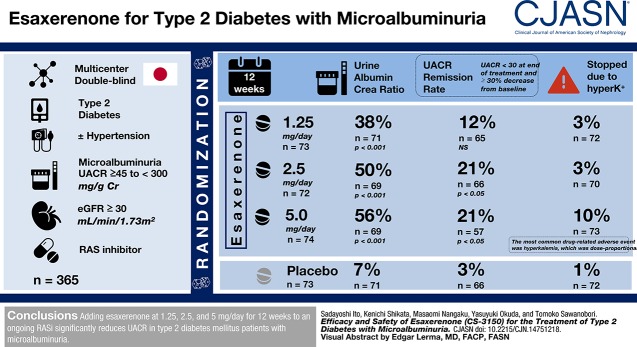
Visual Abstract
Keywords: Esaxerenone, mineralocorticoid receptor antagonist, type 2 diabetes mellitus, microalbuminuria, randomized, placebo-controlled, Urine albumin-to-creatinine ratio, remission
Abstract
Background and objectives
The progression of kidney disease in some patients with type 2 diabetes mellitus may not be adequately suppressed by renin-angiotensin system inhibitors. Esaxerenone (CS-3150) is a nonsteroidal mineralocorticoid receptor blocker that has shown kidney protective effects in preclinical studies, and it is a potential add-on therapy to treat diabetic kidney disease. This phase 2 study evaluated the efficacy and safety of esaxerenone in Japanese patients with type 2 diabetes mellitus and microalbuminuria.
Design, setting, participants, & measurements
This multicenter, randomized, double-blind, placebo-controlled trial enrolled 365 hypertensive or normotensive patients with type 2 diabetes mellitus and microalbuminuria (urinary albumin-to-creatinine ratio ≥45 to <300 mg/g creatinine) treated with renin-angiotensin system inhibitor who had eGFR≥30 ml/min per 1.73 m2. Participants were randomized to receive 0.625, 1.25, 2.5, or 5 mg/d esaxerenone or placebo for 12 weeks. The primary end point was the change in urinary albumin-to-creatinine ratio from baseline to week 12 (with last observation carried forward).
Results
Esaxerenone treatment at 1.25, 2.5, and 5 mg/d significantly reduced urinary albumin-to-creatinine ratio by the end of treatment (38%, 50%, and 56%, respectively) compared with placebo (7%; all P<0.001). The urinary albumin-to-creatinine ratio remission rate (defined as urinary albumin-to-creatinine ratio <30 mg/g creatinine at the end of treatment and ≥30% decrease from baseline) was 21% in the 2.5- and 5-mg/d groups versus 3% for placebo (both P<0.05). Adverse events occurred slightly more frequently with esaxerenone versus placebo, but the frequencies of drug-related adverse events and discontinuation rates were similar in the placebo and the 0.625-, 1.25-, and 2.5-mg/d groups. Drug-related adverse events and treatment discontinuations were marginally higher in the 5-mg/d group. The most common drug-related adverse event was hyperkalemia, which was dose proportional.
Conclusions
Adding esaxerenone at 1.25, 2.5, and 5 mg/d for 12 weeks to an ongoing renin-angiotensin system inhibitor significantly reduces urinary albumin-to-creatinine ratio in patients with type 2 diabetes mellitus and microalbuminuria.
Introduction
Diabetic kidney disease affects 20%–30% of patients with type 1 or type 2 diabetes mellitus, and it is the most common cause of ESKD requiring dialysis (1,2). Current treatment guidelines in Japan recommend a multifactorial therapeutic approach, including controlling blood glucose, lipids, and BP and using renin-angiotensin system inhibitors (RASis) to suppress the onset and progression of early diabetic kidney disease (3–5). Despite the extensive use of RASi to treat diabetic kidney disease, there is insufficient clinical efficacy and therefore, a need for the development of additional treatments. There are reports that, to achieve normoalbuminuria in diabetic kidney disease, treatment should be initiated during the early stages of disease (6–8). Aldosterone binds to the mineralocorticoid receptors in the kidneys (9). Aldosterone-induced mineralocorticoid receptor activation impairs insulin sensitivity and is associated with obesity, hypertension, and diabetes mellitus (10,11). Mineralocorticoid receptor blockers act on mineralocorticoid receptors in kidney tubular epithelial cells and lower BP by inhibition of urinary sodium reabsorption and potassium (K+) excretion. Excessive mineralocorticoid receptor activation can lead to kidney damage independent of BP (12,13). The existing mineralocorticoid receptor blockers spironolactone and eplerenone suppress mineralocorticoid receptor–induced kidney damage (10), and when added to an RASi, they can reduce albuminuria (13,14). However, eplerenone and spironolactone increase the risk of hyperkalemia compared with placebo, and eplerenone is contraindicated for administration in type 2 diabetes mellitus with albuminuria (15).
Esaxerenone (CS-3150; Daiichi-Sankyo), a nonsteroidal mineralocorticoid receptor blocker, showed kidney protective effects in preclinical studies and may be added to existing treatments for patients with diabetic kidney disease (16,17). In a phase 1 study, the safety of a single dose and multiple doses of esaxerenone was confirmed in healthy Japanese subjects (18). The objective of this study was to evaluate the dose-response, efficacy, and safety of esaxerenone and determine the optimal dose for decreasing albuminuria in participants with type 2 diabetes mellitus and microalbuminuria.
Materials and Methods
Study Design
This was a multicenter (71 sites) (Supplemental Appendix), randomized, double-blind, placebo-controlled phase 2 trial conducted in Japan from January 2015 to June 2016. Participants were randomized to receive 0.625, 1.25, 2.5, or 5 mg/d of esaxerenone or placebo orally for 12 weeks, with 6 weeks of follow-up (Supplemental Figure 1). Randomization was conducted by dynamic allocation (minimization method) on the basis of the eGFR and urinary albumin-to-creatinine ratio (UACR) obtained during the observation period.
This was a 12-week trial on the basis of previous trials of eplerenone and spironolactone to ensure sufficient time for the efficacy evaluation to be performed after the decrease in UACR had reached a steady state (19,20). The highest dose (5 mg/d) was on the basis of the safety data from a phase 2a study (unpublished data) and a phase 1 study in healthy adults (18).
Esaxerenone was administered orally once daily after breakfast for 12 weeks, and all participants received the same number of identical tablets to ensure blinding.
Participants meeting the following discontinuation criteria were withdrawn from the study: one measurement of serum K+ ≥6.0 mEq/L or two consecutive measurements of serum K+ ≥5.5 mEq/L, kidney dysfunction (defined as a ≥50% increase in serum creatinine for two consecutive measurements compared with baseline), severe hypotension, persistent sitting systolic BP of ≥180 or <90 mm Hg, and persistent sitting diastolic BP of ≥110 or <50 mm Hg.
Ethics
This study was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and the laws of Japan. The study protocol was approved by the institutional review boards of all participating institutions. All participants provided written informed consent before enrollment. The trial is registered on JapicCTI (JapicCTI-152774) and ClinicalTrials.gov (NCT02345057).
Participants
The inclusion criteria were as follows: hypertensive or normotensive patients with type 2 diabetes mellitus, ages >20 years old, prior treatment with an RASi (at the highest usual dose) for at least 3 months, microalbuminuria (first urine in the morning measured; UACR≥45 to <300 mg/g creatinine on at least two occasions during the observation period), and eGFR calculated from serum creatinine of ≥30 ml/min per 1.73 m2 using the following equation (eGFR =194× serum creatinine−1.904× age−0.287 [multiplied by 0.739 for women]) (21).
The key exclusion criteria were as follows: presence of type 1 diabetes, glycated hemoglobin (National Glycohemoglobin Standardization Program criteria) ≥8.4%, secondary glucose intolerance, nondiabetic kidney disease, nephrotic syndrome, secondary hypertension, or malignant hypertension; sitting systolic BP of ≥160 or <110 mm Hg and sitting diastolic BP of ≥100 or <50 mm Hg measured at the second and third visits; serum K+ level of <3.5 or ≥5.1 mEq/L in participants with eGFR of ≥45 ml/min per 1.73 m2; and a serum K+ level of <3.5 or ≥4.8 mEq/L in participants with eGFR of ≥30 ml/min per 1.73 m2 and <45 ml/min per 1.73 m2. The study permitted the re-enrolment of a single participant withdrawn during the observation period.
End Points
Primary Efficacy End Point.
The primary end point was the change in UACR from baseline to the end of treatment (mean of values at weeks 11 and 12).
Secondary Efficacy End Points.
The secondary end point was the proportion of participants in remission defined as a reversal of UACR to normoalbuminuria (<30 mg/g creatinine) and a reduction in UACR by ≥30% from baseline at both weeks 11 and 12. Other end points assessed included the proportion of participants who sustained remission after treatment, had a ≥30% or ≥50% reduction in UACR, and had a UACR that progressed to ≥300 mg/g creatinine at the last two time points of the observation period. Changes in BP from baseline, plasma aldosterone concentration, plasma renin activity, and levels of creatinine were also analyzed.
Subgroup analyses of the primary end point stratified by body mass index (BMI; ≥25 and <25 kg/m2), systolic BP (<140 and ≥140 mm Hg), eGFR (≥60 and <60 ml/min per 1.73 m2), and concurrent/nonconcurrent use of non-RASi antihypertensive drugs were performed.
Pharmacokinetic End Point.
Pharmacokinetic analysis included the assessment of trough plasma esaxerenone concentrations, and samples were collected on weeks 4 and 12. These plasma samples were analyzed using liquid chromatography-tandem mass spectrometry as described previously (18).
Safety End Points.
The safety end points included assessment of adverse events, laboratory tests, vital signs, and changes in eGFR from baseline. The proportions of participants with serum K+ ≥5.5 mEq/L observed at least once and participants with serum K+ ≥6.0 mEq/L or two consecutive measurements of serum K+ ≥5.5 mEq/L observed during the treatment period were also assessed. Additionally, the proportions of participants with decreased eGFR by ≥30%, ≥40%, and ≥50% at week 12 were assessed. Adverse events were tabulated by frequency as well as by preferred term and system organ class. For serious adverse events, the likelihood of a causal relationship with treatment was assessed.
Statistical Analyses
The planned sample size of 65 participants per group was designed to detect a statistically significant difference in paired comparisons between the placebo and each esaxerenone group at esaxerenone dosages of ≥1.25 mg/d with a type 1 error of 5% (two tailed) and at least 80% statistical power. It assumed that change in UACR from baseline to week 12 of treatment would be −10% in the placebo group and −33% in the esaxerenone 1.25 mg group, that the common SD for logarithmically converted change in UACR would be 0.55, and that dropout rate would be 5%.
Primary efficacy analyses were performed using the full analysis set. For the primary end point, the last observation carried forward method was used to impute missing UACR values. UACR values were log transformed, and the change in UACR from baseline to the end of treatment was calculated. These values were defined as the averages of weeks 11 and 12 after last observation carried forward imputation. We compared each esaxerenone dosage group with the placebo group and calculated the geometric least squares mean ratio to baseline with corresponding 95% confidence intervals (95% CIs) and P values. This was on the basis of an analysis of covariance (ANCOVA) model, which included treatment group as a factor and baseline log-transformed UACR, baseline eGFR, and baseline sitting systolic BP as covariates. When comparing the change in UACR among groups, the fixed sequence procedure was used to adjust for the multiplicity: the comparison was initially made between the high-dose esaxerenone groups and the placebo group with two-sided 5% significance levels. The comparison for the other dosage groups was continued at a 5% significance level in descending order of dosage but only when significance was shown.
For the secondary end point, UACR remission rates were calculated using point estimates for each group with the exact 95% CI on the basis of the F distribution. We also used a multivariable logistic regression model to compare remission ratios. Here, we calculated the adjusted odds ratio, its 95% CI, and P values for achieving remission.
For the primary and secondary end points, the same analyses were conducted for the following subgroups: BMI (≥25 and <25 kg/m2), systolic BP (<140 and ≥140 mm Hg), eGFR (≥60 and <60 ml/min per 1.73 m2), and concurrent use of non-RASi antihypertensive drugs. In addition, we performed an ad hoc analysis to investigate treatment-subgroup interactions using an ANCOVA model.
For eGFR, sitting BP, and renin-angiotensin aldosterone system hormone, the summary statistics and change from baseline were calculated by visit, and the least squares means of the change from baseline were calculated on the basis of the ANCOVA model, which included treatment group as a factor and baseline value as a covariate. In addition, we performed an ad hoc analysis to investigate the association between UACR and sitting BP.
Safety analyses were conducted in a descriptive manner and presented with the appropriate summary statistics by treatment group. Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Participants
In total, 365 participants were randomized; seven of them were excluded due to suspected Good Clinical Practice violations, and 358 participants (men/women: 279/79) were included in the full analysis set (Supplemental Figure 2, Table 1).
Table 1.
Baseline characteristics of participants with type 2 diabetes and CKD in a clinical trial of esaxerenone (efficacy full analysis set)
| Clinical Characteristics/Prior Treatment | Placebo, n=73 | Esaxerenone 0.625 mg/d, n=71 | Esaxerenone 1.25 mg/d, n=72 | Esaxerenone 2.5 mg/d, n=70 | Esaxerenone 5 mg/d, n=72 | All, n=358 |
|---|---|---|---|---|---|---|
| Sex, men | 57 (78) | 54 (76) | 54 (75) | 57 (81) | 57 (79) | 279 (78) |
| Age, yr | 66±10 | 66±9 | 66±8 | 64±11 | 65±9 | 65±9 |
| Weight, kg | 70±15 | 69±12 | 68±15 | 70±12 | 69±13 | 69±13 |
| Body mass index, kg/m2 | 25.9±4.2 | 25.8±3.6 | 25.7±3.9 | 26.0±4.1 | 25.6±3.8 | 25.8±3.9 |
| Sitting systolic BP, mm Hg | 138±11 | 138±11 | 139±10 | 137±13 | 136±12 | 138±11 |
| Sitting diastolic BP, mm Hg | 76±9 | 76±9 | 76±8 | 77±9 | 76±9 | 76±9 |
| UACR (mg/g Cr), geometric mean (95% CI) | 110 (98 to 123) | 104 (92 to 116) | 111 (98 to 125) | 112 (100 to 126) | 110 (101 to 126) | 110 (104 to 115) |
| HbA1c, % | 6.9±0.6 | 6.8±0.7 | 6.8±0.7 | 7.0±0.6 | 6.9±0.6 | 6.9±0.6 |
| Serum K+, mEq/L | 4.3±0.3 | 4.2±0.3 | 4.3±0.3 | 4.3±0.3 | 4.2±0.3 | 4.3±0.3 |
| eGFR, ml/min per 1.73 m2 | 69±19 | 66±14 | 66±18 | 68±19 | 69±18 | 67±18 |
| LDL cholesterol, mg/dl | 109±28 | 101±28 | 107±29 | 107±30 | 109±31 | 107±29 |
| Duration of diabetes, yr | 14±9 | 14±9 | 16±10 | 16±12 | 14±8 | 15±10 |
| Other diabetic complications | 29 (40) | 34 (48) | 39 (54) | 35 (50) | 36 (50) | 173 (48) |
| Hypertension | 71 (97) | 68 (96) | 70 (97) | 68 (97) | 68 (94) | 345 (96) |
| Hyperlipidemia | 56 (77) | 56 (79) | 53 (74) | 52 (74) | 53 (74) | 270 (75) |
| Cardiac disorder | 14 (19) | 10 (14) | 13 (18) | 9 (13) | 13 (18) | 59 (17) |
| Other antihypertensive agents | ||||||
| Calcium channel blocker | 47 (64) | 47 (66) | 47 (65) | 43 (61) | 47 (65) | 231 (65) |
| Diuretics | 8 (11) | 8 (11) | 14 (19) | 8 (11) | 7 (10) | 45 (13) |
| Antihypertensive agents | ||||||
| ARB | 71 (97) | 69 (97) | 70 (97) | 67 (96) | 71 (99) | 349 (97) |
| ACEi | 2 (3) | 2 (3) | 2 (3) | 3 (4) | 1 (1) | 10 (3) |
| No. of antihypertensive agents | ||||||
| Monotherapy | 25 (34) | 19 (27) | 20 (28) | 24 (34) | 22 (31) | 110 (31) |
| Double therapy | 30 (41) | 36 (51) | 32 (44) | 34 (49) | 32 (44) | 164 (46) |
| Triple therapy or more | 18 (25) | 16 (23) | 20 (28) | 12 (17) | 18 (25) | 84 (24) |
| HMG-CoA reductase inhibitor | 38 (52) | 31 (44) | 31 (43) | 38 (54) | 31 (43) | 169 (47) |
| Hypoglycemic agent | 68 (93) | 61 (86) | 64 (89) | 62 (89) | 57 (79) | 312 (87) |
| DPP4 inhibitor | 48 (66) | 44 (62) | 44 (61) | 46 (66) | 46 (64) | 228 (64) |
| SGLT2 inhibitor | 3 (4) | 3 (4) | 4 (6) | 10 (14) | 6 (8) | 26 (7) |
Data are n (%) or mean ± SD unless otherwise stated. UACR, urinary albumin-to-creatinine ratio; Cr, creatinine; 95% CI, 95% confidence interval; HbA1c, glycated hemoglobin; K+, potassium; ARB, angiotensin receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme; DPP4, dipeptidyl peptidase 4; SGLT2, sodium-glucose cotransporter 2.
Participant baseline demographic and clinical characteristics are summarized in Table 1. The mean age, baseline glycated hemoglobin, and sitting systolic BP/diastolic BP were 65 years old, 6.9%, and 138/76 mm Hg, respectively; the geometric mean at baseline UACR was 110 mg/g creatinine. Overall, 96% of participants had hypertension. The proportions of participants with eGFR≥60 and <60 ml/min per 1.73 m2 were 65% and 35%, respectively. There were no notable differences between treatment groups in baseline demographics.
Efficacy
Primary Efficacy End Point.
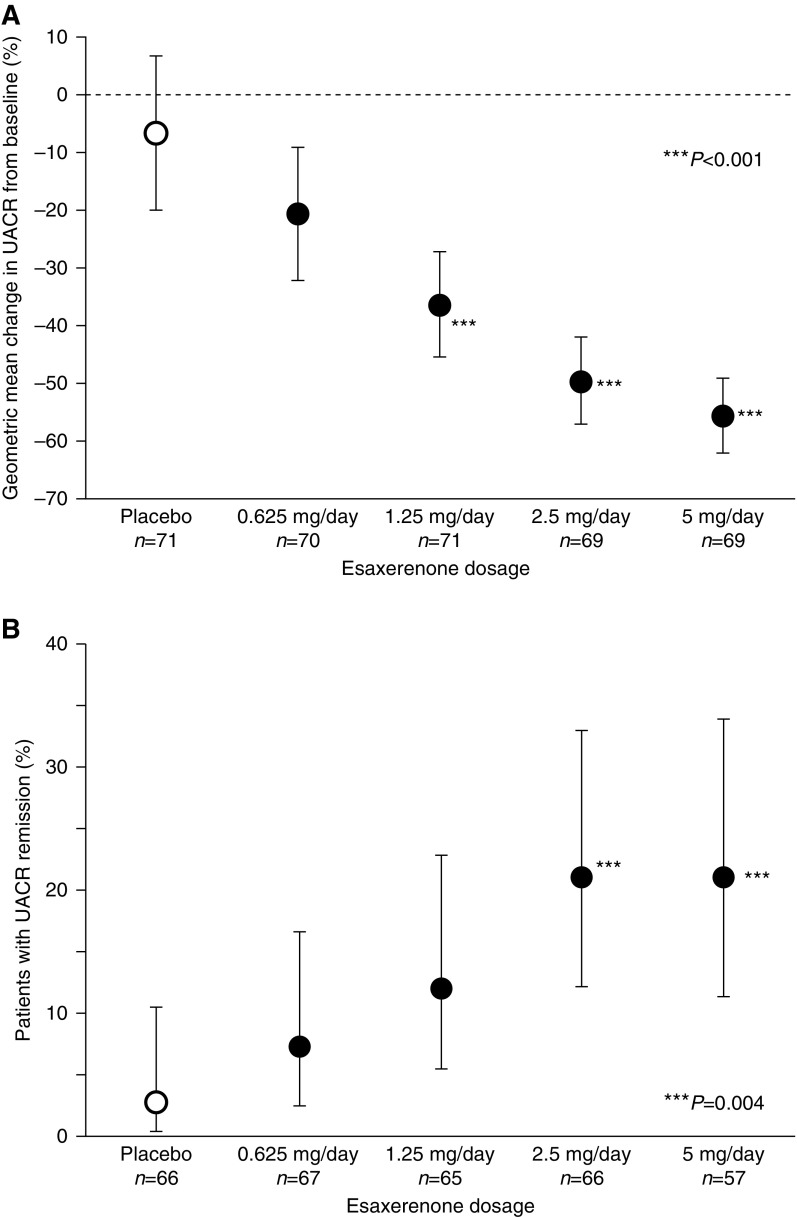
Esaxerenone at 0.625, 1.25, 2.5, and 5 mg/d doses dependently reduced UACR at the end of treatment (21%, 38%, 50%, and 56% from baseline, respectively) compared with placebo (7%) (Figure 1A). The geometric least squares mean ratios (95% CIs) of the value at the end of treatment to the value before the first dose were 0.9 (95% CI, 0.8 to 1.1) for the placebo group, 0.8 (95% CI, 0.7 to 0.9) for the esaxerenone 0.625-mg/d group, 0.6 (95% CI, 0.5 to 0.7) for the 1.25-mg/d group, 0.50 (95% CI, 0.4 to 0.6) for the 2.5-mg/d group, and 0.4 (95% CI, 0.4 to 0.5) for the 5-mg/d group (Table 2). Except for the 0.625-mg/d group, the rate of decrease in UACR for all esaxerenone groups was significantly greater than for the placebo group (all P<0.001). Approximately 10% of participants did not have their primary end point values recorded (Supplemental Table 1). Therefore, we conducted several sensitivity analyses to investigate the effect of missing data on the primary end point analysis and found that the data were consistent (Supplemental Table 2).
Figure 1.
Esaxerenone dose dependently reduced mean changes in urinary albumin-to-creatinine ratio (UACR) from baseline to the end of treatment (A) and higher UACR remission rates were observed with esaxerenone 2.5- and 5-mg/d groups at the end of treatment (B). Data are geometric mean (point estimates) ±95% confidence interval (A); point estimate ± exact 95% CI (B). Among the participants in the full analysis set, those with UACR measurements at both weeks 11 and 12 were included in the calculation of the achievement rate of remission. ***P<0.001 versus placebo (A); ***P=0.004 versus placebo (B).
Table 2.
Mean change from baseline to week 12 for primary and secondary outcomes
| Outcome Measures | Placebo | Esaxerenone 0.625 mg/d | Esaxerenone 1.25 mg/d | Esaxerenone 2.5 mg/d | Esaxerenone 5 mg/d |
|---|---|---|---|---|---|
| UACR, mg/g Cr | n=73a; 66b | n=71a; 68b | n=72a; 65b | n=70a; 66b | n=72a; 58b |
| Baseline, mean (SD) | 123 (58) | 117 (60) | 127 (69) | 125 (58) | 126 (62) |
| Week 12, mean (SD) | 145 (191) | 113 (123) | 96 (110) | 81 (96) | 66 (63) |
| Change from baseline, mean (SD) | 21 (181) | −4 (97) | −30 (104) | −39 (83) | −59 (65) |
| Difference to baseline, geometric LS mean ratio [95% CI]c | 0.9 [0.8 to 1.1] | 0.8 [0.7 to 0.9] | 0.6 [0.5 to 0.7] | 0.5 [0.4 to 0.6] | 0.4 [0.4 to 0.5] |
| Difference to placebo, geometric LS mean ratio [95% CI]c | — | 0.8 [0.7 to 1.0] | 0.7 [0.5 to 0.8] | 0.5 [0.4 to 0.7] | 0.5 [0.4 to 0.6] |
| UACR remission rate, n (%) | 2 (3) | 5 (8) | 8 (12) | 14 (21) | 12 (21) |
| Treatment difference, OR [95% CI]d | — | 2.2 [0.4 to 12] | 4.4 [0.9 to 22] | 10 [2.1 to 49] | 10 [2.1 to 50] |
| eGFR, ml/min per 1.73 m2 | n=73a; 67b | n=71a; 68b | n=72a; 65b | n=70a; 66b | n=72a; 58b |
| Baseline, mean (SD) | 69 (18) | 65 (14) | 66 (18) | 68 (19) | 69 (18) |
| Week 12, mean (SD) | 71 (21) | 66 (15) | 64 (23) | 66 (22) | 65 (19) |
| Change from baseline, mean (SD) | 1 (6) | 0.3 (7) | −2 (8) | −2 (8) | −6 (8) |
| Difference to baseline, LS mean [95% CI]e | 1 [−0.5 to 3.0] | 0.4 [−1.3 to 2.2] | −2 [−3.5 to 0.1] | −2 [−3.5 to 0.1] | −7 [−8.6 to −4.8] |
| Difference to placebo, LS mean [95% CI]e | — | −0.8 [−3.3 to 1.7] | −3 [−5.5 to −0.5] | −3 [−5.5 to −0.5] | −8 [−10 to −5.3] |
| Serum K+, mEq/L | n=72a; 66b | n=71a; 68b | n=72a; 65b | n=70a; 66b | n=73a; 59b |
| Baseline, mean (SD) | 4.3 (0.3) | 4.2 (0.3) | 4.3 (0.3) | 4.3 (0.3) | 4.2 (0.3) |
| Week 12, mean (SD) | 4.2 (0.3) | 4.3 (0.4) | 4.4 (0.5) | 4.4 (0.4) | 4.6 (0.5) |
| Change from baseline, mean (SD) | −0.1 (0.3) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.4) | 0.4 (0.4) |
| Difference to placebo [95% CI] | — | 0.2 [0.1 to 0.3] | 0.3 [0.1 to 0.4] | 0.2 [0.1 to 0.4] | 0.5 [0.4 to 0.6] |
UACR, urinary albumin-to-creatinine ratio; Cr, creatinine; LS, least squares; 95% CI, 95% confidence interval; —, not applicable; OR, odds ratio; K+, potassium.
Number of participants at baseline.
Number of participants at week 12.
On the basis of the analysis of covariance model, with treatment group as a factor and log-transformed baseline values of eGFR and sitting systolic BP as covariates. Estimates are back transformed and expressed as the ratio in the original scale.
Adjusted for baseline UACR, eGFR, and sitting systolic BP.
Adjusted for baseline eGFR.
UACR Remission and Subgroup Analyses.
The UACR remission rates were significantly higher for the esaxerenone 2.5-and 5-mg/d groups (both 21%) compared with the placebo group (3%; both P=0.004; adjusted odds ratios of 10; 95% CI, 2.1 to 49 and 10; 95% CI, 2.1 to 50, respectively) (Figure 1B, Table 2). The number of participants achieving sustained remission ranged from two to 14 participants per group, and no set trend was observed. The UACR progression rate was 0%–4% for all groups. The percentages of participants who achieved a reduction of ≥30% in UACR increased as esaxerenone dose increased and were higher than with placebo group participants (placebo, 14%; 0.625 mg/d, 35%; 1.25 mg/d, 49%; 2.5 mg/d, 57%; and 5 mg/d, 65%). A similar effect was observed for participants who achieved a ≥50% reduction in UACR (placebo, 9%; 0.625 mg/d, 16%; 1.25 mg/d, 28%; 2.5 mg/d, 39%; and 5 mg/d, 40%). There were no significant treatment-subgroup interactions for the reduction in UACR in subgroups stratified by BMI (P=0.12), sitting systolic BP (P=0.34), and eGFR (P=0.19) or groups with and without concurrent use of non-RASi antihypertensive drugs (P=0.89) (Supplemental Figure 3).
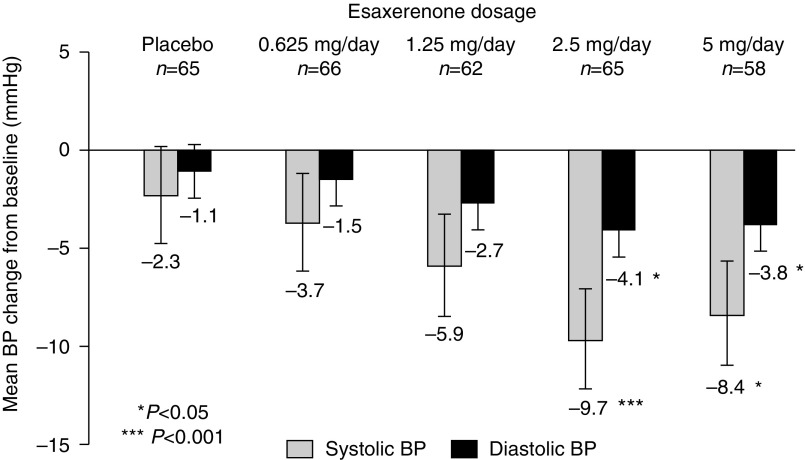
BP.
Sitting BP decreased in all groups from week 2, and all esaxerenone groups showed a steady trend to numerically greater reductions from baseline compared with the placebo group (Figure 2).
Figure 2.
Esaxerenone groups show numerically greater reductions in mean changes in systolic BP and diastolic BP compared with the placebo group at the end of treatment. Data are least square means ±95% confidence interval. *P<0.05 versus placebo; ***P<0.001 versus placebo.
Plasma Renin Activity and Plasma Aldosterone Concentration.
Both plasma renin activity and plasma aldosterone concentration increased dose dependently in all esaxerenone groups in the fourth week of dose administration, with a high level maintained until week 12 (data not shown). The levels decreased almost to the baseline values during the follow-up period.
Pharmacokinetics.
Trough esaxerenone plasma concentrations increased in proportion with esaxerenone dose and were approximately the same in weeks 4 and 12 of the treatment period (Supplemental Table 3).
Safety
The overall rates of treatment-emergent adverse events were 56% (40 of 72) in the placebo group and 54% (38 of 71), 69% (50 of 72), 67% (47 of 70), and 64% (47 of 73) in the esaxerenone 0.625-, 1.25-, 2.5-, and 5-mg/d groups, respectively (Table 3). All reported treatment-emergent adverse events, except for one serious case of intestinal obstruction in a patient in the esaxerenone 1.25-mg/d group, were mild to moderate in severity. The most common treatment-emergent adverse events reported (experienced by ≥5% of participants) were nasopharyngitis, serum K+ increase, bruises, dizziness when standing, and kidney dysfunction. Among these treatment-emergent adverse events, serum K+ increase was observed to be dose dependent. Dizziness when standing and kidney dysfunction were observed only in the esaxerenone 5-mg/d group. No sex hormone–related adverse events (gynecomastia) were reported. Serious treatment-emergent adverse events in patients included one case each of intestinal obstruction (esaxerenone 1.25-mg/d group), cerebral infarction (esaxerenone 2.5-mg/d group), and carotid stenosis (esaxerenone 5-mg/d group). Only the cerebral infarction was causally related to esaxerenone as judged by an investigator. No deaths occurred in the study.
Table 3.
Summary of treatment-emergent adverse events
| Category of Treatment-Emergent Adverse Events | Placebo, n=72 | Esaxerenone 0.625 mg/d, n=71 | Esaxerenone 1.25 mg/d, n=72 | Esaxerenone 2.5 mg/d, n=70 | Esaxerenone 5 mg/d, n=73 | All, n=358 |
|---|---|---|---|---|---|---|
| Participants with at least one treatment-emergent adverse event | 40 (56) | 38 (54) | 50 (69) | 47 (67) | 47 (64) | 222 (62) |
| Participants with at least one drug-related treatment-emergent adverse event | 8 (11) | 6 (9) | 11 (15) | 9 (13) | 21 (29) | 55 (15) |
| Participants with at least one serious treatment-emergent adverse event | 0 (0) | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 3 (0.8) |
| Participants with at least one drug-related serious treatment-emergent adverse event | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 1 (0.3) |
| Participants who discontinued treatment because of a treatment-emergent adverse event | 2 (3) | 2 (3) | 3 (4) | 3 (4) | 11 (15) | 21 (6) |
| Participants who discontinued treatment because of a drug-related treatment-emergent adverse event | 0 (0) | 1 (1) | 1 (1) | 3 (4) | 9 (12) | 14 (4) |
| Participants who discontinued treatment because of increased serum K+ (≥6.0 mEq/L or two consecutive measurements of ≥5.5 mEq/L) | 1 (1) | 2 (3) | 2 (3) | 2 (3) | 7 (10) | 14 (4) |
| Treatment-emergent adverse events reported in ≥3% of participants | ||||||
| Nasopharyngitis | 9 (13) | 11 (16) | 15 (21) | 15 (21) | 11 (15) | 61 (17) |
| Dizziness postural | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 4 (1) |
| Upper respiratory tract inflammation | 1 (1) | 2 (3) | 0 (0) | 2 (3) | 3 (4) | 8 (2) |
| Abdominal discomfort | 0 (0) | 1 (1) | 3 (4) | 0 (0) | 0 (0) | 4 (1) |
| Nausea | 3 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.8) |
| Periodontal disease | 0 (0) | 0 (0) | 3 (4) | 0 (0) | 0 (0) | 3 (0.8) |
| Back pain | 0 (0) | 0 (0) | 3 (4) | 2 (3) | 0 (0) | 5 (1) |
| Blood uric acid increased | 1 (1) | 1 (1) | 3 (4) | 0 (0) | 2 (3) | 7 (2) |
| C-reactive protein increased | 3 (4) | 0 (0) | 0 (0) | 3 (4) | 0 (0) | 6 (2) |
| White blood cell count increased | 1 (1) | 0 (0) | 0 (0) | 3 (4) | 0 (0) | 4 (1) |
| Blood K+ increased | 2 (3) | 2 (3) | 5 (7) | 10 (14) | 15 (21) | 34 (9) |
| Bruises | 1 (1) | 0 (0) | 5 (7) | 2 (3) | 0 (0) | 8 (2) |
| Kidney dysfunction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (6) | 4 (1) |
Data are n (%). System Organ Classes and Preferred Terms are coded using MedDRA/J version 18.0. The percentage was calculated using the number of participants in the column heading as the denominator. K+, potassium.
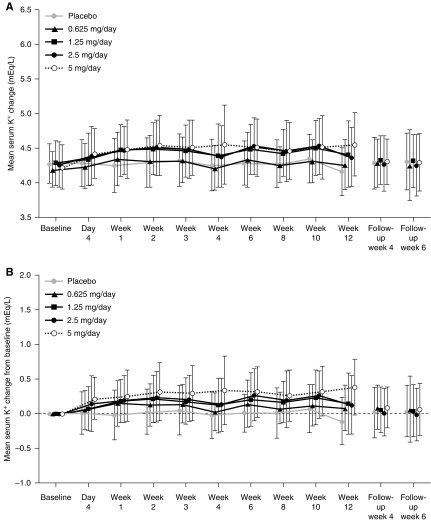
Treatment-emergent adverse events that resulted in discontinuation occurred in 3% (two of 72) of participants in the placebo group and 3% (two of 71), 4% (three of 72), 4% (three of 70), and 15% (11 of 73) in the esaxerenone 0.625-, 1.25-, 2.5-, and 5-mg/d groups, respectively (Table 3). Serum K+ increase was the most commonly reported treatment-emergent adverse event that resulted in discontinuation. Serum K+ levels increased dose dependently; however, these returned to baseline during the follow-up period (Figure 3). Serum K+ increases were reported in 3% (two of 72) of participants in the placebo group, 3% (two of 71) in the esaxerenone 0.625-mg/d group, 7% (five of 72) in the 1.25-mg/d group, 14% (ten of 70) in the 2.5-mg/d group, and 21% (15 of 73) in the 5-mg/d group, showing esaxerenone dose dependency. All events were mild, and participants recovered without any treatment. The percentages of participants with serum K+ ≥6.0 mEq/L or two consecutive measurements of serum K+ ≥5.5 mEq/L were 1% (one of 72) in the placebo group, 3% (two of 71) in the esaxerenone 0.625-mg/d group, 3% (two of 72) in the 1.25-mg/d group, 3% (two of 70) in the 2.5-mg/d group, and 10% (seven of 73) in the 5-mg/d group (Table 3). The maximum serum K+ level observed was 6.9 mEq/L, which occurred in the esaxerenone 5-mg/d group; however, this was reduced to 5.1 mEq/L after esaxerenone treatment was ceased.
Figure 3.
Serum potassium (K+; A) and serum K+ from baseline (B) increased dose dependently with esaxerenone during treatment visits but returned to baseline during the follow-up (safety analysis set). Data are means ± SD.
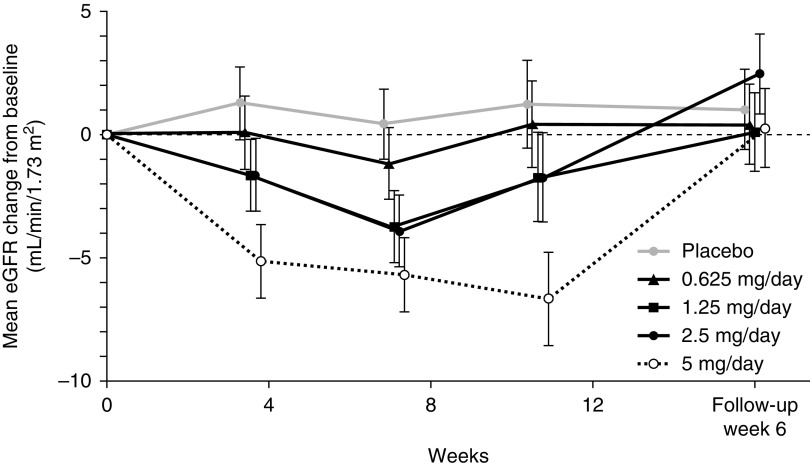
The percentage of participants showing a decrease in eGFR of >30% (kidney dysfunction) after the observation period was higher in the esaxerenone 5-mg/d group compared with the other treatment groups. Although the eGFR decreased after the administration of esaxerenone, it returned to baseline value during the follow-up period (Figure 4).
Figure 4.
Mean change in eGFR from baseline decreased with esaxerenone administration but returned to baseline during the follow-up. Data are least square means ±95% confidence interval.
The mean (SD) changes in weight from baseline to week 12 for participants in the placebo group and the 0.625-, 1.25-, 2.5-, and 5-mg/d esaxerenone groups were 0.04 (2), 0.1 (1), 0.2 (1), −0.08 (1), and 0.1 (1) kg, respectively.
Discussion
Current therapies for the treatment of diabetic kidney disease have limited efficacy, and they are unable to prevent progressive decline in kidney function after the onset of overt diabetic kidney disease. Therefore, there is an unmet need for novel therapies or therapeutic combinations that can slow the progression of diabetic kidney disease.
Esaxerenone is a nonsteroidal mineralocorticoid receptor blocker that has exhibited kidney protective effects superior to eplerenone in preclinical trials (16,17). This study showed that esaxerenone as add-on therapy to RASi in Japanese participants for 12 weeks reduced UACR by approximately one half in a dose-dependent manner. This is the first study to clearly demonstrate a dose-response effect of a mineralocorticoid receptor blocker as an add-on therapy to RASi in Japanese patients with type 2 diabetes mellitus.
The remission of albuminuria has the potential to improve patient outcomes by reducing the risk of kidney and cardiovascular events and preserving kidney function in patients with type 2 diabetes mellitus (6,22,23). Although various limitations have been raised in the literature regarding microalbuminuria changes alone as a surrogate end point of diabetic kidney disease (24), two recent meta-analysis studies report that a >30% reduction in albuminuria confers substantial risk reduction for ESKD (25,26). In this study, significant remission rates were achieved with esaxerenone 2.5 and 5 mg/d. The UACR-lowering effect was also confirmed in groups stratified by BMI, systolic BP, and eGFR or groups with and without concurrent use of non-RASi antihypertensive drugs. Our study showed that the reduction in UACR (ranging from 38% to 56%) was comparable with the findings of Epstein et al. (27), who showed that eplerenone 50 and 100 mg coadministered with enalapril reduced UACR by 41% and 48%, respectively. Furthermore, this reduction in UACR was similar to that in the phase 2 study analyzing the nonsteroidal mineralocorticoid receptor blocker finerenone (28). In the Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study, finerenone (10–20 mg/d) reduced UACR by 27%–42% in a patient subgroup with a baseline UACR of 30–299 mg/g creatinine (28). In addition, as add-on therapy to an RASi, finerenone also significantly reduced systolic BP versus placebo, although this reduction seemed to be inferior to that of esaxerenone when administered at a dose of 2.5 or 5 mg/d.
The mechanism of how esaxerenone induces an antialbuminuric effect is not clear from this study. Because lowering sodium intake or adding a thiazide diuretic to treatment can potentiate an antiproteinuric effect in response to an RASi (29), sodium depletion may be one possible mechanism. Our post hoc analysis revealed that the association between UACR and change in systolic BP at 12 weeks was not statistically significant in the esaxerenone group (Spearman rank correlation coefficient: 0.16–0.20). However, significance was observed in the placebo group (r=0.32, P<0.01), which suggests that the esaxerenone-induced reduction in UACR would be independent of a hemodynamic effect (Supplemental Figure 4). In addition, preclinical studies have shown that the mineralocorticoid receptor is involved in salt-induced organ damage that is independent of plasma aldosterone levels (30). Thus, the observed reductions in UACR may be largely owing to blockade of the mineralocorticoid receptor.
The development of hyperkalemia is associated with diabetic complications, reduced kidney function, and the concomitant use of RASi (31), and a similar trend was also observed in this study. To carefully observe serum K+ levels, 11 measurements were performed during the 12-week administration period and follow-up period, and this revealed a dose-dependent change in serum K+ levels. However, the frequency of withdrawal because of increased serum K+ (serum K+ ≥6.0 mEq/L or two consecutive measurements of serum K+ ≥5.5 mEq/L) with esaxerenone administration up to 2.5 mg/d (3%) was not remarkably different from that of placebo. Esaxerenone up to a dosage of 2.5 mg/d showed a similar risk of a clinically significant increase in serum K+ compared with placebo. It has been reported that the chances of detecting hyperkalemia increase with the number of measurements (32). Nevertheless, serum K+ levels were measured 11 times in this study, and similar incidences of hyperkalemia were observed in the placebo and esaxerenone groups of up to 2.5 mg/d.
A slight decrease in eGFR was observed in the early stages of treatment, but the level plateaued and returned to baseline after cessation of treatment. These changes seemed to correspond with alterations in BP, indicating that the decrease in eGFR was associated with hemodynamic changes. It was surmised that this reduction in eGFR did not represent a safety issue. Overall, these findings indicate that the efficacy of esaxerenone at clinically acceptable doses was confirmed even in an eplerenone-contraindicated population.
Because of the short study period (12 weeks), a longer-term study with a larger sample size is required to evaluate the efficacy of esaxerenone and its safety with regards to hyperkalemia and other concerns in patients with type 2 diabetes mellitus and albuminuria.
In patients with type 2 diabetes mellitus and microalbuminuria currently taking an RASi, esaxerenone resulted in a dose-dependent and significant decrease in UACR. Rates of hyperkalemia at esaxerenone doses of ≤2.5 mg/d were equivalent to those in the placebo group. Efficacy and safety results indicate that esaxerenone 2.5 mg/d could be a suitable dose for use in a phase 3 trial in patients with type 2 diabetes mellitus and microalbuminuria.
Disclosures
Dr. Ito reports grants and personal fees from Daiichi-Sankyo during the conduct of the study and grants and personal fees from Daiichi-Sankyo outside the submitted work. Dr. Nangaku reports grants and personal fees from Daiichi-Sankyo during the conduct of the study. Dr. Shikata reports grants and personal fees from Astellas, Daiichi-Sankyo, Eli Lilly Japan, Kyowa Hakko Kirin, Mitsubishi Tanabe, Novartis, MSD, Ono, and Takeda; personal fees from Nippon Boehringer Ingelheim; and personal fees from AstraZeneca, outside the submitted work. Dr. Okuda and Dr. Sawanobori are employees of Daiichi-Sankyo and report personal fees from Daiichi-Sankyo during the conduct of the study.
Funding
This study was supported by Daiichi-Sankyo.
Supplementary Material
Acknowledgments
Medical writing services were provided by Marion Barnett and Emma Donadieu of Edanz Medical Writing during the development of this manuscript, which was funded by Daiichi-Sankyo.
Deidentified individual participant data and applicable supporting clinical trial documents, which include participant demographics, medical histories, vitals, laboratory test results, adverse events, concomitant medications and final subject statements, may be available on request at https://vivli.org/. Data will be available for sharing after the treatment agent has received marketing approval and after the study results have been accepted for publication. Requests for data will be reviewed by the Independent Review Panel on the basis of the scientific merit of the research proposal and bound by the limitation of participants’ consent. Data requested must execute the Data Use Agreement, and access will be provided for 1 year after the request has been authorized. Requests should be made via the Vivli Platform, through which access to the data will be provided. In cases where clinical trial data and supporting documents are provided pursuant to our company policies and procedures, Daiichi-Sankyo will continue to protect the privacy of our clinical trial participants. Access to data may be declined if there is a potential conflict of interest or competitive risk between Daiichi-Sankyo Co., Ltd. and the requesting party.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14751218/-/DCSupplemental.
Supplemental Appendix. Study sites and investigators participating in this study.
Supplemental Figure 1. Study design.
Supplemental Figure 2. Participant disposition.
Supplemental Figure 3. Change in UACR from baseline stratified by (A) BMI, (B) systolic BP, (C) eGFR, and (D) concurrent use of non-RASi antihypertensive drugs.
Supplemental Figure 4. Association between the ratio to baseline in urinary albumin-to-creatinine ratio (UACR) and the change from baseline in systolic BP at week 12.
Supplemental Table 1. Proportion of participants with missing data.
Supplemental Table 2. Sensitivity analyses for the missing data in the primary end point analysis.
Supplemental Table 3. Plasma esaxerenone concentrations.
References
- 1.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW; American Diabetes Association : Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS: Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev 4: 28–33, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Society of Nephrology : Evidence-based clinical practice guideline for CKD (2013). Clin Exp Nephrol 18: 346–423, 2014 [Google Scholar]
- 4.Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E: Japanese Clinical Practice Guideline for Diabetes 2016. Diabetol Int 9: 1–45, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension : The Japanese society of hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res 37: 253–390, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O: Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 19: 2784–2788, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Ruggenenti P, Fassi A, Ilieva A, Iliev IP, Chiurchiu C, Rubis N, Gherardi G, Ene-Iordache B, Gaspari F, Perna A, Cravedi P, Bossi A, Trevisan R, Motterlini N, Remuzzi G; BENEDICT-B Study Investigators : Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: The BENEDICT-B randomized trial. J Hypertens 29: 207–216, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Schievink B, Kröpelin T, Mulder S, Parving HH, Remuzzi G, Dwyer J, Vemer P, de Zeeuw D, Lambers Heerspink HJ: Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 18: 64–71, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Marver D, Kokko JP: Renal target sites and the mechanism of action of aldosterone. Miner Electrolyte Metab 9: 1–18, 1983 [PubMed] [Google Scholar]
- 10.Hirsch JS, Drexler Y, Bomback AS: Aldosterone blockade in chronic kidney disease. Semin Nephrol 34: 307–322, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Mavrakanas TA, Gariani K, Martin PY: Mineralocorticoid receptor blockade in addition to angiotensin converting enzyme inhibitor or angiotensin II receptor blocker treatment: An emerging paradigm in diabetic nephropathy: A systematic review. Eur J Intern Med 25: 173–176, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Jaisser F, Farman N: Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacol Rev 68: 49–75, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Schwenk MH, Hirsch JS, Bomback AS: Aldosterone blockade in CKD: Emphasis on pharmacology. Adv Chronic Kidney Dis 22: 123–132, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Ruilope LM, Tamargo J: Renin-angiotensin system blockade: Finerenone. Nephrol Ther 13[Suppl 1]: S47–S53, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Vukadinović D, Lavall D, Vukadinović AN, Pitt B, Wagenpfeil S, Böhm M: True rate of mineralocorticoid receptor antagonists-related hyperkalemia in placebo-controlled trials: A meta-analysis. Am Heart J 188: 99–108, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Arai K, Morikawa Y, Ubukata N, Tsuruoka H, Homma T: CS-3150, a novel nonsteroidal mineralocorticoid receptor antagonist, shows preventive and therapeutic effects on renal injury in deoxycorticosterone acetate/salt-induced hypertensive rats. J Pharmacol Exp Ther 358: 548–557, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Arai K, Tsuruoka H, Homma T: CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol 769: 266–273, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F, Ishizuka H: Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol 84: 1821–1829, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi S, Bigazzi R, Campese VM: Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int 70: 2116–2123, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Sato A, Hayashi K, Saruta T: Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens 18: 44–49, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama H, Araki S, Honjo J, Okizaki S, Yamada D, Shudo R, Shimizu H, Sone H, Moriya T, Haneda M: Association between remission of macroalbuminuria and preservation of renal function in patients with type 2 diabetes with overt proteinuria. Diabetes Care 36: 3227–3233, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki S, Haneda M, Koya D, Hidaka H, Sugimoto T, Isono M, Isshiki K, Chin-Kanasaki M, Uzu T, Kashiwagi A: Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 56: 1727–1730, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME: Diabetic kidney disease: A report from an ADA consensus conference. Diabetes Care 37: 2864–2883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AL, Chan TM, Hou FF, Lewis JB, Locatelli F, Praga M, Schena FP, Levey AS, Inker LA; Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 7: 128–139, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, Black C, Brunskill NJ, Carrero JJ, Feldman HI, Fox CS, Inker LA, Ishani A, Ito S, Jassal S, Konta T, Polkinghorne K, Romundstad S, Solbu MD, Stempniewicz N, Stengel B, Tonelli M, Umesawa M, Waikar SS, Wen CP, Wetzels JFM, Woodward M, Grams ME, Kovesdy CP, Levey AS, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 7: 115–127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B: Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1: 940–951, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM; Mineralocorticoid Receptor Antagonist Tolerability Study–Diabetic Nephropathy (ARTS-DN) Study Group : Effect of finerenone on albuminuria in patients with diabetic nephropathy: A randomized clinical trial. JAMA 314: 884–894, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G: Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol 19: 999–1007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagase M, Matsui H, Shibata S, Gotoda T, Fujita T: Salt-induced nephropathy in obese spontaneously hypertensive rats via paradoxical activation of the mineralocorticoid receptor: Role of oxidative stress. Hypertension 50: 877–883, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Khanna A, White WB: The management of hyperkalemia in patients with cardiovascular disease. Am J Med 122: 215–221, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Nilsson E, Gasparini A, Ärnlöv J, Xu H, Henriksson KM, Coresh J, Grams ME, Carrero JJ: Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol 245: 277–284, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.