Abstract
Although the biological mechanisms underlying the beneficial effects of vitamin B6 on cardiovascular disease (CVD) have been reported on, epidemiological studies have yielded controversial results, and data on the Korean population are limited. This study examined the association between dietary vitamin B6 intake and CVD incidence in Koreans. A total of 9142 participants of the Korean Genome and Epidemiology Study, aged 40–69 years, who did not have CVD or cancer at the baseline were included in the analysis. Dietary data were assessed using a validated semi-quantitative food frequency questionnaire. CVD incidence was assessed using biennial questionnaires and confirmed through repeated personal interviews. Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazard regression models. After multivariate adjustment, a higher vitamin B6 intake was significantly associated with a decreased CVD risk in men (HR: 0.44; 95% CI: 0.25–0.78); no such association was observed in women. Dose-response analysis confirmed the presence of inverse linearity between vitamin B6 intake and CVD incidence in men (p for nonlinearity = 0.3). A higher dietary intake level of vitamin B6 was associated with a reduced CVD risk in Korean men. These observations require further verification in other populations.
Keywords: cardiovascular disease, vitamin B6, men, cohort study
1. Introduction
Cardiovascular disease (CVD) is responsible for a third of all global deaths [1], and the prevalence of CVD is increasing worldwide [2] as well as in Korea [3]. As the rise in the CVD incidence poses an economic burden and leads to increases in the number of disability-adjusted life years, indicating a diminished quality of life, it is important to identify and understand the potential risk factors and protective factors that may alleviate the burden of this disease [4].
Certain nutrients have long been highlighted as modifiable risk factors for CVD [5,6]. Previous studies have investigated the possible biological mechanisms underlying the beneficial effects of vitamin B6 on CVD prevention, such as the inhibition of lipoperoxide production and decreases in the levels of homocysteine and inflammation which are known risk factors of CVD [7]. In addition, vitamin B6 deficiency can cause hyperhomocysteinemia [8], which may lead to arterial wall damage [7]. Nevertheless, epidemiological studies investigating the association between vitamin B6 and CVD have shown inconsistent results [9,10,11]. The Nurses’ Health Study in the United States demonstrated an inverse relationship between plasma vitamin B6 levels and the risk of myocardial infarction [9]. Similarly, a Japanese cohort study revealed an inverse association between vitamin B6 intake and coronary heart disease (CHD) incidence [10]. In contrast, a study conducted in Finnish men found no significant association between vitamin B6 intake and CVD risk [11]. In Korea, few studies have investigated the effect of vitamin B6 intake on CVD, and no corresponding prospective analyses have been conducted to date. Two previous case-control studies analyzed the association between vitamin B6 and stroke in the Korean population and yielded conflicting results [12,13]. Those case-control studies were limited by their design, which aggravated the analysis of long-term dietary effects on chronic diseases, as it is practically impossible to recall long-term dietary habits prior to disease diagnosis [14,15].
Further investigation of the longitudinal association between vitamin B6 and CVD is warranted in a prospective cohort study, as is the assessment of dietary intake prior to disease onset. We, therefore, prospectively examined this association among Korean adults enrolled in the Ansung–Ansan cohort study.
2. Materials and Methods
2.1. Study Population
The present study analyzed data obtained in one of the prospective cohort studies that were part of the Korean Genome and Epidemiology Study. The baseline survey of the community-based Ansung–Ansan cohort study was conducted between 2001 and 2002 in 10,030 Korean adults aged 40–69 years who were residing in the Ansung (rural) and Ansan (urban) areas of Gyeonggi province. Participants were randomly recruited from enrolled inhabitants for statistically reliable results, and the general characteristics of participants from both areas were similar to those who were not recruited (Ansung, n = 5018, response rate = 69.6%; Ansan, n = 5012, response rate = 45.7%). The follow-up examination was conducted every two years thereafter. Details on the baseline recruitment and follow-up survey have been presented elsewhere [16]. During each examination, the participants’ demographics, lifestyle, environmental factors, family history of disease, medical history, and diet were assessed with a structured questionnaire. Data collection was performed by trained investigators according to standardized procedures. We used baseline data and data obtained during follow-ups that were conducted until 2012 in this study.
Exclusion criteria were having a history of CVD or cancer, taking medications, or receiving treatments for CVD-related diseases (n = 498) at the baseline. Participants with no available data on vitamin B6 consumption (n = 307) or with an implausible daily energy intake level <500 kcal or >5000 kcal (n = 83) were also excluded [17]. A total of 9142 participants were finally included in the study (Figure S1).
Informed consent was obtained from all participants and the study was approved by the Korea Centers for Disease Control and Prevention Institutional Review Board (IRB number: KU-IRB-15-EX-256-A-1) and the Yeungnam University Institutional Review Board (IRB number: 7002016-E-2016-003).
2.2. General Characteristics and Anthropometric Measurements
The participants’ demographic data (sex, age, residential area, educational levels, monthly household income) and lifestyle data (smoking status, alcohol consumption, physical activity levels) were collected using questionnaires. The monthly household income was classified into four groups, ranging from <1,000,000 Korean Republic Won (KRW) to ≥4,000,000 KRW. The educational levels were categorized into: elementary school graduate or lower, middle school graduate, high school graduate, and college graduate or higher. By smoking status, participants were categorized into the current smoker and non-smoker groups. For alcohol consumption, we used participants’ weekly intake frequencies (glasses/week) and grouped them into the non-drinker and drinker groups. Physical activity levels were quantified using the daily exercise hours and intensity of physical activities (light, moderate, and vigorous) based on metabolic equivalents [18]. Anthropometric measurements such as height and weight were conducted by trained technicians. Body mass index (BMI) was calculated by dividing the body weight (kg) by the height squared (m2).
2.3. Dietary Assessment
Dietary information was assessed during the baseline survey (2001–2002) and the second follow-up survey (2005–2006) using a semi-quantitative food frequency questionnaire (SQFFQ), the validity and reproducibility of which have been proven [19]. In brief, the frequency of intake was assessed by offering nine possible responses for each food, ranging from “almost never” to “3 times per day”. The portion size of each food could be estimated as “0.5 times the reference”, “reference”, or “1.5–2 times the reference”. The food consumption levels were determined by weighing the portion size and multiplying it with the weekly frequency of each food (servings per week). Daily nutrient and total energy intake values were calculated based on dietary information and the food composition database of the Rural Development Administration of Korea [20]. The mean dietary information values from the two surveys were used to minimize misclassification errors. Fully conditional specification imputation procedures were used to handle missing values of dietary data in the second follow-up survey. We imputed missing values of second follow-up dietary data from their posterior predictive distribution using regression equations with baseline data [21]. Data on the use of dietary supplements (yes/no) were obtained from a subset of the dietary questionnaire.
2.4. Ascertainment of CVD
CVD-related data were obtained through biennial self-reported questionnaires and confirmed by trained investigators during individual participant interviews. CVD incidence was established if participants had been newly diagnosed or were taking medications or receiving treatments for one or more of the following diseases: stroke, coronary artery disease, myocardial infarction, and/or cerebrovascular disease.
2.5. Statistical Analysis
The person-years of each participant were computed as the period between the date of baseline registration and study end date, which was defined as the date of the first CVD diagnosis or last known alive for participants without CVD. Study participants were divided into quintiles based on their dietary intake levels of vitamin B6. Continuous and categorical variables were analyzed for each quintile using a general linear model or chi-square test, respectively. All nutrient intakes were adjusted for total energy intake using the residual method [17]. Nutrient patterns were derived from those of 20 nutrients (without vitamin B6) through principal component analysis (PCA) [22], performed using varimax rotation with 20 nutrients. Major nutrient patterns were extracted based on a scree plot as well as eigenvalues >1.0. The factor loading matrix of nutrients is presented in Table S1. In total, three nutrient factors were identified: Factor 1, with high loading for fiber, folate, carotene, vitamin A, potassium, vitamin C, iron, sodium, vitamin E, calcium, and phosphorus; Factor 2, with high loading for carbohydrate, protein, fat, thiamin, and niacin; and Factor 3, with high loading for retinol, cholesterol, calcium, riboflavin, and phosphorus. Cox proportional hazard regression models were employed to estimate hazard ratios (HRs) and their confidence intervals (CIs) for the risk of CVD according to dietary intake levels of vitamin B6. Possible effect modifiers were determined for demographic variables, lifestyle, and history of disease using both linear and Cox proportional hazard regression models. Multiple confounding factors were identified based on preliminary analysis and literature review [23,24,25]. Four covariate models were evaluated: Model 1, unadjusted; Model 2, adjusted for age; Model 3, further adjusted for monthly household income, BMI, educational levels, physical activity levels, residential area, smoking status, alcohol consumption, and hypertension and diabetes (defined as a history of diagnosis or taking medication) at baseline; Model 4, Model 3 with further adjustments for total energy intake, three major nutrient factors identified in the PCA, and use of dietary supplements. The p for trend was calculated using the median of the vitamin B6 intake quintiles as a continuous variable. Nonlinear relationships were examined using restricted cubic splines. The Statistical Analysis System version 9.4 software (SAS Institute, Cary, NC, USA) was used to perform all analyses and the significance level for all tests was defined as α = 0.05.
3. Results
During the mean follow-up period of 7.4 years, 278 and 284 CVD cases were documented among men and women, respectively. The general baseline characteristics of the participants are shown in Table 1. The dietary vitamin B6 intake ranges were 1.01–6.22 mg/day in men and 0.88–7.51 mg/day in women, and the average age was 51.47 ± 0.13 years in men and 52.52 ± 0.13 years in women. Almost two-thirds of the alcohol drinkers were male, including 70.78%, 73.52%, and 71.54% of men in the lowest, mid, and highest dietary vitamin B6 intake groups, respectively. In contrast, female alcohol drinkers accounted for only one-fourth of all the women, including 21.86%, 25.56%, and 27.57% of those in the lowest, mid, and highest dietary vitamin B6 intake groups, respectively. Approximately half the men were current smokers (51.60%, 48.80%, and 50.06% in the lowest, mid, and highest dietary vitamin B6 intake groups, respectively.), while the proportion of female smokers was very low (3.62%, 2.45%, and 4.19% in the lowest, mid, and highest dietary vitamin B6 intake groups, respectively).
Table 1.
Baseline characteristics of participants according to sex stratified by energy-adjusted dietary vitamin B6 intake levels.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Vitamin B6 Intake (Quintile) | Vitamin B6 Intake (Quintile) | |||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| n | 878 | 878 | 878 | 950 | 950 | 950 |
| Range (median), mg/day | 1.01–1.83 (1.70) | 2.01–2.16 (2.08) | 2.40–6.21 (2.62) | 0.88–1.73 (1.58) | 1.93–2.08 (2.00) | 2.30–5.22 (2.49) |
| Age (years) | 54.12 ± 0.29 | 51.26 ± 0.29 | 49.86 ± 0.29 | 55.08 ± 0.29 | 52.54 ± 0.29 | 50.23 ± 0.29 |
| Residential area | ||||||
| Ansung | 596 (67.88) | 320 (36.45) | 329 (37.47) | 642 (67.58) | 419 (44.11) | 495 (52.11) |
| Ansan | 282 (32.12) | 558 (63.55) | 549 (62.53) | 308 (32.42) | 531 (55.89) | 455 (47.89) |
| Educational levels | ||||||
| Elementary school graduation or lower | 248 (28.38) | 142 (16.21) | 130 (14.87) | 549 (58.59) | 416 (44.11) | 316 (33.40) |
| Middle school graduation | 210 (24.03) | 188 (21.46) | 179 (20.48) | 188 (20.06) | 213 (22.59) | 256 (27.06) |
| High school graduation | 285 (32.61) | 346 (39.50) | 346 (39.59) | 164 (17.50) | 249 (26.41) | 287 (30.34) |
| College graduation or higher | 131 (14.99) | 200 (22.83) | 219 (25.06) | 36 (3.84) | 65 (6.89) | 87 (9.20) |
| Monthly household income (KRW) | ||||||
| <1,000,000 | 353 (40.67) | 187 (21.37) | 169 (19.34) | 520 (55.56) | 386 (41.15) | 301 (32.44) |
| 1,000,000-<2,000,000 | 269 (30.99) | 287 (32.80) | 267 (30.55) | 217 (23.18) | 256 (27.29) | 288 (31.03) |
| 2,000,000-<4,000,000 | 201 (23.16) | 302 (34.51) | 332 (37.99) | 174 (18.59) | 237 (25.27) | 272 (29.31) |
| ≥4,000,000 | 45 (5.18) | 99 (11.31) | 106 (12.13) | 25 (2.67) | 59 (6.29) | 67 (7.22) |
| Body mass index (kg/m2) | 23.66 ± 0.10 | 24.29 ± 0.10 | 24.79 ± 0.10 | 24.68 ± 0.11 | 25.01 ± 0.11 | 25.00 ± 0.11 |
| Physical activity levels a | 25.38 ± 0.58 | 19.31 ± 0.58 | 20.31 ± 0.58 | 22.54 ± 0.53 | 19.71 ± 0.53 | 20.23 ± 0.53 |
| Alcohol drinkers | 620 (70.78) | 644 (73.52) | 626 (71.54) | 207 (21.86) | 241 (25.56) | 260 (27.57) |
| Current smokers | 452 (51.60) | 428 (48.80) | 438 (50.06) | 34 (3.62) | 23 (2.45) | 39 (4.19) |
| Dietary supplement users | 119 (13.55) | 130 (14.81) | 149 (16.97) | 162 (17.05) | 236 (24.84) | 258 (27.16) |
| Nutrient factors b | ||||||
| Factor 1 | −0.61 ± 0.03 | −0.10 ± 0.03 | 0.24 ± 0.03 | −0.28 ± 0.03 | −0.12 ± 0.03 | 0.73 ± 0.03 |
| Factor 2 | −0.20 ± 0.03 | 0.29 ± 0.03 | 0.48 ± 0.03 | −0.57 ± 0.03 | −0.20 ± 0.03 | 0.17 ± 0.03 |
| Factor 3 | −0.22 ± 0.03 | −0.14 ± 0.03 | −0.22 ± 0.03 | 0.12 ± 0.03 | 0.16 ± 0.03 | 0.22 ± 0.03 |
Values are mean ± standard error or n (%). Q, quintile; KRW, Korean Republic Won. a Physical activity levels were quantified using the daily exercise hours and intensity of physical activities (light, moderate, and vigorous) based on metabolic equivalents. b Major nutrient factors identified in the principal component analysis.
Table 2 shows the HRs of incident CVD in men and women according to the quintiles of dietary vitamin B6 intake levels. In the multivariable adjusted model (Model 4), men in the highest quintile of dietary vitamin B6 intake were less likely to develop CVD than those in the lowest quintile (HR: 0.44; 95% CI: 0.25–0.78). In contrast, the dietary intake of vitamin B6 was not associated with CVD risk in women.
Table 2.
Hazard ratios (95% confidence intervals) for cardiovascular disease risk according to energy-adjusted dietary vitaminB6 intake levels stratified by sex.
| Vitamin B6 Intake (Quintile) | p for Trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Men | ||||||
| n | 878 | 879 | 878 | 879 | 878 | |
| Case | 72 | 53 | 52 | 65 | 36 | |
| Person year | 7016 | 6002 | 5876 | 6305 | 7340 | |
| Range (median), mg/day | 1.01–1.83 (1.70) | 1.84–2.00 (1.93) | 2.01–2.16 (2.08) | 2.17–2.39 (2.26) | 2.40–6.22 (2.61) | |
| Model 1 | 1 | 0.88 (0.62, 1.25) | 0.88 (0.62, 1.26) | 1.02 (0.73, 1.43) | 0.48 (0.32, 0.71) | 0.001 |
| Model 2 | 1 | 1.04 (0.73, 1.49) | 1.11 (0.77, 1.59) | 1.42 (1.01, 2.00) | 0.68 (0.45, 1.02) | 0.3 |
| Model 3 | 1 | 0.96 (0.67, 1.39) | 0.98 (0.68, 1.43) | 1.27 (0.88, 1.82) | 0.56 (0.36, 0.86) | 0.048 |
| Model 4 | 1 | 0.90 (0.62, 1.32) | 0.89 (0.60, 1.33) | 1.10 (0.72, 1.67) | 0.44 (0.25, 0.78) | 0.02 |
| Women | ||||||
| n | 950 | 950 | 950 | 950 | 950 | |
| Case | 97 | 53 | 47 | 41 | 46 | |
| Person year | 8050 | 6890 | 6410 | 6514 | 7382 | |
| Range (median), mg/day | 0.88–1.73 (1.58) | 1.74–1.92 (1.84) | 1.93–2.08 (2.00) | 2.09–2.29 (2.17) | 2.30–7.51 (2.48) | |
| Model 1 | 1 | 0.65 (0.46, 0.90) | 0.62 (0.44, 0.88) | 0.53 (0.37, 0.77) | 0.52 (0.37, 0.74) | <0.001 |
| Model 2 | 1 | 0.70 (0.50, 0.98) | 0.74 (0.52, 1.05) | 0.74 (0.51, 1.08) | 0.75 (0.53, 1.07) | 0.1 |
| Model 3 | 1 | 0.77 (0.54, 1.09) | 0.84 (0.59, 1.22) | 0.86 (0.58, 1.27) | 0.76 (0.51, 1.13) | 0.2 |
| Model 4 | 1 | 0.78 (0.55, 1.12) | 0.88 (0.60, 1.30) | 0.93 (0.60, 1.43) | 0.87 (0.53, 1.44) | 0.6 |
Q, quintile. Model 1: unadjusted. Model 2: adjusted for age. Model 3: additionally adjusted for monthly household income, body mass index, educational levels, physical activity levels, residential area, smoking status, alcohol consumption, and hypertension and diabetes at baseline. Model 4: model 3 plus additionally adjusted total energy intake, three major nutrient factors identified in the principal component analysis and use of dietary supplement.
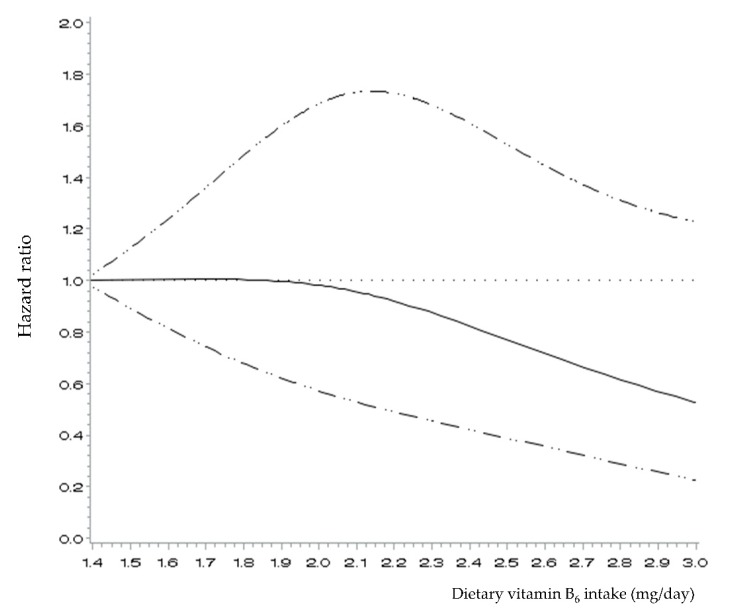
Figure 1 presents the spline curve for the association between dietary vitamin B6 intake and CVD incidence among men. An inverse linear association was observed, in that CVD risk decreased with each increment in the dietary levels of vitamin B6 intake after about 1.9 mg/day (p for nonlinearity = 0.3).
Figure 1.
Hazard ratios (95% confidence intervals) for the non-linear relationship between energy-adjusted dietary vitamin B6 intake levels and incidence of cardiovascular disease in Korean men, evaluated with restricted cubic splines. The model was adjusted for age, monthly household income, body mass index, educational levels, physical activity levels, residential area, smoking status, alcohol consumption, hypertension and diabetes at baseline, total energy intake, three major nutrient factors identified in the principal component analysis, and use of dietary supplements.
4. Discussion
In this prospective study, we evaluated the effect of dietary vitamin B6 intake on the risk of CVD among Korean men and women enrolled in the community-based Ansung–Ansan cohort study. As a result, Korean men with a higher dietary intake of vitamin B6 had a lower incidence of CVD. This association was not significant in women. Dose-response analyses confirmed the presence of a linear association between higher dietary vitamin B6 intake and decreased CVD incidence in men.
Pyridoxal 5′-phosphate (PLP), the active coenzyme form of vitamin B6, is required for more than 140 different enzymatic reactions in carbohydrate, amino acid and lipid metabolism, neurotransmitter synthesis, and steroid hormone receptor modulation [26]. PLP is also involved in reactions pertaining to antioxidant activity, the inflammatory process, homocysteine catabolism, and phosphorylation-all metabolic processes that are related to CVD prevention [27]. Oxidative stress, which represents a state of imbalance between oxidants and antioxidants, may occur due to the excessive accumulation of oxidants in the body or due to a lack of antioxidants, and may lead to vascular inflammation and endothelial dysfunction [28]. Homocysteine, an amino acid synthesized during the conversion of methionine to cysteine in one-carbon metabolism [29], is known to increase oxidative stress when deposited in the body [30]. The results of a meta-analysis of prospective cohort studies demonstrated that elevated homocysteine levels are associated with the incidence of CVD, indicating that homocysteine is an independent risk factor for CVD along with oxidative stress [31]. Vitamin B6 acts as an antioxidant by scavenging free radicals, reducing lipid peroxidation, and preventing damage to the integrity of the mitochondrial membrane [32]. It is also linked to inflammatory-related functions, which play a key part in the pathogenesis of atherosclerosis [33]. PLP regulates inflammatory reactions through involvement in the regulation of cytokine-induced mechanisms of immunocytes for the activation of the inflammatory reaction as well as synthesis of nucleic acids and proteins [33,34]. Several epidemiological studies have shown that low plasma PLP levels are inversely associated with the major bio-markers of inflammation [33,34,35]. In addition, vitamin B6 acts as a cofactor of cystathionine β-synthase and cystathionine γ-cystathionase in the trans-sulfuration of homocysteine to cystathionine and cysteine [36]. A previous study revealed that a higher vitamin B6 intake level was associated with a lower plasma total homocysteine level [37]. In other words, vitamin B6 may lower the risk of CVD by regulating the homocysteine concentrations in the plasma and indirectly regulating the production of cysteine as a precursor of the antioxidant, glutathione.
Previous epidemiological studies evaluating the association between vitamin B6 from diet and plasma PLP levels, and the incidence of CVD yielded inconsistent results [23,24,25,38,39,40]. An inverse association between plasma PLP levels and CHD was previously identified in a cohort study in the United States [40], while a study in the Netherlands reported that plasma PLP concentrations were not associated with CHD risk in women and men [39]. A study in Chinese men demonstrated that a higher vitamin B6 intake level was associated with a decreased CVD risk and total mortality [23]. In addition, a study conducted in Finland revealed a significantly decreased risk of stroke in male smokers with a high dietary vitamin B6 intake after adjustment for age and dietary supplement intake; however, this association was not significant after adjustment for multiple confounding factors such as alcohol consumption, smoking, and BMI [38]. Furthermore, the Health Professional Follow-up Study found no association between vitamin B6 intake and the risk of stroke [25]. A recent meta-analysis demonstrated an inverse association between vitamin B6 intake and CHD risk. In addition, a strong inverse association between the intake of vitamin B6 and CHD incidence was found in a sensitivity analysis after the exclusion of participants with pre-existing CVD [41]. No such association was observed in women, which is consistent with our results.
In the present study, higher dietary vitamin B6 intake levels lowered the CVD risk in men but had no significant effect on the CVD risk in women. This may be attributed to the sex-specific difference in plasma homocysteine levels and the complex interactions between vitamin B6, estrogen, and homocysteine. A sex-related comparison in Europe and the United States showed that men had higher levels of plasma homocysteine than women [42,43]. A previous study assessing homocysteine levels in Koreans also demonstrated that the mean homocysteine concentrations and prevalence of hyperhomocysteinemia were significantly higher in men than women [44]. The effects of the female hormone, estrogen, on the cardiovascular system may also mask the health benefits of vitamin B6 in women. Estrogen leads to enhanced glutathione levels and prevents peroxynitrite (ONOO-) formation by activating cystathionine β-synthase and regulating enzymes in glutathione synthesis [45]. Furthermore, estrogen is known to prevent the accumulation of low-density lipoproteins in the blood vessel walls, promote the bio-availability of nitric oxide, and improve vascular endothelium function [46]. Nevertheless, the mechanism underlying the sex-dependent effect of vitamin B6 on CVD risk is unclear; hence, further research is needed to investigate this association.
Several limitations of this study should be noted. First, the residual confounding effects of unmeasured or unknown variables may have affected the results, although we did adjust for multiple potential confounding factors. Second, we could not obtain data on dietary supplements and plasma homocysteine levels in our study; thus, the interactions between vitamin B6 intake (food and supplement), homocysteine levels, and CVD incidence could not be evaluated. Third, the incidence of CVD was assessed based on responses to self-reported questionnaires, which may have led to diagnostic misclassification. However, a previous study reported that the validity of the CVD cases in this cohort was acceptable, showing a 93% concordance between self-reported CVD and medical records. Finally, as the participants of this study were all residents of the Gyeonggi area in South Korea, it may be difficult to generalize the results to other populations. Nevertheless, we aimed to minimize errors in measurement by using the average dietary values of two repeats of a validated SQFFQ. To our knowledge, this is the first study to prospectively evaluate the association between dietary vitamin B6 intake and CVD risk in Korean adults.
5. Conclusions
In summary, a higher dietary intake level of vitamin B6 was associated with a decreased risk of CVD in Korean men but not Korean women. Further large-scale studies need to examine the effect of dietary and supplemental vitamin B6 intakes on plasma homocysteine levels in men and women as well as to determine the appropriate intake levels for CVD prevention.
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 4851-302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/7/1484/s1, Figure S1: Flow chart of the participants in the study, KoGES, Korean Genome and Epidemiology study, Table S1: Factor loading matrix for the factor analysis of nutrients.
Author Contributions
Conceptualization, K.P.; Methodology, K.P. and J.J.; Software, J.J.; Validation, K.P.; Formal Analysis, J.J.; Data Curation, J.J.; Writing—Original Draft Preparation, J.J.; Writing—Review and Editing, K.P.; Visualization, J.J.; Supervision, K.P.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (grant number: NRF-2017R1A1A3A04069759). The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Cardiovascular Diseases (CVDs) [(accessed on 10 January 2019)]; Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 2.World Health Organization Global Status Report on Noncommunicable Diseases 2014. [(accessed on 7 January 2019)]; Available online: https://www.who.int/nmh/publications/ncd-status-report-2014/en/
- 3.Korean Statistical Information Service Deaths and Death Rates by Cause by Sex and Age(Five-Year Age) [(accessed on 5 January 2019)]; Available online: http://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B34E01&conn_path=I2.
- 4.Benziger C.P., Roth G.A., Moran A.E. The Global Burden of Disease Study and the Preventable Burden of NCD. Glob. Heart. 2016;11:393–397. doi: 10.1016/j.gheart.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Tang G.Y., Meng X., Li Y., Zhao C.N., Liu Q., Li H.B. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients. 2017;9:857. doi: 10.3390/nu9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aune D., Keum N., Giovannucci E., Fadnes L.T., Boffetta P., Greenwood D.C., Tonstad S., Vatten L.J., Riboli E., Norat T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018;108:1069–1091. doi: 10.1093/ajcn/nqy097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friso S., Lotto V., Corrocher R., Choi S.W. Vitamin B6 and cardiovascular disease. Subcell. Biochem. 2012;56:265–290. doi: 10.1007/978-94-007-2199-9_14. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly P., Alam S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page J.H., Ma J., Chiuve S.E., Stampfer M.J., Selhub J., Manson J.E., Rimm E.B. Plasma vitamin B(6) and risk of myocardial infarction in women. Circulation. 2009;120:649–655. doi: 10.1161/CIRCULATIONAHA.108.809038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishihara J., Iso H., Inoue M., Iwasaki M., Okada K., Kita Y., Kokubo Y., Okayama A., Tsugane S. Intake of folate, vitamin B6 and vitamin B12 and the risk of CHD: The Japan Public Health Center-Based Prospective Study Cohort, I. J. Am. Coll. Nutr. 2008;27:127–136. doi: 10.1080/07315724.2008.10719684. [DOI] [PubMed] [Google Scholar]
- 11.Marniemi J., Alanen E., Impivaara O., Seppanen R., Hakala P., Rajala T., Ronnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr. Metab. Cardiovasc. Dis. 2005;15:188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Choe H., Hwang J.Y., Yun J.A., Kim J.M., Song T.J., Chang N., Kim Y.J., Kim Y. Intake of antioxidants and B vitamins is inversely associated with ischemic stroke and cerebral atherosclerosis. Nutr. Res. Pract. 2016;10:516–523. doi: 10.4162/nrp.2016.10.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park Y. Intakes of vegetables and related nutrients such as vitamin B complex, potassium, and calcium, are negatively correlated with risk of stroke in Korea. Nutr. Res. Pract. 2010;4:303–310. doi: 10.4162/nrp.2010.4.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson F.E., Subar A.F. Dietary Assessment Methodology. In: Coulston A.M., Boushey C.J., Ferruzzi M.G., Delahanty L.M., editors. Nutrition in the Prevention and Treatment of Disease. 4th ed. Academic Press; San Diego, CA, USA: 2017. pp. 5–48. [Google Scholar]
- 15.Hennekens C.H., Buring J.E., Mayrent S.L., Doll R. Epidemiology in Medicine. Little, Brown and Company; Boston, MA, USA: 1987. pp. 30–53. [Google Scholar]
- 16.Kim Y., Han B.-G., KoGES Group Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017;46:e20. doi: 10.1093/ije/dyv316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett W. Nutritional Epidemiology, 3rd ed. Oxford University Press; New York, NY, USA: 2012. pp. 274–307. [Google Scholar]
- 18.Ainsworth B.E., Haskell W.L., Leon A.S., Jacobs D.R., Jr., Montoye H.J., Sallis J.F., Paffenbarger R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Ahn Y., Kwon E., Shim J.E., Park M.K., Joo Y., Kimm K., Park C., Kim D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 20.Rural Development Administration Korea . Food Composition Table. 5th ed. Rural Development Administration Korea; Suwon, Korea: 1996. pp. 40–457. [Google Scholar]
- 21.Van Buuren S., Brand J.P., Groothuis-Oudshoorn C.G., Rubin D.B. Fully conditional specification in multivariate imputation. J. Stat. Comput. Simul. 2006;76:1049–1064. doi: 10.1080/10629360600810434. [DOI] [Google Scholar]
- 22.Hu F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L.G., Shu X.O., Li H.L., Gao J., Han L.H., Wang J., Fang J., Gao Y.T., Zheng W., Xiang Y.B. Prospective cohort studies of dietary vitamin B6 intake and risk of cause-specific mortality. Clin. Nutr. 2018 doi: 10.1016/j.clnu.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui R., Iso H., Date C., Kikuchi S., Tamakoshi A. Dietary folate and vitamin b6 and B12 intake in relation to mortality from cardiovascular diseases: Japan collaborative cohort study. Stroke. 2010;41:1285–1289. doi: 10.1161/STROKEAHA.110.578906. [DOI] [PubMed] [Google Scholar]
- 25.He K., Merchant A., Rimm E.B., Rosner B.A., Stampfer M.J., Willett W.C., Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169–174. doi: 10.1161/01.STR.0000106762.55994.86. [DOI] [PubMed] [Google Scholar]
- 26.di Salvo M.L., Contestabile R., Safo M.K. Vitamin B(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta. 2011;1814:1597–1608. doi: 10.1016/j.bbapap.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Schalinske K.L., Smazal A.L. Homocysteine imbalance: A pathological metabolic marker. Adv. Nutr. 2012;3:755–762. doi: 10.3945/an.112.002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review) Vascul. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Skovierova H., Vidomanova E., Mahmood S., Sopkova J., Drgova A., Cervenova T., Halasova E., Lehotsky J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016;17:1733. doi: 10.3390/ijms17101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai W.K., Kan M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015;67:1–12. doi: 10.1159/000437098. [DOI] [PubMed] [Google Scholar]
- 31.Peng H.Y., Man C.F., Xu J., Fan Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J. Zhejiang Univ. Sci. B. 2015;16:78–86. doi: 10.1631/jzus.B1400183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannan K., Jain S.K. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic. Biol. Med. 2004;36:423–428. doi: 10.1016/j.freeradbiomed.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Friso S., Girelli D., Martinelli N., Olivieri O., Lotto V., Bozzini C., Pizzolo F., Faccini G., Beltrame F., Corrocher R. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am. J. Clin. Nutr. 2004;79:992–998. doi: 10.1093/ajcn/79.6.992. [DOI] [PubMed] [Google Scholar]
- 34.Friso S., Jacques P.F., Wilson P.W., Rosenberg I.H., Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation. 2001;103:2788–2791. doi: 10.1161/01.CIR.103.23.2788. [DOI] [PubMed] [Google Scholar]
- 35.Chiang E.P., Bagley P.J., Roubenoff R., Nadeau M., Selhub J. Plasma pyridoxal 5’-phosphate concentration is correlated with functional vitamin B-6 indices in patients with rheumatoid arthritis and marginal vitamin B-6 status. J. Nutr. 2003;133:1056–1059. doi: 10.1093/jn/133.4.1056. [DOI] [PubMed] [Google Scholar]
- 36.Joseph J., Loscalzo J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients. 2013;5:3235–3256. doi: 10.3390/nu5083235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye X., Maras J.E., Bakun P.J., Tucker K.L. Dietary intake of vitamin B-6, plasma pyridoxal 5’-phosphate, and homocysteine in Puerto Rican adults. J. Am. Diet. Assoc. 2010;110:1660–1668. doi: 10.1016/j.jada.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson S.C., Mannisto S., Virtanen M.J., Kontto J., Albanes D., Virtamo J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am. J. Epidemiol. 2008;167:954–961. doi: 10.1093/aje/kwm395. [DOI] [PubMed] [Google Scholar]
- 39.de Bree A., Verschuren W.M., Blom H.J., Nadeau M., Trijbels F.J., Kromhout D. Coronary heart disease mortality, plasma homocysteine, and B-vitamins: A prospective study. Atherosclerosis. 2003;166:369–377. doi: 10.1016/S0021-9150(02)00373-8. [DOI] [PubMed] [Google Scholar]
- 40.Folsom A.R., Nieto F.J., McGovern P.G., Tsai M.Y., Malinow M.R., Eckfeldt J.H., Hess D.L., Davis C.E. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998;98:204–210. doi: 10.1161/01.CIR.98.3.204. [DOI] [PubMed] [Google Scholar]
- 41.Jayedi A., Zargar M.S. Intake of vitamin B6, folate, and vitamin B12 and risk of coronary heart disease: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2018:1–11. doi: 10.1080/10408398.2018.1511967. [DOI] [PubMed] [Google Scholar]
- 42.Dierkes J., Jeckel A., Ambrosch A., Westphal S., Luley C., Boeing H. Factors explaining the difference of total homocysteine between men and women in the European Investigation Into Cancer and Nutrition Potsdam study. Metabolism. 2001;50:640–645. doi: 10.1053/meta.2001.23286. [DOI] [PubMed] [Google Scholar]
- 43.Beydoun M.A., Shroff M.R., Beydoun H.A., Zonderman A.B. Serum folate, vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom. Med. 2010;72:862–873. doi: 10.1097/PSY.0b013e3181f61863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim H.J., Kim M.K., Kim J.U., Ha H.Y., Choi B.Y. Major determinants of serum homocysteine concentrations in a Korean population. J. Korean. Med. Sci. 2010;25:509–516. doi: 10.3346/jkms.2010.25.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimitrova K.R., DeGroot K., Myers A.K., Kim Y.D. Estrogen and homocysteine. Cardiovasc. Res. 2002;53:577–588. doi: 10.1016/S0008-6363(01)00462-X. [DOI] [PubMed] [Google Scholar]
- 46.Bechlioulis A., Naka K.K., Calis K.A., Makrigiannakis A., Michalis L., Kalantaridou S.N. Cardiovascular effects of endogenous estrogen and hormone therapy. Curr. Vasc. Pharmacol. 2010;8:249–258. doi: 10.2174/157016110790886974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.