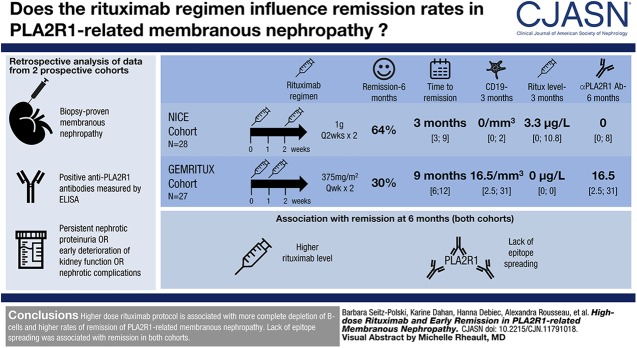
Visual Abstract
Keywords: membranous nephropathy; clinical immunology; Humans; Rituximab; lomerulonephritis, Membranous; B-lymphocytes; Cohort Studies; Recurrence; Epitopes
Abstract
Background and objectives
Different rituximab protocols are used to treat membranous nephropathy. We compared two rituximab protocols in patients with membranous nephropathy.
Design, setting, participants, & measurements
Twenty-eight participants from the NICE cohort received two infusions of 1-g rituximab at 2-week intervals, whereas 27 participants from the Prospective Randomized Multicentric Open Label Study to Evaluate Rituximab Treatment for Membranous Nephropathy (GEMRITUX) cohort received two infusions of 375 mg/m2 at 1-week interval. We measured serum rituximab levels and compared remission at month 6 and before any treatment modification and analyzed factors associated with remission and relapses.
Results
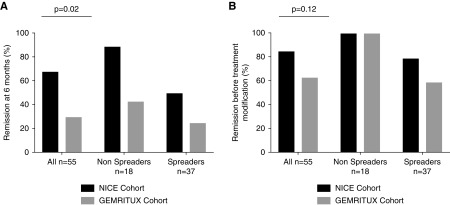
Remissions occurred in 18 (64%) versus eight (30%) from the NICE and GEMRITUX cohort (P=0.02) at month 6, respectively, and in 24 (86%) versus 18 (67%) participants (P=0.12) before treatment modification, respectively. Median time to remission was 3 [interquartile range (IQR), 3–9] and 9 [IQR, 6–12] months for NICE and GEMRITUX cohorts respectively (P=0.01). Participants from the NICE cohort had higher circulating level of rituximab and lower CD19 counts (3.3 µg/L [IQR, 0.0–10.8] versus 0.0 [IQR, 0.0–0.0] P<0.001 and 0.0 [IQR, 0.0–2.0] versus 16.5 [IQR, 2.5–31.0] P<0.001) at month 3, lower level of anti-PLA2R1 antibodies at month 6 (0.0 [IQR, 0.0–8.0] versus 8.3 [IQR, 0.0–73.5] P=0.03). In the combined study population, lower epitope spreading at diagnosis and higher rituximab levels at month 3 were associated with remissions at month 6 (13/26 (50%) versus 22/29 (76%) P=0.05 and 2.2 µg/ml [IQR, 0.0–10.9] versus 0.0 µg/ml [IQR, 0.0–0.0] P<0.001 respectively). All non-spreaders entered into remission whatever the protocol. Eight of the 41 participants who reached remission had relapses. Epitope spreading at diagnosis (8/8 (100%) versus 16/33 (48%) P=0.01) and incomplete depletion of anti-PLA2R1 antibodies at month 6 (4/8 (50%) versus 5/33 (9%) P=0.05) were associated with relapses.
Conclusions
Our work suggests that higher dose rituximab protocol is more effective on depletion of B-cells and lack of epitope spreading is associated with remission of membranous nephropathy.
Introduction
Membranous nephropathy is defined by the presence of subepithelial immune complex deposits with alteration of the glomerular basement membrane. Most cases of membranous nephropathy are associated with antibodies against podocyte antigens such as neutral endopeptidase in the neonate, and M-type phospholipase A2 receptor (PLA2R1) or thrombospondin type-1 domain-containing 7A (THSD7A) in 70%–80% and <5% of adult patients, respectively (1–3). The pathogenic role of anti-PLA2R1 antibodies is not yet proven, but antibody titers rise during clinically active phases and decrease before clinical remission (4–7), which led to the proposal of a serology-based approach for the treatment of PLA2R1-related membranous nephropathy (8). Spontaneous remissions and ESKD may both occur in about a third of the patients (9). High titers of anti-PLA2R1 antibodies at presentation are associated with poor clinical outcome (10,11). Therefore, reducing anti-PLA2R1 antibody levels has become an important goal of therapy (12).
We identified reactive epitopes in the CysR, CTLD1, and CTLD7 domains of PLA2R1 (13) and found that patients with anti-CysR–restricted activity (nonspreaders) had lower proteinuria and better prognosis, with higher rate of spontaneous remission and lower rate of kidney failure progression, compared with patients with epitope spreading beyond the CysR domain (13).
Rituximab, an anti-CD20 chimeric antibody, can trigger B cell death by apoptosis (14,15), complement-mediated cytotoxicity (16), and antibody-dependent cellular cytotoxicity (17,18). Rituximab induced clinical remission in 60%–80% of patients with primary membranous nephropathy in several nonrandomized studies (19–21), and its efficacy was established in our recent randomized, controlled clinical trial, Prospective Randomized Multicentric Open Label Study to Evaluate Rituximab Treatment for Membranous Nephropathy (GEMRITUX), using two doses of 375 mg/m2 at 1-week intervals (22). We confirmed in this trial that PLA2R1 epitope spreading at baseline was independently associated with a lower rate of remission in this trial (23).
There are still uncertainties regarding the rituximab protocol that should be used in nephrotic patients (24). Cravedi et al. (25) proposed titrating rituximab to circulating CD20 cells. Fervenza et al. (26) showed that four doses of rituximab resulted in more effective B cell depletion. Moroni et al. (27) demonstrated that a single low dose of rituximab (375 mg/m2) was poorly effective in patients with membranous nephropathy. The reason for these discrepancies may be that rituximab pharmacokinetics studies have demonstrated a large interindividual variability, related to either disease or genetic factors, which could explain differences in the clinical response (28–31). Residual serum levels of rituximab were detected in several patients up to 6–9 months after the first infusion, owing to recycling from endothelial cells via FcRn receptors (32). In patients with membranous nephropathy, the rituximab t1/2 was 11.5 days, as compared with 18.0 days in patients with rheumatoid arthritis (26). In previous studies where 1-g rituximab infusions were given at 2-week intervals, Fervenza et al. (20,26) did not find differences in serum rituximab levels between responders and nonresponders at any time point until day 15 postdose.
The aim of this study was to compare two protocols of rituximab in two prospective cohorts of anti-PLA2R1–positive patients.
Materials and Methods
Study Design and Patient Population
Patients were enrolled after signing informed consent from the two prospective studies. The NICE cohort recruited consecutive participants with primary membranous nephropathy in the Department of Nephrology at Pasteur Hospital in Nice (Clinicaltrials.gov identifier NCT02199145), testing epitope profile at diagnosis to predict the need of immunosuppressive therapy (rituximab); only participants recruited in Nice Hospital, treated by rituximab and followed at least 1 year were included in this study. The GEMRITUX cohort is part of a French multicenter, randomized, controlled trial (Clinicaltrials.gov identifier NCT01508468) testing rituximab added to antiproteinuric therapy against antiproteinuric therapy alone (22); only participants with anti-PLA2R1 antibodies treated with rituximab were included in this study. The inclusion criteria were (1) biopsy-proven diagnosis; (2) primary membranous nephropathy defined by the absence of anti-nuclear antibodies, negative hepatitis B and C serologies, and negative cancer workup; (3) positive anti-PLA2R1 antibodies measured by ELISA; and (4) persistent nephrotic proteinuria (i.e., urinary protein-to-creatinine ratio >3.5 g/g) after maximal tolerated antiproteinuric treatment, early deterioration of kidney function, or complications of nephrotic syndrome.
Serum and morning spot urine samples were prospectively collected at the first infusion and every 3 months after the first rituximab infusions (i.e., month 3, month 6). Remissions were defined according to the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. Complete remission was defined by a urinary protein-to-creatinine ratio <0.3 g/g, accompanied by a normal serum albumin concentration and a preserved kidney function. Partial remission was defined by urinary protein-to-creatinine ratio <3.5 g/g with >50% reduction of proteinuria, accompanied by an improvement or normalization of the serum albumin concentration and preserved kidney function. A relapse was defined by an increase of urinary protein-to-creatinine ratio >3.5 g/g after remission.
Outcome
The first aim of this study was to compare the efficacy of rituximab in term of clinical remission at 6 months and before treatment modifications in two cohorts of PLA2R1-related membranous nephropathy treated with two different regimens of rituximab.
The second aim was to identify factors associate with remission at month 6 and before treatment modifications in the two combined cohorts.
Measurement of Anti-PLA2R1 Antibodies by ELISA
Serum levels of total IgG anti-PLA2R1 antibodies were measured by the ELISA test developed by EUROIMMUN AG (Lübeck, Germany) (33). Participants were considered as anti-PLA2R1–positive when levels were >14 RU/ml.
Measurement of PLA2R1 Epitope Spreading by ELISA
CysR (Ala-26 to Lys-164), CTLD1 (Thr-223 to Asn-359), and CTLD7 (Thr-1102 to Glu-1237) PLA2R1 domains were produced in HEK293 cells and reactivity of sera of patients with membranous nephropathy toward these domains were analyzed essentially as previously described for the GEMRITUX cohort (23). Participants with only anti-CysR reactivity were defined as nonspreaders, whereas participants with additional anti-CTLD1 and/or anti-CTLD7 reactivity were defined as spreaders (i.e., participants with intramolecular epitope spreading within PLA2R1).
Measurement of Rituximab by ELISA
Serum levels of rituximab were measured by ELISA according to the manufacturer’s instructions (LISA-TRACKER Duo Rituximab; Theradiag, Croissy Beaubourg, France). This assay measures only free rituximab. The limit of detection for rituximab defined by the manufacturer was 2 μg/ml, with an intrarun variability of 8% and interrun variability of 10%.
Statistical Analyses
Categorical variables were expressed as frequencies and compared using Fisher exact test. Continuous variables were expressed as medians and interquartile ranges and compared using Wilcoxon–Mann–Whitney test or Kruskall–Wallis test for multilevel variables. Correlation between residual serum rituximab level and CD19 counts were assessed by Spearman rank correlation coefficient. Adjusted analysis was performed using logistic regression. Survival curves were calculated using Kaplan–Meier estimates for survival distribution. A categorical variable combining treatment and spreading was built to investigate the effect of the interaction between treatment and spreading on the response. All tests were two-sided and a P value <0.05 indicated statistical significance. Analyses were performed using SAS software v.9.3 (SAS Institute, Cary, NC).
Results
Comparison of the NICE and GEMRITUX Cohorts at Baseline
The NICE cohort included 28 consecutive PLA2R1-positive participants who received two 1-g infusions of rituximab at 2-week intervals after 6 months of symptomatic treatment, or earlier in the case of acute complications according to the KDIGO guidelines. Seven participants were treated after <6 months of symptomatic treatment: two presented with thromboembolic complications at diagnosis and five participants developed progressive kidney failure. The GEMRITUX cohort included 27 PLA2R1-positive participants from the French GEMRITUX clinical trial (22) who were given two 375 mg/m2 infusions of rituximab at 1-week intervals at least 6 months after kidney biopsy. Participants’ baseline characteristics were similar, apart from an older age in the NICE cohort (63 [interquartile range (IQR), 51–71] versus 51 [IQR, 40–63] years; P=0.03). Proteinuria tended to be lower in the NICE cohort, although this did not reach statistical significance (5.9 [IQR, 4.9–7.6] versus 8.4 [IQR, 4.4–11.0]; P=0.13), (Table 1). All other baseline characteristics were similar, including anti-PLA2R1 antibody titers (NICE: 165.0 RU/ml [IQR, 67.0–245.5]; GEMRITUX: 102.5 RU/ml [IQR, 36.1–672.5]; P=0.65) and epitope spreading as defined by anti-CysR reactivity with additional anti-CTLD1 and/or anti-CTLD7 activities in addition to anti-CysR reactivity (61% epitope spreading in NICE versus 74% in GEMRITUX; P=0.39) (Table 1).
Table 1.
Baseline characteristics and outcome data in rituximab-treated participants from NICE and GEMRITUX cohorts
| Patient Characteristics | NICE Cohort (n=28) | GEMRITUX Cohort (n=27) |
|---|---|---|
| Baseline characteristics | ||
| Agea | 63 [51; 71] | 51 [40; 63] |
| Sex ratio (F/M) | 7/21 | 6/21 |
| Body mass index (kg/m2) | 25.4 [23.3; 27.2] | 25.4 [23.5; 28.4] |
| Proteinuria (g/g of creatinine) | 5.9 [4.9; 7.6] | 8.4 [4.4; 11.0] |
| Serum albumin (g/dl) | 2.0 [1.5; 2.5] | 2.1 [1.8; 2.6] |
| Dose of rituximab received (g)b | 2 [2; 2] | 1.45 [1.44; 1.46] |
| Serum creatinine (mg/dl) | 1.2 [0.9; 1.5] | 1.1 [0.9; 1.3] |
| BP (mm Hg) | ||
| Systolic | 121 [114; 130] | 124 [110; 140] |
| Diastolic | 82 [66; 80] | 77 [64; 84] |
| Time from kidney biopsy to rituximab infusion | 6.0 [6.0; 12.0] | 8.0 [6.0; 13.0] |
| Anti-PLA2R1 titer (RU/ml) at first infusion | 165.0 [67.0; 245.5] | 102.5 [36.1; 672.5] |
| Spreading | ||
| No | 11 (39%) | 7 (26%) |
| Yes | 17 (61%) | 20 (74%) |
| Characteristics at month 3 | ||
| Serum rituximab (μg/ml)b | 3.3 [0.0; 10.8] | 0.0 [0.0; 0.0] |
| Proteinuria (g/g of creatinine)b | 2.6 [1.1; 5.0] | 4.8 [3.2; 7.4] |
| Serum albumin (g/dl) | 2.7 [2.0; 3.0] | 2.7 [2.1; 3.1] |
| Anti-PLA2R1 (RU/ml) titer | 0.0 [0.0; 19.0] | 0.0 [0.0; 60.5] |
| Rate of PLA2R1 antibody depletion | 16/27 (59%)c | 14/25 (56%) |
| Spreading | ||
| No | 17 (61%) | 13 (54%) |
| Yes | 11 (39%) | 11 (45%) |
| CD19 count (/mm3)b | 0.0 [0.0; 2.0] | 16.5 [2.5; 31.0]d |
Quantitative values are medians [interquartile ranges]; qualitative values are numbers. F/M, female/male; PLA2R1, M-type phospholipase A2 receptor.
P<0.05.
P<0.001.
One point is missing.
Two points are missing.
Serum Rituximab Levels, CD19 Counts, and Anti-PLA2R1 Depletion
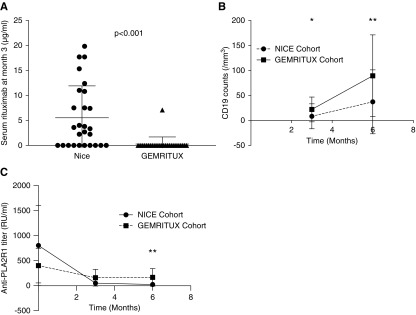
Rituximab serum levels were measured at month 3 in all participants. Residual levels were detected in 18 of the 28 NICE participants at month 3, but in only one of the 27 GEMRITUX participants at month 3. The median residual rituximab level at month 3 was higher with the NICE regimen (3.3 µg/ml [IQR, 0.0–10.8]) compared with the GEMRITUX regimen (0.0 µg/ml [IQR, 0.0–0.0]; P<0.001) (Figure 1A, Table 1). CD19 counts were lower at month 3 (0.0 [IQR, 0.0–2.0] in the NICE cohort versus 16.5 [IQR, 2.5–31.0] in the GEMRITUX cohort; P<0.001), and month 6 (5.0 [IQR, 1.8–48.5] versus 63.0 [IQR, 37.0–115.0]; P<0.001) (Figure 1B, Tables 1 and 2). Furthermore, immunologic remission defined by anti-PLA2R1 depletion at month 6 was more frequent with the NICE protocol (78% versus 50%; P=0.05) and anti-PLA2R1 titer was lower at month 6 in NICE cohort (0.0 [IQR, 0.0–8.0] versus 8.3 [IQR, 0.0–73.5]; P=0.03) (Figure 1C, Table 2).
Figure 1.
Temporal changes of circulating intermediate markers of rituximab effect in the NICE and GEMRITUX cohorts. (A) Comparison of serum residual rituximab level at month 3 between the NICE (n=28, two infusions of 1 g at 2-week intervals) and GEMRITUX (n=27, two infusions of 375 mg/m2 at 1-week intervals) cohorts. Note variability of residual rituximab concentrations and lower concentrations in the GEMRITUX participants (P<0.001). Threshold of detection was 2 μg/ml. (B) Evolution of CD19 count at month 3 and month 6 according to the regimens received. *CD19 count was lower in NICE cohort at month 3 (P>0.001). **CD19 count was lower at month 6 in NICE cohort (P=0.03). (C) Evolution of anti-PLA2R1 titer at baseline, month 3, and month 6 according to the regimens received. **Anti-PLA2R1 titer was lower at month 6 in NICE cohort (P>0.001).
Table 2.
Outcomes of rituximab-treated participants in the NICE and GEMRITUX cohorts
| Patients Characteristics | NICE Cohort (n=28) | GEMRITUX Cohort (n=27) | P Values |
|---|---|---|---|
| Characteristics at month 6 | |||
| Proteinuria (g/g of creatinine) | 2.0 [0.7; 3.2] | 3.7 [1.8; 6.5] | 0.001 |
| Serum albumin (g/dl) | 3.2 [2.8; 3.5] | 2.9 [2.4; 3.4] | 0.24 |
| Anti-PLA2R1 titer (RU/ml) | 0.0 [0.0; 8.0] | 8.3 [0.0; 73.5] | 0.03 |
| Rate of PLA2R1 antibody depletion | 21/27(78%) | 13/26 (50%) | 0.05 |
| Spreading | |||
| No | 25 (93%) | 17 (71%) | 0.07 |
| Yes | 2 (7%) | 7 (29%) | |
| CD19 counta | 5.0 [1.8; 48.5] | 63.0 [37.0; 115.0]a | <0.001 |
| Remission (KDIGO guidelines) | 18/28 (64%) | 8/27 (30%) | 0.02 |
| Complete remissionb | 5/28 (18%) | 0/27 (0%) | 0.05 |
| Characteristics at last follow-up | |||
| Remission before any treatment modification | 24/28 (86%) | 18/27 (67%) | 0.12 |
| Median time to remission (mo) | 3 [3; 9] | 9 [6; 12] | 0.01 |
| Complete remission | 9/28 (32%) | 6/27 (22%) | 0.55 |
| SAEs related to rituximab | 1/28 (0.03%) | 1/27 (0.04%) | 1 |
Quantitative values are medians [interquartile ranges]; qualitative values are numbers. PLA2R1, M-type phospholipase A2 receptor; KIDGO, Kidney Disease: Improving Global Outcomes; SAEs, serious adverse events.
Three points are missing.
Outcome
Outcome was analyzed at month 6 and before treatment modification.
At month 6, complete or partial remission was obtained at month 6 in 18 of the 28 (64%) NICE participants, but in only eight of the 27 (30%) GEMRITUX participants (P=0.02), (Table 1). Complete remissions at month 6 were only observed in the NICE cohort (n=5; P=0.05). Fourteen of the 18 NICE participants who achieved remission at month 6 had a residual rituximab concentration >2 µg/ml.
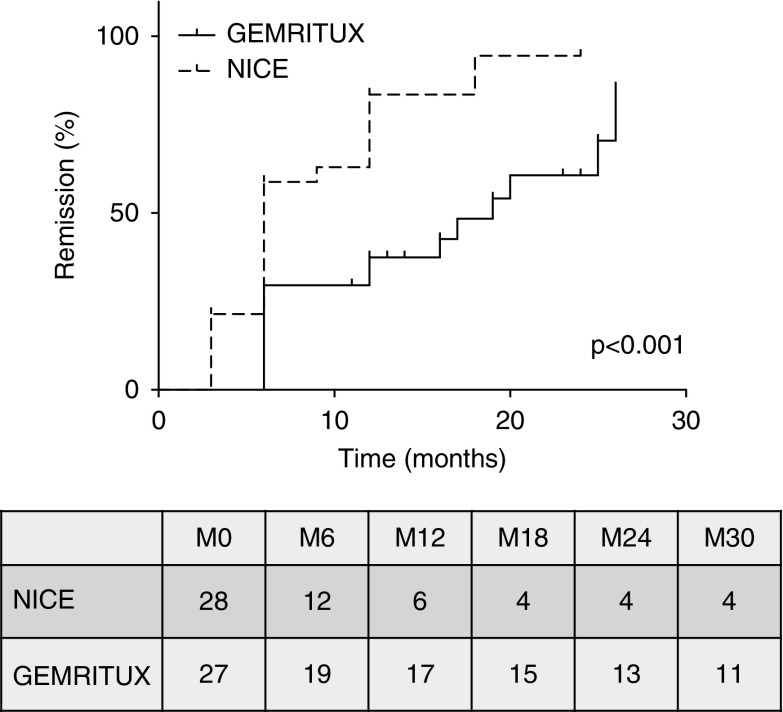
After a median follow-up of 15 [IQR, 11–19] (NICE cohort) and 24 [IQR, 22–25] (GEMRITUX cohort) months before any therapeutic modification, remissions occurred in 24 out of 28 (86%) NICE participants and 18 out of 27 (67%) GEMRITUX participants (P=0.12). Complete remission occurred in nine out of 28 (32%) and six out of 27 (22%) participants of the NICE and GEMRITUX cohorts, respectively. Median time to remission was 3 months [IQR, 3–9] in the NICE cohort versus 9 months [IQR, 6–12] in the GEMRITUX cohort (P=0.01) (Table 2), and Kaplan–Meier analysis demonstrates faster time to remission using the NICE protocol (P<0.001; Figure 2).
Figure 2.
Time from initiation of rituximab to remission of membranous nephropathy in the NICE and GEMRITUX cohorts. Note that NICE the log-rank P value for remission was P<0.001. Numbers in the table are number at risk.
Factors associated with Remission
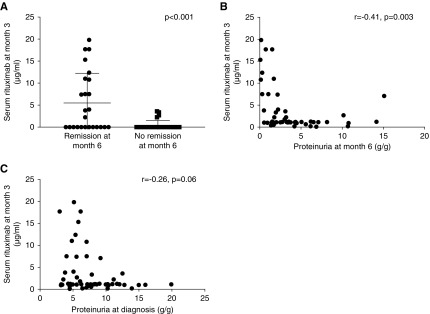
Clinical remissions at month 6 were associated with lower rate of epitope spreading at diagnosis (13/26 (50%) versus 22/29 (76%); P=0.05), and higher serum rituximab levels at month 3 (2.2 µg/ml [IQR, 0.0–10.9] versus 0.0 µg/ml [IQR, 0.0–0.0]; P<0.001) (Figure 3A, Table 2). Residual serum rituximab levels at month 3 were inversely correlated with proteinuria at month 3 and month 6 (r=0.42; P=0.003 and r=−0.41; P=0.003, respectively; Figure 3B), and tended to correlate with proteinuria at baseline (r=−0.26; P=0.06; Figure 3C) and with CD19 counts at month 3 and month 6 (r=−0.32; P=0.03 and r=−0.41; P=0.008, respectively). The clinical remission rate at month 6 was different according to the rituximab protocol combined with epitope spreading at baseline (P=0.003) (Figure 4A, Table 3). In adjusted analysis, remission rates were analyzed according to age, anti-PLA2R1 titers, epitope spreading at diagnosis, and the rituximab protocol. Epitope spreading at diagnosis and the rituximab protocol (1 g at the day of rituximab infusion and 2 weeks after rituximab infusion versus 375 mg/m2 at the day of rituximab infusion and 7 days after rituximab infusion) were independent risk factors for remission at month 6 (odds ratio, 4.34; 95% confidence interval, 1.07 to 17.5; P=0.04 and odds ratio, 5.08; 95% confidence interval, 1.3 to 19.6; P=0.02, respectively) (Table 4).
Figure 3.
Intermediate outcomes according to serum rituximab concentration at 3 months. (A) Comparison of serum rituximab concentrations according to remission (n=26) or absence of remission (n=29) at month 6 in the pooled NICE and GEMRITUX cohorts. Note higher concentration of residual rituximab concentrations at month 3 in participants who achieved remission (P<0.001). (B) Correlation of serum rituximab concentrations at month 3 and proteinuria at month 6 in the pooled NICE and GEMRITUX cohorts (r=−0.41; P=0.003). (C) Correlation of serum rituximab concentrations at month 3 and proteinuria at baseline in the pooled NICE and GEMRITUX cohorts (r=0.26; P=0.06).
Figure 4.
Remission of membranous nephropathy in the NICE and GEMRITUX cohorts according to epitope spreading at baseline. (A) Remission at month 6 according to epitope spreading at baseline and rituximab protocol received. A categorical variable combining treatment and spreading was built to investigate the impact of the interaction between treatment and spreading on the response (P=0.003; comparing the null hypothesis that there are no differences among groups). Note that the lowest remission rate is observed in participants with epitope spreading treated by GEMRITUX protocol. (B) Remission at last follow-up according to epitope spreading at baseline and rituximab protocol received. A categorical variable combining treatment and spreading was built to investigate the effect of the interaction between treatment and spreading on the response (P=0.01; comparing the null hypothesis that there are no differences among groups). Note that all participants without epitope spreading are in remission at last follow-up whatever the protocol received and participants with epitope spreading benefit from the NICE protocol (remission rate 79% versus 55%).
Table 3.
Prognosis factors of proteinuria remission at 6 months in the pooled NICE and GEMRITUX participants
| Participant Characteristics | No Remission at Month 6, n=29 | Remission at Month 6, n=26 |
|---|---|---|
| Baseline characteristics | ||
| Age | 50 [38; 65] | 61 [51; 70] |
| Sex | ||
| Men | 21 (75) | 19 (86) |
| Women | 8 (25) | 3 (14) |
| Body mass index (kg/m2) | 25.2 [23.4; 28.6] | 25.5 [23.5; 26.9] |
| Proteinuria (g/g of creatinine) | 7.8 [4.5; 10.3] | 6.0 [4.7; 8.0] |
| Serum creatinine (mg/dl) | 1.1 [0.9; 1.4] | 1.2 [0.9; 1.3] |
| Serum albumin (g/dl) | 2.0 [1.5; 2.7] | 2.1 [1.8; 2.5] |
| Anti-PLA2R1 antibody (RU/ml) | 192.0 [45.9; 1227.0] | 125.0 [62.0; 194.0] |
| Time to rituximab (mo) | 8.0 [6.0; 12.0] | 7.0 [6.0; 13.0] |
| Spreadinga | ||
| No | 7 (24) | 13 (50) |
| Yes | 22 (76) | 13 (50) |
| Epitope profile | ||
| CysR | 7 (24) | 13 (50) |
| CysRC1 | 7 (24) | 7 (27) |
| CysRC1C7 | 15 (52) | 6 (23) |
| Rituximab dose and spreadinga | ||
| GEMRITUX protocol without spreading | 4 (14) | 3 (12) |
| NICE protocol without spreading | 1 (3) | 10 (38) |
| GEMRITUX protocol with spreading | 15 (52) | 5 (19) |
| NICE protocol with spreading | 9 (31) | 8 (31) |
| Characteristics at month 3 | ||
| Anti-PLA2R1 antibody (RU/ml)a | 18.0 [0.0; 60.5] | 0.0 [0.0; 13.5] |
| Spreadingb | ||
| No | 10 | 22 |
| Yes | 16 | 3 |
| Rituximab, µg/mlb | 0.0 [0.0; 0.0] | 2.2 [0.0; 10.9] |
| CD19a | 12.1 [1.0; 25.0] | 0.0 [0.0; 2.7] |
| Characteristics at month 6 | ||
| Anti-PLA2R1 antibody (RU/ml)b | 9.0 [0.0; 75.5] | 0.0 [0.0; 1.2] |
| CD19 | 47.5 [17.5; 92.0] | 15.0 [2.0; 70.5] |
PLA2R1, M-type phospholipase A2 receptor.
P<0.05.
P<0.001.
Table 4.
Adjusted analysis for remission at month-6
| Prognosis Factors Tested | P Value | Odds Ratio | 95% Wald Confidence Limits |
|---|---|---|---|
| Age | 0.86 | 1.00 | 0.96 to 1.05 |
| Anti-PLA2R1 titer at diagnosis | 0.08 | 0.99 | 0.99 to 1.00 |
| Spreading at diagnosis | 0.04 | 4.34 | 1.07 to 17.54 |
| Rituximab regimens (NICE versus GEMRITUX) | 0.02 | 5.08 | 1.32 to 19.6 |
PLA2R1, M-type phospholipase A2 receptor.
Before treatment modification, clinical remission was associated with lower anti-PLA2R1 titers and absence of epitope spreading at baseline (101.5 RU/ml [IQR, 35.9–212.3] versus 508.0 [IQR, 144.9–1620.0]; P=0.003 and 18 out of 42 (43%) versus zero out of 13 (0%); P<0.001, respectively) (Supplemental Table 1), higher residual rituximab levels at month 3 (0.0 μg/ml [IQR, 0.0–5.7] versus 0.0 μg/ml [IQR, 0.0–0.0]; P=0.02) (Supplemental Table 1). The clinical remission rate at last follow-up was different according to the rituximab protocol combined with epitope spreading at baseline (P=0.01) (Figure 4B, Supplemental Table 1). All participants with anti-CysR–restricted activity at baseline entered into remission. Because each site measured remission at last follow-up at different time points, we performed a time-to-event analysis of remission according to the two different protocols for determining epitope spreading at diagnosis (CysR profile versus CysRC1 versus CysRC1C7). Remissions occurred earlier in participants treated by the NICE protocol (rituximab 1 g the day of rituximab infusion and 2 weeks after rituximab infusion; P<0.001) (Figure 2) or in participants with anti-CysR–restricted activity (P=0.01; Supplemental Figure 1A), with no difference according to proteinuria at diagnosis (P=0.64; Supplemental Figure 1B) or anti-PLA2R1 titers (P=0.26; Supplemental Figure 1C).
We found a strong association between epitope spreading and anti-PLA2R1 titer (Supplemental Figure 2A). The clinical remission rate at month 6 was different according to the rituximab protocol combined with anti-PLA2R1 titer at baseline (P=0.02) (low titer was defined as <70 RU/ml) and tended to be different at last follow-up (P=0.07) (Supplemental Figures 3 and 4 A and B). We established receiver operating characteristic curve (area under the curve =0.68 [IQR, 0.53–0.83]; P=0.03) to define a threshold of anti-PLA2R1 titer associated with epitope spreading: a titer >321 RU/ml was associated with spreading, with a sensitivity of 32.4% [IQR, 19.6%–48.5%] and a specificity of 94.4% [IQR, 74.2%–99.7%] (Supplemental Figure 2, B–D).
Relapses
During follow-up, 41 participants achieved partial or complete remission and eight participants relapsed (Supplemental Table 2), with a median time to relapse of 12 months [IQR, 7–17]. There was no significant difference between participants with (n=8) or without relapse (n=33) for age, proteinuria, anti-PLA2R1 antibodies titers before rituximab, time from diagnosis to treatment, and residual serum rituximab levels at month 3. Only epitope spreading at diagnosis and an incomplete PLA2R1 antibody depletion at month 6 were significantly associated with relapses (eight out of eight (100%) versus 16 out of 33 (48%); P=0.01, and four out of eight (50%) versus five out of 33 (9%); P=0.05, respectively) (Table 5).
Table 5.
Baseline characteristics and outcome data in relapsing and nonrelapsing participants from NICE and GEMRITUX cohorts treated with rituximab
| Participants Characteristics | Relapse (n=8) | No Relapse (n=33) |
|---|---|---|
| Baseline characteristics | ||
| Age | 60 [51; 70] | 57 [46; 68] |
| Sex ratio (F/M) | 2/6 | 9/24 |
| Proteinuria (g/g of creatinine) | 6.3 [5.2; 12.7] | 7.0 [4.7; 9.3] |
| Serum albumin (g/dl) | 2.0 [1.1; 2.2] | 2.3 [1.8; 2.7] |
| Serum creatinine (mg/dl) | 1.1 [0.8; 1.4] | 1.1 [0.9; 1.4] |
| BP (mm Hg) | ||
| Systolic | 120 [ 108; 127] | 120 [114; 140] |
| Diastolic | 63 [60; 80] | 80 [68; 81] |
| Anti-PLA2R1 titer (RU/ml) at first infusion | 113.8 [35.3; 249.4] | 100.5 [38.3; 213.7] |
| Spreadinga | ||
| No | 0 (0) | 17 (52) |
| Yes | 8 (100) | 16 (48) |
| Characteristics at month 3 | ||
| Serum rituximab (μg/ml) | 3.1 [0.0; 6.6] | 0.0 [0.0; 7.5] |
| Anti-PLA2R1 titer (RU/ml) | 7.5 [0.0; 49.1] | 0.0 [0.0; 11.0] |
| CD19 count | 0.0 [0.0; 13.7] | 1.0 [0.0; 17.25]b |
| Characteristics at month 6 | ||
| Anti-PLA2R1 titer (RU/ml) | 9.5 [0.0; 37.0] | 0 [0.0; 3.0] |
| CD19 count | 19.0 [11.7; 64.2] | 34.0 [5.9; 83.5]c |
| Rate of PLA2R1 antibody depletiona | 4/8 (50) | 5/33 (9) |
Quantitative values are medians [interquartile ranges]; qualitative values are numbers. F/M, female/male; PLA2R1, M-type phospholipase A2 receptor.
P<0.05.
Two points are missing.
Three points are missing.
Discussion
The first aim of this study was to compare the efficacy of rituximab in term of clinical remission at 6 months and before treatment modification in two cohorts of PLA2R1-related membranous nephropathy treated with two different regimens of rituximab. We show that higher cumulative doses of rituximab (2 g in NICE cohort versus 1.4 g in GEMRITUX) combined with different timing of infusion (2-week intervals instead of 1-week intervals) induce earlier remission and higher rate of remission with more complete remissions at month 6. A shorter time to remission is clinically meaningful to reduce the risk of complications of nephrotic syndrome, particularly venous thromboembolic disease. These findings are associated with higher residual serum rituximab levels at month 3 and lower CD19 counts at month 3 and month 6, and with a greater decline in anti-PLA2R1 antibodies titer at month 6. The lack of significant difference in the outcome at last follow-up between the two treatment groups might be explained by the small cohort size, because there was a trend toward a higher remission rate in the NICE cohort (86% versus 67%; P=0.12).
Little attention has been paid to rituximab pharmacokinetics in nephrotic patients except in one study by Fervenza et al., who showed a shorter rituximab t1/2 (11.5 days versus 18.0 days in patients with rheumatoid arthritis) with four doses of 375 mg/m2 (day 1, day 8, day 15, and day 22). Of note, the median rituximab serum level was four-fold higher with the NICE protocol despite a substantial dispersion of individual serum rituximab concentrations, and only one of the 27 GEMRITUX participants had detectable rituximab serum levels at month 3. Residual rituximabemia, residual serum level of rituximab, levels are dependent on proteinuria loss, rituximab dosing, and genetic factors. Although rituximabemia was not correlated with baseline proteinuria in our study, there was a trend to lower levels of proteinuria in the NICE cohort, which might account for a greater loss of rituximab antibody in the urine in the GEMRITUX cohort. It has recently been suggested that such loss in urine should modify the formulation or modality of rituximab delivery to ensure efficacy of the therapy (34). Cravedi et al. (25) have shown that low-dose rituximab can reduce cost, whereas other groups (24,27) have found that low-dose regimens are poorly effective. Our results suggest that low doses may delay remission even if B cell depletion is achieved. Absence of remission at last follow-up was associated with lower residual serum rituximab levels, higher CD19 count, and higher anti-PLA2R1 antibody levels at month 3. These findings make a case for the use of the NICE regimen, which was also used in the Multicenter Randomized Controlled Trial of Rituximab versus Cyclosporine in the Treatment of Idiopathic Membranous Nephropathy (MENTOR), instead of the GEMRITUX regimen. They also suggest that the dose of rituximab might be insufficient in patients with heavy proteinuria in whom we have recently shown that reinfusion of rituximab could induce remission in patients that were considered “refractory” to rituximab (35).
The second aim was to identify factors associated with remission at month 6 and last follow-up in the two combined cohorts. We confirmed that the absence of spreading at baseline was associated with higher rate of clinical remission (23). We found that the percentage of epitope spreaders tended to be lower at month 6 with the NICE protocol, which suggests that high-dose rituximab may reverse spreading. Further studies are needed to determine if this improves outcome as compared with patients that fail to experience spreading reversal. A combined analysis of rituximab protocol and spreading suggested that the NICE protocol blunted the effects of spreading on clinical remission at last follow-up. As long as epitope spreading is not routinely available, we propose that patients with anti-PLA2R1 titer >321 RU/ml (using Euroimmun ELISA) should receive high-dose rituximab. We showed that in patients with anti-PLA2R1 titer >321 RU/ml, 95% are spreaders. We previously demonstrated in the GEMRITUX cohort that 100% of patients with a titer >369.5 RU/ml are spreaders (23). Another point to notice concerns the possibility that the cut-off of anti-PLA2R1 antibodies at a low level according to the Euroimmun ELISA may need to be redefined with more sensitive assays. In fact, an indirect immunofluorescence test is still necessary for low titers (3–14 RU/ml) to confirm anti-PLA2R1 antibody positivity (36). It should be useful to add a construct CysR-deleted domain to diagnose spreader and nonspreader in this kit. Overall, these results confirm that analysis of epitope spreading may provide an added value to quantitative serology.
This study has several limitations. First, it is a retrospective study but uses systematically collected prospective data and samples. Second, the number of participants is relatively small. Third, there is a trend for higher proteinuria in the GEMRITUX cohort (P=0.13), which could contribute to the better outcome in the NICE cohort. We cannot formally exclude a higher rate of early spontaneous remissions in the NICE cohort compared with GEMRITUX, although this is unlikely because we previously showed that spontaneous remission occurred mainly in nonspreader patients (45% versus 0.05% in spreaders) (13), and we had the same number of nonspreader patients in the two cohorts.
In conclusion, we have made several clinically relevant observations. First, rituximab dose seems to affect the early remission rate at month 6, particularly in patients with PLA2R1 epitope spreading. Second, higher residual serum rituximab levels at month 3 are associated with a higher rate of clinical remission. Third, epitope spreading is confirmed as a factor negatively associated with clinical remission, and is now potentially associated positively with relapse.
Disclosures
Dr. Dahan reports personal fees from Amicus Therapeutics. Dr. Lambeau has a patent for Diagnosis for Membranous Nephropathy licensed with and with royalties paid to Euroimmun and a patent for Methods and Kits for Monitoring Membranous Nephropathy licensed to Euroimmun issued. Dr. Seitz-Polski and Dr. Lambeau have a patent for Prognosis and Monitoring of Membranous Nephropathy Based on the Analysis of PLA2R1 Epitope Profile and Spreading pending. Dr. Andreani, Dr. Benzaken, Dr. Bernard, Dr. Debiec, Dr. Esnault, Dr. Ronco, Dr. Rosenthal, Dr. Rousseau, Dr. Ticchioni, and Dr. Zaghrini have nothing to disclose.
Funding
Dr. Esnault and Dr. Seitz-Polski are supported by grants from the Centre Hospitalier Universitaire de Nice and the Direction générale de l’Offre de soins of the French Ministry of Health (PHRC2011-A01302-39, NCT01897961). Dr. Lambeau is supported by grants from CNRS, the National Research Agency (ANR) through the Investments for the Future LABEX SIGNALIFE programme (MNaims reference #ANR-17-CE17-0012-01) and the Fondation pour la Recherche Médicale (FRMING20140129210, SPF20150934219, FDT201805005509, and DEQ20180339193). Dr. Ronco is supported by grants from the European Research Council ERC-2012-ADG_20120314 (grant agreement 322947) and the Seventh Framework Programme of the European Community contract 2012-305608 (European Consortium for High-Throughput Research in Rare Kidney Diseases). Dr. Seitz-Polski is supported by a grant of Les Amis de la faculté de médecine de Nice. Dr. Zaghrini is supported by grants the National Research Agency (ANR) through the Investments for the Future LABEX SIGNALIFE programme (reference #ANR-11-LABX-0028-01). The sponsor of the GEMRITUX cohort (NCT01508468) was Assistance Publique-Hôpitaux de Paris (Département de la Recherche Clinique et du Développement). The GEMRITUX study received legal, monitoring, and administrative management support from the Assistance Publique-Hôpitaux de Paris. The sponsor of the NICE cohort was Centre Hospitalier de Nice (NCT02199145). This work has been developed and supported through the FHU Oncoage, Nice.
Supplementary Material
Acknowledgments
Rituximab was given by Hoffmann–La Roche to Dr. Ronco for the GEMRITUX trial.
Footnotes
B.S.-P., K.D., and H.D., and G.L., P.R., and V.L.M.E. are cofirst and colast authors.
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Does Epitope Spreading Influence Responsiveness to Rituximab in PLA2R-Associated Membranous Nephropathy?,” on pages 1122–1124.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11791018/-/DCSupplemental.
Supplemental Table 1. Prognosis factors of proteinuria remission before treatment modification in the pooled NICE and GEMRITUX participants.
Supplemental Table 2. Individual biological data of participants with membranous nephropathy relapses.
Supplemental Figure 1. (A) Time from initiation of rituximab to remission of membranous nephropathy in participants according to epitope profile. (B) Time from initiation of rituximab to remission of membranous nephropathy in participants according to proteinuria level at baseline (T1: low tertile, T2: medium tertile, T3: high tertile). (C) Time from initiation of rituximab to remission of membranous nephropathy in participants according to PLA2R1 titer at baseline (T1: low tertile versus T2-T3: medium and high tertile).
Supplemental Figure 2. Correlation between anti-PLA2R1 titer and epitope spreading.
Supplemental Figure 3. (A) Remission at month 6 according titer of PLA2R1 (T1: low tertile versus T2-T3: medium and high tertile) at baseline and rituximab protocol received. (B) Remission before treatment modification according to titer of PLA2R1 at baseline and rituximab protocol received.
Supplemental Figure 4. (A) Remission at month 6 according titer of PLA2R1 (at baseline and rituximab protocol received. (B) Remission before treatment modification according to titer of PLA2R1 at baseline and rituximab protocol received.
References
- 1.Debiec H, Guigonis V, Mougenot B, Decobert F, Haymann JP, Bensman A, Deschênes G, Ronco PM: Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med 346: 2053–2060, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, Ronco P, Remuzzi G: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glassock RJ: Diagnosis and natural course of membranous nephropathy. Semin Nephrol 23: 324–332, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA: Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25: 1357–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruggenenti P, Fervenza FC, Remuzzi G: Treatment of membranous nephropathy: Time for a paradigm shift. Nat Rev Nephrol 13: 563–579, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semac I, Palomba C, Kulangara K, Klages N, van Echten-Deckert G, Borisch B, Hoessli DC: Anti-CD20 therapeutic antibody rituximab modifies the functional organization of rafts/microdomains of B lymphoma cells. Cancer Res 63: 534–540, 2003 [PubMed] [Google Scholar]
- 15.Bonavida B: Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene 26: 3629–3636, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, Sotto JJ, Leroux D, Bensa JC, Plumas J: In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood 101: 949–954, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Wang SY, Racila E, Taylor RP, Weiner GJ: NK-cell activation and antibody-dependent cellular cytotoxicity induced by rituximab-coated target cells is inhibited by the C3b component of complement. Blood 111: 1456–1463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roccatello D, Sciascia S, Di Simone D, Solfietti L, Naretto C, Fenoglio R, Baldovino S, Menegatti E: New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: A prospective study and a review of the literature. Autoimmun Rev 15: 529–538, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P: Rituximab for idiopathic membranous nephropathy. Lancet 360: 923–924, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P; GEMRITUX Study Group : Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol 28: 348–358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seitz-Polski B, Debiec H, Rousseau A, Dahan K, Zaghrini C, Payré C, Esnault VLM, Lambeau G, Ronco P: Phospholipase A2 Receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol 29: 401–408, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cattran D, Brenchley P: Membranous nephropathy: Thinking through the therapeutic options. Nephrol Dial Transplant 32[Suppl 1]: i22–i29, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G: Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 2: 932–937, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroni G, Depetri F, Del Vecchio L, Gallelli B, Raffiotta F, Giglio E, Brunini F, D’Amico M, Longhi S, Radice A, Messa P, Sinico RA: Low-dose rituximab is poorly effective in patients with primary membranous nephropathy. Nephrol Dial Transplant 32: 1691–1696, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Piro LD, White CA, Grillo-López AJ, Janakiraman N, Saven A, Beck TM, Varns C, Shuey S, Czuczman M, Lynch JW, Kolitz JE, Jain V: Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 10: 655–661, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Cartron G, Blasco H, Paintaud G, Watier H, Le Guellec C: Pharmacokinetics of rituximab and its clinical use: Thought for the best use? Crit Rev Oncol Hematol 62: 43–52, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Cartron G, Trappe RU, Solal-Céligny P, Hallek M: Interindividual variability of response to rituximab: From biological origins to individualized therapies. Clin Cancer Res 17: 19–30, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Thurlings RM, Teng O, Vos K, Gerlag DM, Aarden L, Stapel SO, van Laar JM, Tak PP, Wolbink GJ: Clinical response, pharmacokinetics, development of human anti-chimaeric antibodies, and synovial tissue response to rituximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 69: 409–412, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Boye J, Elter T, Engert A: An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol 14: 520–535, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, Hofstra JM, Hoxha E, Stahl RA, Lambeau G, Stöcker W, Schlumberger W: Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421: 213–218, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Jacobs R, Langer-Jacobus T, Duong M, Stahl K, Haller H, Schmidt RE, Schiffer M: Detection and quantification of rituximab in the human urine. J Immunol Methods 451: 118–121, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Dahan K, Johannet C, Esteve E, Plaisier E, Debiec H, Ronco P: Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int 95: 233–234, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Li X, Ma C, Wang P, Liu J, Su H, Zhuo H, Kong X, Xu D, Xu D: Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: The optimal cut-off value for Chinese patients. Clin Chim Acta 476: 9.–, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.