Diuretics are among the most commonly prescribed drugs and, although effective, they are often used to treat patients at substantial risk for complications, making it especially important to understand and appreciate their pharmacokinetics and pharmacodynamics (see recent review by Keller and Hann [1]). Although the available diuretic drugs possess distinctive pharmacokinetic and pharmacodynamic properties that affect both response and potential for adverse effects, many clinicians use them in a stereotyped manner, reducing effectiveness and potentially increasing side effects (common diuretic side effects are listed in Table 1). Diuretics have many uses, but this review will focus on diuretics to treat extracellular fluid (ECF) volume expansion and edema; the reader is referred elsewhere for discussion of diuretic treatment of hypertension, kidney stones, and other conditions.
Table 1.
Common side effects of diuretics
| Loop diuretics |
|---|
| Hypersensitivity reactions |
| Extracellular fluid volume depletion |
| Hypokalemic alkalosis |
| Hypomagnesemia |
| Ototoxicity |
| Distal convoluted tubule diuretics |
| Hypersensitivity reactions |
| Hyponatremia |
| Hypokalemic alkalosis |
| hyperglycemia/diabetes |
| Hyperuricemia/gout |
| Hypomagnesemia |
| Hypokalemia and prerenal azotemia, when combined with loop diuretics |
| Potassium-sparing diuretics |
| Hypersensitivity |
| Hyperkalemia |
| Metabolic acidosis |
| Azotemia |
| Gynecomastia, vaginal bleeding (spironolactone) |
Classification and Mechanisms of Action
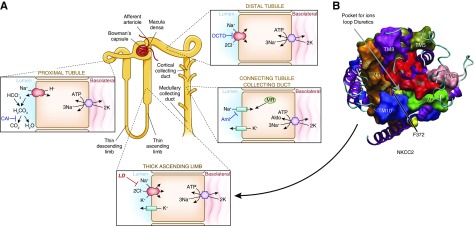
Diuretic drugs are typically classified first according to their predominant site of action along the nephron and second by the mechanism by which they inhibit transport (Figure 1A). The loop diuretics furosemide, bumetanide, and torsemide act from the lumen to inhibit the Na-K-2Cl cotransporter (NKCC2, encoded by SLC12A1) along the thick ascending limb and macula densa. As organic anions, they bind within the translocation pocket on the transport protein by interacting with the chloride-binding site (2) (Figure 1B, see below for clinical relevance). Because they are larger than chloride, they are not transported through the pocket, and thereby inhibit the transporter. Distal convoluted tubule diuretics (thiazides and thiazide-like drugs) are also organic anions that act in much the same manner, but bind to the thiazide-sensitive NaCl cotransporter (NCC, encoded by SLC12A3) along the distal convoluted tubule (Figure 1A). This mechanism of action accounts for a key aspect of loop and distal convoluted tubule diuretic action; these drugs both exert their effect from the luminal side of the tubule.
Figure 1.
Sites of sodium reabsorption and diuretic action along the nephron. (A) Nephron figure showing percentages of sodium reabsorption by associated segment. (B) Homology structural model of the loop diuretic–sensitive NKCC2 viewed from the extracellular surface. The pocket for ion translocation and diuretic binding is shown by the arrow. Mutation of a key phenylalanine (F372) alters diuretic binding (reconstruction adapted from Somasekharan et al. [2]). Aldo, aldosterone; Aml, amiloride (and triamterene); CAI, carbonic anhydrase inhibitors; DCTD, distal convoluted tubule diuretic; LD, loop diuretics; MR, mineralocorticoid receptor, site of spironolactone and eplerenone action (not shown).
Potassium-sparing diuretics include drugs that block apical sodium channels (amiloride and triamterene) and those that antagonize mineralocorticoid receptors (spironolactone and eplerenone). A new nonsteroidal mineralocorticoid blocker, finerenone, is currently in phase 3 clinical trials. The mineralocorticoid blockers and perhaps ethacrynic acid, a more toxic loop diuretic, act within cells and do not require secretion into the tubule lumen.
Gastrointestinal Absorption of Diuretics
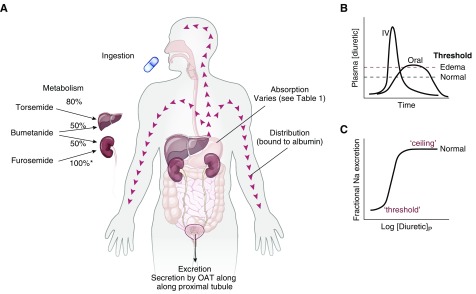
The normal metabolism of loop diuretics is shown in Figure 2A. Furosemide, bumetanide, and torsemide are absorbed relatively quickly after oral administration (see Figure 2B), reaching peak concentrations within 0.5–2 hours (3,4); when administered intravenously, their effects are nearly instantaneous. The oral bioavailability of bumetanide and torsemide typically exceeds 80%, whereas that of furosemide is substantially lower, at approximately 50% (see Table 2) (5). Although the t1/2 of furosemide is short, its duration of action is longer when administered orally, as its gastrointestinal absorption may be slower than its elimination t1/2. This is a phenomenon called “absorption-limited kinetics” (3) and may explain the mnemonic that this drug “lasts 6 hours” (6). This is not the case for bumetanide and torsemide, where oral absorption is rapid (7). On the basis of oral bioavailability, when a patient is switched from intravenous to oral loop diuretic, the dose of bumetanide or torsemide should be maintained, whereas the dose of furosemide should be doubled (7); in practice, however, and as discussed further below, other factors affect diuretic efficacy, and a fixed intravenous/oral conversion cannot be given (8).
Figure 2.
(A) Features of absorption, distribution, metabolism, and excretion (so-called ADME) of drugs. (B) Comparing the plasma diuretic concentration as a function of time after oral or intravenous diuretic administration. The dashed lines show natriuretic thresholds in normal individuals and in those with edema. Note that the primary determinant of natriuresis is the time above the threshold, indicating why route of administration has different effects in stable patients and in those with severe edema. In a normal individual, an oral dose may be effective, whereas it may not be in edema despite retained bioavailability. (C) Classic dose-response curve, plotted versus the logarithm of the plasma concentration. Note the threshold for natriuresis and the maximal level, often called the ceiling. IV, intravenous.
Table 2.
Pharmacokinetics of commonly used diuretics
| Diuretic | Oral Bioavailability, % | Elimination t1/2, h | |||
|---|---|---|---|---|---|
| Normal | CKD | Cirrhotic Ascites | Heart Failure | ||
| Furosemide | 50 (10–100) | 1.5–2 | 2.8 | 2.5 | 2.7 |
| Bumetanide | 80–100 | 1 | 1.6 | 2.3 | 1.3 |
| Torsemide | 68–100 | 3–4 | 4–5 | 8 | 6 |
| Hydrochlorothiazide | 55–77 | 6–15 | Prolonged | ||
| Chlorthalidone | 61–72 | 40–60 | Prolonged | ||
| Metolazone | 70–90a | 14–20 | Prolonged | ||
| Amiloride | ∼50b | 6–26 | 100 | Not changed | |
| Spironolactone | >90 | 1.5c | d | ||
Data are presented as single reported values or range of reported values. Values for furosemide are given as the mean (range). When precise values were not provided, descriptive terms are provided.
Absorption may be decreased in heart failure.
Decreased by food.
Active metabolites of spironolactone have t1/2 of >15 hours.
Active metabolites accumulate in CKD. Adapted from Karin (82).
The loop diuretics have steep dose-response curves. This property, although typically taught to students and residents, is often neglected in clinical practice but is crucial to optimal use. Figure 2C shows a typical natriuretic response plotted versus the logarithm of the plasma diuretic concentration. Inspection reveals that there is little diuretic or natriuretic effect below a given plasma concentration (identified as the “threshold”), above which the response increases rapidly. Although such relations are typically plotted as the logarithm of the diuretic concentration or dose, clinicians do not typically “think” in logarithmic terms. This underlies the reasoning behind the common recommendation to “double the dose,” if no response is obtained. At higher concentrations, a plateau or “ceiling” is reached, with progressively higher plasma concentrations failing to elicit more natriuresis. Although this fact has been used to invoke the concept of ceiling doses of loop diuretics, we will argue that increasing a diuretic dose above this ceiling often elicits more natriuresis, owing to pharmacokinetic considerations (see below).
As should be evident from Figure 2C, a diuretic dose must exceed the threshold to be effective; yet the failure to give a dose that exceeds the threshold is one of the most common errors in diuretic usage. The problem is that the threshold is not easily estimated in an individual, especially an individual with kidney or heart disease. Although nearly all healthy individuals will respond to 20 mg furosemide (or its equivalent), given orally, healthy individuals are not typically treated. As discussed below, conditions that predispose to ECF volume expansion and edema alter both the pharmacokinetics and pharmacodynamics of diuretics. It is little wonder that an empirically selected dose may be ineffective. Below, we will provide broad generalizations about dose adjustments for individuals with a variety of edematous disorders. Yet, adherence to algorithms may lead to diuretic failure. Instead, it is often best to approach a patient as an “n of one trial,” that is, start with a dose consistent with the clinical guidelines (more aggressive for acute edema, more conservative for more chronic processes) and then adjust the dose according to the response.
Although limited bioavailability is a concern with furosemide, a larger problem may be its inconsistent bioavailability. Furosemide absorption varies from day to day in an individual, and between individuals (9,10). Absorption is also affected by food consumption, unlike that of bumetanide or torsemide (11,12), although the clinical significance of this effect has been doubted (3). The more consistent bioavailability of torsemide, compared with furosemide, and its relatively longer t1/2, have suggested that it may be a superior loop diuretic, as suggested by two small, clinical trials (13–16). A recent post hoc analysis of the large Effect of Nesiritide in Patients with Acute Decompensated Heart Failure study suggested that patients with heart failure discharged on torsemide might have lower mortality (17). Yet, none of these studies is sufficiently powered or rigorous enough to be considered definitive, and some other studies do not suggest such a benefit (18).
Gastrointestinal absorption can be slowed, especially during exacerbations of edematous disorders such as heart failure, although again, this may be true primarily of furosemide (19). Although total bioavailability is typically maintained in these situations, natriuresis may be impaired when absorption is slowed, especially given a concomitant increase in natriuretic threshold, as shown in Figure 2B. As an example, the areas under the curves for arbitrary intravenous and doubled oral furosemide doses may be similar, but the time above the natriuretic threshold may be different when the natriuretic threshold is increased by disease. This is likely to explain the common observation that intravenous doses of loop diuretics, which achieve higher peak levels, may be effective when oral doses lose their effectiveness, especially if the natriuretic threshold is increased.
Volumes of Distribution, Metabolism, and t1/2
Loop diuretics are organic anions that circulate tightly bound to albumin (>95%). Thus, their volumes of distribution are low, except during extreme hypoalbuminemia (20). This has suggested that severe hypoalbuminemia might impair diuretic effectiveness, owing to impaired delivery to the kidney, and that albumin administration might enhance natriuresis. This conjecture was supported in an early proof-of-concept study (20), but subsequent larger studies have produced mixed results. A relatively recent meta-analysis concluded that the existing data, albeit of poor quality, suggest transient effects of modest clinical significance for coadministration of albumin with furosemide in hypoalbuminemic patients (21). A similar assessment is reflected in the Kidney Disease Improving Global Outcomes guidelines for diuretic treatment of GN (22). Nevertheless, most recent studies have enrolled patients whose serum albumin concentrations exceeded 2 g/dl, so that these considerations may not apply for severely hypoalbuminemic patients. Some guidelines continue to suggest that albumin infusion should be used as an adjunct to diuretics when nephrotic patients appear to have vascular volume depletion (or appear to be “underfilled”) (23).
Approximately 50% of an administered furosemide dose is excreted unchanged into the urine. The remainder appears to be eliminated by glucuronidation, predominantly also in the kidney. Torsemide and bumetanide are eliminated both by hepatic processes and urinary excretion, although hepatic metabolism may predominate, especially for torsemide (24). The differences in metabolic fate mean that the t1/2 of furosemide is prolonged in kidney failure, where both excretion by the kidney and kidney-mediated glucuronidation are slowed. In contrast, the t1/2 of torsemide and bumetanide tend to be preserved in CKD (25). Although the ratio of equipotent doses of furosemide-to-bumetanide is 40:1 in normal individuals, that ratio declines as kidney disfunction progresses (26). Although this apparent increase in furosemide potency may seem beneficial, it also likely increases the toxic potential of furosemide in the setting of AKI. Deafness and tinnitus from loop diuretics appear to result primarily from high serum concentrations, which inhibit an Na-K-2Cl isoform (NKCC1, encoded by SLC12A2). This transport protein, which is different from that expressed along the thick ascending limb, is expressed by the stria vascularis and participates in secretion of potassium-rich endolymph (27,28). This complication was seen more frequently in the past when very large bolus doses of loop diuretics were used to forestall dialysis (29). In one meta-analysis of furosemide use for patients with AKI, the odds ratio for hearing loss was more than three when high-dose furosemide was used; it should be noted, however, that the doses cited in that analysis (1–3 g daily) exceeded those currently recommended (30). The tendency of bolus infusion to lead to high peak furosemide concentrations is one reason that many investigators recommend continuous infusions instead (1).
Loop diuretics exert their actions by binding to transport proteins along the luminal membrane of thick ascending limb cells. To gain access to the tubular fluid and therefore to their sites of activity, they must be secreted across the proximal tubule, as their protein binding in plasma largely prevents glomerular filtration. Although some data suggest that bumetanide is also delivered into the tubule lumen by filtration (31), a preponderance of evidence suggests that it also gains entry primarily via secretion (32). Peritubular uptake is mediated by the organic anion transporters OAT1 and OAT3, whereas the apically located multidrug resistance-associated protein 4 (Mrp-4) appears to mediate at least a portion of secretion into the tubular fluid. Mice lacking OAT1, OAT3, or Mrp-4 are resistant to loop and thiazide diuretics, illustrating the functional importance of these proteins (31,33).
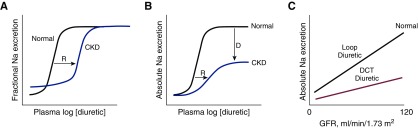
Although human mutations in OAT1 have not been described, these pathways may be inhibited by drugs and endogenous toxins, thereby causing diuretic resistance (31). Nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit diuretic secretion and alter diuretic responsiveness, and because of their frequent use, are an important cause of heart failure exacerbations (34). Yet other classes of drugs, including antihypertensives, antibiotics, and antivirals, may also interact with these transporters and cause resistance (35). Endogenous metabolites also compete for diuretic secretion, including indoxyl sulfate, carboxy-methyl-propyl-furanpropionate, p-cresol sulfate, and kynurenate, which accumulate in CKD (36). In all of these situations, the natriuretic dose-response curve is shifted to the right (Figure 3A).
Figure 3.
Pharmacokinetics and pharmacodynamics of diuretic action. (A) Effects of CKD on diuretic actions. Note that in CKD, baseline fractional sodium excretion is high, to maintain absolute rates of sodium excretion equal to intake. There is a shift in the dose-response curve to the right (R), primarily owing to impaired diuretic secretion, but no change in the ceiling effect. (B) The same relationship plotted versus absolute rates of sodium excretion. The same rightward shift is evident, but the ceiling is lower, owing to the GFR reduction (as indicated by D). (C) Comparing effects of loop diuretics and distal convoluted tubule (DCT) diuretics on absolute sodium excretion, given a retained effect on fractional excretion.
There are additional reasons that CKD is a loop diuretic–resistant state. Metabolic acidosis, which is frequently observed in uremia, depolarizes the membrane potential of proximal tubule cells (37), which also decreases organic anion secretion, an effect that may explain why diuretic secretion is enhanced by alkalosis (38). In addition to a shift in the dose-response curve, patients with CKD and those taking NSAIDs have a downward shift of the ceiling natriuresis, when expressed as absolute sodium excretion (rather than fractional). The mechanism for resistance attributable to NSAIDs is complex. Loop diuretic inhibition of NaCl reabsorption at the macula densa stimulates both renin secretion and prostaglandin (PG) production, the latter predominantly via cyclooxygenase-2 (39). When this happens, PG E2 feeds back on tubules, contributing to the resulting natriuresis by inhibiting NaCl transport along the thick ascending limb and collecting duct (40,41). NSAIDs block this PG-mediated antinatriuresis. When used chronically, NSAIDs increase the abundance and activity of NKCC2 along the thick ascending limb (42). Additionally, loop diuretics inhibit the second transporter isoform, NKCC1, mentioned above, which is also expressed by vascular smooth muscle cells; loop diuretics contribute to afferent arteriolar vasodilation by blocking this transporter (43), thus helping to maintain GFR despite a lower ECF volume. Again, this compensatory adaptation is largely dependent on PG production and can be blocked by NSAIDs. The clinical consequence of these effects is evident in the association between recent use of NSAIDs and risk for hospitalization in patients with heart failure (34). In fact, the combination of three classes of drugs that affect hemodynamics of the kidney, loop diuretics, angiotensin-converting inhibitors (or receptor blockers), and NSAIDs, is associated with AKI (44).
CKD also impairs the natriuretic response to diuretics through a different mechanism. It is frequently noted that the maximal natriuretic capacity of loop diuretics is maintained in the face of CKD, when natriuresis is measured as a fraction of filtered load (Figure 3A). Yet the maximal natriuretic effect of these diuretics, when measured as the more clinically relevant absolute rate, is markedly reduced (Figure 3B). This is because, as GFR and filtered sodium load decrease, kidneys suppress sodium reabsorption by the tubule to maintain the balance between dietary salt intake and urinary salt excretion. This suppression occurs along the thick ascending limb, so that even when a diuretic reaches the segment and inhibits the transporter, its net effect is reduced. Thus, NSAIDs and CKD cause diuretic resistance both by shifting the diuretic dose-response curve to the right (which can be overcome by higher doses) and by reducing maximal natriuresis (which cannot; compare Figure 3, A and B). This phenomenon likely explains the reduced effectiveness of distal convoluted tubule diuretics in CKD. If, like loop diuretics, maximal fractional sodium excretion remains constant as GFR declines, then their already modest ceiling will appear minimal when GFR is low (Figure 3C).
Loop diuretics are characterized by relatively short t1/2 (see Table 2). Thus, the initial natriuresis typically wanes within 3–6 hours, so that a single daily dose leaves some 16–21 hours for the kidneys to compensate for salt and water losses. For individuals in steady state, the phenomenon of “postdiuretic NaCl retention” defines that fact that urinary NaCl excretion declines below the baseline when the diuretic effect wears off. This is typically true until another dose of diuretic is administered (45). It should be noted, however, that although this relationship applies to patients who are at steady state (and thereby excreting their daily intake of salt), it is altered in patients with decompensated edema, who may present during a period of positive NaCl balance, with urinary [NaCl] very low, even without diuretic administration. In this case, any increase in urinary NaCl excretion will be beneficial.
Regardless of these differences, the net NaCl loss from a diuretic typically results from a short period of natriuresis and a longer period of antinatriuresis. This accounts for the usual recommendation to use loop diuretics twice daily; clearly, from inspection of the t1/2, this imperative is most important when using bumetanide and least so with torsemide. As noted above, when CKD progresses, the t1/2 of furosemide is prolonged, increasing its apparent relative potency versus bumetanide. Even when administered twice daily, however, long internatriuretic periods limit drug efficacy; this is most important when dietary NaCl intake is high, as NaCl retention by the kidneys will lead to more positive NaCl balance.
One strategy to address t1/2 issues, at least for hospitalized patients, is to infuse loop diuretics continuously. Although the advantages of this approach over high-dose bolus treatment remain largely speculative (46), the physiologic basis for this approach is appealing, and recent stepped care guidelines (see below) recommend continuous infusions (47). Along these lines, an investigational extended release formulation of torsemide that delivers torsemide to the circulation over 8–12 hours was reported recently to double salt and water losses in normal volunteers after a single dose, without increasing potassium excretion (48). If such a formulation, which should avoid some of the obvious pharmacokinetic limitations of short acting loop diuretics, works as well in patients with heart failure or nephrotic syndrome, it may change the standard approach to treatment.
Somewhat different considerations apply to patients with cirrhotic ascites. Here, relative gastrointestinal absorption tends to be preserved (49). Coupled with the tendency for relative underfilling in this setting, it is typically recommended to avoid intravenous diuretics, if possible (50). In this situation, a combination of furosemide with spironolactone, in a ratio of 40 mg furosemide to 100 mg spironolactone, is recommended in most patients, to balance efficacy and safety, although in patients with concomitant kidney disease, this ratio may need to be adjusted, with the goal of maintaining normokalemia (51).
Using Diuretics Effectively to Treat ECF Volume Expansion
When diuretics are initiated to treat edema, whether in a patient with normal or abnormal kidney function, it is essential to confirm that the dose provides a tubule concentration that exceeds the threshold (Figure 1B). That this threshold has been reached can be detected by moss ambulatory patients, who should notice an increase in urine volume within 2–4 hours of an oral dose. A discrepancy between diuresis and weight loss in outpatients suggests that excessive NaCl consumption is limiting effectiveness; in this case, measuring 24-hour urine sodium excretion, using creatinine to confirm collection adequacy, may confirm excessive NaCl intake, although single urine [Na+] collections may not give fully accurate results (52). For hospitalized patients, a dose reaching the threshold should lead to an increase in urine volume during the 6 hours that follow a dose. On the basis of the relationship of plasma diuretic concentration and time shown in Figure 2B, diuresis should occur more promptly after an intravenous dose. This difference may be especially pronounced if furosemide is the diuretic chosen. If an effect is not observed during this period, it is customary to double the dose, for example from 20 to 40 mg of furosemide or from 80 to 160 mg of furosemide, a recommendation predicated on the dose-response curve shown in Figure 2C. The dose is then escalated to a maximal safe level, as discussed below. Although loop diuretics are typically administered twice daily, there is no reason to introduce a second daily dose if the first dose does not exceed the threshold. Once a threshold has been reached, however, most patients will require two daily doses.
Although dose recommendations for loop diuretics have been published, on the basis of pharmacokinetic and pharmacodynamic considerations (24) or expert consensus (53), several more specific dose ranges have been tested in clinical trials. For acute decompensated heart failure, Felker and colleagues compared doses 2.5-times the home daily dose with one-times the home daily dose, given intravenously. Although differences in the primary outcome were not observed using the higher dose in this trial, prespecified secondary outcomes were encouraging, and negative consequences were not observed. Importantly, this and other recent trials, including those for patients with cardiorenal syndrome, aimed for 3–5 L of diuresis per day for initial treatment (47), rates that are more aggressive than often targeted. These studies emphasize that, for hospitalized patients, an aggressive approach to diuresis is often safe as well as effective. Prior concerns that diuretic drugs might be harmful to the kidney or the system overall, therefore, likely reflected confounding by indication when determined in observational trials (54). In fact, post hoc analyses of large trials suggest that those who experience a moderate increase in creatinine (worsening kidney function) may actually have better prognosis than those who do not (55,56).
The net or therapeutic natriuretic response to a diuretic is determined by the difference between the net sodium excreted in the urine and the sodium consumed. Although increasing a diuretic dose above the ceiling does not increase the maximal minute-natriuresis (the maximal rate of NaCl excretion per given time, see Figure 2C), it often increases the net natriuresis by prolonging the period during which the diuretic concentration exceeds the threshold (see Figure 2A). This is one reason that current guidelines for heart failure may recommend doses that exceed ceiling doses and are multiples of prior or home doses (see below and Ellison and Felker [45]).
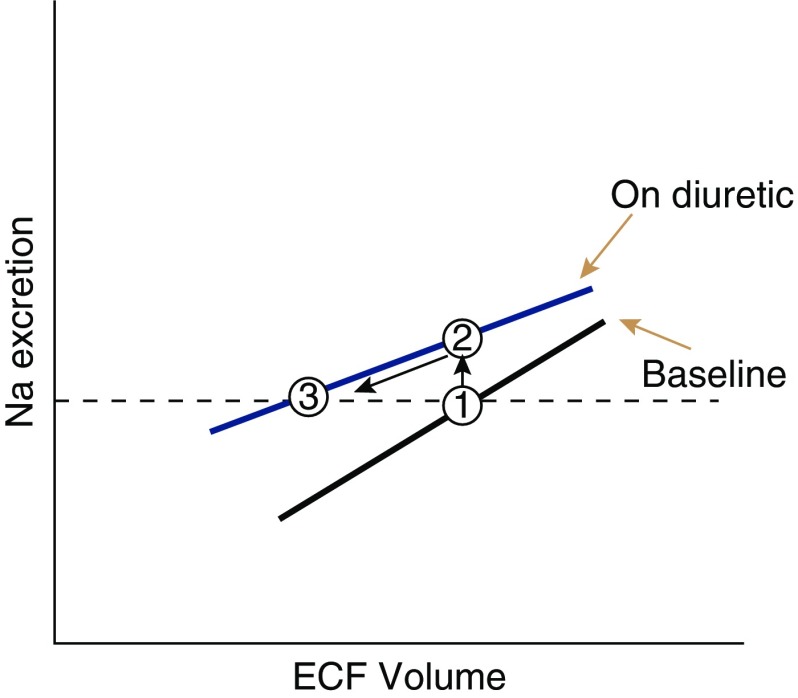
In both normal individuals and in patients with ECF volume expansion, there is a linear relationship between ECF volume and sodium excretion (UNaV), elegantly elucidated by Walser (57). This is similar to, but distinct from, the pressure natriuresis, which describes the relationship between mean arterial pressure and UNaV. Diuretics are recommended universally to treat symptomatic ECF volume expansion, with rare exceptions, and therapeutic success is considered to be reduction in ECF. This invariably requires initial sodium and water losses, induced by diuretic doses that exceed the threshold (Figure 4). Yet the situation changes as initial treatment moves toward successful chronic treatment. At any therapeutically active dose, natriuresis wanes as ECF declines, an effect often called the “braking phenomenon” (58). This means that, at steady state, the individual returns to NaCl balance, during which urinary NaCl excretion is equal to dietary NaCl intake once again. This occurs, however, at a lower ECF volume than before treatment. Functionally, then, chronic diuretic treatment shifts the relationship between ECF volume and UNaV to the left (see Figure 4), thereby permitting NaCl excretion rates to again equal intake, albeit with lower ECF volume. It should be noted, however, that although daily NaCl excretion normalizes, the pattern of salt and water loss remains more episodic, so that a patient may complain that the diuretic regimen is increasing urine output.
Figure 4.
Relationship between ECF volume and sodium excretion, based on (57). Diuretics shift this curve upward (blue line), but may make it shallower. The baseline sodium excretion rate (which equals intake) is shown by the dashed line. After a diuretic is started, urinary sodium excretion rises by shifting to a new curve (from point 1 to point 2). Gradually (through the braking phenomenon) urinary sodium excretion declines back to the baseline level, but at a new and reduced ECF volume (from point 2 to point 3).
Although the braking phenomenon is adaptive once ECF volume has been reduced successfully, it is maladaptive, when it occurs in the setting of persistent ECF volume expansion. Many factors resulting primarily from changes in ECF volume, such as stimulation of nerves innervating the kidney and activation of the renin-angiotensin system, likely contribute to braking (59,60), but it is now recognized that adaptive changes in segments other than the thick ascending limb also play an important role (61,62). Remodeling of the distal nephron occurs (63), leading to hypertrophy and hyperplasia, especially of distal segments. This results from increased salt delivery (64), increased angiotensin II (65) and aldosterone concentrations (66), and changes in potassium balance. The consequences of remodeling are that distal tubules increase their transport capacity to rival that of thick ascending limbs; for this reason, more of the NaCl that escapes the loop of Henle is reabsorbed distally, and net natriuresis is reduced.
Adding a thiazide or thiazide-like drug will help to treat, and may even prevent, this type of adaptation and restore diuretic efficacy. Most commonly, especially in patients with CKD, metolazone is chosen as the second agent, although other thiazides may be equally effective (67). Interestingly, at least three factors may contribute to these beneficial effects. First, by blocking transport along the distal tubule, a site exhibiting transport activation, the potency of these normally weak diuretics will be increased (68). Second, when oral metolazone or chlorthalidone is used in this situation, its longer t1/2 (approximately 14 and 50 hours [69]) means that postdiuretic NaCl retention may be attenuated. Third, these drugs may mitigate distal nephron remodeling and activation of the thiazide-sensitive NCC (70). Nevertheless, a key hazard of this approach is the substantial potential for hypokalemia (71). As hypokalemia is now recognized as the dominant factor activating NCC (72), such secondary effects counteract the goal of adding a second class of diuretic. In this situation, lower or less frequent doses may gain the benefits as well as limit the risks.
Evidence-Based Diuretic Dosing for ECF Volume Expansion
Although recommendations for loop diuretic dosing have traditionally been made on the basis of pharmacological properties, some more recent studies of acute decompensated heart failure have focused on patient-centered outcomes. The Diuretic Strategies in Patients with Acute Decompensated Heart Failure trial compared high and low doses of loop diuretics for acute decompensated heart failure and showed that the higher dose (2.5 times the home daily dose) is well tolerated and effective. One concern about aggressive diuretic approaches in this situation is worsening kidney function, which was used as a harm signal in this study. Yet worsening kidney function in this trial, as indicated by a rise in creatinine, is actually associated with better, rather than worse, prognosis (55). When adequate diuresis does not occur, a stepped care approach, shown in Table 3, has been recommended (47). Although not compared directly with other approaches, this algorithm was used successfully in randomized trials and proved at least as effective as invasive techniques, such as ultrafiltration (73).
Table 3.
Stepped pharmacologic care algorithm for heart failure
| Level | Current Daily Furosemide Dosea, mg | Bolus | Infusion Rate, mg/h | Metolazone (Oral) |
|---|---|---|---|---|
| 1 | ≤80 | 40 | 5 | 0 |
| 2 | 81–160 | 80 | 10 | 5 mg daily |
| 3 | 161–240 | 80 | 20 | 5 mg twice daily |
| 4 | ≥240 | 80 | 30 | 5 mg twice daily |
Diuretic equivalents: 40 mg furosemide is considered equivalent to 1 mg bumetanide 20 mg torsemide. Adapted from Grodin et al. (47) and Bart et al. (73). The full algorithm provided in the references includes additional considerations for vasodilator, inotropic, or mechanical therapy for patients who fail to respond within 48 h.
More limited but compelling data suggest that patients with cirrhotic ascites are best treated with a combination of furosemide and spironolactone, at a ratio of 40:100 mg (74). This preserves the plasma potassium concentration in most patients, although it may need to be adjusted if abnormalities occur. For patients with nephrotic syndrome, diuretic binding was previously suggested to contribute to resistance. Yet a study comparing the natriuretic effect of loop diuretics with and without protein displacement indicated clearly that this factor was not contributing (75). Another contributor in this situation is the cleavage of the epithelial sodium channel by filtered proteases (76); recent animal data suggest that this may be a target for intervention, with either protease inhibitors or amiloride (77).
Diuretics for AKI
Recommendations for and against diuretic use in AKI have varied widely. At the end of the 20th century, extremely high diuretic doses were often used, which can convert oliguric to nonoliguric AKI, but were found to be associated with deafness and no change in mortality in controlled trials (78). A later retrospective trial suggested that diuretic use in patients with AKI is associated with increased mortality, and suggested that “the widespread use of diuretics in critically ill patients with acute renal failure should be discouraged” (79). Yet, statistical approaches cannot overcome the inherent limitations in such retrospective studies. To address this concern and reduce confounding by indication, Grams et al. performed a post hoc analysis of data for patients with AKI from the Fluid and Catheter Treatment Trial (80). In this trial, patients with adult respiratory distress syndrome were randomized to liberal or restrictive fluid policies; for those randomized to restricted fluid, diuretics were used aggressively. The results of this trial suggested that patients who developed AKI who were randomized to a strategy that involved more diuretic administration had a lower adjusted odds ratio for death (80). Although even this trial is not definitive, it suggested that prior reported adverse outcomes from diuretic use in AKI likely did reflect confounding by indication. At this point, it seems reasonable to use diuretics as an adjunct in AKI to maintain euvolemia. It is generally best, however, to avoid very high doses, and avoid using diuretics to delay more definitive treatments, such as dialysis.
Summary
Diuretic drugs, agents that target solute transport along the nephron, are used commonly in individuals with normal or reduced kidney function. Each diuretic drug has a unique pharmacokinetic profile, but such differences may not receive sufficient consideration when the drugs are used therapeutically. Recent large, clinical trials now provide an evidence base for diuretic treatment of heart failure. Yet, even when such evidence is available, a deep understanding of diuretic pharmacokinetics and pharmacodynamics enhances the clinical approach to diuresis. As the drugs have substantial ability to ameliorate breathlessness and edema, the goal of optimizing their use should improve patient-focused clinical outcomes. The development of diuretic drugs has been one of the greatest accomplishments of scientific medicine; the persistence of disorders of ECF volume into the 21st century means that these drugs will continue to play central roles in medical practice for the foreseeable future.
Disclosures
Dr. Ellison has nothing to disclose.
Funding
Dr. Ellison was supported by a grant from the National Center for Advancing Translational Science (U54TR001628).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Keller F, Hann A: Clinical pharmacodynamics: Principles of drug response and alterations in kidney disease. Clin J Am Soc Nephrol 13: 1413–1420, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Somasekharan S, Tanis J, Forbush B: Loop diuretic and ion-binding residues revealed by scanning mutagenesis of transmembrane helix 3 (TM3) of Na-K-Cl cotransporter (NKCC1). J Biol Chem 287: 17308–17317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammarlund MM, Paalzow LK, Odlind B: Pharmacokinetics of furosemide in man after intravenous and oral administration. Application of moment analysis. Eur J Clin Pharmacol 26: 197–207, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Brater DC, Day B, Burdette A, Anderson S: Bumetanide and furosemide in heart failure. Kidney Int 26: 183–189, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Shankar SS, Brater DC: Loop diuretics: From the Na-K-2Cl transporter to clinical use. Am J Physiol Renal Physiol 284: F11–F21, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Huang X, Dorhout Mees E, Vos P, Hamza S, Braam B: Everything we always wanted to know about furosemide but were afraid to ask. Am J Physiol Renal Physiol 310: F958–F971, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Brater DC: Diuretic pharmacokinetics and pharmacodynamics. In: Diuretic Agents: Clinical Physiology and Pharmacology, edited by Seldin DW, Giebisch G, San Diego, Academic Press, 1997, pp 189–208 [Google Scholar]
- 8.Brater DC: Pharmacodynamic considerations in the use of diuretics. Annu Rev Pharmacol Toxicol 23: 45–62, 1983 [DOI] [PubMed] [Google Scholar]
- 9.Murray MD, Haag KM, Black PK, Hall SD, Brater DC: Variable furosemide absorption and poor predictability of response in elderly patients. Pharmacotherapy 17: 98–106, 1997 [PubMed] [Google Scholar]
- 10.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC: Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther 57: 601–609, 1995 [DOI] [PubMed] [Google Scholar]
- 11.McCrindle JL, Li Kam Wa TC, Barron W, Prescott LF: Effect of food on the absorption of frusemide and bumetanide in man. Br J Clin Pharmacol 42: 743–746, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer WG: Effect of food on the pharmacokinetics and pharmacodynamics of torsemide. Am J Ther 2: 499–503, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Murray MD, Ferguson JA, Bennett SJ, Adams LD, Forrhofer MM, Minick SM, Tierny WM, Brater DC: Fewer hospitalizations for heart failure by using a completely and predictably absorbed loop diuretic. J Gen Intern Med 16: 45–52, 1998 [Google Scholar]
- 14.Murray MD, Deer MM, Ferguson JA, Dexter PR, Bennett SJ, Perkins SM, Smith FE, Lane KA, Adams LD, Tierney WM, Brater DC: Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med 111: 513–520, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Bikdeli B, Strait KM, Dharmarajan K, Partovian C, Coca SG, Kim N, Li SX, Testani JM, Khan U, Krumholz HM: Dominance of furosemide for loop diuretic therapy in heart failure: Time to revisit the alternatives? J Am Coll Cardiol 61: 1549–1550, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiNicolantonio JJ: Should torsemide be the loop diuretic of choice in systolic heart failure? Future Cardiol 8: 707–728, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Mentz RJ, Hasselblad V, DeVore AD, Metra M, Voors AA, Armstrong PW, Ezekowitz JA, Tang WH, Schulte PJ, Anstrom KJ, Hernandez AF, Velazquez EJ, O’Connor CM: Torsemide versus furosemide in patients with acute heart failure (from the ASCEND-HF trial). Am J Cardiol 117: 404–411, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasavada N, Saha C, Agarwal R: A double-blind randomized crossover trial of two loop diuretics in chronic kidney disease. Kidney Int 64: 632–640, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC: Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med 102: 314–318, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Inoue M, Okajima K, Itoh K, Ando Y, Watanabe N, Yasaka T, Nagase S, Morino Y: Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int 32: 198–203, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Kitsios GD, Mascari P, Ettunsi R, Gray AW: Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: A meta-analysis. J Crit Care 29: 253–259, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Radhakrishnan J, Cattran DC: The KDIGO practice guideline on glomerulonephritis: Reading between the (guide)lines--application to the individual patient. Kidney Int 82: 840–856, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Pasini A, Benetti E, Conti G, Ghio L, Lepore M, Massella L, Molino D, Peruzzi L, Emma F, Fede C, Trivelli A, Maringhini S, Materassi M, Messina G, Montini G, Murer L, Pecoraro C, Pennesi M: The Italian society for Pediatric Nephrology (SINePe) consensus document on the management of nephrotic syndrome in children: Part I - diagnosis and treatment of the first episode and the first relapse. Ital J Pediatr 43: 41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brater DC: Diuretic therapy. N Engl J Med 339: 387–395, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Brater DC: Disposition and response to bumetanide and furosemide. Am J Cardiol 57: 20A–25A, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Voelker JR, Cartwright-Brown D, Anderson S, Leinfelder J, Sica DA, Kokko JP, Brater DC: Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int 32: 572–578, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Delpire E, Lu J, England R, Dull C, Thorne T: Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22: 192–195, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Flagella M, Clarke LL, Miller ML, Erway LC, Giannella RA, Andringa A, Gawenis LR, Kramer J, Duffy JJ, Doetschman T, Lorenz JN, Yamoah EN, Cardell EL, Shull GE: Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem 274: 26946–26955, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Dormans TP, van Meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P: Diuretic efficacy of high dose furosemide in severe heart failure: Bolus injection versus continuous infusion. J Am Coll Cardiol 28: 376–382, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Ho KM, Sheridan DJ: Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 333: 420, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V: Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10: 2039–2049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau HS, Shih LJ, Smith DE: Effect of probenecid on the dose-response relationship of bumetanide at steady state. J Pharmacol Exp Ther 227: 51–54, 1983 [PubMed] [Google Scholar]
- 33.Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK: Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol 294: F867–F873, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Heerdink ER, Leufkens HG, Herings RM, Ottervanger JP, Stricker BHC, Bakker A: NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med 158: 1108–1112, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Burckhardt G: Drug transport by Organic Anion Transporters (OATs). Pharmacol Ther 136: 106–130, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Wu W, Bush KT, Nigam SK: Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep 7: 4939, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cemerikić D, Wilcox CS, Giebisch G: Intracellular potential and K+ activity in rat kidney proximal tubular cells in acidosis and K+ depletion. J Membr Biol 69: 159–165, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Loon NR, Wilcox CS: Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin Sci (Lond) 94: 287–292, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Mann B, Hartner A, Jensen BL, Kammerl M, Krämer BK, Kurtz A: Furosemide stimulates macula densa cyclooxygenase-2 expression in rats. Kidney Int 59: 62–68, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Stokes JB: Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest 64: 495–502, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hébert RL, Jacobson HR, Breyer MD: Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest 87: 1992–1998, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Llama P, Ecelbarger CA, Ware JA, Andrews P, Lee AJ, Turner R, Nielsen S, Knepper MA: Cyclooxygenase inhibitors increase Na-K-2Cl cotransporter abundance in thick ascending limb of Henle’s loop. Am J Physiol 277: F219–F226, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Oppermann M, Hansen PB, Castrop H, Schnermann J: Vasodilatation of afferent arterioles and paradoxical increase of renal vascular resistance by furosemide in mice. Am J Physiol Renal Physiol 293: F279–F287, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S: Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 346: e8525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellison DH, Felker GM: Diuretic treatment in heart failure. N Engl J Med 377: 1964–1975, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvador DR, Rey NR, Ramos GC, Punzalan FE: Continuous infusion versus bolus injection of loop diuretics in congestive heart failure. Cochrane Database Syst Rev (3): CD003178, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grodin JL, Stevens SR, de Las Fuentes L, Kiernan M, Birati EY, Gupta D, Bart BA, Felker GM, Chen HH, Butler J, Dávila-Román VG, Margulies KB, Hernandez AF, Anstrom KJ, Tang WH: Intensification of medication therapy for cardiorenal syndrome in acute decompensated heart failure. J Card Fail 22: 26–32, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah S, Pitt B, Brater DC, Feig PU, Shen W, Khwaja FS, Wilcox CS: Sodium and fluid excretion with torsemide in healthy subjects is limited by the short duration of diuretic action. J Am Heart Assoc 6: e006135, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawhney VK, Gregory PB, Swezey SE, Blaschke TF: Furosemide disposition in cirrhotic patients. Gastroenterology 81: 1012–1016, 1981 [PubMed] [Google Scholar]
- 50.Daskalopoulos G, Laffi G, Morgan T, Pinzani M, Harley H, Reynolds T, Zipser RD: Immediate effects of furosemide on renal hemodynamics in chronic liver disease with ascites. Gastroenterology 92: 1859–1863, 1987 [DOI] [PubMed] [Google Scholar]
- 51.Runyon BA; Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD): Management of adult patients with ascites due to cirrhosis. Hepatology 39: 841–856, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Titze J: Estimating salt intake in humans: Not so easy! Am J Clin Nutr 105: 1253–1254, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL: 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American college of cardiology Foundation/American heart association task force on practice guidelines. Circulation 128: 1810–1852, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM: Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147: 331–338, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM: Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: Insights from the DOSE trial. J Card Fail 22: 753–760, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG, Testani JM: Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation 137: 2016–2028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walser M: Phenomenological analysis of renal regulation of sodium and potassium balance. Kidney Int 27: 837–841, 1985 [DOI] [PubMed] [Google Scholar]
- 58.Wilcox CS, Mitch WE, Kelly RA, Skorecki K, Meyer TW, Friedman PA, Souney PF: Response of the kidney to furosemide. I. Effects of salt intake and renal compensation. J Lab Clin Med 102: 450–458, 1983 [PubMed] [Google Scholar]
- 59.Wilcox CS, Guzman NJ, Mitch WE, Kelly RA, Maroni BJ, Souney PF, Rayment CM, Braun L, Colucci R, Loon NR: Na+, K+, and BP homeostasis in man during furosemide: Effects of prazosin and captopril. Kidney Int 31: 135–141, 1987 [DOI] [PubMed] [Google Scholar]
- 60.Kelly RA, Wilcox CS, Mitch WE, Meyer TW, Souney PF, Rayment CM, Friedman PA, Swartz SL: Response of the kidney to furosemide. II. Effect of captopril on sodium balance. Kidney Int 24: 233–239, 1983 [DOI] [PubMed] [Google Scholar]
- 61.Loon NR, Wilcox CS, Unwin RJ: Mechanism of impaired natriuretic response to furosemide during prolonged therapy. Kidney Int 36: 682–689, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Rao VS, Planavsky N, Hanberg JS, Ahmad T, Brisco-Bacik MA, Wilson FP, Jacoby D, Chen M, Tang WHW, Cherney DZI, Ellison DH, Testani JM: Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol 28: 3414–3424, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Subramanya AR, Ellison DH: Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang YS, Xie J, Yang SS, Lin SH, Huang CL: Differential roles of WNK4 in regulation of NCC in vivo. Am J Physiol Renal Physiol 314: F999–F1007, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castañeda-Bueno M, Gamba G: Mechanisms of sodium-chloride cotransporter modulation by angiotensin II. Curr Opin Nephrol Hypertens 21: 516–522, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH: Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(-)-cotransporter abundance: Role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Fliser D, Schröter M, Neubeck M, Ritz E: Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int 46: 482–488, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Ellison DH: The physiologic basis of diuretic synergism: Its role in treating diuretic resistance. Ann Intern Med 114: 886–894, 1991 [DOI] [PubMed] [Google Scholar]
- 69.Kountz DS, Goldman A, Mikhail J, Ezer M: Chlorthalidone: The forgotten diuretic. Postgrad Med 124: 60–66, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA: SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jentzer JC, DeWald TA, Hernandez AF: Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol 56: 1527–1534, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH: Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E; Heart Failure Clinical Research Network: Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367: 2296–2304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Runyon BA; AASLD Practice Guidelines Committee: Management of adult patients with ascites due to cirrhosis: An update. Hepatology 49: 2087–2107, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Agarwal R, Gorski JC, Sundblad K, Brater DC: Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. J Am Soc Nephrol 11: 1100–1105, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Svenningsen P, Bistrup C, Friis UG, Bertog M, Haerteis S, Krueger B, Stubbe J, Jensen ON, Thiesson HC, Uhrenholt TR, Jespersen B, Jensen BL, Korbmacher C, Skøtt O: Plasmin in nephrotic urine activates the epithelial sodium channel. J Am Soc Nephrol 20: 299–310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bohnert BN, Menacher M, Janessa A, Wörn M, Schork A, Daiminger S, Kalbacher H, Häring HU, Daniel C, Amann K, Sure F, Bertog M, Haerteis S, Korbmacher C, Artunc F: Aprotinin prevents proteolytic epithelial sodium channel (ENaC) activation and volume retention in nephrotic syndrome. Kidney Int 93: 159–172, 2018 [DOI] [PubMed] [Google Scholar]
- 78.Brown CB, Ogg CS, Cameron JS: High dose frusemide in acute renal failure: A controlled trial. Clin Nephrol 15: 90–96, 1981 [PubMed] [Google Scholar]
- 79.Mehta RL, Pascual MT, Soroko S, Chertow GM; PICARD Study Group: Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 288: 2547–2553, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network: Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 6: 966–973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milionis HJ, Alexandrides GE, Liberopoulos EN, Bairaktari ET, Goudevenos J, Elisaf MS: Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur J Heart Fail 4: 167–173, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Karim A: Spironolactone: Disposition, metabolism, pharmacodynamics, and bioavailability. Drug Metab Rev 8: 151–188, 1978 [DOI] [PubMed] [Google Scholar]