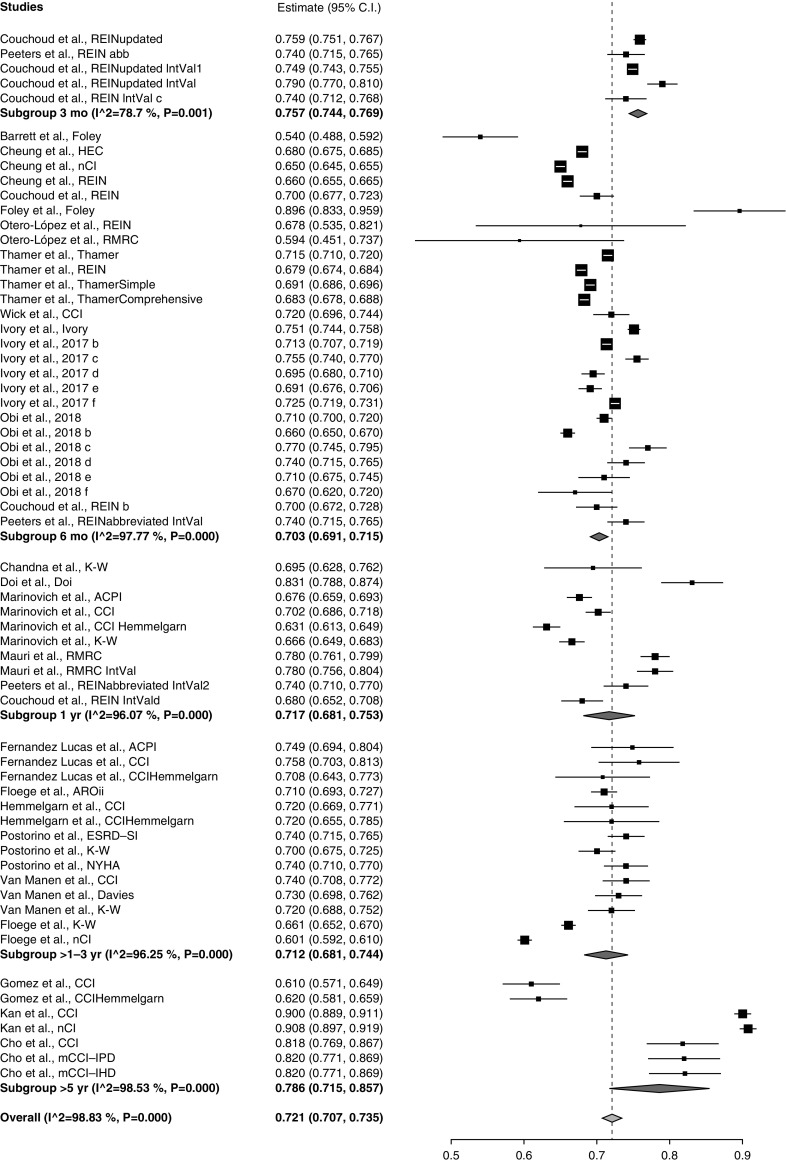
Preplanned meta-analysis of discrimination by index showed that the c-statistic or AUC was highest for the Obi low GFR (0.74; 95% CI, 0.70 to 0.78) and Ivory indices (0.74; 95% CI, 0.71 to 0.77), followed by the CCI (0.74; 95% CI, 0.65 to 0.83) and new comorbidity index (nCI) (0.72; 95% CI, 0.57 to 0.87) (Figure 2). Heterogeneity was high (I2=98.89; P<0.001) for the overall meta-analysis with a c-statistic of 0.71 (0.69 to 0.73). Figure 3 shows comparative meta-analyses of the existing predictors by predictive window showing the best discrimination in the >5 year (AUC, 0.79; CI, 0.72, 0.86) and 3-month windows (AUC, 0.76; 95% CI, 0.74, 0.77). Meta-analysis not surprisingly showed the highest AUC for development cohorts (0.75; 95% CI, 0.77 to 0.78) followed by internal validation (0.74; 95% CI, 0.72 to 0.76), and indices performed worst in external validation (0.7; 95% CI, 0.68 to 0.71) (Supplemental Figure 1). When analyzed by cohort size the largest studies had the best discrimination (Supplemental Figure 2). A separate meta-analysis was done for the five papers that had cohorts with average age >75 years showing worse discrimination (0.69; 0.67 to 0.70), with I2=91.57 (Supplemental Figure 3). Heterogeneity did not decrease when we limited this analysis to only the four papers reporting the performance of the REIN index.
Figure 2.
Meta-analysis of discrimination score of mortality prediction by index for those where three or more results were available. Weights are from random-effects analysis. 95% CI, 95% confidence interval; K-W, Kahn-Wright.
Figure 3.
Meta-analysis by predictive window, categorized to within 3 months, within 6 months, 1–5 years, or >5 years. Weights are from random-effects analysis. 95% CI, 95% confidence interval; ACPI, age-comorbidity prognostic index; AROii, Analyzing Data, Recognizing Excellence, and Optimizing Outcomes; ESRD-SI, End stage Renal Disease Severity Index; HEC, hospice eligibility criteria; K-W, Kahn-Wright; mCCI-IHD, Modified Charlson comorbidity index (CCI) - Intermittent Hemodialysis; mCCI-IPD, Modified CCI Intermittent Peritoneal Dialysis; NYHA, New York Heart Association; RMRC, Registre de Malalts Renals de Catalunya.
Heterogeneity was high for all meta-analyses (Figures 2 and 3, Supplemental Figures 1 and 2). Sources of clinical heterogeneity included variable sample size, demographics, distribution of dialysis type, predictive window, predictive grouping, and predictors used in the different indices (Supplemental Tables 2 and 3, Table 1). Sensitivity analyses omitting the three studies with sample sizes of <100 patients and the two studies with the longest predictive window (8 and 10 years) did not alter results or decrease heterogeneity.
We attempted a meta-analysis of calibration, however only one paper gave the slope for the calibration curve, most of the papers only presented the observed versus expected data in graphs and none gave confidence intervals.
Variables in Death Prediction Indices
The variables or risk predictors used by the prediction indices varied between the indices (Supplemental Tables 2 and 3). Most included age, only two included sex and one included race (41). There was variability in included comorbidities, some including severity weighting. Functional status was used in five studies (33,40,43,45,46). Socioeconomic variables were not used as predictors in any of the studies. Clinical context was included in some indices, i.e., unplanned dialysis initiation (33), temporary vascular access (45), complicated uremia (39), number of hospital days (28), and ventilator dependency (39). Only one index used the type of primary kidney disease as a variable (40).
Risk of Bias Across Studies
Most studies had at least a moderate risk of bias, limiting the level of certainty in the estimates (Table 2). Most indices omitted important risk factors, such as race and socioeconomic status, raising concerns about confounding in the majority of the studies.
Table 2.
Risk of bias of the included studies
| Indices Evaluated | Study Participationa | Study Attritionb | Prognostic Factor Measurementc | Outcome Measurementd | Confounding Measurement and Accounte | Statistical Analysesf |
|---|---|---|---|---|---|---|
| Foley | g | g | h | h | i | h |
| CCI (Beddhu) | g | h | h | g | – | – |
| Kahn-Wright | g | – | g | g | h | g |
| nCI-Liu | g | g | g | g | h | g |
| REIN | ||||||
| HEC | ||||||
| CCI mCCI-IPD mCCI-IHD | g | g | g | g | h | g |
| REIN score | g | h | g | h | – | g |
| REIN | g | g | g | g | h | g |
| Kahn-Wright | g | – | h | g | ? | g |
| Davies | ||||||
| CCI-H | ||||||
| CCI (Di Iorio) | g | h | h | g | – | – |
| Doi | – | – | g | h | h | g |
| CCI | g | h | h | g | – | g |
| ACPI | ||||||
| AROii | g | – | g | g | h | g |
| Foley | g | g | h | h | – | g |
| CCI | g | – | h | h | – | g |
| CCI Davies | g | ? | — | g | h | g |
| CCI-H Rule-based model | g | h | h | h | g | g |
| CCI | – | – | h | – | g | g |
| CCI-H | ||||||
| CCI-H | g | g | h | h | – | g |
| CCI | ||||||
| Barthel | g | g | g | g | – | g |
| Ivory | g | g | g | g | h | g |
| REIN | ||||||
| Wagner | ||||||
| CCI | g | – | g | g | – | g |
| Kahn-Wright | g | ? | h | g | ? | g |
| Kahn-Wright | g | ? | h | g | ? | g |
| Multifactorial Frailty Score | g | g | g | g | — | g |
| SF-36 PCS | — | h | g | g | — | g |
| SF-36 MCS | ||||||
| Karnofsky scale | ||||||
| CCI | _ | ? | h | h | h | g |
| CCI-H | ||||||
| nCI-Liu | ||||||
| CCI | g | g | h | h | – | g |
| ACPI | ||||||
| Kahn-Wright | ||||||
| CCI-H | ||||||
| RMRC score | g | h | h | h | g | g |
| ICED | h | ? | g | g | h | g |
| Obi low GFR | g | g | g | g | h | g |
| Obi high GFR | ||||||
| REIN score | g | g | h | g | – | g |
| RMRC score | ||||||
| REIN | g | ? | h | g | ? | g |
| NYHA class | g | h | – | g | g | g |
| ESRD-SI (RDSS) | ||||||
| Kahn-Wright | ||||||
| Simple | g | h | g | g | g | g |
| Comprehensive | ||||||
| REIN | ||||||
| Wagner | ||||||
| Kahn-Wright | g | – | h | g | h | g |
| Davies | ||||||
| CCI | ||||||
| Wick | g | g | g | g | h | g |
CCI, Charlson comorbidity index; –, does not meet low risk of bias criteria; nCI, new comorbidity index; REIN, Renal Epidemiology and Information Network; HEC, hospice eligibility criteria; mCCI-IPD, Modified CCI Intermittent Peritoneal Dialysis; mCCI-IHD, Modified CCI Intermittent Hemodialysis; ?, unknown; CCI-H, Charlson comorbidity index (Hemmelgarn);ACPI, age-comorbidity prognostic index; AROii, Analyzing data, Recognizing excellence, and Optimizing outcomes; SF-36, Short Form 36; PCS, physical component score; MCS, mental component score; RMRC, Registre de Malalts Renals de Catalunya; ICED, Index of Coexisting Disease, NYHA, New York Heart Association; ESRD-SI, End-Stage Renal Disease Severity Index; RDSS, Renal Disease Severity Score.
The study sample represents the population of interest on key characteristics sufficient to limit potential bias to the results.
Loss to follow-up (from sample to study population) is not associated with key characteristics sufficient to limit potential bias (i.e., the study data adequately represent the sample).
The prognostic factor of interest is adequately measured in study participants to sufficiently limit potential bias.
The outcomes of interest are adequately measured in study participants to sufficiently limit potential bias.
Important potential confounders are appropriately accounted for, limiting potential bias with respect to the prognostic factor of interest.
The statistical analysis is appropriate for the design of the study, limiting potential for presentation of invalid results.
Meets low risk of bias criteria.
Unclear; ? unknown/NR.
Does not meet low risk of bias criteria.
Clinical Utility and Usability of the Included Studies
Clinical utility and usability was limited for many of the indices. The age-comorbidity prognostic index; Analyzing Data, Recognizing Excellence, and Optimizing Outcomes, Doi, Foley, Kahn-Wright, REIN, Updated REIN, Thamer, and Wick indices provided risk categories and/or mortality percentiles for easy translation (31,33,37,39,43,45,47,48). Geddes et al. (44) reported calibration for the CCI predicting 1- and 5-year mortality (PPV, 78.7% and 79.4%; negative predictive value, 40.8% and 70.4%; and likelihood ratio, 1.1 and 7.0, respectively) and defined survival-based cutoffs for the Hemmelgarn index. Other studies have provided cutoffs for the nCI (42); and for the nCI, REIN, and Hospice Eligibility Criteria indices (23). In the study by Cheung et al. on elderly patients on dialysis, a REIN score of ≥9 had a specificity of 80%–92% for predicting death (23). The 3-, 6-, and 12-month mortality rates associated with different REIN index scores have also been reported (30). Others reported less useful data.
One of the studies referred to an online calculators (www.DialysisScore.com) and one gave a nomogram to help with bedside translation (30,49). The CCI can be calculated with several online calculators, the updated REIN (https://qxmd.com/calculate/calculator_286/3-month-mortality-in-incident-elderly-esrd-patients), Thamer (http://www.pmidcalc.org/?sid=26123861&newtest=Y), and Obi (www.DialysisScore.com) are available online (47).
Discussion
Our systematic review identified several well validated indices for predicting survival of incident ESKD that show promise for bedside translation. The CCI was most commonly used and delivered the best and most consistent discrimination performance. Calibration was rarely reported and not consistently enough to allow for meta-analysis. There was a lack of information on predictive performance of specific score cutoffs and risk thresholds that could inform clinical practice. A third of the tools lacked external validation and statistical heterogeneity was very high, suggesting lack of reproducibility and/or transportability as well as case-mix effects. Clinical heterogeneity was also substantial in the chosen prognostic variables, prognostic window, and risk cutoffs and there was significant risk of bias. Few scores were easy to calculate (31,33,38,39,50). The indices were developed and validated in cohorts that underrepresented patients of races and ethnicity other than white and Asian, affecting generalizability.
Implications for Practice
Dialysis is a prime example of a preference-sensitive decision with a strong technological and moral imperative to treat despite clinical equipoise regarding the balance of risks and benefits. Treatment burden is high and strong arguments have been made for a more palliative approach to dialysis treatment of patients who have a limited life expectancy (51,52). However, patients with good prognosis stand to benefit from proactive vascular access placement and consideration of preemptive kidney transplantation (8).
Dialysis patients also receive more aggressive end-of-life care than most other patient groups, and hospice is underused (53,54). A major barrier to earlier hospice transition is that most hospices require patients to discontinue dialysis because it is not covered under the Medicare hospice benefit. We found eight indices that could predict 6-month mortality (23,28,33,39,42,47,48,55) and five that could predict 3-month mortality (30,37,44,45,47) with dialysis treatment. For the oldest patients, a REIN index score of ≥9 predicted over 70% mortality at 6 months (specificity, 80%–92%) (23). Currently, Medicare requires >50% expected 6-month mortality, thus a strong argument could be made for a policy change allowing for concurrent Medicare reimbursement for dialysis and hospice for this high-risk subgroup of patients to promote earlier hospice enrollment and avoid costly and burdensome hospitalizations and interventions at the end of life.
Implications for Research
We did not find any prospective studies testing the performance or utility of these indices in clinical practice. We also did not identify any studies testing the effect of mortality prediction on improving shared decision making. Patients, surrogates, and clinicians tend to be overly optimistic in the face of a poor prognosis (10,56,57). We do not know if providing patients with prognostic information at dialysis start will help patients develop more insight into their prognosis. Clinicians may hesitate to use prognostic indices with discrimination of only 0.7–0.75 and feel that those are not accurate enough. Furthermore, even a near-perfect model for mortality, validated externally in numerous populations and settings, would provide no evidence for its utility in improving shared decision making at the bedside. Indeed, qualitative research suggests that a major barrier for clinicians to discussing prognosis with patients is fear of taking away hope and giving patients a wrong estimate (58). In addition these conversations are emotionally charged and can be uncomfortable for clinicians who often lack communication skills training to facilitate these conversations (58). More research is needed to determine the value of risk prediction to patients and their family members and clinicians, the utility and usability of existing tools at the bedside, and their effect on shared decision making and other patient important outcomes.
Strengths and Limitations
Our study has limitations. Most importantly, our decision to limit the review to patients on incident dialysis and exclude studies that did not report early mortality resulted in the exclusion of many large studies utilizing the prevalent population in the US Renal Data System and other large population databases. This was deliberate, as we feel strongly that excluding early mortality presents a biased survival estimate to starting dialysis and that there is a need to present a more “realistic” estimate for patients trying to decide whether to start dialysis. We did not use the recommended screening filters suggested by the checklist for critical appraisal and data extraction for systematic reviews of prediction modelling studies (CHARMS) checklist as our work started before this publication (59). However, we worked closely with a librarian with decades of experience in systematic reviews (P.J.E.) who developed a very sensitive search strategy, yielding 12,132 titles for screening and followed the PRISMA statement. We included all prognostic indices that we could confirm had been validated, either internally during index development or subsequent to the index development, but we cannot be certain that none were missed. Several of the studies did not report key performance data, prohibiting direct comparison. Our review did not evaluate the use of the surprise question, which has been shown to perform well in prevalent dialysis patients. (60) In fact, clinician intuition outperformed the index in one of the included studies (55). Strengths include a comprehensive and sensitive search strategy that covered several databases without language restrictions. We used a validated tool to assess bias and a previously defined assessment of clinical usability. We also focused our review on the time of starting dialysis, when these decisions are most likely to add value to therapeutic recommendations and shared decision making.
In conclusion, prognosis is a key element of shared decision making (9), yet poorly integrated into dialysis decision making. Several validated indices with good prognostic ability are available to predict mortality at the start of dialysis. Little attention has been paid to bedside translation of these indices to determine the value of sharing prognostic information with patients. Further research is needed to understand how best to utilize these indices in patient care to support shared decision making.
Disclosures
Dr. Tangri reports grants and personal fees from Astra Zeneca Inc. related to SGLT2 inhibitors and hyperkalemia treatments, personal fees from Otsuka Inc. related to tolvaptan and ADPKD, personal fees from Janssen related to SGLT2 inhibitors, personal fees from Boehringer Ingelheim and Eli Lilly related to SGLT2 inhibitors, and grants and personal and other fees, including consulting fees and stock options related to veverimer, from Tricida Inc., outside the submitted work. Dr. Anderson, Dr. Bellolio, Dr. Cleek, Dr. Erwin, Dr. Feely, Dr. Giddings Connolly, Dr. Hart, Dr. Hickson, Dr. Majzoub, Dr. Mayukha, Dr. Pajouhi, Dr. Giddings Connolly, Dr. Thorsteinsdottir, and Dr. Wilson have nothing to disclose.
Funding
This project was supported by a Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery award (to Thorsteinsdottir and Hickson); the Norman S. Coplon Extramural Grant Program of Satellite Healthcare, a not-for-profit renal care provider (to Thorsteinsdottir and Hickson); National Institute on Aging grant K23AG051679 (to Thorsteinsdottir); and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant K23 DK109134 (to Hickson). Additional support was provided by the National Center for Advancing Translational Sciences grant UL1 TR000135.
Supplementary Material
Acknowledgments
We appreciate the help of Susan Curtis, Annika Beck and Anna Jones in preparing the manuscript for submission and Dr. Adelaide Arruda-Olson, Dr. Sorita Atsushi, and Dr. s. Grzegorz Nowakowski for their help with foreign language full-text manuscript screening. We thank Dr. Fares Alahdab for his help with the use of OpenMeta for meta-analysis and figure editing, and Dr. Victor Montori for help in framing the presentation of our data.
Dr. Thorsteinsdottir conceived and designed the study, obtained funding, oversaw and assisted with acquisition and interpretation of data, drafted sections of the manuscript, and provided critical revisions to the manuscript. Dr. Anderson contributed to the conception and design of the study, assisted with acquisition of data, analyzed and interpreted the data, and drafted the manuscript. Dr. Erwin crafted the search strategy and assisted with acquisition of data. Dr. Cleek, Dr. Mayukha, Dr. Majzoub, Dr. Hart, Dr. Connolly, Dr. Feely., and Dr. Wilson assisted with acquisition of data and provided critical revisions to the manuscript. Dr. Hickson and Dr. Tangri contributed to the conception and design of the study and provided critical revisions to the manuscript. Dr. Bellolio helped with methodologic questions and data interpretation, performed analysis of raw data when needed, and provided critical revisions to the manuscript. All authors read and approved the final manuscript and the decision to submit the manuscript for publication.
The interpretation and reporting of the data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government. The funding source had no role in the study design, data collection, analysis and interpretation of data, writing the report, or the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Present address: Dr. Hailey Cleek, School of Law, Wake Forest University, Winston-Salem, North Carolina.
Present address: Dr. Ananya Mayukha, Williams College, Williamstown, Massachusetts.
Present address: Dr. Ryan T. Anderson, College of Medicine and Science, Mayo Clinic, Rochester, Minnesota.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00050119/-/DCSupplemental.
Supplemental Appendix 1. Search strategy.
Supplemental Appendix 2. Data abstraction form.
Supplemental Appendix 3. Data Abstraction form: study quality.
Supplemental Table 1. Papers excluded after full-text review.
Supplemental Table 2. Overview of predictive variables by index.
Supplemental Table 3. Predictive variables and scoring of included indices.
Supplemental Figure 1. Meta-analysis by validation status.
Supplemental Figure 2. Meta-analysis by cohort size.
Supplemental Figure 3. Meta-analysis for cohorts with >75 years mean age.
References
- 1.Thorsteinsdottir B, Montori VM, Prokop LJ, Murad MH: Ageism vs. the technical imperative, applying the GRADE framework to the evidence on hemodialysis in very elderly patients. Clin Interv Aging 8: 797–807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Hare AM, Armistead N, Schrag WL, Diamond L, Moss AH: Patient-centered care: An opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD program. Clin J Am Soc Nephrol 9: 2189–2194, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 361: 1539–1547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murtagh FEM, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Davison SN: End-of-life care preferences and needs: Perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 195–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russ AJ, Shim JK, Kaufman SR: “Is there life on dialysis?”: Time and aging in a clinically sustained existence. Med Anthropol 24: 297–324, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russ AJ, Kaufman SR: Discernment rather than decision-making among elderly dialysis patients. Semin Dial 25: 31–32, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Thorsteinsdottir B, Swetz KM, Albright RC: The ethics of chronic dialysis for the older patient: Time to reevaluate the norms. Clin J Am Soc Nephrol 10: 2094–2099, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michel DM, Moss AH: Communicating prognosis in the dialysis consent process: A patient-centered, guideline-supported approach. Adv Chronic Kidney Dis 12: 196–201, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Wachterman MW, Marcantonio ER, Davis RB, Cohen RA, Waikar SS, Phillips RS, McCarthy EP: Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med 173: 1206–1214, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combs SA, Culp S, Matlock DD, Kutner JS, Holley JL, Moss AH: Update on end-of-life care training during nephrology fellowship: A cross-sectional national survey of fellows. Am J Kidney Dis 65: 233–239, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ameling JM, Auguste P, Ephraim PL, Lewis-Boyer L, DePasquale N, Greer RC, Crews DC, Powe NR, Rabb H, Boulware LE: Development of a decision aid to inform patients’ and families’ renal replacement therapy selection decisions. BMC Med Inform Decis Mak 12: 140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis JL, Davison SN: Hard choices, better outcomes: A review of shared decision-making and patient decision aids around dialysis initiation and conservative kidney management. Curr Opin Nephrol Hypertens 26: 205–213, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Thorsteinsdottir B, Swetz KM, Tilburt JC: Dialysis in the frail elderly--a current ethical problem, an impending ethical crisis. J Gen Intern Med 28: 1511–1516, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moss AH: Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis : Clinical Practice Guideline, 2nd Ed., Rockville, MD, Renal Physicians Association, 2010 [Google Scholar]
- 16.Williams AW, Dwyer AC, Eddy AA, Fink JC, Jaber BL, Linas SL, Michael B, O’Hare AM, Schaefer HM, Shaffer RN, Trachtman H, Weiner DE, Falk AR; American Society of Nephrology Quality, and Patient Safety Task Force : Critical and honest conversations: The evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol 7: 1664–1672, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, Murtagh FE, Naicker S, Germain MJ, O’Donoghue DJ, Morton RL, Obrador GT; Kidney Disease: Improving Global Outcomes : Executive summary of the KDIGO Controversies Conference on supportive care in chronic kidney disease: Developing a roadmap to improving quality care. Kidney Int 88: 447–459, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J Clin Epidemiol 62: e1–e34, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB Sr: Evaluating discrimination of risk prediction models: The C statistic. JAMA 314: 1063–1064, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW: Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 21: 128–138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayden JA, Côté P, Bombardier C: Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 144: 427–437, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Tangri N, Kitsios GD, Inker LA, Griffith J, Naimark DM, Walker S, Rigatto C, Uhlig K, Kent DM, Levey AS: Risk prediction models for patients with chronic kidney disease: A systematic review. Ann Intern Med 158: 596–603, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Cheung KL, Montez-Rath ME, Chertow GM, Winkelmayer WC, Periyakoil VS, Kurella Tamura M: Prognostic stratification in older adults commencing dialysis. J Gerontol A Biol Sci Med Sci 69: 1033–1039, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S, editors: Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://www.handbook.cochrane.org. Accessed January 28, 2019 [Google Scholar]
- 25.Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH: Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Software 49: 1–15, 2013 [Google Scholar]
- 26.Beddhu S, Bruns FJ, Saul M, Seddon P, Zeidel ML: A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 108: 609–613, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Cho H, Kim MH, Kim HJ, Park JY, Ryu DR, Lee H, Lee JP, Lim CS, Kim KH, Oh KH, Joo KW, Kim YS, Kim DK: Development and validation of the modified Charlson Comorbidity Index in incident peritoneal dialysis patients: A national population-based approach. Perit Dial Int 37: 94–102, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Di Iorio B, Cillo N, Cirillo M, De Santo NG: Charlson Comorbidity Index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int J Artif Organs 27: 330–336, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Peeters P, Van Biesen W, Veys N, Lemahieu W, De Moor B, De Meester J: External Validation of a risk stratification model to assist shared decision making for patients starting renal replacement therapy. BMC Nephrol 17: 41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan IH, Catto GR, Edward N, Fleming LW, Henderson IS, MacLeod AM: Influence of coexisting disease on survival on renal-replacement therapy. Lancet 341: 415–418, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, Stengel B; French Renal Epidemiology and Information Network (REIN) registry : A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant 24: 1553–1561, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies SJ, Phillips L, Naish PF, Russell GI: Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 17: 1085–1092, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Fried L, Bernardini J, Piraino B: Charlson Comorbidity Index as a predictor of outcomes in incident peritoneal dialysis patients. Am J Kidney Dis 37: 337–342, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Fried L, Bernardini J, Piraino B: Comparison of the Charlson Comorbidity Index and the Davies score as a predictor of outcomes in PD patients. Perit Dial Int 23: 568–573, 2003 [PubMed] [Google Scholar]
- 37.Couchoud CG, Beuscart JBR, Aldigier JC, Brunet PJ, Moranne OP; REIN registry : Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int 88: 1178–1186, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Fernandez Lucas M, Teruel JL, Zamora J, Lopez Mateos M, Rivera M, Ortuno J: A Mediterranean age-comorbidity prognostic index for survival in dialysis populations. J Nephrol 20: 696–702, 2007 [PubMed] [Google Scholar]
- 39.Foley RN, Parfrey PS, Hefferton D, Singh I, Simms A, Barrett BJ: Advance prediction of early death in patients starting maintenance dialysis. Am J Kidney Dis 23: 836–845, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Mauri JM, Clèries M, Vela E; Catalan Renal Registry : Design and validation of a model to predict early mortality in haemodialysis patients. Nephrol Dial Transplant 23: 1690–1696, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Wagner M, Ansell D, Kent DM, Griffith JL, Naimark D, Wanner C, Tangri N: Predicting mortality in incident dialysis patients: An analysis of the United Kingdom renal registry. Am J Kidney Dis 57: 894–902, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kan WC, Wang JJ, Wang SY, Sun YM, Hung CY, Chu CC, Lu CL, Weng SF, Chio CC, Chien CC: The new comorbidity index for predicting survival in elderly dialysis patients: A long-term population-based study. PLoS One 8: e68748, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doi T, Yamamoto S, Morinaga T, Sada KE, Kurita N, Onishi Y: Risk score to predict 1-year mortality after haemodialysis initiation in patients with stage 5 chronic kidney disease under predialysis nephrology care. PLoS One 10: e0129180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geddes CC, van Dijk PC, McArthur S, Metcalfe W, Jager KJ, Zwinderman AH, Mooney M, Fox JG, Simpson K: The ERA-EDTA cohort study--comparison of methods to predict survival on renal replacement therapy. Nephrol Dial Transplant 21: 945–956, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S, Pisoni RL, Robinson BM, Marcelli D, Froissart M, Eckardt KU: Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 87: 996–1008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaguma D, Tanaka A, Shinjo H: Physical function at the time of dialysis initiation is associated with subsequent mortality. Clin Exp Nephrol 21: 425–435, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H: Predicting early death among elderly dialysis patients: Development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis 66: 1024–1032, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wick JP, Turin TC, Faris PD, MacRae JM, Weaver RG, Tonelli M, Manns BJ, Hemmelgarn BR: A clinical risk prediction tool for 6-month mortality after dialysis initiation among older adults. Am J Kidney Dis 69: 568–575, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Obi Y, Nguyen DV, Zhou H, Soohoo M, Zhang L, Chen Y, Streja E, Sim JJ, Molnar MZ, Rhee CM, Abbott KC, Jacobsen SJ, Kovesdy CP, Kalantar-Zadeh K: Development and validation of prediction scores for early mortality at transition to dialysis. Mayo Clin Proc 93: 1224–1235, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Postorino M, Marino C, Tripepi G, Zoccali C; Calabrian Registry of Dialysis and Transplantation : Prognostic value of the New York Heart Association classification in end-stage renal disease. Nephrol Dial Transplant 22: 1377–1382, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal SV, Perl J, Weiner DE, Mehrotra R; Dialysis Advisory Group of the American Society of Nephrology : A palliative approach to dialysis care: A patient-centered transition to the end of life. Clin J Am Soc Nephrol 9: 2203–2209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jassal SV: Four plus forty-four: Hours to modify, theirs to enjoy. Clin J Am Soc Nephrol 10: 169–171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong SP, Kreuter W, O’Hare AM: Treatment intensity at the end of life in older adults receiving long-term dialysis. Arch Intern Med 172: 661–663, discussion 663–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH: Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol 1: 1248–1255, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Barrett BJ, Parfrey PS, Morgan J, Barré P, Fine A, Goldstein MB, Handa SP, Jindal KK, Kjellstrand CM, Levin A, Mandin H, Muirhead N, Richardson RM: Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis 29: 214–222, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Zier LS, Sottile PD, Hong SY, Weissfield LA, White DB: Surrogate decision makers’ interpretation of prognostic information: A mixed-methods study. Ann Intern Med 156: 360–366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hole B, Salem J: How long do patients with chronic disease expect to live? A systematic review of the literature. BMJ Open 6: e012248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schell JO, Patel UD, Steinhauser KE, Ammarell N, Tulsky JA: Discussions of the kidney disease trajectory by elderly patients and nephrologists: A qualitative study. Am J Kidney Dis 59: 495–503, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, Reitsma JB, Collins GS: Critical appraisal and data extraction for systematic reviews of prediction modelling studies: The CHARMS checklist. PLoS Med 11: e1001744, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White N, Kupeli N, Vickerstaff V, Stone P: How accurate is the ‘Surprise Question’ at identifying patients at the end of life? A systematic review and meta-analysis. BMC Med 15: 139, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandna SM, Schulz J, Lawrence C, Greenwood RN, Farrington K: Is there a rational for rationing chronic dialysis? A hospital based cohort study of factors affecting survival and morbidity. BMJ 318: 217–223, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez AT, Kiberd BA, Royston JP, Alfaadhel T, Soroka SD, Hemmelgam BR, Tennankore KK: Comorbidity burden at dialysis initiation and mortality: A cohort study. Can J Kidney Health Dis 2: 34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivory SE, Polkinghorne KR, Khandakar Y, Kasza J, Zoungas S, Steenkamp R, Roderick P, Wolfe R: Predicting 6-month mortality risk of patients commencing dialysis treatment for end-stage kidney disease. Nephrol Dial Transplant 32: 1558–1565, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Khan IH, Campbell MK, Cantarovich D, Catto GR, Delcroix C, Edward N, Fontenaille C, van Hamersvelt HW, Henderson IS, Koene RA, Papadimitriou M, Ritz E, Ramsay C, Tsakiris D, MacLeod AM: Comparing outcomes in renal replacement therapy: How should we correct for case mix? Am J Kidney Dis 31: 473–478, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Lee SW, Lee A, Yu MY, Kim SW, Kim KI, Na KY, Chae DW, Kim CH, Chin HJ: Is Frailty a Modifiable Risk Factor of Future Adverse Outcomes in Elderly Patients with Incident End-Stage Renal Disease? Journal of Korean medical science 32: 1800–1806, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez Revuelta K, Garcia Lopez FJ, de Alvaro Moreno F, Alonso J: Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end-stage renal disease (CALVIDIA Study). Nephrol Dial Transplant 19: 2347–2353, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Ma TK, Chow KM, Kwan BC, Ng JK, Pang WF, Leung CB, Li PK, Szeto CC: The choice of comorbidity scoring system in Chinese peritoneal dialysis patients. Clin Exp Nephrol 22: 159–166, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Marinovich S, Lavorato C, Morinigo C, Celia E, Bisignano L, Soratti M, Hansen-Krogh D: A new prognostic index for one-year survival in incident hemodialysis patients. Int J Artif Organs 33: 689–699, 2010 [PubMed] [Google Scholar]
- 69.Nicolucci S, Cubasso D, Labbrozzi D, Mari E, Impicciatore P, Procaccini DA, Forcella M, Stella I, Querques M, Pappani A, Strippoli P: Effect of coexistent diseases on survival of patients undergoing dialysis. ASAIO Journal 38: M291–M295, 1992 [DOI] [PubMed] [Google Scholar]
- 70.Otero-López MS, Martínez-Ocaña JC, Betancourt-Castellanos L, Rodríguez-Salazar E, García-García M: Two prognostic scores for early mortality and their clinical applicability in elderly patients on haemodialysis: Poor predictive success in individual patients. Nefrologia 32: 206–212, 2012 [DOI] [PubMed] [Google Scholar]
- 71.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT: Dialysis NSGNC-oSotAo: How to adjust for comorbidity in survival studies in ESRD patients: a comparison of different indices. Am J Kidney Dis 40: 82–89, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.