Abstract
Over the years, a broad spectrum of applications of the radionuclide holmium-166 as a medical isotope has been established. The isotope holmium-166 is attractive as it emits high-energy beta radiation which can be used for a therapeutic effect and gamma radiation which can be used for nuclear imaging purposes. Furthermore, holmium-165 can be visualized by MRI because of its paramagnetic properties and by CT because of its high density. Since holmium-165 has a natural abundance of 100%, the only by-product is metastable holmium-166 and no costly chemical purification steps are necessary for production of nuclear reactor derived holmium-166. Several compounds labelled with holmium-166 are now used in patients, such Ho166-labelled microspheres for liver malignancies, Ho166-labelled chitosan for hepatocellular carcinoma (HCC) and [166Ho]Ho DOTMP for bone metastases. The outcomes in patients are very promising, making this isotope more and more interesting for applications in interventional oncology. Both drugs as well as medical devices labelled with radioactive holmium are used for internal radiotherapy. One of the treatment possibilities is direct intratumoural treatment, in which the radioactive compound is injected with a needle directly into the tumour. Numerous other applications have been developed, like patches for treatment of skin cancer and holmium labelled antibodies and peptides. The second major application that is currently clinically applied is selective internal radiation therapy (SIRT, also called radioembolization), a novel treatment option for liver malignancies. This review discusses medical drugs and medical devices based on the therapeutic radionuclide holmium-166.
Keywords: Holmium-166, Holmium, Lanthanide, Radiation therapy, SIRT, Microspheres, Chitosan, DOTMP
Introduction
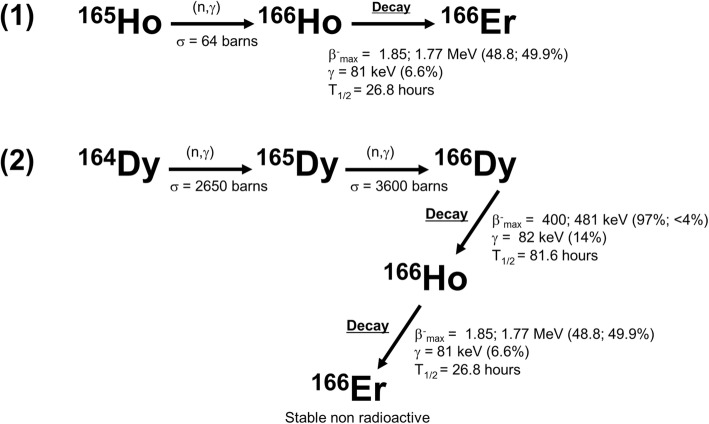
Holmium is one of the 15 rare earth elements called lanthanides, a group of elements that has become an established source of radionuclides for nuclear diagnostic and therapeutic applications (Nayak and Lahiri 1999). Holmium-166 (166Ho) can be produced by two methods; neutron activation by (n, γ) irradiation in a nuclear reactor (Nayak and Lahiri 1999; Nijsen et al. 1999) or by neutron activation of dysprosium-164 (164Dy) (Nijsen et al. 2007) (Fig. 1). Because holmium-165 (165Ho) has a natural abundance of 100% and a cross section of 64 b (Foote Jr et al. 1953), it can be neutron activated in a relatively short neutron activation time resulting in 166Ho with a high purity of the isotope (Nijsen et al. 1999). The only by-product is metastable holmium-166 (166mHo), approximately a factor 7 × 106 times less than 166Ho. 166mHo has a half-life of 1200 years and emits beta radiation and a number of gamma rays between 80 and 1563 keV (Nijsen et al. 2007; Hino et al. 2000; Bernardes 2001). The cross section of the 165Ho(n, γ)166Ho reaction is 64 b (Foote Jr et al. 1953) and the cross section of the 165Ho(n, γ)166mHo reaction is around 3.4 b for thermal neutrons (Nethaway and Missimer 1968).
Fig. 1.
Diagrams of the production methods of (1) 166Ho and (2) 166Dy. Reactor neutron activated 165Ho will result in 166Ho with a high purity (1). The second method is via neutron activation of 164Dy by two neutrons. Dysprosium-164 has a natural abundance of 28.2% and enriched material will have a purity of over 90%. By capture of two neutrons, 164Dy will be converted into 166Dy which will decay into carrier-free 166Ho as the daughter radionuclide (166Dy/166Ho generator) (data were collected from the International Atomic Energy Agency Database: https://www-nds.iaea.org/)
The other production option is via neutron activation of 164Dy by two neutrons following a (2n, γ) reaction forming dysprosium-166 (166Dy). The cross section of 164Dy is extremely high (2650 b). A second neutron irradiation on this instable nuclide is necessary to result in 166Dy. The radionuclide 166Dy will decay with a half-life of 81.5 h into 166Ho, during which two beta particles up to 481 keV are emitted (Smith et al. 1995).
The radionuclide 166Ho emits high-energy beta particles (1774.32 keV; yield 48.8% and 1854.9 keV; yield 49.9%) and gamma rays (80.57 keV; yield 6.7% and 1379.40 keV; yield 0.9% (only emissions are given with yield higher than 0.5%) and has a half-life of 26.8 h (Nijsen et al. 2007) (Fig. 1). The high energetic beta particles are responsible for the therapeutic effect and the gamma ray of 80.57 keV can be used for nuclear imaging purposes. Furthermore, 165Ho can be visualized by MRI because of its paramagnetic properties (Nijsen et al. 2004; Seevinck et al. 2007; Smits et al. 2012) and by CT because of its high density (Seevinck et al. 2007; Bakker et al. 2018).
As the half-life of 166Ho is 26.8 h, over 90% of the radiation is deposited in less than 4 days. The maximum tissue range of the beta particles in soft tissue is 8.7 mm, the average range 2.2 mm and 90% of the total radiation dose will be delivered in the first 2.1 mm (Johnson and Yanch 1991). This results in an interesting relatively high dose-rate if the same cumulative dose is given compared to other often used radioisotopes for cancer treatment, such as phosphor-32 (32P), yttrium-90 (90Y), iodine-131 (131I), lutetium-177 (177Lu) and rhenium-186 (186Re), with half-lives between 2.7 and 14.3 days.
Over the last 30 years, 150 articles have been published on the use of 166Ho as a medical isotope. Especially since the start of this century interest in this radioisotope has been growing, resulting in around 6 publications per year (Fig. 2) and translated into the development of many systems loaded or conjugated to holmium (Table 1). Since the production of 166Ho has become more and more standardized, the number of clinical applications (Fig. 3) and clinical trials (Table 2) has been growing and several compounds are now used in patients: 166Ho-labelled microspheres for liver malignancies (Smits et al. 2012; Prince et al. 2018), 166Ho-labelled chitosan for hepatocellular carcinoma (HCC) (Sohn et al. 2009) and [166Ho]Ho DOTMP (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene-phosphonic acid) for bone metastases (Denis-Bacelar et al. 2018). Thus, it can be expected that the significance of the use of this radioisotope will continue to grow rapidly. In this review, the many therapeutic applications of 166Ho that have been developed over the years are discussed.
Fig. 2.
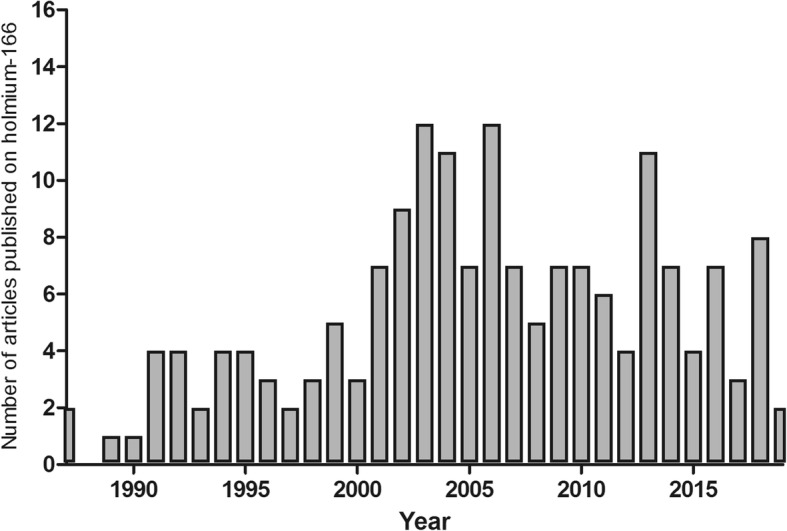
Number of 166Ho related publications over the years (search through PubMed)
Table 1.
Representative examples of carriers that have been loaded or conjugated to holmium and to the dysprosium/holmium generator
| Bone seeking agents | DOTMP | (Bayouth et al. 1995a; Bayouth et al. 1995b; Parks et al. 1993; Giralt et al. 2003; Breitz et al. 2006; Rajendran et al. 2002; Ueno et al. 2009; Breitz et al. 2003a) |
| EDTMP | (Sohaib et al. 2011; Bahrami-Samani et al. 2010; Appelbaum et al. 1992; Louw et al. 1996) | |
| PAM | (Vaez-Tehrani et al. 2016) | |
| TTHMP | (Yousefnia et al. 2014) | |
| APDDMP | (Marques et al. 2006; Zeevaart et al. 2001) | |
| Antibodies | DO3A-4B4 | (Ballard et al. 2011) |
| CHX-A"DTPA-6D2 | (Thompson et al. 2014) | |
| DOTA-CC49 MeO-DOTA-CC49 | (Mohsin et al. 2006; Mohsin et al. 2011) | |
| Other complexes | DTPA | (Majali et al. 2001; Hong et al. 2002) |
| DOTA | (Das et al. 2003) | |
| Chitosan | (Ha et al. 2013; Huh et al. 2005; Kim et al. 2006; Kwak et al. 2005; Suzuki et al. 1998; Lee et al. 2006; Cho et al. 2010; Song et al. 2001; Lee et al. 2003) | |
| Oxine lipiodol | (Das et al. 2009b) | |
| PMMA | (Hirsch et al. 2008) | |
| Microparticles | Glass | (Costa et al. 2009; Brown et al. 1991) |
| Resin | (Turner et al. 1994; Subramanian et al. 2018; Costa and Osso Junior 2008) | |
| Alginate | (Zielhuis et al. 2007) | |
| Lipiodol-alginate | (Oerlemans et al. 2015) | |
| AcAc-PLLA | (Nijsen et al. 1999; Mumper et al. 1992) | |
| Polyester | (Mumper and Jay 1992) | |
| AcAc | (Bult et al. 2012; Bult et al. 2009) | |
| PO4 | (Bult et al. 2012; Arranja et al. 2018) | |
| Hydroxiapatite | (Das et al. 2009a; Unni et al. 2002) | |
| Ferric hydroxide (FHMA) | (Makela et al. 2004; Makela et al. 2003b; Vuorela et al. 2005; Kraft et al. 2007; Cho et al. 2010; Makela et al. 2004; Ofluoglu et al. 2002) | |
| Nanoparticles | Mesoroporous silica nanoparticles | (Di Pasqua et al. 2013) |
| Mesoroporous carbon nanoparticles | (Kim et al. 2017) | |
| AcAc-DSPE-PEG | (Di Pasqua et al. 2012) | |
| AcAc-PLLA | (Hamoudeh et al. 2008) | |
| AcAc | (Bult et al. 2010) | |
| Liposomes | DPPC:Chol:PEG-DSPE | (Zielhuis et al. 2006) |
| Patches | Tape | (Lee et al. 1997; Chung et al. 2000) |
| Nanofibers | (Munaweera et al. 2014) | |
| Ceramic materials | Seeds | (Diniz et al. 2017; Valente et al. 2011; Nogueira and Campos 2011; Nogueira and de Campos 2012; Nogueira and Campos 2016; Hosseini et al. 2013; Valente and Campos 2010; Won et al. 2005) |
| Membranes | (Nogueira and de Campos 2012) | |
| Generator | DTPA complex | (Smith et al. 1995) |
| Macroaggregates | (Makela et al. 2003a; Park et al. 1996; McLaren et al. 1990; Sledge et al. 1977) | |
| DTPA-Biotin | (Ferro-Flores et al. 2004; Ferro-Flores et al. 2003) | |
| EDTMP | (Pedraza-Lopez et al. 2004a) | |
| MOFDOTMP | (Vosoghi et al. 2016) | |
| Chitosan microspheres | (Cho and Choi 2018) |
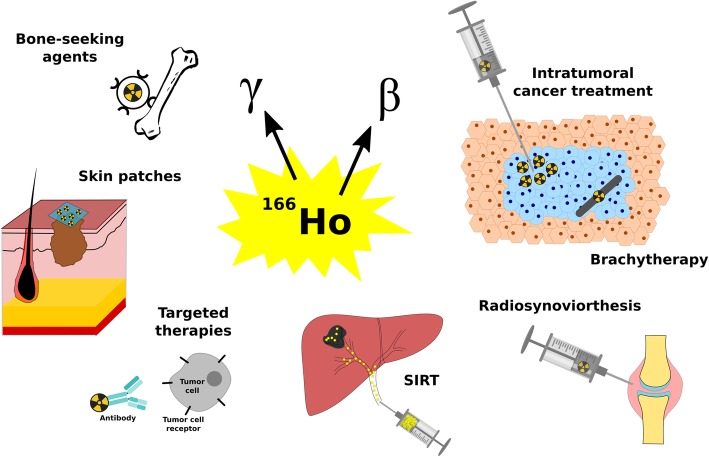
Fig. 3.
Schematic overview of medical applications of the isotope 166Ho
Table 2.
Clinical studies published at www.clinicaltrials.gov in which a compound with holmium-166 is used
| Study title | Device/ drug | Tumour type | Compound | Sponsor | Time path | ClinicalTrials.gov Identifier | Reference(s) |
|---|---|---|---|---|---|---|---|
| QuiremSpheres Observational Study | device | Liver cancer, non-resectable tumors | Holmium-166 polylactic microspheres | Terumo Europe N.V. | 2018 - (recruiting) | NCT03563274 | |
| Holmium Radioembolization as Adjuvant Treatment to RFA for Early Stage HCC: Dose Finding Study | device | hepatocellular carcinoma | RFA + Holmium-166 polylactic microspheres | Leiden University Medical Center The Netherlands | 2018 - (recruiting) | NCT03437382 | |
| HEPAR Primary: Holmium-166-radioembolization in Hepatocellular Carcinoma Patients | device | hepatocellular carcinoma |
SIRT Holmium-166 PLLA microspheres |
Erasmus Medical Center Rotterdam, University Medical Center Utrecht The Netherlands | 2017 - (recruiting) | NCT03379844 | |
| Feasibility of Holmium-166 Micro Brachytherapy in Head and Neck Tumors | device | head-and-neck neoplasms |
SIRT Holmium-166 PLLA microspheres |
University Medical Center Utrecht The Netherlands |
2016–2018 (terminated, slow accrual) |
NCT02975739 | |
| Holmium-166-radioembolization in NET After Lutetium-177-dotatate; an Efficacy Study | device | neuroendocrine tumours in the liver |
SIRT Holmium-166 PLLA microspheres |
University Medical Center Utrecht, The Netherlands | 2016 - (recruiting) | NCT02067988 | (Braat et al. 2018b; van Nierop et al. 2018) |
| Surefire Infusion System vs. Standard Microcatheter Use During Holmium-166 Radioembolization | device | colorectal metastases in the liver |
SIRT Holmium-166-PLLA microspheres |
University Medical Center Utrecht The Netherlands | 2016 - (recruiting) | NCT02208804 | (van Nierop et al. 2018; van den Hoven et al. 2016) |
| Radioactive Holmium Microspheres for the Treatment of Unresectable Liver Metastases | device | liver neoplasms |
SIRT Holmium-166-PLLA microspheres |
University Medical Center Utrecht The Netherlands |
2012–2015 (completed) |
NCT01612325 | (Prince et al. 2018; Braat et al. 2018a; Prince et al. 2015; van Nierop et al. 2018) |
| Radioactive Holmium Microspheres for the Treatment of Liver Metastases | device |
liver metastases liver tumours |
SIRT Holmium-166-PLLA microspheres |
University Medical Center Utrecht Utrecht, Netherlands |
2009–2012 (completed) |
NCT01031784 | (Smits et al. 2012; van de Maat et al. 2013; Smits et al. 2013; Smits et al. 2010; Prince et al. 2014; Elschot et al. 2014; Braat et al. 2018a; van Nierop et al. 2018) |
| Radiation Therapy Using Holmium Ho 166 DOTMP Plus Melphalan and Peripheral Stem Cell Transplantation in Treating Patients With Multiple Myeloma | drug | multiple myeloma and plasma cell neoplasm |
melphalan + Holmium-166- DOTMP |
Fred Hutchinson Cancer Research CenterSeattle, Washington, United States |
2004–2010 (completed) |
NCT00004158 | |
| Melphalan With or Without Holmium Ho 166 DOTMP Followed by Peripheral Stem Cell Transplantation in Treating Patients With Multiple Myeloma | drug | multiple myeloma and plasma cell neoplasm |
melphalan + Holmium-166- DOTMP |
Fred Hutchinson Cancer Research Center Seattle, Washington, United States |
2004–2010 (completed) |
NCT00008229 | |
| Study Comparing STR (Skeletal Targeted Radiotherapy) Plus Melphalan to Melphalan Alone, With Stem Cell Transplant in Multiple Myeloma | drug | multiple myeloma | Holmium-166- DOTMP | Poniard Pharmaceuticals |
2004–2009 (terminated) |
NCT00083564 | (Giralt et al. 2003; Breitz et al. 2003b) |
| Holmium Ho 166 DOTMP Followed by Peripheral Stem Cell Transplantation in Treating Patients With Metastatic Ewing's Sarcoma or Rhabdomyosarcoma That Has Spread to the Bone | drug | metastatic cancer, sarcoma | Holmium-166- DOTMP | Fred Hutchinson Cancer Research Center Seattle, Washington, United State |
2003–2011 (completed) |
NCT00006234 | |
| Chemotherapy, Holmium Ho 166 DOTMP, and Peripheral Stem Cell Transplantation in Treating Patients With Multiple Myeloma | drug | multiple myeloma and plasma cell neoplasm | Holmium-166- DOTMP | Poniard Pharmaceuticals |
2003–2009 (completed) |
NCT00045136 | |
| A Trial of Skeletal Targeted Radiotherapy Using Holmium-166-DOTMP in Patients With Multiple Myeloma | drug | multiple myeloma | Holmium-166-DOTMP | Poniard Pharmaceuticals |
2002–2009 (terminated, business reasons) |
NCT00039754 |
Intratumoural applications
Chitosan
The medical isotope 166Ho is gaining more and more interest from nuclear physicians in the treatment of tumours. Both drugs and medical devices labelled with radioactive 166Ho are used for internal radiotherapy. One of the therapy possibilities is the direct intratumoural treatment, in which the radioactive compound is injected with a needle directly into the tumour (Fig. 3). Such an intratumoural application can be found in for instance the 166Ho-labelled chitosan complex, which has been studied in different types of tumours such as brain (Ha et al. 2013; Huh et al. 2005), liver (Sohn et al. 2009; Kim et al. 2006) and prostate cancer (Kwak et al. 2005). Chitosan is a polymer obtained from the deacetylation of chitin which can form chelates with heavy metals (Suzuki et al. 1998; Park 1997). Its solubility in water is pH-dependent. Below pH 4.0, it is readily soluble in water. However, under neutral or basic conditions, it is converted into a gelatinized material enabling the retention of the complex at the administration site. The 166Ho-labelled chitosan complex was developed as a radiopharmaceutical for cancer therapy by the Korean Atomic Energy Research Institute. Preparation of this complex for injection involves vigorously mixing a solution composed of [166Ho]Ho nitrate and chitosan for 2 to 3 min (Sohn et al. 2009; Kim et al. 2006; Kwak et al. 2005). A retention of 166Ho at the injection site of around 90% has been demonstrated using the 166Ho-labelled chitosan complex due to the formation of a gel in the microenvironment of the injection site (Kwak et al. 2005; Suzuki et al. 1998; Park 1997; Muzzarelli et al. 1988). In animal studies, it was shown that a significant decrease in tumour volume was measured in the treated group compared to the control group (Huh et al. 2005; Kwak et al. 2005; Suzuki et al. 1998). In clinical research, positive effects have been observed as well: Kim et al. demonstrated complete tumour necrosis in 80.8% (42 of 52) of the patients with small hepatocellular carcinoma (Kim et al. 2006). In a study of Ha et al., 70% of the patients with recurrent cystic brain tumours responded positively to treatment with 166Ho-labelled chitosan, without systematic absorption or leakage (Ha et al. 2013; Huh et al. 2005).
166Ho-labelled chitosan has also been studied in order to treat renal cysts. Seventeen patients with renal cysts were injected with 166Ho-labelled chitosan under ultrasonographic guidance. At the end of the follow-up, 90% of the treated cysts had underwent a complete or near complete regression (Kim et al. 2004).
Ceramic materials
Another type of intratumoural treatment is brachytherapy (Fig. 3), in which radioactive ceramic materials are implanted in the tumour. These devices can form a variety of shapes and sizes and have been widely used because they can deliver a high local dose to the tumour while the surrounding tissue is spared, and they have shown to be biocompatible and biodegradable (Roberto et al. 2003; Campos et al. 2008). They are usually synthesized by the sol-gel technique which is a chemical method using temperatures below those used in traditional methods to process glass and ceramics (Hench and West 1990). This technique involves the homogenization of several components, pouring into moulds and then following a specific procedure of gelation, aging, drying and heat treatment (Hench and West 1990). The preparation of seeds (Diniz et al. 2017; Valente et al. 2011; Nogueira and Campos 2011; Nogueira and de Campos 2012; Nogueira and Campos 2016) and membranes (Nogueira and de Campos 2012) composed of 165Ho have been reported in the literature. Seeds usually have dimensions ranging from 0.3 to 0.8 mm in diameter and 1.5 to 1.8 mm in length and a density of 2 to 3.7 g/cm3 (Valente et al. 2011; Nogueira and Campos 2011). Holmium concentrations of 20 wt% (Valente et al. 2011) up to 30 wt% (Nogueira and Campos 2016) are achieved in these materials and it has been shown that the radionuclides are homogeneously distributed in the seeds matrix (Nogueira and Campos 2011). Neutron activation is performed on the final material and, although different elements in the matrix can be neutron activated, it has been shown that the radionuclide impurities are negligible due to low neutron cross-section of the materials used in their fabrication (Nogueira and Campos 2011). Insertion of other elements in the holmium-based ceramic materials such as barium (Nogueira and de Campos 2012) and zirconium (Nogueira and Campos 2016) to improve the radiological contrast allowing visualization of the seeds using conventional X-rays and mammography has also been investigated.
166Ho –labelled ceramic seeds have been studied in brain tissue in an animal study and also in a simulation study in human breast tissue (Diniz et al. 2017; de Campos et al. 2016). The advantages of the 166Ho-labelled seeds are the high dose rate and not having to remove the seeds after the treatment. Diniz et al. investigated the safety of degradable seeds in rat brains, using non-neutron activated seeds. The results showed no neurological or brain architectural changes, indicating a safe procedure (Diniz et al. 2017). In a study on brachytherapy in breast cancer by de Campos et al. a computational simulation was performed to provide a dosimetric analysis of temporary 192Ir-labelled seeds compared to permanent 166Ho-labelled seeds. It was shown that the spatial dose distribution of 166Ho was confined to the implanted volume, whereas this is not the case for brachytherapy using iridium-192, which is frequently used (de Campos et al. 2016). Monte Carlo simulations (Hosseini et al. 2013) and MIRD methodology (Valente and Campos 2010) have been used to determine the range and total absorbed dose of 166Ho-labelled ceramic seeds. The results were compared with seeds containing 32P, 90Y (Hosseini et al. 2013) or samarium-132 (132Sm). The radial dose values of the 166Ho-labelled seeds were concluded to be higher than other seeds at distances smaller than 5 mm (Hosseini et al. 2013; Valente and Campos 2010).
Microspheres
166Ho-labelled microspheres (166Ho-MS) have been developed for SIRT (also called radioembolization) (see paragraph on Selective internal radiation therapy) (Nijsen et al. 1999; Nijsen et al. 2004; Nijsen et al. 2001), but may potentially be used in microbrachytherapy (Bakker et al. 2017) for treatment of liver tumours (Bult et al. 2013a), kidney tumours (Bult et al. 2012) and head-and-neck tumours (van Nimwegen et al. 2018; Bakker et al. 2018). Moreover, this intratumoural approach can be used also for other tumour types such as pancreas tumours and even lung tumours. The intratumoural potential of 166Ho-MS has been investigated in both veterinary and human patients (Bakker et al. 2018; Bult et al. 2013a; van Nimwegen et al. 2018; Bult et al. 2013b; Van de Bovenkamp et al. 2009). Currently, two types of 166Ho-MS for intratumoural application, i.e., 166Ho-labelled acetylacetonate microspheres (166Ho-AcAc-MS) and 166Ho-labelled poly(L-lactic acid) microspheres (166Ho-PLLA-MS) were tested in animals. The 166Ho-AcAc-MS are prepared by a conventional oil in water (o/w) emulsification and solvent evaporation method by dissolving 165Ho-labelled AcAc crystals in chloroform and adding this organic solution to an aqueous phase containing an emulsifier. The emulsion formed is stirred until complete evaporation of the chloroform. The method enables preparation of microspheres with different sizes by varying for instance the stirring speed (Bult et al. 2012; Bult et al. 2009). The 166Ho-PLLA-MS are prepared using a similar method with the exception that the polymer (PLLA) is added to the organic solution before emulsification (Nijsen et al. 1999). After preparation, both microspheres have to be neutron activated to obtain radioactive 166Ho-AcAc-MS and 166Ho-PLLA-MS which can later be used for intratumoural treatments. In 2013, the first two papers on 166Ho-AcAc-MS in microbrachytherapy were published by Bult et al. (Bult et al. 2013a; Bult et al. 2013b). In the first study, the feasibility of applying 166Ho-AcAc-MS as an intratumoural treatment for renal tumours was demonstrated (Bult et al. 2013b). In the second study three domestic cats with spontaneous liver cancer were treated by administration of 166Ho-AcAc-MS via ultrasound guided percutaneous injections. In all cats the treatment was well tolerated and life was extended with good quality of life (Bult et al. 2013a). Van Nimwegen et al. treated 13 cats with inoperable squamous cell carcinoma by intratumoural injection of 166Ho-PLLA-MS. A response rate of 55% was observed with minimal side effects (van Nimwegen et al. 2018). Bakker et al. used 166Ho-PLLA-MS as a palliative treatment for patients with recurrent head-and-neck squamous cell carcinoma. Due to technical difficulties, a low patient dose and non-homogeneous distribution was observed which is probably the reason that a relatively low therapeutic efficacy was seen, however in none of the cases adverse effects were observed (Bakker et al. 2018).
Holmium solutions
The possibility of using dissolved 166Ho has also been studied. Suzuki et al. injected dissolved 166Ho salts into the liver of a male rat, resulting in a high uptake in blood and other tissue, however there was no retention of 166Ho at the injection site (Suzuki et al. 1998). In a mouse model of melanoma, free 166Ho was injected into the tumours with one single injection. The injection fluid was not always uniformly distributed throughout the tumours causing tumour regrowth. It was suggested that free 166Ho may be used for solid and firm tumours, although hypervascularized tumours may not retain the soluble form (Lee et al. 2002). Tumour growth was increasingly impaired when intratumoural injection of free 166Ho was combined with the injection of dendritic cells 1 week after 166Ho injection, compared to 166Ho injection alone. Nineteen days after 166Ho injection, the average tumour size in the 166Ho injection alone group was 1658 mm2, against 444 mm2 in the 166Ho and dendritic cell group (Lee et al. 2006).
Intravenous applications
Antibodies and peptides
Targeted therapy with radionuclides is an upcoming form of cancer treatment, in which radioisotopes are labelled to antibodies or other tumour-seeking peptides (Fig. 3). Different antibodies (Table 1) have been conjugated to 166Ho. For this, (monoclonal) antibodies (mAbs) are first conjugated to an appropriate chelator such as DO3A-4B4 (Ballard et al. 2011), DOTA, methoxy-DOTA (MeO-DOTA) (Mohsin et al. 2006) or CHX-A" DTPA (DTPA = diethylenetriaminepentaacetic acid) (Thompson et al. 2014). Radiolabeling with 166Ho is then performed in an appropriate buffer by mixing the antibody-chelator conjugate with the radioactive 166Ho dissolved in hydrochloric acid. The complex is then purified typically by size exclusion chromatography (Mohsin et al. 2006).
Melanoma is an example of a disease in which labelled antibodies or peptides with 166Ho have been used as a treatment. Ballard et al. labelled a 4B4 peptide with three types of isotopes, i.e. 166Ho, 177Lu and samarium-153 (153Sm), as a potential therapy for melanoma. In vitro binding assays demonstrated a lower binding efficiency and specific activity of 166Ho and 153Sm compared to 177Lu, as a result 166Ho and 153Sm have not been included in further in vivo experiments (Ballard et al. 2011). In a study by Thompson et al., a comparison was made between 166Ho-, 90Y-, and 188Re-labelled 6D2 mAbs as a potential treatment for melanoma. The results showed a comparable therapeutic effect between 166Ho- and 188Re-labelled 6D2 mAbs after injection of an activity of 37 MBq. Additionally, no toxic effect was observed as a result of injection of 166Ho or 188Re complex, whereas the 90Y complex was toxic to mice and did not produce an antitumour effect (Thompson et al. 2014).
Over the years, a strong interest in targeted therapies for colorectal cancer has been developed. In a preclinical study, mice were intravenously injected with 166Ho-, promethium-149 (149Pm)- or 177Lu-labelled CC49 mAbs 14 days after implantation of human colon tumours. Biodistribution results showed a maximum tumour uptake of 166Ho at 96 h post injection (Mohsin et al. 2006), which is rather late. Since the half-life of 166Ho is only 26.8 h, the therapeutic effect may then be limited. In another study the same mAbs and radionuclides were compared and the 166Ho-labelled mAbs yielded the least favourable results in terms of tumour doubling time and survival (Mohsin et al. 2011). These unfavourable results may also be the result of the combination of short half-life (26.8 h) and late (96 h) maximum tumour uptake, as the 166Ho may already be decayed when arriving to the tumour. Khorami-Moghada et al. provided a study on a colon cancer model in mice, labelling 166Ho to the VEGF-A antibody bevacizumab. The biodistribution results show high uptake in the liver, blood, kidneys and in the tumours (Khorami-Moghadam et al. 2013). Unfortunately, the article is not very clear in its material and methods and therefore it is difficult to estimate the real value.
Bone-seeking agents
Bone marrow transplantation poses a potentially curative treatment to various hematologic malignancies. Prior to the autologous or allogeneic transplantation high local radiation doses are needed to destroy the old bone marrow. High doses generated by external radiation may cause damage to other organs, which is unfavourable. Therefore, bone seeking radiopharmaceuticals have been developed to generate a high local dose and limiting dose to other tissues.
An efficient and safe radiopharmaceutical for bone marrow ablation requires the formation of a stable complex between the bone-seeking agent and the radionuclide. Phosphonate chelates are commonly used for this purpose due to their excellent specificity for bone localization. When combined with radioactive isotopes, the complexes can deliver high levels of radiation to bone and bone marrow, leaving normal tissues unaffected. The isotope 166Ho has been conjugated to several bone-seeking agents for bone marrow ablation such as EDTMP (ethylene-diamine-tetramethylene phosphonic acid) (Sohaib et al. 2011; Bahrami-Samani et al. 2010; Appelbaum et al. 1992; Louw et al. 1996), DOTMP (Bayouth et al. 1995a; Bayouth et al. 1995b; Parks et al. 1993; Giralt et al. 2003; Breitz et al. 2006; Rajendran et al. 2002; Ueno et al. 2009; Breitz et al. 2003a), TTHMP (triethylene tetramine hexa (methylene phos- phonic acid)) (Yousefnia et al. 2014), PAM (pamidronate) (Vaez-Tehrani et al. 2016), APDDMP (N,N-dimethylenephosphonate-1-hydroxy-4-aminopropylidene-diphosphonate) (Marques et al. 2006; Zeevaart et al. 2001), among others. The preparation of the 166Ho-radiolabelled complexes is performed very similarly with the different bone-seeking agents. In general, a target of holmium oxide is neutron activated which is afterwards dissolved in hydrochloric acid to form 166HoCl3. Then, a solution containing the dissolved chelator complex (e.g. DOTMP) is added and the pH is adjusted to 7–8. Complexation occurs within typically 1 h at room temperature with high radiochemical purity (more than 99%) (Sohaib et al. 2011; Bahrami-Samani et al. 2010; Bayouth et al. 1995a; Yousefnia et al. 2014).
Appelbaum et al. showed that after 24 h post injection of the bone seeking phosphonate EDTMP labelled with 166Ho, the [166Ho]Ho EDTMP concentration was 200-fold higher in bone than in other organs (Appelbaum et al. 1992). The results of a study performed by Bahrami-Samani et al. in wild-type rats demonstrated significant bone accumulation (> 70%) of [166Ho]Ho EDTMP after 48 h (Bahrami-Samani et al. 2010). However, in a study by Louw et al., [166Ho]Ho EDTMP turned out to be significantly inferior to [153Sm]Sm EDTMP in terms of pharmacokinetics, biodistribution and skeletal localization (Louw et al. 1996). These results differ considerably from the results found by Appelbaum et al. (Appelbaum et al. 1992). The discrepancy might, among others, be due to the use of S-values for human children by Appelbaum et al., whereas adult human S-values were used by Louw et al. (Louw et al. 1996). Sohaib et al. also demonstrated inferiority of [166Ho]Ho EDTMP compared to [90Y]Y EDTMP, with a skeletal accumulation of only approximately 27% (46% in [90Y]Y EDTMP) (Sohaib et al. 2011).
Pedraza-Lopez et al. have labelled 166Dy/166Ho with the bone seeker EDTMP to treat haematological malignancies. 166Dy2O3 was neutron activated and converted to 166DyCl3 which was then added to a solution containing EDTMP in phosphate buffer (pH 8.0). A radiochemical purity of 99.3 ± 0.6% was achieved. Animal studies showed a fast blood clearance of [166Dy]Dy/[166Ho]Ho EDTMP and a skeletal uptake of 22.32 ± 1.86% ID/g at 2 h and 20.12 ± 1.94% ID/g after 10 days. Theoretical bone marrow absorbed dose calculations indicate that the [166Dy]Dy/[166Ho]Ho EDTMP in vivo generator system produced 3.47 times more dose than [166Ho]Ho DOTMP per unit of initial activity in the skeleton (Pedraza-Lopez et al. 2004a). The same group also demonstrated that the [166Dy]Dy/[166Ho]Ho EDTMP system induced considerable cytotoxicity, genotoxicity and severe myelosuppression in mice at bone marrow absorbed doses of 18–23 Gy, suggesting that this system could potentially be a good agent for use in humans (Pedraza-Lopez et al. 2004b).
In an animal study on splenectomized young adult beagle dogs, a complete ablation of hematopoietic marrow was seen within 7 days after receiving a radiopharmaceutical dosage of 370 MBq/kg body weight of [166Ho]Ho DOTMP (Parks et al. 1993). The pharmacokinetics, biodistribution and absorbed dose estimation of the [166Ho]Ho DOTMP complex has also been studied in six patients suffering from multiple myeloma. A high uptake in the skeleton and rapid clearance from the blood was observed (Bayouth et al. 1995a). Two larger phase I/II dose escalation studies in human patients showed a fast bone uptake, rapid clearance from the blood, no retention in soft tissue and minimal systemic toxicity (Giralt et al. 2003; Rajendran et al. 2002). A therapeutic dose of [166Ho]Ho DOTMP was given to 83 patients with multiple myeloma. In 35% of the patients complete remission was achieved and no acute toxicity issues were seen (Giralt et al. 2003). In this cohort study, dosimetry and toxicity were also assessed. Doses in the marrow, bladder and kidney range from 13 to 59 Gy, 4.7 to 157 Gy, and 0.5 to 7.9 Gy, respectively. Hemorrhagic cystitis was observed in a number of patients who received a dose of more than 40 Gy to the bladder wall. Renal toxicity related to [166Ho]Ho DOTMP was observed in 7 patients (12%) (Breitz et al. 2003a). Ueno et al. treated 6 women with bone-only metastasized breast cancer with [166Ho]Ho DOTMP. After a follow-up time of 6 years, 2 of the 6 patients sustained complete response. An acceptable toxicity profile was also described in this paper (Ueno et al. 2009). Since the results are very promising, five clinical trials have been performed on [166Ho]Ho DOTMP, of which no papers have been published yet, with the following clinicaltrials.gov identifier numbers: NCT00045136, NCT00004158, NCT00008229, NCT00039754, NCT00006234 (Table 2).
Vosoghi et al. developed a highly-stable metallic organic framework (MOF) as a bone seeking agent composed of DOTMP, 166Dy/166Ho generator and CuCl2. The MOF was formed by stirring neutron activated [166Dy]Dy/[166Ho]Ho nitrate, CuCl2 and DOTMP at room temperature. Purification of the 166Dy from the 166Ho and other isotopes was achieved by chromatography before preparation of the MOF. The particles obtained had a size ranging from 60 to 100 nm. The product has shown to have high affinity for simulated bone matrix and comparable to that of the FDA-approved radiopharmaceutical Quadramet ([153Sm]Sm EDTMP) (Vosoghi et al. 2016).
Two other bone-seeking agents are nano-hydroxyapatite dopped with 166Ho (da Silva et al. 2017) and [166Ho]Ho PDTMP (Zolghadri et al. 2013). In both studies, a significant bone uptake was demonstrated, but no further research has been done.
Nanoparticles
Nanoparticles containing holmium have been used for different applications with different compositions (Table 1). Munaweera et al. developed magnetic nanoparticles containing both 166Ho and a platinum-based chemotherapeutic to treat lung cancer. This study demonstrated that the nanoparticles are toxic to the tumour cells and that tumour to liver ratios increases when an external magnetic field was applied (Munaweera et al. 2015). Zielhuis et al. prepared nanosized liposomes (~ 120 nm) using a conventional thin-film hydration technique and loaded the liposomes with the radioactive [166Ho]Ho DTPA-lipid complex which was incorporated in the liposomal bilayer. The high stability and paramagnetic properties make them suitable for treatment and multimodal imaging with SPECT and MRI (Zielhuis et al. 2006).
Selective internal radiation therapy
Selective internal radiation therapy (SIRT, also called radioembolization) is a form of internal radiation therapy, during which millions of microspheres are administered into an artery, close to a target lesion. These microspheres of around 30 μm in diameter are administered through a catheter and carried by the blood flow until they lodge at the arteriolar level. Clinically, SIRT is applied as novel treatment for liver malignancies. The rationale is that liver tumours mainly depend on the hepatic artery for their blood supply, whereas the healthy liver tissue is mainly supplied by the portal vein. Therefore, microspheres in the hepatic artery will especially target liver tumours. This will result in a higher radiation dose to the liver tumours, whilst sparing the healthy liver tissue (Nijsen et al. 2002a).
Currently, the only commercially available and clinically used microspheres for SIRT based on the isotope 166Ho are 166Ho-PLLA-MS (QuiremSpheres, Quirem Medical, the Netherlands). Other microspheres with 166Ho that were investigated for application within SIRT procedures are MS-PDLLA-HoAcAc (Nayak and Lahiri 1999) and resin-based microspheres from Turner et al. (Turner et al. 1994; Zielhuis et al. 2007; Mumper and Jay 1992). The polymer-based microspheres used for SIRT are typically prepared by the emulsification and solvent evaporation method (see section 3.3) and have the advantage of near blood plasma density and biocompatibility (Nijsen et al. 2002a; Mumper et al. 1992). However, polymers are usually sensitive to neutron irradiation which can lead to damage of the microspheres after long neutron activation (Nijsen et al. 1999; Nijsen et al. 2002b). Nevertheless, the 166Ho-PLLA-MS microspheres can be neutron activated till high amounts of activity, sufficient for transportation to the hospital and clinical application (Vente et al. 2009). Resin-based microspheres present the advantage of easier preparation performed through the incubation of the radioactive 166Ho with commercially available resins. Bio-Rex resins which are made of an acrylic polymer with carboxylic groups were used to bind 166Ho with high-yield (94.53% at pH 8.5) by Subramanian et al. (Subramanian et al. 2018). Aminex A-5 resin, based on styrene divinylbenzene copolymer with sulphonic acid, has also been conjugated to 166Ho by Turner et al. (Turner et al. 1994). Alginate microspheres can also be loaded with 166Ho after neutron activation (Das et al. 2009a). Last, chitosan microspheres containing the 166Dy/166Ho in vivo generator have also been prepared. Stability studies showed that 5–10% of the radioactivity was released in human serum (Cho and Choi 2018).
Imaging (SPECT, MRI and CT)
Smits et al. described that the activity for using holmium microspheres in SIRT can be calculated with the following formula: AHo166 (MBq) = Liver dose × 63 (MBq/J) × LW (kg) where AHo166 is the administered activity, LW is the tissue weight, and dose is the intended absorbed radiation dose in Gy (Smits et al. 2012; van de Maat et al. 2013). When imaging the 166Ho distribution with SPECT, medium-energy collimators are often used with energy windows of 80.6 keV (15% window width) for the 166Ho photopeak and 118 keV (12%) for correction for down-scattered high-energy photons. A typical protocol will be 120 projections of 30 s orbits around the liver. Data for the distribution of 166Ho-PLLA-MS deposition in liver SIRT treatments have been reconstructed to a 128 · 128 · 128 matrix with an isotropic voxel size of 4.7 mm or 4.8 mm using an ordered-subsets expectation maximization algorithm including resolution recovery and a hybrid method for scatter and attenuation correction (Elschot et al. 2011; Smits et al. 2013).
The holmium in the microspheres can be used for quantitative MR imaging, since it is a highly paramagnetic metal. The imaging is usually performed on clinical whole-body MR systems of 1.5 or 3 T (Smits et al. 2013). For quantitative measurements of the 166Ho-microsphere biodistribution, a multi-slice multi-gradient echo sequence is used, sampling the MR imaging signal of the free induction decay. The holmium quantification is performed though T2*-weighted turbo spin echo imaging (Seevinck et al. 2007; van de Maat et al. 2013; Smits et al. 2013; Seevinck et al. 2012).
Agglomerations of holmium microspheres can be visualized through CT imaging, in practice especially holmium microspheres that contain relatively high amounts of holmium. If these microspheres are concentrated, which is the case in intratumoural injections, CT can be used for quantitative detection (Seevinck et al. 2007; Bakker et al. 2018). Moreover, novel microspheres with a much higher holmium content are currently developed (Bult et al. 2009; Arranja et al. 2018). These microspheres are expected to be visible on CT in even lower concentrations.
Head-and-neck tumours
Van Es et al. performed two animal studies in which Vx2 cells were subcutaneously injected into the auricles of rabbits. After reaching a tumour size of 4 cm2, 165/166Ho-PLLA-MS (van Es et al. 2001; Van Es et al. 2001) or 99mTc-labelled Dextran hydrogel (Dex) MS (van Es et al. 2001) were injected into the caudal auricular artery. In the first study a comparison was made between injecting 165Ho-PLLA-MS and 166Ho-PLLA-MS. Complete remission of 79% and 86% was obtained by injecting 165Ho-PLLA-MS and 166Ho-PLLA-MS, respectively. Although there was a high remission rate, the limited additional effect of irradiation might be explained by the high sensitivity of the Vx2 tumour model to vascular occlusion. In addition, retention of approximately 40% of the MS into the infusion system may have led to an ineffective dosage (Van Es et al. 2001). In the second study, injection of 166Ho-PLLA-MS was compared to Dex MS. 51% of the 166Ho-PLLA-MS, with a diameter of 19 μm, shunted to the lungs, whereas 95% of the Dex MS were retained in the tumour (van Es et al. 2001).
Liver tumours
In a biodistribution study in which SIRT was performed with 166Ho-PLLA-MS on rats with implanted liver tumours, the radioactivity in the tumours was six time higher than in the non-targeted liver tissue, indicating the 166Ho-PLLA-MS deposition was confined to the liver (Nijsen et al. 2001). In order to assess the clinical effects of the microspheres, healthy pigs received an activity up to 6.5 GBq through the liver artery, which corresponds with an estimated absorbed liver dose of 120 Gy. After 2 months of follow-up, it was concluded that the toxicity profile of holmium microspheres is low and that administration of doses up to 100 Gy is not associated with clinically relevant side effects (Vente et al. 2008).
Seppenwoolde and his colleagues demonstrated the feasibility of fully MR-guided SIRT with holmium microspheres in pigs. Whereas the positioning of the catheter normally takes place under X-ray guidance, in this study a passive tracking sequence was used to visualize paramagnetic markers on the catheter. Although some drawbacks are described (such as difficulty in visualization of the catheter due to respiratory motion artefacts) this article is the first to report on real-time imaging of holmium microspheres in an anthropomorphic in vivo model as a proof of principle that real-time image guided treatment is possible under MRI (Seppenwoolde et al. 2005).
The first clinical trial in which 166Ho-PLLA-MS were administered to patients was the phase 1 HEPAR 1 trial (2009–2011) (Smits et al. 2012; Smits et al. 2010) (Table 2). In this study, 15 patients with liver metastases of any origin were treated by SIRT at different whole-liver absorbed dose levels ranging from 20 to 80 Gy (Fig. 4). In the 80 Gy cohort, dose-limiting toxicity occurred in two out of three patients. Therefore, the maximum tolerated radiation dose was identified as 60 Gy. Exposure rates (μSv/h) from patients were measured at 1.0 m distance from a lateral and frontal position at 0, 3, 6, 24, and 48 h after infusion. The total effective dose equivalent to a contact of patients treated with 166Ho-labelled MS did not exceed the NRC limit of 5 mSv. Contact restrictions 6 h after treatment are unnecessary for infused activities < 7 GBq (Prince et al. 2014).
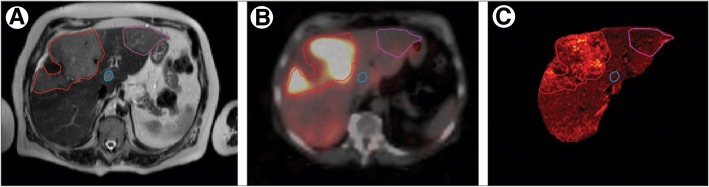
Fig. 4.
Intrahepatic visualisation of 166Ho-microspheres after SIRT. T2-weighted MRI of the liver in a patient with several ocular melanoma liver metastases, outlined by coloured regions of interest (a). After SIRT, the distribution of 166Ho-PLLA-microspheres within the liver was visualized by single-photon-emission CT (b) and R2-weighted MRI (c). Reprinted from The Lancet Oncology, Vol. 13, Smits et al., Holmium-166 SIRT in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study, 1025–1034, Copyright 2012, with permission from Elsevier
After SIRT of liver metastases with 166Ho-PLLA-MS was deemed safe in the HEPAR 1 trial, the HEPAR 2 trial was performed to investigate the efficacy of the 166Ho SIRT (Prince et al. 2018) (Table 2). In this trial, 38 patients with liver metastases refractory to systemic therapy and ineligible for surgical resection were treated with 166Ho-PLLA-MS. In 73% of the patients, the target lesions showed complete response, partial response, or stable disease (disease control) at 3 months. There was an acceptable toxicity profile. The most common adverse events during follow-up were gastrointestinal complaints, i.e. nausea, abdominal pain and fatigue. In a subgroup analysis on the patients with liver-dominant colorectal cancer metastases, a median survival of 13.4 months (95% CI, 8.2–15.7 mo) was seen, which is comparable to the reported range of 8.3–15.2 mo after 90Y SIRT (Prince et al. 2018).
In order to prevent any unintentional deposition of the microspheres in tissue other than the liver, a safety procedure is typically performed before the actual treatment. During this procedure, relevant extrahepatic arteries are coiled and a scout dose of technetium-99 m-labelled macro-aggregated albumin (99mTc-MAA) is administered. SPECT-imaging is then performed in order to estimate the extrahepatic shunting of activity. In literature, it has been argued multiple times that this 99mTc-MAA scout dose is not an accurate estimation of the actual microsphere distribution, due to morphological (different non-spherical shapes, large size distribution) and biochemical differences between 99mTc-MAA and microspheres such as 166Ho-labelled MS and 90Y-labelled MS (Wondergem et al. 2013; Elschot et al. 2014).
For this matter, studies have been performed to investigate the possibility of using a scout dose of 166Ho-labelled MS (166Ho-SD) before treatment. Braat et al. (Braat et al. 2018a) used a scout dose (250 MBq) in 82 patients. In 6 patients there were extrahepatic depositions, however, no adverse events related to this were observed during a median follow-up of 4 months (range 1–12 months). In another study, the extrahepatic depositions of 99mTc-MAA were objectified in 166 patients, and then the absorbed dose was calculated as if these patients had received a 166Ho-SD. Only in 2 cases the theoretical dose exceeded 50 Gy (Prince et al. 2015). These results support the safety of a 250 MBq 166Ho-SD in a clinical setting.
Radosa et al. investigated the clinical feasibility, technical success and toxicity of SIRT with 166Ho-labelled MS in 9 patients with HCC (Radosa et al. 2019). The median administered activity was 3.7 GBq. The treatment was found to be feasible and safe, with no significant hepatotoxicity, as 4 patients suffered from mild and transient post radioembolization syndrome and no indicators of SIRT induced liver disease (REILD) were observed. Eight patients showed a good response and only one patient had a progressive disease at 6 months follow-up. All in all, the results with holmium microspheres were deemed at least comparable to yttrium microspheres in terms of safety.
Radiosynoviorthesis
Ferric hydroxide macroaggregates
Besides cancer treatment, 166Ho may also be used as a radionuclide in a technique called radiosynoviorthesis to treat chronic synovitis or recurrent hemarthrosis in coagulation disorders. In this treatment, a 166Ho-labelled ferric hydroxide macroaggregates (FHMA) complex is injected into the affected joints (Fig. 3) to generate local irradiation. Various studies have been performed on this type of treatment. In a Monte Carlo simulation study by Ferro-Flores et al. it was shown that 166Ho generates a favourable radiation dose to the articular cartilage and bone surface (Ferro-Flores et al. 2004). In an animal study, 30 rabbits received intra-articular injections with 166Ho-labelled FHMA, resulting in acute focal radiation necrosis without hyperplasia of synoviocytes. Autoradiography showed an uneven distribution of the radiotherapeutical along the synovial lining. After 3 days, the majority of the 166Ho-labelled FHMA complex had leaked out of the joint or was phagocytized by the synoviocytes (Makela et al. 2002). In a study by Makela et al., six horses were treated with 166Ho-labelled FHMA, with a mean activity of 1000 MBq/joint. 165Ho-labelled FHMA was used as a control. In this study, acute focal radiation necrosis (of the synovium) was observed as well, but in contrast to the study on rabbits, no radiopharmaceutical leakage was found (Makela et al. 2003a). The articular cartilage of the horses showed only mild signs of degeneration as a result of this therapy (Makela et al. 2004). In another study, the short and long-term effects of radiosynoviorthesis with 166Ho-labelled FHMA were observed in mature and growing rabbits. The radiation effects in the growing rabbits included mild cartilage fibrillation and downregulation of cartilage-specific genes (Makela et al. 2003b). In a study by Vuorela et al., SPECT and MRI images of human patients treated with 166Ho-labelled FHMA were fused. SPECT images were used to localize the 166Ho, whereas the T1- weighted Gd-DTPA MR images were used to visualize the inflamed synovium. A higher uptake of 166Ho in regions associated with a higher level of synovitis was seen (Vuorela et al. 2005). Kraft et al. studied the therapeutic effect of 166Ho-labelled boron macroaggregates. In this study, 17 knees of 15 human patients with chronic synovitis were treated with a mean activity of 972 MBq. Only an insignificant leakage of radiopharmaceuticals was observed. Six months after treatment, 73% of the patients experienced a lower sense of pain. In addition, 2 patients had no knee exudation anymore and a decrease of knee exudation was seen in 4 patients (Kraft et al. 2007).
Chitosan
Synovitis can also be treated with intra-articularly injected 166Ho-labelled chitosan. The preparation of the complex is similar to the one used for intratumoural application (section 3.1). In a study on human patients with rheumatoid arthritis, most of the injected radioactivity was localized within the injected cavity and limited radioactive excretion and negligible extra-articular leakage were observed (Cho et al. 2010; Song et al. 2001). Cho et al. stated that 166Ho-labelled chitosan is a favourable agent compared to the conventional treatment of synovititis using other radioisotopes, based on a higher permeability to soft tissue, smaller extra-articular leakage and higher amount of induced synovial necrosis (Cho et al. 2010). Lee et al. evaluated the response using MR imaging and found decreased joint effusion at 4 months after the treatment with 166Ho-labelled chitosan (Lee et al. 2003).
Microspheres
Next to SIRT, 166Ho-PLLA-MS have also been studied as an agent for radionuclide synovectomy. In a study by Mumper et al., 166Ho-PLLA-MS were injected in the knee joints of six healthy rabbits. At 120 h post injection, intra-articular 166Ho retention of approximately 98% was found with no uptake in the lymph nodes (Mumper et al. 1992).
166Dy/166Ho macroaggregates
Several 166Dy/166Ho-labelled macroaggregates have also been prepared for radiation synovitis (Park et al. 1996; McLaren et al. 1990; Sledge et al. 1977) with promising results in patients (Edmonds et al. 1994). Ferro-Flores et al. also developed 166Dy/166Ho-labelled hydroxide macroaggregates (HM) for radiation synovectomy. For this, 164Dy2O3 was neutron activated which was then converted into 166DyCl3 and the 166Dy isotope purified. The 166DyCl3 was then incubated with a sodium hydroxide solution in an ultrasonic bath to form 166Dy/166Ho-labelled HM with sizes ranging from 2 to 5 μm and more than 99.5% of radiochemical purity. In vivo studies in rats showed that the 166Dy/166Ho-labelled HM were retained at the administration site even after 7 days (Ferro-Flores et al. 2004).
Miscellaneous applications
Intraluminal irradiation
Another application of 166Ho is for intraluminal irradiation, in which typically stenoses or other blood vessel wall abnormalities are treated. Different approaches for intraluminal irradiation have been studied, e.g. 166Ho –impregnated polyurethane coating on a stent (Won et al. 2002; Won et al. 2005), 166Ho-coated surface of a dilatation balloon (Kim et al. 2003; Hong et al. 2003) or dilatation balloons filled with 166Ho conjugated to DTPA (Hong et al. 2004; Park et al. 2007; Park et al. 2003; Kim et al. 2000). Won et al. developed self-expandable stents covered with 166Ho in a canine model. Both studies demonstrate the formation of fibrosis on the adjacent wall. No serious complications have been reported and it was indicated that this type of stent could be used as an alternative treatment (Won et al. 2002; Won et al. 2005). Hong et al. developed a procedure in which the surface of a dilatation balloon could be coated with 166Ho. Herein is was stated that coated balloons are more resistant to leaking radioactivity as opposed to liquid [166Ho]Ho DTPA filled balloons and a higher dose may be delivered. However, there may be a chance of peeling off of the 166Ho-coated surface and the activity can be unevenly distributed (Hong et al. 2003). Kim et al. delivered a dose of 20 Gy, using a dilatation balloon with 166Ho coated on the surface, in a porcine coronary stent restenosis model and the irradiated group demonstrated a significantly decreased stenosis area compared to the control group (Kim et al. 2003). As already mentioned, 166Ho is also used in combination with DTPA. In a study in rats and rabbits it was shown that after intravenous injection [166Ho]Ho DTPA was excreted relatively quickly to the urine bladder. Furthermore, a relatively low absorption in vital organs was seen (Hong et al. 2002). In a phantom study, in which clinically relevant irradiation and duration of exposure were tested, it was shown that [166Ho]Ho DTPA is a good source for endovascular irradiation (Joh et al. 2000). This was confirmed in a study in 12 pigs, in which an average dose of 30 Gy was delivered to treat pseudointimal hyperplasia (Park et al. 2007). The feasibility of the treatment to prevent restenosis was also demonstrated by Kim et al. in an animal study on 34 pigs (Kim et al. 2000). Park et al. conducted a study on 56 patients with in-stent restenosis. A balloon filled with liquid [166Ho]Ho DTPA which was a little longer than the stenosed stent was placed inside the lesion. For all patients the treatment was successful, and in 93% of the patients no restenosis occurred within the time of follow-up (approximately 19 months) (Park et al. 2003). Hong et al. tested whether it would be beneficial to combine [166Ho]Ho DTPA with a CT contrast medium. It was demonstrated that a CT contrast medium may have an added value to detect radiation leakage of the balloon on real-time basis (Hong et al. 2004). Majali et al. studied the potential of [166Ho]Ho dimethyl di ethylene triamine penta acetic acid (DMDTPA) for intraluminal irradiation in mice. Biodistribution data demonstrated a faster clearance of the complex compared to [166Ho]Ho DTPA, almost no accumulation in bone or any other vital organs was seen (Majali et al. 2002).
Patches
Patches for the treatment of skin cancer and Bowen’s disease (a precursor of squamous cell skin cancer) containing 166Ho have also been fabricated. Lee et al. created patches in which 165Ho-labelled macroaggregates were attached to an adhesive tape followed by coating with a polyethylene film, where after the patch was neutron-activated. These were then applied to mice with induced skin tumours (Lee et al. 1997). Chung et al. also created a patch to treat skin malignancies by dissolving holmium-nitrate and polyurethane in a solvent mixture of dimethylformamide and tetrahydrofuran and the solution was casted on an aluminium dish until solvent evaporation and consequent formation of a dried film. Particles of 30 to 50 μm were obtained from this cast, neutron activated and afterwards attached to adhesive tape. These patches were applied to patients with Bowen’s disease (Chung et al. 2000). The results of both studies were positive and complete remission was observed. However, they also described several disadvantages, such as the limited penetration depth of the beta radiation, which makes it hard to irradiate bulky tumours. Another disadvantage of patches to which macroaggregates are attached is the chance of isotope leakage, which may lead to contamination. Manuweera et al. developed a bandage of nanofibers containing homogeneously incorporated holmium nanoparticles (Munaweera et al. 2014). First, 165Ho-labelled iron garnet nanoparticles (165HoIG) were prepared by mixing iron nitrate and holmium nitrate with ethylene glycol followed by precipitation of the 165HoIG nanoparticles with sodium hydroxide. This resulted in nanoparticles with a length of 55 nm and a width of 28 nm. Then, electrospinning of a solution containing polyacrylonitrile (PAN) and the 165HoIG nanoparticles dispersed in dimethylformamide resulted in a bandage containing nanofibers with the nanoparticles incorporated (Fig. 5). The 165HoIG/PAN nanofibers were neutron activated and showed to be stable up to a thermal neutron-flux of approximately 3.5 × 1012 neutrons/cm2 s for at least 4 h at 100 °C. Stability studies using simulated body fluid revealed no leakage of the nanoparticles from the nanofibers after 8 h incubation (Munaweera et al. 2014).
Fig. 5.
Holmium polyacrylonitrile patch. Reprinted (adapted) with permission from Munaweera et al.. Radiotherapeutic bandage based on electrospun polyacrylonitrile containing holmium-166-labelled iron garnet nanoparticles for the treatment of skin cancer, Copyright 2014 American Chemical Society
Radiovertebroplasty
In order to treat bone malignancies a new type of treatment named radiovertebroplasty has been developed. In this therapy, radionuclides are incorporated in bone cement to generate a local high dose. For example, Hirsch et al. developed an acrylic cement of polymethyl methacrylate (PMMA) and uniformly incorporated 166Ho. The authors concluded 166Ho is a potential radionuclide for radiovertebroplasty based on dosimetry (Hirsch et al. 2008). Donanzam et al. managed to generate stable bioceramics using the sol-gel technique (see section 3.2) in which 166Ho is incorporated in calcium phosphate. Despite the described potential of the developed biomaterial, clinical trials are necessary to further investigate the bioactivity and efficacy in bone malignancies (Donanzam et al. 2013).
Conclusion
In this review, we have described the broad spectrum of medical applications of 166Ho as a result of the growing research interest over the years. 166Ho is among the most promising relatively new therapeutic radioisotopes. Holmium has very interesting physical properties: a high energy beta particle emission for treatment, a low energy gamma photon emission for imaging and a high magnetic susceptibility for MR imaging. Therefore, 166Ho labelled systems are attractive for radionuclide treatment. Furthermore, because of its natural abundancy of 100% and a high cross section of 64 b it can be produced in a straightforward manner, both fast and therefore relatively low-priced. In the radionuclide therapies, it is observed that quantitative analyses and dosimetry are gaining more and more importance to predict treatment efficacy. The value of treatment verification as an integral part of any radiotherapeutic treatment is also underpinned by the Council Directive 2013/59/EURATOM, which came into effect in February 2018, stating: “For all medical exposure of patients for radiotherapeutic purposes, exposures of target volumes shall be individually planned, and their delivery appropriately verified …”. Imaging and dosimetry can be used for better patient selection and to improve treatment dose calculations. The multimodal imaging medical isotope 166Ho can be a good response to the increasing demand of more personalized treatments in patients.
Acknowledgements
N.a.
Abbreviations
- 166Ho-SD
A scout dose of 166Ho-MS
- 99mTc-MAA
Technetium-99 m-labelled macro-aggregated albumin
- AcAc
Acetylacetonate
- APDDMP
N,N- dimethylenephosphonate- 1-hydroxy-4-aminopropylidenediphosphonate
- CT
Computed tomography
- Dex
Dextran hydrogel
- DMDTPA
Di ethylene triamine penta acetic acid
- DMF
Dimethylformamide
- DOTMP
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylene-phosphonic acid
- DTPA
Diethylenetriaminepentaacetic acid
- EBRT
External beam radiotherapy
- EDTMP
Ethylene-diamine-tetramethylene phosphonic acid
- FHMA
Ferric hydroxide macroaggregates
- HCC
Hepatocellular carcinoma
- HM
Hydroxide macroaggregates
- mAbs
monoclonal antibodies
- MOF
Metallic organic framework
- MRI
Magnetic resonance imaging
- MS
Microspheres
- PAM
Pamidronate
- PAN
Polyacrylonitrile
- PLLA
Poly(L-lactic acid)
- PMMA
Polymethyl methacrylate
- REILD
Radioembolization induced liver disease
- SIRT
Selective internal radiation therapy
- SPECT
Single photon emission computed tomography
- TTHMP
Triethylene tetramine hexa (methylene phos- phonic acid)
Authors’ contributions
NJMK, MJA, AGA and JFWN conceived the general outline of the review. All authors performed the literature search on their topic, reviewed the literature at specific chapters and wrote the manuscript. All authors read and approved of the final manuscript.
Funding
N.a.
Availability of data and materials
N.a.
Ethics approval and consent to participate
N.a.
Consent for publication
N.a.
Competing interests
J.F.W. Nijsen is inventor on the patents related to holmium microspheres which are assigned to University Medical Center Utrecht Holding BV and/or Quirem Medical. He is co-founder and chief scientific officer of Quirem Medical, and has a minority share in the company Quirem Medical. The activities of J.F.W. Nijsen within Quirem Medical are approved and supported by Dirkjan Masman (Director Technology Transfer Office Radboudumc) and Mathias Prokop (Head of Radiology and Nuclear Medicine at Radboudumc).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nienke J. M. Klaassen, Mark J. Arntz, Alexandra Gil Arranja, Joey Roosen and J. Frank W. Nijsen contributed equally to this work.
Contributor Information
Nienke J. M. Klaassen, Email: nienke.klaassen@radboudumc.nl
Mark J. Arntz, Email: mark.arntz@radboudumc.nl
Alexandra Gil Arranja, Email: alexandra.gilarranja@radboudumc.nl.
Joey Roosen, Email: joey.roosen@radboudumc.nl.
J. Frank W. Nijsen, Email: frank.nijsen@radboudumc.nl
References
- Appelbaum FR, Brown PA, Sandmaier BM, Storb R, Fisher DR, Shulman HM, Graham TC, Schuening FG, Deeg HJ, Bianco JA, et al. Specific marrow ablation before marrow transplantation using an aminophosphonic acid conjugate 166Ho-EDTMP. Blood. 1992;80(6):1608–1613. [PubMed] [Google Scholar]
- Arranja AG, et al. Radioactive holmium phosphate microspheres for cancer treatment. Int J Pharm. 2018;548(1):73–81. doi: 10.1016/j.ijpharm.2018.06.036. [DOI] [PubMed] [Google Scholar]
- Bahrami-Samani A, Bagheri R, Jalilian AR, Shirvani-Arani S, Ghannadi-Maragheh M, Shamsaee M. Production, quality control and pharmacokinetic studies of Ho-EDTMP for therapeutic applications. Sci Pharm. 2010;78(3):423–433. doi: 10.3797/scipharm.1004-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RC, van Es RJJ, Rosenberg AJWP, van Nimwegen SA, Bastiaannet R, de Jong HWAM, Nijsen JFW, Lam MGEH. Intratumoral injection of radioactive holmium-166 microspheres in recurrent head and neck squamous cell carcinoma: preliminary results of first use. Nucl Med Commun. 2018;39(3):213–221. doi: 10.1097/MNM.0000000000000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RC, et al. Intratumoral treatment with radioactive beta-emitting microparticles: a systematic review. J Radiat Oncol. 2017;6(4):323–341. doi: 10.1007/s13566-017-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard B, Jiang Z, Soll CE, Revskaya E, Cutler CS, Dadachova E, Francesconi LC. In vitro and in vivo evaluation of melanin-binding decapeptide 4B4 radiolabeled with 177Lu, 166Ho, and 153Sm radiolanthanides for the purpose of targeted radionuclide therapy of melanoma. Cancer Biother Radiopharm. 2011;26(5):547–556. doi: 10.1089/cbr.2011.0954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayouth JE, et al. Pharmacokinetics, dosimetry and toxicity of holmium-166-DOTMP for bone marrow ablation in multiple myeloma. J Nucl Med. 1995;36(5):730–737. [PubMed] [Google Scholar]
- Bayouth JE, et al. Radiation dose distribution within the bone marrow of patients receiving holmium-166-labeled-phosphonate for marrow ablation. Med Phys. 1995;22(6):743–753. doi: 10.1118/1.597491. [DOI] [PubMed] [Google Scholar]
- Bernardes EMDO. Holmium-166m: multi-gamma standard to determine the activity of radionuclides in semiconductor detectors. Int Nucl Inf Syst. 2001;43(50):1–76.
- Braat A, et al. Safety analysis of holmium-166 microsphere scout dose imaging during radioembolisation work-up: a cohort study. Eur Radiol. 2018;28(3):920–928. doi: 10.1007/s00330-017-4998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat A, et al. Additional hepatic Ho-166-radioembolization in patients with neuroendocrine tumours treated with Lu-177-DOTATATE; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial) BMC Gastroenterol. 2018;18:84. doi: 10.1186/s12876-018-0817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitz H, Wendt R, Stabin M, Bouchet L, Wessels B. Dosimetry of high dose skeletal targeted radiotherapy (STR) with 166Ho-DOTMP. Cancer Biother Radiopharm. 2003;18(2):225–230. doi: 10.1089/108497803765036391. [DOI] [PubMed] [Google Scholar]
- Breitz H, et al. Dosimetry of high dose skeletal targeted radiotherapy (STR) with ho-166-DOTMP. Cancer Biother Radiopharm. 2003;18(2):225–230. doi: 10.1089/108497803765036391. [DOI] [PubMed] [Google Scholar]
- Breitz HB, et al. 166Ho-DOTMP radiation-absorbed dose estimation for skeletal targeted radiotherapy. J Nucl Med. 2006;47(3):534–542. [PubMed] [Google Scholar]
- Brown RF, Lindesmith LC, Day DE. 166Holmium-containing glass for internal radiotherapy of tumors. International journal of radiation applications and instrumentation. Part B. Nucl Med Biol. 1991;18(7):783–790. doi: 10.1016/0883-2897(91)90018-g. [DOI] [PubMed] [Google Scholar]
- Bult W, Kroeze SG, Elschot M, Seevinck PR, Beekman FJ, de Jong HW, Uges DR, Kosterink JG, Luijten PR, Hennink WE, van het Schip AD, Bosch JL, Nijsen JF, Jans JJ. Intratumoral administration of holmium-166 acetylacetonate microspheres: antitumor efficacy and feasibility of multimodality imaging in renal cancer. PLoS One. 2013;8(1):e52178. doi: 10.1371/journal.pone.0052178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult W, Vente MA, Vandermeulen E, Gielen I, Seevinck PR, Saunders J, van Het Schip AD, Bakker CJ, Krijger GC, Peremans K, Nijsen JF. Microbrachytherapy using holmium-166 acetylacetonate microspheres: a pilot study in a spontaneous cancer animal model. Brachytherapy. 2013;12(2):171–177. doi: 10.1016/j.brachy.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Bult W, et al. Microspheres with ultrahigh holmium content for radioablation of malignancies. Pharm Res. 2009;26(6):1371–1378. doi: 10.1007/s11095-009-9848-8. [DOI] [PubMed] [Google Scholar]
- Bult W, et al. Holmium nanoparticles: preparation and in vitro characterization of a new device for radioablation of solid malignancies. Pharm Res. 2010;27(10):2205–2212. doi: 10.1007/s11095-010-0226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult W, et al. Radioactive holmium acetylacetonate microspheres for interstitial microbrachytherapy: an in vitro and in vivo stability study. Pharm Res. 2012;29(3):827–836. doi: 10.1007/s11095-011-0610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos TPR, et al. Study of the Sm-153 seeds degradation and evaluation of the absorbed dose in rabbit’s liver implants. Prog Nucl Energy. 2008;50(7):757–766. doi: 10.1016/j.pnucene.2008.01.007. [DOI] [Google Scholar]
- Cho B-B, Choi K. Preparation of chitosan microspheres containing 166Dy/166Ho in vivo generators and their theranostic potential. J Radioanal Nucl Chem. 2018;317(2):1123–1132. doi: 10.1007/s10967-018-5984-0. [DOI] [Google Scholar]
- Cho YJ, Kim K, Chun YS, Rhyu KH, Kwon BK, Kim DY, Yoo MC. Radioisotope synoviorthesis with Holmium-166-chitosan complex in haemophilic arthropathy. Haemophilia. 2010;16(4):640–646. doi: 10.1111/j.1365-2516.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- Chung YL, Lee JD, Bang D, Lee JB, Park KB, Lee MG. Treatment of Bowen's disease with a specially designed radioactive skin patch. Eur J Nucl Med. 2000;27(7):842–846. doi: 10.1007/s002590000262. [DOI] [PubMed] [Google Scholar]
- Costa RF, Osso Junior JA. Cation exchange resins labeled with holmium-166 for treatment of liver malignancy. Conference proceedings: DOSIMN 2008: 1 Symposium of internal dosimetry applied to nuclear medicine. Brazil; 2008.
- Costa RF, et al. Production of microspheres labeled with holmium-166 for liver cancer therapy: the preliminary experience at IPEN/CNEN-SP. in INAC 2009: International nuclear atlantic conference Innovations in nuclear technology for a sustainable future. Brazil; 2009.
- da Silva FMR, de Almeida JC, Oliveira EEM, de Souza Albernaz M, Rossi AM, Santos-Oliveira R. Nano-hydroxyapatite doped with Ho-166 as drug delivery system for bone cancer therapy and diagnosis: developing a theragnostic radiopharmaceuticals. Anticancer Agents Med Chem. 2017;17(3):355–358. doi: 10.2174/1871520616666151110130428. [DOI] [PubMed] [Google Scholar]
- Das T, et al. Preparation and animal biodistribution of 166Ho labeled DOTA for possible use in intravascular radiation therapy (IVRT) J Label Compd Radiopharm. 2003;46(3):197–209. doi: 10.1002/jlcr.657. [DOI] [Google Scholar]
- Das T, et al. 166Ho-labeled hydroxyapatite particles: a possible agent for liver cancer therapy. Cancer Biother Radiopharm. 2009;24(1):7–14. doi: 10.1089/cbr.2008.0545. [DOI] [PubMed] [Google Scholar]
- Das T, et al. Preparation of 166Ho-oxine-lipiodol and its preliminary bioevaluation for the potential application in therapy of liver cancer. Nucl Med Commun. 2009;30(5):362–367. doi: 10.1097/MNM.0b013e328329981a. [DOI] [PubMed] [Google Scholar]
- de Campos TP, Nogueira LB, Trindade B, Cuperschmid EM. Dosimetric intercomparison of permanent Ho-166 seed's implants and HDR Ir-192 brachytherapy in breast cancer. Rep Pract Oncol Radiother. 2016;21(3):240–249. doi: 10.1016/j.rpor.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis-Bacelar AM, et al. Bone lesion absorbed dose profiles in patients with metastatic prostate cancer treated with molecular radiotherapy. Br J Radiol. 2018;91(1084):20170795. [DOI] [PMC free article] [PubMed]
- Di Pasqua AJ, et al. Preparation of neutron-activatable holmium nanoparticles for the treatment of ovarian cancer metastases. Small. 2012;8(7):997–1000. doi: 10.1002/smll.201102488. [DOI] [PubMed] [Google Scholar]
- Di Pasqua AJ, et al. Neutron-Activatable holmium-containing mesoporous silica nanoparticles as a potential radionuclide therapeutic agent for ovarian Cancer. J Nucl Med. 2013;54(1):111–116. doi: 10.2967/jnumed.112.106609. [DOI] [PubMed] [Google Scholar]
- Diniz MF, Ferreira DM, de Lima WG, Pedrosa ML, Silva ME, de Almeida Araujo S, Sampaio KH, de Campos TPR, Siqueira SL. Biodegradable seeds of holmium don't change neurological function after implant in brain of rats. Rep Pract Oncol Radiother. 2017;22(4):319–326. doi: 10.1016/j.rpor.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donanzam BA, Campos TP, Dalmazio I, Valente ES. Synthesis and characterization of calcium phosphate loaded with Ho-166 and Sm-153: a novel biomaterial for treatment of spine metastases. J Mater Sci Mater Med. 2013;24(12):2873–2880. doi: 10.1007/s10856-013-5024-0. [DOI] [PubMed] [Google Scholar]
- Edmonds J, et al. A comparative study of the safety and efficacy of dysprosium-165 hydroxide macro-aggregate and yttrium-90 silicate colloid in radiation synovectomy--a multicentre double blind clinical trial. Australian dysprosium trial group. Br J Rheumatol. 1994;33(10):947–953. doi: 10.1093/rheumatology/33.10.947. [DOI] [PubMed] [Google Scholar]
- Elschot M, et al. Quantitative evaluation of scintillation camera imaging characteristics of isotopes used in liver radioembolization. PLoS One. 2011;6(11):e26174. doi: 10.1371/journal.pone.0026174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elschot M, et al. ((9)(9)m)Tc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with (1)(6)(6)ho-microspheres. Eur J Nucl Med Mol Imaging. 2014;41(10):1965–1975. doi: 10.1007/s00259-014-2784-9. [DOI] [PubMed] [Google Scholar]
- Ferro-Flores G, Hernández-Oviedo O, Arteaga de Murphy C, Tendilla JI, Monroy-Guzman F, Pedraza-Lopez M, Aldama-Alvarado K. [166Dy]Dy/166Ho hydroxide macroaggregates: an in vivo generator system for radiation synovectomy. Appl Radiat Isot. 2004;61(6):1227–1233. doi: 10.1016/j.apradiso.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Ferro-Flores G, et al. Labeling of biotin with [166Dy]Dy/166Ho as a stable in vivo generator system. Int J Pharm. 2003;255(1–2):129–138. doi: 10.1016/S0378-5173(03)00052-8. [DOI] [PubMed] [Google Scholar]
- Foote H, Jr, Landon H, Sailor V. Slow neutron resonances in holmium, thulium, and lutetium. Phys Rev. 1953;92(3):656. doi: 10.1103/PhysRev.92.656. [DOI] [Google Scholar]
- Giralt S, Bensinger W, Goodman M, Podoloff D, Eary J, Wendt R, Alexanian R, Weber D, Maloney D, Holmberg L, Rajandran J, Breitz H, Ghalie R, Champlin R. 166Ho-DOTMP plus melphalan followed by peripheral blood stem cell transplantation in patients with multiple myeloma: results of two phase 1/2 trials. Blood. 2003;102(7):2684–2691. doi: 10.1182/blood-2002-10-3250. [DOI] [PubMed] [Google Scholar]
- Ha EJ, et al. Intracavitary radiation therapy for recurrent cystic brain tumors with holmium-166-Chico: a pilot study. J Korean Neurosurg Soc. 2013;54(3):175. doi: 10.3340/jkns.2013.54.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoudeh M, et al. Holmium-loaded PLLA nanoparticles for intratumoral radiotherapy via the TMT technique: preparation, characterization, and stability evaluation after neutron irradiation. Drug Dev Ind Pharm. 2008;34(8):796–806. doi: 10.1080/03639040801918623. [DOI] [PubMed] [Google Scholar]
- Hench LL, West JK. The sol-gel process. Chem Rev. 1990;90(1):33–72. doi: 10.1021/cr00099a003. [DOI] [Google Scholar]
- Hino Y, et al. Absolute measurement of 166mHo radioactivity and development of sealed sources for standardization of γ-ray emitting nuclides. Appl Radiat Isot. 2000;52(3):545–549. doi: 10.1016/S0969-8043(99)00208-0. [DOI] [PubMed] [Google Scholar]
- Hirsch AE, Medich DC, Rosenstein BS, Martel CB, Hirsch JA. Radioisotopes and vertebral augmentation: dosimetric analysis of a novel approach for the treatment of malignant compression fractures. Radiother Oncol. 2008;87(1):119–126. doi: 10.1016/j.radonc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Hong YD, Choi SJ, Choi SM, Jang BS. The availability of contrast media in the application of Holmium-166-DTPA for vascular brachytherapy. Nucl Med Biol. 2004;31(2):225–230. doi: 10.1016/j.nucmedbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hong YD, Choi SJ, Kim KH, Shin BC, Park WW, Park SH, Park KB. 166Ho-coated balloon for vascular brachytherapy. Appl Radiat Isot. 2003;58(4):455–462. doi: 10.1016/S0969-8043(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Hong YD, Park KB, Jang BS, Choi SJ, Choi SM, Kim YM. Holmium-166-DTPA as a liquid source for endovascular brachytherapy. Nucl Med Biol. 2002;29(8):833–839. doi: 10.1016/S0969-8051(02)00329-3. [DOI] [PubMed] [Google Scholar]
- Hosseini SH, Enferadi M, Sadeghi M. Dosimetric aspects of 166Ho brachytherapy biodegradable glass seed. Appl Radiat Isot. 2013;73:109–115. doi: 10.1016/j.apradiso.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Huh R, et al. Therapeutic effects of Holmium-166 chitosan complex in rat brain tumor model. Yonsei Med J. 2005;46(1):51–60. doi: 10.3349/ymj.2005.46.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh CW, Park CH, Kang HJ, Oh YT, Chun, Kim HS, Choi BI, Park KB, Kim YM, Kim KH, Vahc YW, Jang JS, Lee BK. Measurement of radiation absorbed dose in endovascular Ho-166 brachytherapy using a balloon angio-catheter. Nucl Med Commun. 2000;21(10):959–964. doi: 10.1097/00006231-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Johnson LS, Yanch JC. Absorbed dose profiles for radionuclides of frequent use in radiation synovectomy. Arthritis Rheum. 1991;34(12):1521–1530. doi: 10.1002/art.1780341208. [DOI] [PubMed] [Google Scholar]
- Khorami-Moghadam A, Bolouri B, Jalilian AR, Bahrami-Samani NM, Mazidi SM, Alirezapour B. Preclinical evaluation of holmium-166 labeled anti-VEGF-A(Bevacizumab) J Labelled Comp Radiopharm. 2013;56(8):365–369. doi: 10.1002/jlcr.3044. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho YH, Kim JS, Oh YT, Kang HJ, Chun MS, Joh CW, Park CH, Park KB, Tahk SJ, Choi BW. Effect of transcatheter endovascular radiation with holmium-166 on neointimal formation after balloon injury in porcine coronary artery. J Nucl Cardiol. 2000;7(5):478–483. doi: 10.1067/mnc.2000.107427. [DOI] [PubMed] [Google Scholar]
- Kim J, et al. In-situ formation of holmium oxide in pores of mesoporous carbon nanoparticles as substrates for neutron-Activatable radiotherapeutics. Carbon N Y. 2017;117:92–99. doi: 10.1016/j.carbon.2017.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JT, Kim EK, Won JY, Kim MJ, Lee JD, Hong SJ. Percutaneous sclerotherapy of renal cysts with a beta-emitting radionuclide, holmium-166-chitosan complex. Korean J Radiol. 2004;5(2):128–133. doi: 10.3348/kjr.2004.5.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, et al. Long-term clinical outcome of phase IIb clinical trial of percutaneous injection with holmium-166/chitosan complex (Milican) for the treatment of small hepatocellular carcinoma. Clin Cancer Res. 2006;12(2):543–548. doi: 10.1158/1078-0432.CCR-05-1730. [DOI] [PubMed] [Google Scholar]
- Kim W, Jeong MH, Park OY, Rhew JY, Bom HS, Choi SJ, Park KB, Kim EH, Kim JH, Ahn YK, Park JT, Cho JG, Park JC, Kang JC. Effects of beta-radiation using a holmium-166 coated balloon on neointimal hyperplasia in a porcine coronary stent restenosis model. Circ J. 2003;67(7):625–629. doi: 10.1253/circj.67.625. [DOI] [PubMed] [Google Scholar]
- Kraft O, Kaspárek R, Ullmann V, Melichar F, Kropacek M, Mirzajevova M. Radiosynoviorthesis of knees by means of 166Ho-holmium-boro-macroaggregates. Cancer Biother Radiopharm. 2007;22(2):296–302. doi: 10.1089/cbr.2006.312. [DOI] [PubMed] [Google Scholar]
- Kwak C, et al. Effective local control of prostate cancer by intratumoral injection of 166 ho-chitosan complex (DW-166HC) in rats. Eur J Nucl Med Mol Imaging. 2005;32(12):1400–1405. doi: 10.1007/s00259-005-1892-y. [DOI] [PubMed] [Google Scholar]
- Lee JD, Park KK, Lee MG, Kim EH, Rhim KJ, Lee JT, Yoo HS, Kim YM, Park KB, Kim JR. Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med. 1997;38(5):697–702. [PubMed] [Google Scholar]
- Lee JD, Yang WI, Lee MG, Ryu YH, Park JH, Shin KH, Kim GE, Suh CO, Seong JS, Han BH, Choi CW, Kim EH, Kim KH, Park KB. Effective local control of malignant melanoma by intratumoural injection of a beta-emitting radionuclide. Eur J Nucl Med Mol Imaging. 2002;29(2):221–230. doi: 10.1007/s00259-001-0696-y. [DOI] [PubMed] [Google Scholar]
- Lee SH, Suh JS, Kim HS, Lee JD, Song J, Lee SK. MR evaluation of radiation synovectomy of the knee by means of intra-articular injection of holmium-166-chitosan complex in patients with rheumatoid arthritis: results at 4-month follow-up. Korean J Radiol. 2003;4(3):170–178. doi: 10.3348/kjr.2003.4.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Cho YH, Lee JD, Yang WI, Shin JL, Lee MG. Enhanced antitumor effect of dendritic cell based immunotherapy after intratumoral injection of radionuclide Ho-166 against B16 melanoma. Immunol Lett. 2006;106(1):19–26. doi: 10.1016/j.imlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Louw WK, et al. Evaluation of samarium-153 and holmium-166-EDTMP in the normal baboon model. Nucl Med Biol. 1996;23(8):935–940. doi: 10.1016/S0969-8051(96)00117-5. [DOI] [PubMed] [Google Scholar]
- Majali MA, Debnath MC, Saxena SK, Joshi SH. Preparation and evaluation of [166Ho] holmium-dimethyl diethylenetriaminepentaaceticacid (DMDTPA) as potential radiopharmaceutical for endovascular radiation therapy (EVRT) Appl Radiat Isot. 2002;56(6):863–869. doi: 10.1016/S0969-8043(02)00057-X. [DOI] [PubMed] [Google Scholar]
- Majali MA, et al. Potential 166Ho radiopharmaceuticals for endovascular radionuclide therapy. II. Preparation and evaluation of 166Ho-DTPA. Nucl Med Commun. 2001;22(1):97–103. doi: 10.1097/00006231-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Makela O, Penttilä P, Kolehmainen E, Sukura A, Sankari S, Tulamo RM. Experimental radiation synovectomy in rabbit knee with holmium-166 ferric hydroxide macroaggregate. Nucl Med Biol. 2002;29(5):593–598. doi: 10.1016/S0969-8051(02)00317-7. [DOI] [PubMed] [Google Scholar]
- Makela O, Sukura A, Penttila P, Hiltunen J, Tulamo RM. Radiation synovectomy with holmium-166 ferric hydroxide macroaggregate in equine metacarpophalangeal and metatarsophalangeal joints. Vet Surg. 2003;32(4):402–409. doi: 10.1053/jvet.2003.50039. [DOI] [PubMed] [Google Scholar]
- Makela OT, Lammi MJ, Uusitalo H, Hyttinen MM, Vuorio E, Helminen HJ, Tulamo RM. Analysis of lapine cartilage matrix after radiosynovectomy with holmium-166 ferric hydroxide macroaggregate. Ann Rheum Dis. 2003;62(1):43–49. doi: 10.1136/ard.62.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela OT, Lammi MJ, Uusitalo H, Viitanen M, Hyttinen MM, Jurvelin JS, Vuorio E, Helminen HJ, Tulamo RM. Effect of radiosynovectomy with holmium-166 ferric hydroxide macroaggregate on adult equine cartilage. J Rheumatol. 2004;31(2):321–328. [PubMed] [Google Scholar]
- Marques F, et al. 13- and 14-membered macrocyclic ligands containing methylcarboxylate or methylphosphonate pendant arms: chemical and biological evaluation of their 153Sm and 166Ho complexes as potential agents for therapy or bone pain palliation. J Inorg Biochem. 2006;100(2):270–280. doi: 10.1016/j.jinorgbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- McLaren A, et al. Dysprosium (165Dy) hydroxide macroaggregates for radiation synovectomy — animal studies. Eur J Nucl Med. 1990;16(8):627–632. doi: 10.1007/BF00998160. [DOI] [PubMed] [Google Scholar]
- Mohsin H, Jia F, Bryan JN, Sivaguru G, Cutler CS, Ketring AR, Miller WH, Simon J, Frank RK, Theodore LJ, Axworthy DB, Jurisson SS, Lewis MR. Comparison of pretargeted and conventional CC49 radioimmunotherapy using 149Pm, 166Ho, and 177Lu. Bioconjug Chem. 2011;22(12):2444–2452. doi: 10.1021/bc200258x. [DOI] [PubMed] [Google Scholar]
- Mohsin H, Jia F, Sivaguru G, Hudson MJ, Shelton TD, Hoffman TJ, Cutler CS, Ketring AR, Athey PS, Simon J, Frank RK, Jurisson SS, Lewis MR. Radiolanthanide-labeled monoclonal antibody CC49 for radioimmunotherapy of cancer: biological comparison of DOTA conjugates and 149Pm, 166Ho, and 177Lu. Bioconjug Chem. 2006;17(2):485–492. doi: 10.1021/bc0502356. [DOI] [PubMed] [Google Scholar]
- Mumper RJ, Jay M. Biodegradable radiotherapeutic polyester microspheres: optimization and in-vitro/in-vivo evaluation. J Control Release. 1992;18(3):193–203. doi: 10.1016/0168-3659(92)90165-N. [DOI] [Google Scholar]
- Mumper RJ, Mills BJ, Ryo UY, Jay M. Polymeric microspheres for radionuclide synovectomy containing neutron-activated holmium-166. J Nucl Med. 1992;33(3):398–402. [PubMed] [Google Scholar]
- Munaweera I, Levesque-Bishop D, Shi Y, Di Pasqua AJ, Balkus KJ., Jr Radiotherapeutic bandage based on electrospun polyacrylonitrile containing holmium-166 iron garnet nanoparticles for the treatment of skin cancer. ACS Appl Mater Interfaces. 2014;6(24):22250–22256. doi: 10.1021/am506045k. [DOI] [PubMed] [Google Scholar]
- Munaweera I, Shi Y, Koneru B, Saez R, Aliev A, Di Pasqua AJ, Balkus KJ., Jr Chemoradiotherapeutic magnetic nanoparticles for targeted treatment of nonsmall cell lung cancer. Mol Pharm. 2015;12(10):3588–3596. doi: 10.1021/acs.molpharmaceut.5b00304. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988;9(3):247–252. doi: 10.1016/0142-9612(88)90092-0. [DOI] [PubMed] [Google Scholar]
- Nayak D, Lahiri S. Application of radioisotopes in the field of nuclear medicine - I. lanthanide series elements. J Radioanal Nucl Chem. 1999;242(2):423–432. doi: 10.1007/BF02345573. [DOI] [Google Scholar]
- Nethaway D, Missimer M. The half-lives of 166Ho, 167Tm, 168Tm and the 165Ho (n, γ) 166mHo cross-section. J Inorg Nucl Chem. 1968;30(1):15–18. doi: 10.1016/0022-1902(68)80056-9. [DOI] [Google Scholar]
- Nijsen F, et al. Targeting of liver tumour in rats by selective delivery of holmium-166 loaded microspheres: a biodistribution study. Eur J Nucl Med. 2001;28(6):743–749. doi: 10.1007/s002590100518. [DOI] [PubMed] [Google Scholar]
- Nijsen JF, Krijger GC, van Het Schip AD. The bright future of radionuclides for cancer therapy. Anti Cancer Agents Med Chem. 2007;7(3):271–290. doi: 10.2174/187152007780618207. [DOI] [PubMed] [Google Scholar]
- Nijsen JF, et al. Advances in nuclear oncology: microspheres for internal radionuclide therapy of liver tumours. Curr Med Chem. 2002;9(1):73–82. doi: 10.2174/0929867023371454. [DOI] [PubMed] [Google Scholar]
- Nijsen JF, et al. Influence of neutron irradiation on holmium acetylacetonate loaded poly(L-lactic acid) microspheres. Biomaterials. 2002;23(8):1831–1839. doi: 10.1016/S0142-9612(01)00309-X. [DOI] [PubMed] [Google Scholar]
- Nijsen JF, et al. Liver tumors: MR imaging of radioactive holmium microspheres--phantom and rabbit study. Radiology. 2004;231(2):491–499. doi: 10.1148/radiol.2312030594. [DOI] [PubMed] [Google Scholar]
- Nijsen JFW, et al. Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: effects of preparation and neutron activation techniques. Eur J Nucl Med. 1999;26(7):699–704. doi: 10.1007/s002590050440. [DOI] [PubMed] [Google Scholar]
- Nogueira LB, Campos TPR. Nuclear characterization and investigation of radioactive bioglass seed surfaces for brachytherapy via scanning electron microscopy. J Sol-Gel Sci Technol. 2011;58(1):251–258. doi: 10.1007/s10971-010-2385-4. [DOI] [Google Scholar]
- Nogueira LB, Campos TPR. Synthesis, chemical characterization and radiological response of ho and HoZr bioglass seeds. J Sol-Gel Sci Technol. 2016;77(3):688–698. doi: 10.1007/s10971-015-3900-4. [DOI] [Google Scholar]
- Nogueira LB, de Campos TP. Radiological response of ceramic and polymeric devices for breast brachytherapy. Appl Radiat Isot. 2012;70(4):663–669. doi: 10.1016/j.apradiso.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Oerlemans C, et al. Holmium-lipiodol-alginate microspheres for fluoroscopy-guided embolotherapy and multimodality imaging. Int J Pharm. 2015;482(1–2):47–53. doi: 10.1016/j.ijpharm.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Ofluoglu S, et al. Radiation synovectomy with (166)ho-ferric hydroxide: a first experience. J Nucl Med. 2002;43(11):1489–1494. [PubMed] [Google Scholar]
- Park JS, Oh JH, Kim DY, Park YK, Park SJ, Kim SJ. Effects of intraluminal irradiation with Holmium-166 for TIPS stenosis: experimental study in a swine model. Korean J Radiol. 2007;8(2):127–135. doi: 10.3348/kjr.2007.8.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KB. Therapeutic feasibility of various diseases using Holmium-166 chitosan complex. 핵의학분자영상. 1997;31(2):123. [Google Scholar]
- Park KB, Kim JR, Lambrecht R. Preparation of dysprosium-165/holmium-166 macroaggregate for radiation synovectomy. J Radioanal Nucl Chem. 1996;206(1):5–16. doi: 10.1007/BF02040036. [DOI] [Google Scholar]
- Park S, Kang WC, Rhee JH, Ko YG, Choi D, Jang Y, Lee JD, Shim WH, Cho SY. Intracoronary 166Holmium brachytherapy combined with cutting balloon angioplasty for the treatment of in-stent restenosis. Cardiovasc Radiat Med. 2003;4(3):119–125. doi: 10.1016/S1522-1865(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Parks NJ, Kawakami TG, Avila MJ, White R, Cain GR, Raaka SD, Hornoff W, Fisher P, Moore P, Seibert JA, et al. Bone marrow transplantation in dogs after radio-ablation with a new Ho-166 amino phosphonic acid bone-seeking agent (DOTMP) Blood. 1993;82(1):318–325. [PubMed] [Google Scholar]
- Pedraza-Lopez M, et al. Preparation of (166)Dy/(166)ho-EDTMP: a potential in vivo generator system for bone marrow ablation. Nucl Med Commun. 2004;25(6):615–621. doi: 10.1097/01.mnm.0000126516.57329.07. [DOI] [PubMed] [Google Scholar]
- Pedraza-Lopez M, et al. Cytotoxic and genotoxic effect of the [166Dy]Dy/166Ho-EDTMP in vivo generator system in mice. Nucl Med Biol. 2004;31(8):1079–1085. doi: 10.1016/j.nucmedbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Prince JF, et al. Radiation emission from patients treated with holmium-166 radioembolization. J Vasc Interv Radiol. 2014;25(12):1956–1963. doi: 10.1016/j.jvir.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Prince JF, et al. Safety of a scout dose preceding hepatic Radioembolization with ho-166 microspheres. J Nucl Med. 2015;56(6):817–823. doi: 10.2967/jnumed.115.155564. [DOI] [PubMed] [Google Scholar]
- Prince JF, et al. Efficacy of Radioembolization with (166)ho-microspheres in salvage patients with liver metastases: a phase 2 study. J Nucl Med. 2018;59(4):582–588. doi: 10.2967/jnumed.117.197194. [DOI] [PubMed] [Google Scholar]
- Radosa CG, et al. Holmium-166 Radioembolization in hepatocellular carcinoma: feasibility and safety of a new treatment option in clinical practice. Cardiovasc Intervent Radiol. 2019;42(3):405–412. doi: 10.1007/s00270-018-2133-7. [DOI] [PubMed] [Google Scholar]
- Rajendran JG, et al. High-dose 166Ho-DOTMP in myeloablative treatment of multiple myeloma: pharmacokinetics, biodistribution, and absorbed dose estimation. J Nucl Med. 2002;43(10):1383–1390. [PubMed] [Google Scholar]
- Roberto WS, Pereira MM, Campos TPR. Structure and Dosimetric analysis of biodegradable glasses for prostate Cancer treatment. Artif Organs. 2003;27(5):432–436. doi: 10.1046/j.1525-1594.2003.07253.x. [DOI] [PubMed] [Google Scholar]
- Seevinck PR, et al. Factors affecting the sensitivity and detection limits of MRI, CT, and SPECT for multimodal diagnostic and therapeutic agents. Anti Cancer Agents Med Chem. 2007;7(3):317–334. doi: 10.2174/187152007780618153. [DOI] [PubMed] [Google Scholar]
- Seevinck PR, et al. Magnetic resonance imaging-based radiation-absorbed dose estimation of 166Ho microspheres in liver radioembolization. Int J Radiat Oncol Biol Phys. 2012;83(3):e437–e444. doi: 10.1016/j.ijrobp.2011.12.085. [DOI] [PubMed] [Google Scholar]
- Seppenwoolde JH, et al. Internal radiation therapy of liver tumors: qualitative and quantitative magnetic resonance imaging of the biodistribution of holmium-loaded microspheres in animal models. Magn Reson Med. 2005;53(1):76–84. doi: 10.1002/mrm.20320. [DOI] [PubMed] [Google Scholar]
- Sledge CB, et al. Experimental radiation synovectomy by 165Dy ferric hydroxide macroaggregate. Arthritis Rheum. 1977;20(7):1334–1342. doi: 10.1002/art.1780200706. [DOI] [PubMed] [Google Scholar]
- Smith SV, et al. [166Dy]dysprosium/[166Ho]holmium in vivo generator. Appl Radiat Isot. 1995;46(8):759–764. doi: 10.1016/0969-8043(94)00149-T. [DOI] [PubMed] [Google Scholar]
- Smits ML, et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: design of the phase I HEPAR trial. J Exp Clin Cancer Res. 2010;29:70. doi: 10.1186/1756-9966-29-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits ML, et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13(10):1025–1034. doi: 10.1016/S1470-2045(12)70334-0. [DOI] [PubMed] [Google Scholar]
- Smits ML, et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med. 2013;54(12):2093–2100. doi: 10.2967/jnumed.113.119768. [DOI] [PubMed] [Google Scholar]
- Sohaib M, Ahmad M, Jehangir M, Perveen A. Ethylene diamine tetramethylene phosphonic acid labeled with various beta(−)-emitting radiometals: labeling optimization and animal biodistribution. Cancer Biother Radiopharm. 2011;26(2):159–164. doi: 10.1089/cbr.2010.0846. [DOI] [PubMed] [Google Scholar]
- Sohn JH, et al. Phase II study of transarterial holmium-166-chitosan complex treatment in patients with a single, large hepatocellular carcinoma. Oncology. 2009;76(1):1–9. doi: 10.1159/000173735. [DOI] [PubMed] [Google Scholar]
- Song J, Suh CH, Park YB, Lee SH, Yoo NC, Lee JD, Kim KH, Lee SK. A phase I/IIa study on intra-articular injection of holmium-166-chitosan complex for the treatment of knee synovitis of rheumatoid arthritis. Eur J Nucl Med. 2001;28(4):489–497. doi: 10.1007/s002590000470. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Vimalnath KV, Dash A. Preparation and preliminary in vivo evaluation of (166) ho-labeled microspheres for possible use in radioembolic therapy of liver cancer. J Labelled Comp Radiopharm. 2018;61(6):509–514. doi: 10.1002/jlcr.3616. [DOI] [PubMed] [Google Scholar]
- Suzuki YS, et al. Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. J Nucl Med. 1998;39:2161–2166. [PubMed] [Google Scholar]
- Thompson S, Ballard B, Jiang Z, Revskaya E, Sisay N, Miller WH, Cutler CS, Dadachova E, Francesconi LC. 166Ho and 90Y labeled 6D2 monoclonal antibody for targeted radiotherapy of melanoma: comparison with 188Re radiolabel. Nucl Med Biol. 2014;41(3):276–281. doi: 10.1016/j.nucmedbio.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JH, et al. 166Ho-microsphere liver radiotherapy: a preclinical SPECT dosimetry study in the pig. Nucl Med Commun. 1994;15(7):545–553. doi: 10.1097/00006231-199407000-00009. [DOI] [PubMed] [Google Scholar]
- Ueno NT, de Souza JA, Booser D, Nakayama K, Madewell J, Wendt RE, 3rd, Hortobagyi GN, Podoloff D, Champlin RE. Pilot study of targeted skeletal radiation therapy for bone-only metastatic breast cancer. Clin Breast Cancer. 2009;9(3):173–177. doi: 10.3816/CBC.2009.n.028. [DOI] [PubMed] [Google Scholar]
- Unni PR, et al. Preparation and bioevaluation of 166Ho labelled hydroxyapatite (HA) particles for radiosynovectomy. Nucl Med Biol. 2002;29(2):199–209. doi: 10.1016/S0969-8051(01)00303-1. [DOI] [PubMed] [Google Scholar]
- Vaez-Tehrani M, et al. Estimation of human absorbed dose for (166)ho-PAM: comparison with (166)Ho-DOTMP and (166)Ho-TTHMP. Br J Radiol. 2016;89(1066):15. doi: 10.1259/bjr.20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente ES, Campos TPR. Gamma spectrometry and chemical characterization of ceramic seeds with samarium-153 and holmium-166 for brachytherapy proposal. Appl Radiat Isot. 2010;68(12):2157–2162. doi: 10.1016/j.apradiso.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Valente ES, Cuperschmid EM, de Campos TPR. Evaluation of Hela cell lineage response to β radiation from holmium-166 embedded in ceramic seeds. Braz Arch Biol Technol. 2011;54(5):957–964. doi: 10.1590/S1516-89132011000500013. [DOI] [Google Scholar]
- Van de Bovenkamp CG, Meij BP, Stassen QE, Nijsen JF, Kirpensteijn J. Administration of radioactive holmium-166 microsphere in domestic animals with inoperable tumors. Tijdschr Diergeneeskd. 2009;134(12):532–533. [PubMed] [Google Scholar]
- van de Maat GH, et al. MRI-based biodistribution assessment of holmium-166 poly(L-lactic acid) microspheres after radioembolisation. Eur Radiol. 2013;23(3):827–835. doi: 10.1007/s00330-012-2648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoven AF, et al. Surefire infusion system versus standard microcatheter use during holmium-166 radioembolization: study protocol for a randomized controlled trial. Trials. 2016;17:520. doi: 10.1186/s13063-016-1643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Es R, et al. Intra-arterial embolization of head-and-neck cancer with radioactive holmium-166 poly (L-lactic acid) microspheres: an experimental study in rabbits. Int J Oral Maxillofac Surg. 2001;30(5):407–413. doi: 10.1054/ijom.2001.0129. [DOI] [PubMed] [Google Scholar]
- van Es RJ, et al. Tumour embolization of the Vx2 rabbit head and neck cancer model with dextran hydrogel and holmium-poly (L-lactic acid) microspheres: a radionuclide and histological pilot study. J Cranio-Maxillofac Surg. 2001;29(5):289–297. doi: 10.1054/jcms.2001.0234. [DOI] [PubMed] [Google Scholar]
- van Nierop BJ, et al. Accuracy of SPECT/CT-based lung dose calculation for Holmium-166 hepatic radioembolization before OSEM convergence. Med Phys. 2018;45(8):3871–3879. doi: 10.1002/mp.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen SA, Bakker RC, Kirpensteijn J, van Es RJJ, Koole R, Lam MGEH, Hesselink JW, Nijsen JFW. Intratumoral injection of radioactive holmium ( (166) Ho) microspheres for treatment of oral squamous cell carcinoma in cats. Vet Comp Oncol. 2018;16(1):114–124. doi: 10.1111/vco.12319. [DOI] [PubMed] [Google Scholar]
- Vente MA, et al. Clinical effects of transcatheter hepatic arterial embolization with holmium-166 poly(L-lactic acid) microspheres in healthy pigs. Eur J Nucl Med Mol Imaging. 2008;35(7):1259–1271. doi: 10.1007/s00259-008-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vente MA, et al. Neutron activation of holmium poly(L-lactic acid) microspheres for hepatic arterial radio-embolization: a validation study. Biomed Microdevices. 2009;11(4):763–772. doi: 10.1007/s10544-009-9291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosoghi S, et al. Synthesis, characterization, and in vitro evaluation of a radio-metal organic framework composed of in vivo generator 166Dy/166Ho and DOTMP as a novel agent for bone marrow ablation, in Radiochimica Acta. 2016. p. 715. [Google Scholar]
- Vuorela J, Kauppinen T, Sokka T. Distribution of radiation in synovectomy of the knee with 166Ho-FHMA using image fusion. Cancer Biother Radiopharm. 2005;20(3):333–337. doi: 10.1089/cbr.2005.20.333. [DOI] [PubMed] [Google Scholar]
- Won JH, Lee JD, Wang HJ, Han JH, Kim JH, Kim KH, Park KB, Itkin M. Effects of a holmium-166 incorporated covered stent placement in normal canine common bile ducts. J Vasc Interv Radiol. 2005;16(5):705–711. doi: 10.1097/01.RVI.0000153113.87723.43. [DOI] [PubMed] [Google Scholar]
- Won JH, Lee JD, Wang HJ, Kim GE, Kim BW, Yim H, Han SK, Park CH, Joh CW, Kim KH, Park KB, Shin KM. Self-expandable covered metallic esophageal stent impregnated with beta-emitting radionuclide: an experimental study in canine esophagus. Int J Radiat Oncol Biol Phys. 2002;53(4):1005–1013. doi: 10.1016/S0360-3016(02)02837-7. [DOI] [PubMed] [Google Scholar]
- Wondergem M, et al. 99mTc-macroaggregated albumin poorly predicts the intrahepatic distribution of 90Y resin microspheres in hepatic radioembolization. J Nucl Med. 2013;54(8):1294–1301. doi: 10.2967/jnumed.112.117614. [DOI] [PubMed] [Google Scholar]
- Yousefnia H, et al. Preliminary dosimetric evaluation of (166)ho-TTHMP for human based on biodistribution data in rats. Appl Radiat Isot. 2014;94:260–265. doi: 10.1016/j.apradiso.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Zeevaart JR, et al. Metal-ion speciation in blood plasma incorporating the tetraphosphonate, N,N-dimethylenephosphonate-1-hydroxy-4-aminopropilydenediphosphonate (APDDMP), in therapeutic radiopharmaceuticals. J Inorg Biochem. 2001;83(1):57–65. doi: 10.1016/S0162-0134(00)00125-2. [DOI] [PubMed] [Google Scholar]
- Zielhuis SW, Seppenwoolde JH, Mateus VA, Bakker CJ, Krijger GC, Storm G, Zonnenberg BA, van het Schip AD, Koning GA, Nijsen JF. Lanthanide-loaded liposomes for multimodality imaging and therapy. Cancer Biother Radiopharm. 2006;21(5):520–527. doi: 10.1089/cbr.2006.21.520. [DOI] [PubMed] [Google Scholar]
- Zielhuis SW, et al. Characterization of holmium loaded alginate microspheres for multimodality imaging and therapeutic applications. J Biomed Mater Res A. 2007;82(4):892–898. doi: 10.1002/jbm.a.31183. [DOI] [PubMed] [Google Scholar]
- Zolghadri S, Jalilian AR, Naseri Z, Yousefnia H, Bahrami-Samani A, Ghannadi-Maragheh M, Afarideh H. Production, quality control and biological evaluation of (166)Ho-PDTMP as a possible bone palliation agent. Iran J Basic Med Sci. 2013;16(5):719–725. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N.a.