Abstract
Active species for synthetic and catalytic applications are formed from well defined complexes or mixtures of compounds. For group 4 metallocenes, three pathways for the formation of the reactive complex fragment [Cp′2M] are known: (i) reductive mixtures and well defined complexes which are able to form the metallocene fragments either by (ii) addition or (iii) substitution reactions. In this account for each of theses systems (i)–(iii) a prominent example will be discussed in detail, (i) the Negishi reagent Cp2ZrCl2/n‐BuLi, (ii) bis(η5 : η1‐pentafulvene) complexes and (iii) metallocene bis(trimethylsilyl)acetylene complexes, to show the advantages and the disadvantages for each of these methods for synthetic applications. This account summarizes some main advantages of group 4 metallocene bis(trimethylsilyl)acetylene complexes as metallocene generating agents over other synthetically used systems. For each of the special purposes, all described systems have advantages as well as disadvantages. The aim of this overview is to help synthetic chemists in selecting the most effective system on the basis of [Cp′2M] (M=Ti, Zr) for synthetic or catalytic puposes.
Keywords: organometallic synthesis, group 4 transition metals, metallocenes, reactivity, selectivity
1. Introduction
The chemistry of group 4 metallocenes has been reviewed in the past on several occasions, e. g. by Chirik, who presented a historic overview of titanocene chemistry long with more recent examples1a as well as by Xi and Li who focussed on the construction of carbocycles via zircona‐ and titanacycles.1b Sato and coworkers have reviewed the synthesis of organaotitanium complexes and their synthetic applications.1c Very recently Tonks and coworkers published an overview about novel applications of low‐valent early transition metals in synthesis and catalysis.1d
To realize such effective stoichiometric and catalytic reactions it is generally very important to form coordinatively and electronically unsaturated complex fragments. For this the group 4 metallocene bis(trimethylsilyl)acetylene complexes became more and more interesting during the last years in stoichiometric [2–22] and catalytic23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 reactions. Typical examples for several methods to obtain compounds of this type were published and will be described in detail in this account.35
Most transformations start from bench‐stable complexes and form the catalytically active species by different methods of activation. Three groups exist for the formation of reactive metallocenes [Cp′2M]: (i) reductive mixtures, and well defined complexes, which form the metallocene fragments either by (ii) addition or (iii) substitution reactions. In this paper for each of theses systems a significant example will be discussed to show the advantages and the disadvantages in synthetic applications.
1.1. General
Several metallocene [Cp′2M] generating reductive mixtures are known, including combinations of Cp′2MCl2 and reduction agents like Zn, Al, Mg, Sm36 and Cp2TiCl2/EtMgCl2 37 or the most popular Negishi reagent Cp2ZrCl2/n‐BuLi.38 For these examples a broad scope of synthetical and catalytical applications was published. Several methods for the preparation of Cp2Zr(olefin) complexes and of various Cp2Zr(olefin)(PMe3) complexes exist, for which either resonance hybrids or equilibrating mixtures of Cp2Zr(olefin) complexes and the corresponding zirconacyclopropanes were discussed. They show a variety of chemical transformations like the alkene substitution, the ring expansion to give five‐membered zirconacycles by C−C bond formation, the ring contraction of formed five‐membered zirconacycles, several skeletal rearrangements and the stereoisomerization of alkenes.
Additionally, well defined stable precursor complexes were described, in which the coordinatively and electronically unsaturated complex fragments [Cp′2M] are generated during the reaction either by addition or substitution. For example Beckhaus and coworkers have developed a broad range of bis(η 5 : η1‐pentafulvene) metal complexes that undergo substrate addition of element‐H bonds (e. g. molecular hydrogen, N−H and C−H bonds), which leads to the formation of metallocene hydride, imide, hydrazide and aziridine complexes.39
Systems that are more prone to ligand substitution1b include those possessing neutral placeholder ligands such as Cp2Ti(PMe3)2,40 Cp2Ti(P[OEt)3]2,41 [Cp*2Ti(N2)]2N2 42 and others like Cp2Ti(CO)2 43 as well as alkyne complexes of the type Cp′2M(L)(η2‐btmsa) (btmsa=bis(trimethylsilyl)acetylene; with or without L, L=pyridine or THF).2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
Generally, most reductive mixture systems are easily prepared from readily available starting materials but show some disadvantages in synthesis and reactions. In contrast to this, well defined complexes are in some cases less easily obtained but show reasonable to good stabiliy at room temperature and generate the metallocene units [Cp′2M] more selectively. For well defined complexes some side reactions are prevented, which were often observed for mixtures of precursor complexes. As an important prerequisite, the used substrate must have the ability to substitute the stabilizing ligands. For these widely used systems it is not possible to summarize all known examples within this account. For this reason, for each group of metallocene sources only one example is discussed here (Scheme 1).
Scheme 1.
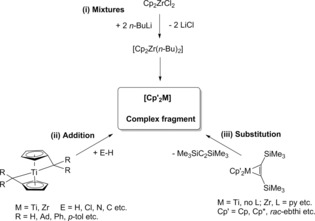
Overview on the formation of [Cp′2M] by (i) reductive mixtures, and well defined complexes, either by (ii) addition or (iii) substitution reactions.
2. Example for Mixtures
2.1. The Negishi Reagent Cp2ZrCl2/n‐BuLi
For the Negishi reagent Cp2ZrCl2/n‐BuLi as the most prominent example for the first group, Dioumaev and Harrod44 showed that under conditions typically used with this system, no [Cp2Zr] is present in solution. Instead, a mixture of zirconocene complexes including butylzirconocene(III), zirconocene(III) hydride, butenylzirconocene(IV) hydride dimer and 1,1‐bis(cyclopentadienyl)‐2‐methyl‐3‐(zirconocenyl hydride)‐1‐zirconacyclobutane(IV) dimer are formed upon warming of dibutylzirconocene to room temperature.
Considering the frequent use of this system Cp2ZrCl2/2 RLi (R=alkyl) in organic synthesis and catalysis the nature of the reactive species is still unclear. Generally, for mixtures Cp2ZrCl2/2 RLi (R=alkyl) as the first step the formation of the zirconocene dialkyl complexes was identified, which in most cases are thermally unstable (Scheme 2).
Scheme 2.
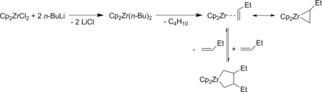
The Negishi reagent Cp2ZrCl2/n‐BuLi and its use in C−C bond formation.
In the thermal decomposition of the dialkylzirconocenes ill‐defined mixtures of several compounds are formed. As a result of this and former investigations it was shown that the main gaseous product is the corresponding alkane RH. Additionally, upon heating the mixture above room temperature, the formation of alkenes was indicated. By the addition of phosphines the zirconocene alkene complexes are stabilized and the complexes Cp2Zr(RCH=CH2)(PR3)1b were isolated. Because for Cp* the phosphine‐free titanocene complex, Cp*2Ti(CH2=CH2) was isolated one can assume that zirconocene alkene complexes may exist in the mixtures. This leads to the conclusion that zirconocene dialkyl complexes formed in the first step undergo β‐hydrogen abstraction, liberation of alkane and formation of the zirconocene alkene complexes. Summarizing the process of the thermal decomposition of zirconocene dibutyl at room temperature Dioumaev and Harrod identified the paramagnetic butylzirconocene(III), zirconocene(III) hydride, the diamagnetic butenylzirconocene(IV) hydride dimer and 1,1‐bis(cyclopentadienyl)‐2‐methyl‐3‐(zirconocenyl hydride)‐1‐zirconacyclobutane(IV) dimer. Additionally the crotylzirconocene(IV) hydride, 1,1‐bis(cyclopentadienyl)‐2‐ethyl‐1‐zirconacyclopropane(IV) and 1,1‐bis(cyclopentadienyl)‐3,4‐diethyl‐1‐zirconacyclopentane(IV) were identified by several analytical methods and a mechanistic proposal for the formation of all these products was made (Scheme 3).
Scheme 3.
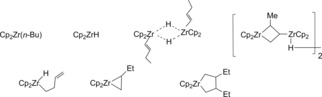
Examples for some by‐products of the Negishi reagent Cp2ZrCl2/n‐BuLi, published by Dioumaev and Harrod.
Most importantly, no [Cp2Zr] is present in solution. Only some of the formed products are able to react by substitution with substrates to the desired products whereas the others can open reaction channels for undesirable formation of by‐products, thus lowering the selectivity. Despite of these disadvantages, the Negishi reagent Cp2ZrCl2/n‐BuLi was used very successfully in many synthetical projects.38 Nevertheless, in some cases it was described that for example zirconocene bis(trimethylsilyl)acetylene complexes show some advantages compared to the Negishi reagent Cp2ZrCl2/n‐BuLi (see the details below).
Advantages for the Negishi reagent as a mixture are, that the starting materials Cp2ZrCl2 and n‐BuLi are commercially available and the mixture is simply prepared. This system was used for many examples, giving broad synthetic applications.
Disadvantages of the system are the procedure, which is restricted to low temperatures and only THF as a solvent as well as sometimes the problems of selectivity and to realize exact stoichiometric relations. Some functional groups are not tolerated by the strong base and in some cases it is a problem to separate the LiCl by‐product from formed products of low solubility.
3. Examples for Substrate Addition
3.1. Beckhaus’ bis(η5 : η1‐pentafulvene) Complexes
Beckhaus presented in a very recent review39 a survey of the chemistry of group 4 metal pentafulvene complexes. He described that in these bis(η5 : η1‐pentafulvene) complexes due to the “Umpolung” of the coordinated pentafulvene ligand the Cexo‐atom becomes strongly nucleophilic and many element‐H bond activation reactions become possible, which lead in the first step to the formation of [Cp′2Ti] complexes and in the second to further interesting synthetic applications (Scheme 4).
Scheme 4.
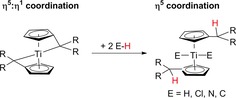
Reaction of bis(η5 : η1‐pentafulvene) titanium complexes to [Cp′2Ti] complexes.
Due to the aforementioned strongly nucleophilic character of the exocyclic C atoms the bis(η5 : η1‐pentafulvene) complexes show compared to the the titanocene dialkyl complexes like Cp2TiMe2 no kinetic hindrance in the reactions with electrophilic substrates. By protonation of the nucleophilic exo C atoms of substituted bis(η5 : η1‐pentafulvene) complexes the complexes (η5‐C5H4−CHR2)TiX2 are easily formed for R=Ph, p‐Tol, adamantly and X=Cl, Br, I.45, 47 In principle, this reaction scheme is possible in a similar manner for other bond activation reactions of molecular hydrogen and N−H as well as C−H bond splitting reactions leading to titanium hydrides, imides and hydrazides as well as titanaaziridines.39
In reactions of substituted bis(η5 : η1‐pentafulvene) titanium complexes with molecular dihydrogen dinuclear hydride bridged titanium complexes were formed by a complete conversion of four η5 : η1‐pentafulvene ligands to the η5‐Cp′ ligands (Scheme 5). For zirconium in a similar reaction also M2H2 dinuclear complexes were formed under conversion of only two pentafulvene [C5H4=CR2]2− to Cp′ ligands [C5H4−CHR2] − whereas the other two remain as [C5H4=CR2]2− ligands.48
Scheme 5.
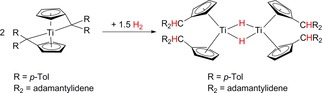
Reaction with molecular hydrogen and complete conversion of η5 : η1‐pentafulvene ligands to η5‐Cp′ ligands.
Titanocene imido complexes were formed in the reaction of the substituted bis(η5 : η1‐pentafulvene) titanium complexes with amines. These complexes exist in equilibrium with the monoamide complexes and were stabilized by coordination of pyridine. They were used synthetically as excellent starting material for reactions with acetylenes, carbodiimides and isocyanates to give heterometallacycles (Scheme 6).49 If 1,1‐diphenylhydrazine was used instead of the amines, interesting titanocene hydrazido complexes were obtained.50
Scheme 6.

Reaction of η5 : η1‐pentafulvene titanium complexes with amines to titanocene imide complexes (η5‐Cp′)2Ti(=NR’)(py).
With this complex as starting material several [2+2] cycloaddition and bond activation reactions with carbodiimides, nitriles, CS2, CO and 9‐borabicyclo[3.3.1]nonane were realized, giving a broad range of synthetical applications.39, 49
Simultaneous N−H and C−H bond activation occurs in reactions with N‐methylaniline to furnish titanocene η2‐imine complexes as titanaaziridines, which are important in the titanium catalyzed hydroaminoalkylation of alkenes (Scheme 7).51 The obtained titanocene η2‐imine complexes were further used as starting materials for ring enlargement reactions with acetylenes, carbonyl compounds, nitriles and other unsaturated complexes.39
Scheme 7.
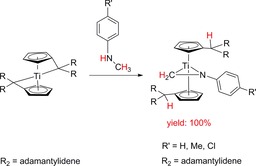
Reaction of η5 : η1‐pentafulvene titanium complexes with N‐methylaniline to titanocene η2‐imine complexes as titanaaziridines.
The η5 : η1‐pentafulvene complexes of titanium with secondary allylamines again with simultaneous N−H and C−H bond activations, however, in this case formation of titanocene 1‐azabutadiene complexes occurs (Scheme 8).52
Scheme 8.
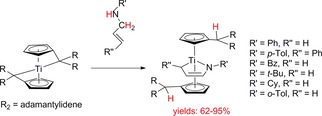
Reaction of η5 : η1‐pentafulvene titanium complexes with allylamines to titanocene 1‐azabutadiene complexes.
The main features of these reactions is the possibility to slelectively obtain in the first step the η5‐Cp′ ligands substituted metallocene complexes by conversion of η5 : η1‐pentafulvene ligands which in a second step yield synthetically very interesting metallacycles. Aditionally, by other reactions the conversion of only one pentafulvene [C5H4=CR2]2− to Cp′ ligands [C5H4−CHR2]1− was described whereas the other remains as [C5H4=CR2]2− ligand. This opens the way to a lot of other synthetically very interesting reactions.53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71
The most spectacular example for unexpected results was the reaction of η5 : η1‐pentafulvene titanium complexes with the allylidenephosphorylide Ph3P=C(H)−C(H)=CH2, giving a titanabutatriene complex by a spontaneous double C−H bond activation via an intermediate Ph3P=C=C=CH2. After dimerization the titanabutatriene complex formed a binuclear zig‐zag hexapentaene titanium complex (Scheme 9).53
Scheme 9.
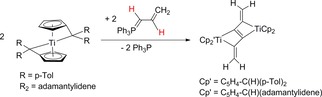
Reactions of η5 : η1‐pentafulvene titanium complexes with the allylidenephosphorylide Ph3P=C(H)−C(H)=CH2 to binuclear zig‐zag hexapentaene titanium complexes.
In summary for the bis(η5 : η1‐pentafulvene) complexes (M: Ti, Zr) complexes electrophilic attack, haptotropic shift, insertion of multiple bonds of e. g. nitriles, isonitriles, imines or carbonyl compounds) and E−H bond activation reactions were found. N−H/C−H and double C−H bond activation reactions under mild conditions show the special character of these complexes compared to metallocene methyl complexes Cp2TiMe2.39 Beckhaus described the weak M−Cexo interaction in these complexes as the first examples of heteroatom free organometallic frustrated Lewis pairs in comparison to classical heteroatom containing Lewis pairs.39, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61
Advantages of η5 : η1‐pentafulvene complexes in reactions with selected substrates are the high yields of products, formed at room temperature (Scheme 5–10) with a very high selectivity. This means that for selected substrates in a two step process the selective reaction of two or even more substrates is possible.
Due to the mild conditions the elementary steps of catalytic cycles can be studied.51a In many reactions the high electronical flexibility allowed the primary substrate coordination by a haptotropic shift from η5 : η1→η4 as shown for the coordination of NHC.51b
Disadvantages are, that the synthetic applications are limited in the first step mostly to E−H substrates. By this process only product complexes of Cp′2M with substituted Cp′ ligands are formed, which give in one or more subsequent reaction the desired products. The starting complexes are not commercially available and require previous organometallic synthesis.
4. Examples for Substrate Substitution
4.1. Metallocene Complexes with bis(trimethylsilyl)acetylene Cp′2M(η2‐btmsa)
As mentioned above, the examples for ligand substitutions in the complexes Cp′2ML2 such as Cp2Ti(PMe3)2,40 Cp2Ti(P[OEt)3]2,41 [Cp*2Ti(N2)]2N2,42 Cp2Ti(CO)2, Cp2TiR2 etc.43 generate the [Cp′2M] fragment by a ligand exchange reaction. The reactivity for these complexes depends on ability of the system for the substitution of the Cp′2M stabilizing ligands L and the reaction conditions. The disadvantage of these complexes is, that the substitution of L sometimes is not complete or the leaving ligands couple with the substrates to yield undesired by‐products. In this context, the complexes Cp′2M(L)(η2‐btmsa) (btmsa=bis(trimethylsilyl)acetylene, with or without L like pyridine or THF) are for better suited for many reactions, because the btmsa shows a greater tendency for substitution even at room temperature without the necessity for further activation and mostly no tendency for coupling.
Several methods were described to obtain such bis(trimethylsilyl)acetylene complexes Cp′2M(η2‐Me3SiC2SiMe3) as starting materials.35 The compound Cp2Ti(η2‐Me3SiC2SiMe3) as the typical examples for complexes with unsubstituted Cp ligands was first obtained by reduction of Cp2TiCl2 with magnesium in THF in the presence of Me3SiC≡CSiMe3,35a later by the reduction of Cp2TiCl2 with n‐butyllithium in n‐hexane and adding Me3SiC≡CSiMe3 35b as well as by the reaction of Cp2TiMe2 with Me3SiC≡CSiMe2H at higher temperature in n‐hexane.35c The zirconocene complex Cp2Zr(py)(η2‐Me3SiC2SiMe3) was synthesized from the complex Cp2Zr(THF)(η2‐Me3SiC2SiMe3)35d which was obtained by reduction of Cp2ZrCl2 with magnesium in the presence of Me3SiC≡CSiMe3 in THF and the substitution of THF by pyridine.35e Later a more simplified alternative procedure by starting from Cp2ZrCl2/2 n‐BuLi via the intermediate [Cp2Zr(THF)(η2‐butene)], followed by addition of Me3SiC≡CSiMe3 and pyridine was used by Tilley and co‐workers.35f One can understand this procedure on one side as an effective purification of the Negishi system and a simplified procedure to avoid the isolation of the intermediate Cp2Zr(THF)(η2‐btmsa). Higher yield of 95 % of this alternative method compared to 49 % of the procedure via the isolation of Cp2Zr(THF)(η2‐btmsa) are remarkable and made the complex a convenient starting material for many selective reactions with high yields.
The first example of a group 4 metallocene alkyne complex without additional ligands Cp2Ti(η2‐btmsa) with bis(trimethylsilyl)acetylene and a series of similar complexes Cp′2M(η2‐btmsa) (M=Ti, Zr, Hf; Cp′=Cp, Cp*=η5‐pentamethylcyclopentadienyl and Cp′2=rac‐(ebthi) as rac‐1,2‐ethylene‐1,1′‐bis(η5‐tetrahydroindenyl) and others were intensively investigated (Scheme 10).2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22
Scheme 10.

Examples for group 4 metallocene bis(trimethylsilyl)acetylene complexes.
The first investigated reaction of Cp2Ti(η2‐btmsa) with tolane PhC≡CPh surprisingly gave no coupling of Me3SiC≡CSiMe3 with PhC≡CPh to a titanacyclopentadiene but the substitution of bis(trimethylsilyl)acetylene by tolane and via the assumed alkyne complex Cp2Ti(η2‐PhC2Ph) another C−C coupling reaction only of two molecules of tolane to the tetraphenylsubstituted titanacyclopentadiene.35a Based on this simple substitution reaction, Cp2Ti(η2‐btmsa) and similar complexes were applied as excellent sources for the generation of the very reactive coordinatively and electronically unsaturated complex fragments [Cp′2M]. These were used in many synthetic and catalytic reactions, summarized before in several papers and some reviews.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Nevertheless, some very view examples exist in which the substitution of the bis(trimethylsilyl)acetylene ligand did not work and a coupling of the alkyne with other substrates was found.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 17, 22
The main advantages of one complex of this group, Cp2Zr(py)(η2‐btmsa) compared to other [Cp2Zr] generating systems, were summarized by Tilley et al. and discussed below in detail. All these advantages are not only restricted to the zirconium complex Cp2Zr(pyr)(η2‐btmsa) but were described in a similar manner for other group 4 metallocene bis(trimethylsilyl)acetylene complexes for several synthetic and catalytic reactions. Because it is impossible to present here all these results, only some impressive examples for typical uses are summarized in the following chapter.
Reactions of Group 4 metallocene bis(trimethylsilyl)acetylene complexes with alkynes and 1,3‐butadiynes were described in detail. In particular 1,3‐butadiynes gave high yields of different products by complexation, cleavage and coupling reactions (Scheme 11).3, 10, 14, 16
Scheme 11.
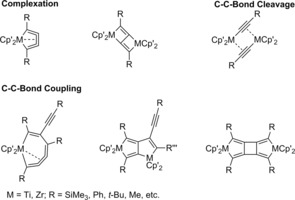
Products of reactions of group 4 metallocene bis(trimethylsilyl)acetylene complexes with 1,3‐butadiynes.
Group 4 metallocene bis(trimethylsilyl)acetylene complexes were investigated in reactions with different alkynes to more special metallacyclopentadienes.21 For example, the formation of macrocycles by zirconocene mediated C−C coupling reactions of many di‐ and oligo‐diynes to large polycyclic aromatic hydrocarbons (PAH's) was described by Tilley et al.35f, 62, 63, 64, 65, 66, 67 by using in addition to the Negishi system consisting of Cp2ZrCl2/2 n‐BuLi the complex Cp2Zr(py)(η2‐btmsa) as a well‐defined source of “Cp2Zr”.
The reaction of Cp2Zr(py)(η2‐btmsa) with 4,4’‐bis(trimethylsilylethynyl)biphenyl for example gave the trinuclear macrocyle in 99 % isolated yield whereas by using Cp2ZrCl2/2 n‐BuLi the yield was only 90 % after separation of the product from the formed LiCl. Protolysis of the zirconacyclopentadiene gave the corresponding substituted butadiene (Scheme 12).
Scheme 12.
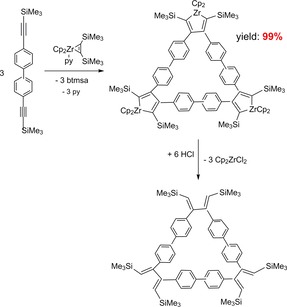
Reaction of Cp2Zr(py)(η2‐btmsa) with 4,4’‐bis(trimethylsilylethynyl)biphenyl and acidolysis of the product.
Such selective zirconocene mediated C−C bond formations were described for many other examples. In most cases very high yields and clean products were obtained. For these macrocycles reversible C−C bond formation was investigated as well (Scheme 13). Starting from these macrocycles by protolysis many compounds with butadiene subunits were described.
Scheme 13.
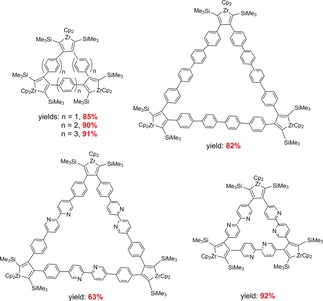
Examples for products obtained from coupling of bis(trimethylsilylethynyl)‐substrates at Cp2Zr(py)(η2‐btmsa.
In more recent investigations as substrates phenanthrene substituted diynes and oligodiynes were used for the intramolecular reductive cyclization by Cp2Zr(py)(η2‐btmsa). These gave several examples of zirconacyclopentadiene‐annulated PAH's with up to 16 fused rings and five zirconacyclopentadiene rings. Using this concept, several PAH's with exocyclic diene functional groups were obtained,67a, 67b involving a general strategy to obtain expanded helicene molecules of this type (Scheme 14).67b
Scheme 14.
Intramolecular reductive cyclization of phenanthrene substituted diynes by Cp2Zr(py)(η2‐btmsa) as a general synthetic strategy.
Generally, dienes with the ortho‐quinodimethane structure exhibit high reactivity but are here stabilized by incorporation into the formed PAH framework. Modification by selective hydrogenation gave highly alkylated PAH's or by the Diels‐Alder reaction the fusion to additional rings. By in situ protodemetalation of the zirconacyclopentadienes without a dienophile other products were formed, compared to the protodemetallation in the presence of N‐ethylmaleimide. Several multifold couplings of tris(diyne) and pentakis(diyne) substrates were realized with Cp2Zr(py)(η2‐btmsa). The intermediate zirconacyclopentadiene produced with an excess of benzoic acid further products of protodemetallation in high yields (Scheme 15).67
Scheme 15.
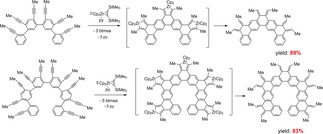
Examples for multifold couplings of tris‐ and pentakis(diynes) with Cp2Zr(py)(η2‐btmsa) to PAH's and protodemetallation.
These and the other reported examples present a highly efficient method to produce new PAH's via fused zirconacyclopentadienes and subsequent protodemetalation. The authors published very selective reactions and high yields with Cp2Zr(py)(η2‐btmsa) and suggested its suitability for the preparation of even larger PAH's and graphene‐type nanostructures.
Going from the C≡C to the C≡N triple bonds of substrates, the reactions of the complexes Cp*2M(η2‐Me3SiC2SiMe3) with nitriles including PhC≡N, p‐tolC≡N, o‐TolC≡N and FcC≡N (Fc=ferrocenyl) resulted in the formation of 1‐metalla‐2,5‐diaza‐cyclopenta‐2,4‐dienes which were isolated in high yields (Scheme 16).68, 69, 70, 71, 72
Scheme 16.
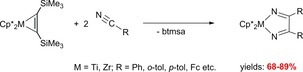
Reactions of Cp*2M(η2‐Me3SiC2SiMe3) (M=Ti, Zr) with nitriles to 1‐metalla‐2,5‐diaza‐cyclopenta‐2,4‐dienes.
Similar reactions were found when using Cp*2Ti(η2‐Me3SiC2SiMe3) and 1,2‐ or 1,3‐dicyanobenzene in a selective intermolecular nitrile‐nitrile C−C coupling in which depending on the substrate tri‐ or tetranuclear macrocycles were formed (Scheme 17).70
Scheme 17.
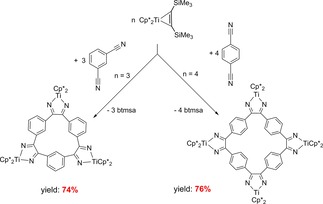
Reactions of Cp*2Ti(η2‐Me3SiC2SiMe3) with dicyanobenzenes.
Such coupling reactions were later described for 2,6‐dicyanopyridine71 and very recently by Reiß, Beweries and coworkers for 2‐cyanofuran as well as 2‐cyanothiophene, giving several mixed tri‐ and tetranuclear complexes by homo‐ and heterocoupling using Cp*2M(η2‐btmsa) (M=Ti, Zr).72b When changing from aryl‐dinitriles to dicyanoalkyl adiponitrile, by a nitrile‐nitrile C−C coupling and subsequent protonation no 1‐titana‐2,5‐diaza‐cyclopenta‐2,4‐diene but a 1,4‐diazadiene complex was formed in a yield of 46 % (Scheme 18).70
Scheme 18.

Reactions of Cp*2Ti(η2‐Me3SiC2SiMe3) with adiponitrile.
Tilley and co‐workers used73 the concept of C−C‐coupling reactions of nitriles for a new general synthetic strategy to obtain large PAHs (N‐containing polycyclic aromatic hydrocarbons) by using Cp2Ti(η2‐Me3SiC2SiMe3) as a cheaper and more readily accessible reagent compared to the permethyltitanocene Cp*2Ti(η2‐Me3SiC2SiMe3). Phenanthrene substituted dinitriles gave di(aza)titanacyclopentadienes by titanocene‐mediated reductive cyclization. Subsequent reactions of these products gave compounds with one or more o‐quinone, diazole, or pyrazine units (Scheme 19).
Scheme 19.
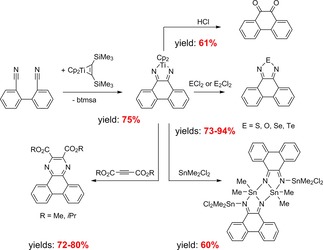
Intramolecular reductive cyclization of phenanthrene substituted dinitriles using Cp2Ti(η2‐Me3SiC2SiMe3) and demetalation reactions of the obtained product (yields as isolated yields).
Such investigations on the reductive coupling of two nitrile units to 2,5‐di(aza)metallacyclopentadienes were not reported for the synthesis of N‐containing polycyclic aromatic hydrocarbons before. The above mentioned basic reaction to several 2,5‐di(aza)metallacyclopentadienes by coupling using Cp*2M(Me3SiC2SiMe3) (M=Ti or Zr, Scheme 16) was modified here by using the complex Cp2Ti(Me3SiC2SiMe3) in combination with phenanthrene substituted dinitriles. Subsequent reactions of the obtained compounds with aqueous acid, main‐group dihalides and acetylene dicarboxylates gave π‐extended o‐quinones, diazoles or pyrazines by very effective metallacycle transfer (Scheme 20).19
Scheme 20.
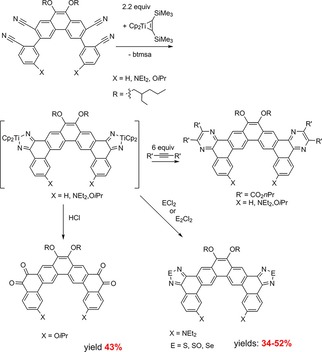
Twofold coupling reactions for synthesis of large N‐containing polycyclic aromatic hydrocarbons (PAHs).
Also by using phenanthrene substituted dinitriles with functional groups 7,10‐substituted dibenzo[f,h]quinoxalines were synthesized as well as examples for larger PAHs with two or more quinone, pyrazine, or diazole units (Scheme 20).
All these reactions were possible only by using the complex Cp2Ti(η2‐btmsa) as an excellent starting material giving reactions with high selectivity and surprisingly high tolerance of several functional groups in multistep reactions.
Staubitz and coworkers presented for the zirconocene complex Cp2Zr(py)(η2‐btmsa) another example to synthesize tin containing conjugated heterocycles that can be used for the introduction of stannole units in the main chain of main group polymers. In an intermolecular coupling reaction the bis(thiophenyl)substituted octadiyne was converted into the zirconacyclopentadiene. In this reaction the iodide substituents remain intact and the obtained products reacted to the stannole compounds. The thiophene substituted stannole monomer gave with the diiodide the desired polymer by Stille‐cross‐coupling in very high selectivity (Scheme 21).74, 75
Scheme 21.
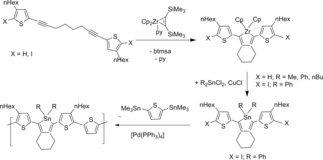
Reaction of the bis(thiophenyl)substituted octadiyne to the zirconacyclopentadiene and conversion to the stannol compounds as well as the Stille‐cross‐coupling of the diiodide to the the polymer.
Very recently Staubitz an coworkers75c compared reactions of Negishi's reagent and Cp2Zr(py)(η2‐btmsa) with several disubstituted alkynes and octadiynes R−C≡C‐(CH2)−C≡C−R (R=SnMe3, Bpin, 4‐thiophenyl, 2‐metoxy‐, 2‐bromo‐ and 2‐iodo‐4‐thiophenyl etc.) to zirconacyclopentadienes. Most of the published reports before described the synthesis of such zirconacyclopentadienes by using Negishi's reagent. The efficiency of both reagents toward substituted diynes was evaluated and compared by kinetic studies on the basis of by 1H NMR measurements. As a result, Cp2Zr(py)(Me3SiC≡CSiMe3) was faster, more reliable and led to higher yields. This complex was described to be more efficient for the synthesis of zirconacyclopentadienes with respect to yield and reaction time when compared to Negishi's reagent (Scheme 21 and 22). Additionally, it is a very functional group tolerant reagent as even aryl‐iodides are not attacked in the reaction. Worth to mention that these investigations are the first systematically conducted experiments to compare the efficiency of both systems.
Scheme 22.
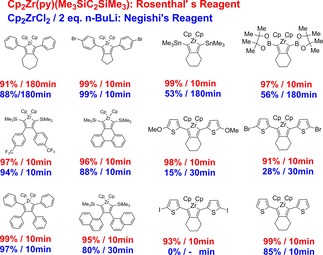
Compared efficiency of Negishi's and Rosenthal's reagent Cp2Zr(py)(Me3SiC≡CSiMe3) in the formation of zirconacyclopentadienes from substituted alkynes and diynes.
Very recently Sindlinger and Heitkemper obtained similar results by comparison of the in situ generated Negishi reagent Cp2Zr(η2‐butene) (51 % yield) with Rosenthal's complex Cp2Zr(py)(η2‐btmsa) (97 % yield) in the formation of a zirconacyclopentadiene by coupling of Ph*C≡CPh* (Ph*=3,5‐(t‐Bu)2C6H3)).75
Self‐assembly reactions to multinuclear complexes using group 4 bis(trimethylsilyl)acetylene complexes are possible and were described in detail.76, 77, 78, 79, 80, 81, 82, 83, 84 Some older examples of C−C coupling reactions with or without C−H bond activation to give tri‐ or tetranuclear complexes were published.76, 77, 78, 79 Beckhaus and co‐workers reported coupling reactions of various N‐heterocycles by Cp2Ti(η2‐btmsa) and Cp*2Ti(η2‐btmsa) (Scheme 23). Pyrazine and Cp*2Ti(η2‐btmsa) gave by threefold C−C coupling a trinuclear and Cp2Ti(η2‐btmsa) with pyrimidine an tetranuclear complex.76 Other aromatic N‐heterocycles like pyrazine,80 4,4’‐bipyridine and tetrazine were investigated, too. The interaction of Cp2Ti(η2‐btmsa) with quinoxalines resulted in a C−H bond activation/dehydroaromatisation reaction along with C−C‐coupling.78 The obtained hexaazatrinaphthylene (HATN) titanium complexes and similar products were investigated in detail.
Scheme 23.
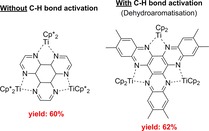
Typical products of C−C‐coupling reaction without and with C−H activation (dehydroaromatisation).
The hexaphenyl substituted derivative (Cp2Ti)3(μ 3‐HATNPh6) was formed by dehydrogenative coupling of 6,7‐diphenylquinoxaline in the presence of Cp2Ti(η2‐btmsa).83 By dissociation of the alkyne the highly reactive and reductive Cp2Ti(II) fragment is formed, giving the dehydrogenative C−C coupling reaction to the same product (Cp2Ti)3(μ 3‐HATNPh6) which was also obtained by direct coordination of the Cp2Ti(II) fragment to the HATNPh6 ligand (Scheme 24).
Scheme 24.
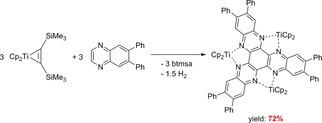
Reactions of Cp2Ti(η2‐btmsa) with 6,7‐diphenylquinoxaline by dehydrogenative coupling to (Cp2Ti)3(μ 3‐HATNPh6).
Reactions of Cp2M(L)(η2‐btmsa) (M=Ti, no py and M=Zr, L=py) and the corresponding permethylmetallocene complexes Cp*2M(η2‐btmsa) (M=Ti, Zr) with imidazole and 4,5‐diphenylimidazole gave trinuclear complexes. This is a new type of self‐assembly reactions to multinuclear titanium(III)‐ and zirconium(III)‐complexes in which the metals are bridged by imidazolate ligands. Tetranuclear complexes were only obtained with the Cp complexes Cp2M(L)(η2‐Me3SiC2SiMe3) (M=Ti, no py and M=Zr, L=py) and imidazole and 4,5‐dimethylbenzimidazole. Starting from Cp2Ti(η2‐Me3SiC2SiMe3) and benzimidazole or 5,6‐dimethylbenzimidazole tetranuclear complexes were formed. The reason could be that with an increased sterical demand of the ligands Cp* and the substituents R=Ph only tri‐ and with the smaller Cp‐ligands and the substituents H or Me the tetranuclear complexes were obtained (Scheme 25).84
Scheme 25.
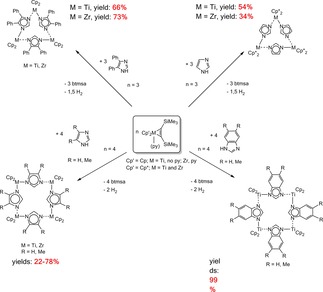
Formation of tri‐ and tetranuclear complexes by reactions of Group 4 bis(trimethylsilyl)acetylene complexes with substituted imidazoles and benzimidazoles.
Advantages of group 4 bis(trimethylsilyl)acetylene complexes are, that these can be readily prepared in large quantities directly from commercially available compounds. They are stable compounds at room temperature, allow a control of the stoichiometry, make the reactions possible in nonpolar solvents at room temperature and allow to remove the formed side products Me3SiC≡CSiMe3 (and in some cases pyridine) easily from the reaction products. Additionally, they tolerate several functional groups giving a higher selectivity for the obtained products and it is possible to influence the reactions of these complexes Cp′2M(L)(η2‐btmsa) by using different ligands Cp′ (Cp, Cp* etc.), metals M (Ti, Zr etc.), the used L and S as the substituents of the substrates. Changing of these different influences makes a fine‐tuning of the reactivity and products of reactions possible.
Disadvantages are the sometimes complicated special synthesis of these complexes under strictly anaerobic conditions. These problems were reduced for the case of Cp2Zr(py)(η2‐btmsa) by the alternative synthesis via Negishi's reagent. Nevertheless, there is the need to use anaerobic conditions, but in principle this is the case for the other presented examples, too.
5. Conclusions
All the presented examples make group 4 bis(trimethylsilyl)acetylene complexes to the most favoured sytems compared to the other here mentioned systems. The disadvantage of the sometimes complicated synthesis of these complexes was compensated for the case of Cp2Zr(py)(η2‐btmsa) by the alternative synthesis via the Negishi‐system as described by Tilley and coworkers. The main advantages of group 4 metallocene bis(trimethylsilyl)acetylene complexes were summarized before for Cp2Zr(py)(η2‐btmsa) as one example:35f
This complex can be readily prepared in large quantities directly from commercially available Cp2ZrCl2 by different methods.
It is stable at room temperature, can be stored under an inert atmosphere, and allows a precise control of the stoichiometry.
In contrast to the reagent Cp2ZrCl2/n‐BuLi for which the formed LiCl is not easily removed after reactions and its limited to tetrahydrofuran as solvent only at low temperatures, this complex allows to remove the formed side products pyridine and Me3SiC≡CSiMe3, which are soluble and volatile. The solubility of this complex allows reactions in nonpolar solvents like pentane.
The complex Cp2Zr(pyr)(η2‐btmsa) tolerates several functional groups giving a higher selectivity for the obtained products.
This picture is more or less empirically supported by many examples, but was promoted by recent kinetic studies from Staubitz et al. In all cases, the route using Rosenthal's reagent Cp2Zr(py)(η2‐btmsa) was faster, more reliable and led to higher yields. It is more efficient for the synthesis of zirconacyclopentadienes with respect to yield and reaction time when compared to Negishi's reagent. Additionally, it tolerates functional groups like aryl‐iodides which were not attacked during the reaction.
All these advantages mentioned here are not only restricted to the zirconium complex Cp2Zr(pyr)(η2‐btmsa) but were described in a similar manner for other group 4 metallocene complexes with bis(trimethylsilyl)acetylene in several synthetic and catalytic reactions as well as the examples presented herein. In addition to these advantages one can directly influence the reactions of these complexes Cp′2M(L)(η2‐btmsa) by variation of the ligands Cp′ (Cp, Cp* etc.), the metals M (Ti, Zr etc.), the used L (with and without, type: THF, py etc.) as well as of S as the substrate substituents. By these changes a fine‐tuning of the reactivity and products becomes possible. One can use mutual effects of these factors to influence the formation of the desired products.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Uwe Rosenthal studied chemistry (1968–72), received his Ph.D. under supervision of E. Kurras (1976), and completed his habilitation (1991) at the University of Rostock. After postdoctoral work at the A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences in Moscow with M. E. Vol′pin and V. B. Shur (1988) he was a visiting research scientist at the Max Planck Institute of Kohlenforschung in Mülheim/Ruhr with G. Wilke and K. Pörschke (1990–91), headed the Max Planck Research group “Complex Catalysis” (1992–96) and became Professor of Inorganic Chemistry at the University of Rostock (1993). From 2003 until 2016 he was the Deputy Director of the Leibniz Institute of Catalysis at the University of Rostock (LIKAT). His scientific interests are the basics of Organometallic Chemistry (unusual metallacycles) for applications in homogeneous catalysis (e. g. selective oligomerization of ethene).

Acknowledgements
I would like to particularly thank all my former PhD students, postdocs, assistants, cooperation partners and other people whose names are mentioned in the list of references, for their excellent scientific results, giving the basis of this review. Scientific discussions with PD Dr. Torsten Beweries are gratefully acknowledged.
U. Rosenthal, ChemistryOpen 2019, 8, 1036.
Dedicated to Prof. Dr. Dirk Walther on the occasion of his 80th birthday
References
- 1.
- 1a. Chirik P. J., Organometallics 2010, 29, 1500–1517; [Google Scholar]
- 1b. Xi Z., Li Z., Top. Organomet. Chem. 2004, 8, 27–56; [Google Scholar]
- 1c. Sato F., Urabe H., Okamoto S., Chem. Rev. 2000, 100, 2835–2886; [DOI] [PubMed] [Google Scholar]
- 1d. Beaumier E. P., Pearce A. J., See X. Y., Tonks I. A., Nat. Chem. Rev. 2019, 3, 15–34; [DOI] [PMC free article] [PubMed] [Google Scholar]; and refs. cited therein. [Google Scholar]
- 2. Ohff A., Pulst S., Lefeber C., Peulecke N., Arndt P., Burlakov V. V., Rosenthal U., Synlett 1996, 111–118. [Google Scholar]
- 3. Rosenthal U., Pellny P.-M., Kirchbauer F. G., Burlakov V. V., Acc. Chem. Res. 2000, 33, 119–129. [DOI] [PubMed] [Google Scholar]
- 4. Rosenthal U., Burlakov V. V. in Titanium and Zirconium in Organic Synthesis, Ed. I. Marek, Wiley-VCH, Weinheim, 2002, 355–389. [Google Scholar]
- 5. Rosenthal U., Angew. Chem. 2003, 115, 1838–1842; [Google Scholar]; Angew. Chem. Int. Ed. 2003, 42, 1794–1798. [DOI] [PubMed] [Google Scholar]
- 6. Rosenthal U., Burlakov V. V., Arndt P., Baumann W., Spannenberg A., Organometallics 2003, 22, 884–900. [Google Scholar]
- 7. Rosenthal U., Burlakov V. V., Arndt P., Baumann W., Spannenberg A., Eur. J. Inorg. Chem. 2004, 22, 4739–4932. [Google Scholar]
- 8. Rosenthal U., Angew. Chem. 2004, 116, 3972–3977; [Google Scholar]; Angew. Chem. Int. Ed. 2004, 43, 3882–3887. [DOI] [PubMed] [Google Scholar]
- 9. Rosenthal U. in Acetylene Chemistry – Chemistry, Biology, and Material Science, Eds. F. Diederich, P. Stang, R. R. Tykwinski, Wiley-VCH, Weinheim, 2004, 139–171. [Google Scholar]
- 10. Rosenthal U., Burlakov V. V., Arndt P., Baumann W., Spannenberg A., Organometallics 2005, 24, 456–471. [Google Scholar]
- 11. Rosenthal U., Burlakov V. V., Bach M. A., Beweries T., Chem. Soc. Rev. 2007, 36, 719–728. [DOI] [PubMed] [Google Scholar]
- 12. Rosenthal U., Angew. Chem. 2008, 120, 5196–5199; [Google Scholar]; Angew. Chem. Int. Ed. 2008, 47, 5118–5121. [DOI] [PubMed] [Google Scholar]
- 13. Rosenthal U., Burlakov V. V., Arndt P., Spannenberg A., Jäger-Fiedler U., Klahn M., Hapke M., Activating Unreactive Bonds, Eds. C. Bolm, F. E. Hahn, Wiley-VCH, Weinheim, 2009, 165 pp. [Google Scholar]
- 14. Beweries T., Rosenthal U., Sci. Synth. 2011, 4, Recent updates in the organometallic chemistry of group 4 metallocene complexes with bis(trimethylsilyl)acetylene. [Google Scholar]
- 15. Beweries T., Rosenthal U., Nat. Chem. 2013, 5, 649; [DOI] [PubMed] [Google Scholar]; and refs. cited therein. [Google Scholar]
- 16. Roy S., Rosenthal U., Jemmis E. D., Acc. Chem. Res. 2014, 47, 2917–2930. [DOI] [PubMed] [Google Scholar]
- 17. Beweries T., Hähnel M., Rosenthal U., Catal. Sci. Technol. 2013, 3, 18–28. [Google Scholar]
- 18. Linshoeft J., Synlett 2014, 25, 2671–2672. [Google Scholar]
- 19. Rosenthal U., Izv. Akad. Nauk Ser. Khim. 2014, 2577–2582, [Google Scholar]; Engl. Transl.: Russ. Chem. Bull., 2014, 2577–2582. [Google Scholar]
- 20. Becker L., Rosenthal U., Coord. Chem. Rev. 2017, 345, 137–149. [Google Scholar]
- 21. Rosenthal U., Angew. Chem. 2018, 130, 14932–14950; [Google Scholar]; Angew. Chem. Int. Ed., 2018, 57, 14718–14735. [DOI] [PubMed] [Google Scholar]
- 22. Rosenthal U., Eur. J. Inorg. Chem. 2019, 895–919. [Google Scholar]
- 23. Isomerization of olefines: Ohff A., Burlakov V. V., Rosenthal U. , J. Mol. Catal. A 1996, 105, 103–110. [Google Scholar]
- 24. Polymerisation of acetylene: Ohff A., Burlakov V. V., Rosenthal U., J. Mol. Catal. A 1996, 108, 119–123. [Google Scholar]
- 25. Ring opening of lactames: Arndt P., Lefeber C., Kempe R., Tillack A., Rosenthal U., Chem. Ber. 1996, 129, 1281–1285. [Google Scholar]
- 26. Ring opening of lactones:
- 26a. Arndt P., Thomas D., Rosenthal U., Tetrahedron Lett. 1997, 38, 5467–5468; [Google Scholar]
- 26b. Thomas D., Arndt P., Peulecke N., Spannenberg A., Kempe R., Rosenthal U., Eur. J. Inorg. Chem. 1998, 1351–1357; [Google Scholar]
- 26c. Arndt P., Spannenberg A., Baumann W., Becke S., Rosenthal U., Eur. J. Inorg. Chem. 2001, 2885–2890. [Google Scholar]
- 27. Hydrosilylation of ald- and ketimines: Tillack A., Lefeber C., Peulecke N., Thomas D., Rosenthal U., Tetrahedron Lett. 1997, 38, 1533–1534. [Google Scholar]
- 28. Polymerisation of ethene: Mansel S., Thomas D., Lefeber C., Heller D., Kempe R., Baumann W., Rosenthal U., Organometallics, 1997, 16, 2886–2890. [Google Scholar]
- 29. Dehydrocoupling of silanes: Peulecke N., Thomas D., Baumann W., Fischer C., Rosenthal U., Tetrahedron Lett. 1997, 38, 6655–6656. [Google Scholar]
- 30. Dehydrocoupling of hydrosilanes: Lunzer F., Marschner C., Winkler B., Peulecke N., Baumann W., Rosenthal U., Monatsh. Chem., 1999, 130, 215–219. [Google Scholar]
- 31. Hydrodefluorination of pentafluoropyridines: Jäger-Fiedler U., Klahn M., Arndt P., Baumann W., Spannenberg A., Burlakov V. V., Rosenthal U., J. Mol. Catal. A, 2007, 261, 184–189. [Google Scholar]
- 32. Hydrodefluorination: Rosenthal U., Klahn M., Organometallics 2012, 31, 1235–1244. [Google Scholar]
- 33. Hydroamination: Tillack A., Garcia Castro I., Hartung C. G., Beller M., Angew. Chem. 2002, 114, 2646; [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002, 41, 2541. [Google Scholar]
- 34. Dehydrogenation of dimethylaminoborane and hydrazineborane:
- 34a. Klahn M., Hollmann D., Spannenberg A., Brückner A., Beweries T., Dalton Trans. 2015, 44, 12103–12111; [DOI] [PubMed] [Google Scholar]
- 34b. Beweries T., Hansen S., Keßler M., Klahn M., Dalton Trans. 2011, 40, 7689–7692; [DOI] [PubMed] [Google Scholar]
- 34c. Beweries T., Thomas J., Klahn M., Heller D., Schulz A., Rosenthal U., ChemCatChem 2011, 3, 1865; [Google Scholar]
- 34d. Thomas J., Klahn M., Spannenberg A., Beweries T., Dalton Trans. 2013, 42, 14668–14672. [DOI] [PubMed] [Google Scholar]
- 35.
- 35a. Burlakov V. V., Rosenthal U., Petrowskij P. V., Shur V. B., Vol'pin M. E., Organomet. Chem. 1988, 1, 526–527; [Google Scholar]
- 35b. Binger P., Müller P., Langhauser F., Sandmeyer F., Philipps P., Gabor B., Mynott R., Chem. Ber. 1993, 126, 1541–1550; [Google Scholar]
- 35c. Lamac M., Spannenberg A., Baumann W., Jiao H., Fischer Ch., Hansen S., Arndt P., Rosenthal U., J. Am. Chem. Soc. 2010, 132, 4369–4380. [DOI] [PubMed] [Google Scholar]
- 35d. Rosenthal U., Ohff A., Michalik M., Görls H., Burlakov V. V., Shur V. B., Angew. Chem. Int. Ed. Engl. 1993, 32, 1193; [Google Scholar]
- 35e. Rosenthal U., Ohff A., Baumann W., Tillack A., Görls H., Burlakov V. V., Shur V. B., Z. Anorg. Allg. Chem. 1995, 621, 77–83; [Google Scholar]
- 35f. Nitschke J. R., Zuercher S., Don Tilley T., J. Am. Chem. Soc. 2000, 122, 10345–10352; [Google Scholar]
- 35g. Hiller J., Thewalt U., Polasek M., Petrusova L., Varga V., Sedmera P., Mach K., Organometallics 1996, 15, 3752–3759. [Google Scholar]
- 36.
- 36a. Huang Y., Liao P., Zhang Y., Wang Y., Synth. Commun. 1997, 27, 1059–1063; [Google Scholar]
- 36b. Bousrez G., Dechamps I., Vasse J.-L., Jaroschik F., Dalton Trans. 2015, 44, 9359–9362; [DOI] [PubMed] [Google Scholar]
- 36c. Yan X., Xi C., Acc. Chem. Res. 2015, 48, 925–946; [DOI] [PubMed] [Google Scholar]; and refs. cited therein. [Google Scholar]
- 37.
- 37a. Xi C., Yan X., Lai C., Kanno K., Takahashi T., Organometallics 2008, 27, 3834–3839; [Google Scholar]
- 37b. Takahashi T., Kageyama M., Denisov V., Hara R., Negishi E., Tetrahedron Lett. 1993, 34, 686–690; [Google Scholar]
- 37c. Takahashi T., Li Y., in Titanium and Zirconium in Organic Synthesis, Ed. I. Marek, Wiley-VCH, Weinheim, 2002, 50–85; and refs. cited therein. [Google Scholar]
- 38.
- 38a. Negishi E., Cederbaum F. E., Takahashi T., Tetrahedron Lett., 1986, 27, 2829; [Google Scholar]
- 38b. Negishi E., Takahashi T., Rev. Heteroat. Chem. 1992, 6, 177–201; [Google Scholar]
- 38c. Negishi E., Holmes S. J., Tour J. M., Miller J. A., Cederbaum F. E., Swanson D. R., Takahashi T., J. Am. Chem. Soc. 1989, 111, 3336–3346; [Google Scholar]
- 38d. Negishi E., Huo S., in Titanium and Zirconium in Organic Synthesis, Ed. I. Marek, Wiley-VCH, Weinheim, 2002, 1–49; and refs. cited therein. [Google Scholar]
- 39. Beckhaus R., Coord. Chem. Rev. 2018, 376, 467–477; [Google Scholar]; and refs. cited therein. [Google Scholar]
- 40. Alt H. G., Engelhardt H. E., Rausch M. D., Kool L. B., J. Am. Chem. Soc. 1985, 107, 3717. [Google Scholar]
- 41. Takeda T., Chem. Rev. 2007, 7, 24; [Google Scholar]; and refs. cited therein. [Google Scholar]
- 42. Manriquez J. M., McAlister D. R., Rosenberg E., Shiller A. M., Williamson K. L., Chan S. I., Bercaw J. E., J. Am. Chem. Soc. 1978, 100, 3078–3083. [Google Scholar]
- 43. Beckhaus R. in Metallocenes, Synthesis, Reactivity, Applications, Vol. 1, Wiley- VCh, 1998, 153–230, Eds. A. Togni, R. L. Halterman. [Google Scholar]
- 44. Dioumaev V. K., Harrod J. F., Organometallics 1997, 16, 1452–1464. [Google Scholar]
- 45. Diekmann M., Bockstiegel G., Lützen A., Friedemann M., Haase D., Saak W., Beckhaus R., Organometallics 2006, 25, 339–348. [Google Scholar]
- 46. Oswald T., Oslage A., Schmidtmann M., Beckhaus R., Acta Crystallogr. Sect. E 2017, E73, 691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oswald T., Lauterbach N., Schmidtmann M., Beckhaus R., Acta Crystallogr. Sect. C, 2018, C74, 442–451. [DOI] [PubMed] [Google Scholar]
- 48. Ebert H., Timmermann V., Oswald T., Saak W., Schmidtmann M., Friedemann M., Haase D., Beckhaus R., Organometallics, 2014, 33, 1440–1452. [Google Scholar]
- 49. Manßen M., de Graff S., Meyer M.-F., Schmidtmann M., Beckhaus R., Organometallics 2018, 37, 4506–4514. [Google Scholar]
- 50. Manßen M., Meyer M.-F., Schmidtmann M., Beckhaus R., Organometallics 2018, 37, 4515–4520. [Google Scholar]
- 51.
- 51a. Manßen M., Lauterbach N., Dörfler J., Schmidtmann M., Saak W., Doye S., Beckhaus R., Angew. Chem. Int. Ed. 2015, 54, 4383–4287; [DOI] [PubMed] [Google Scholar]
- 51b. Manßen M., Adler C., Beckhaus R., Chem. Eur. J. 2016, 22, 4405–4407. [DOI] [PubMed] [Google Scholar]
- 52. Manßen M., Töben I., Kahrs C., Bölte J.-H., Schmidtmann M., Beckhaus R., Organometallics 2017, 36, 2973–2981. [Google Scholar]
- 53. Oswald T., Gelert T., Lasar C., Schmidtmann M., Beckhaus R., Angew. Chem. 2017, 129, 12465–12469; [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2017, 56, 12297–12301. [DOI] [PubMed] [Google Scholar]
- 54. Janssen T., Severin R., Diekmann M., Friedemann M., Haase D., Saak W., Doye S., Beckhaus R., Organometallics 2010, 29, 1806–1817. [Google Scholar]
- 55. Manßen M., Adler C., Beckhaus R., Chem. Eur. J. 2016, 22, 4405–4407. [DOI] [PubMed] [Google Scholar]
- 56. Manßen M., Weimer I., Adler C., Fischer M., Schmidtmann M., Beckhaus R., Eur. J. Inorg. Chem. 2018, 131–136. [Google Scholar]
- 57. Beckhaus R., Lützen A., Haase D., Saak W., Stroot J., Becke S., Heinrichs J., Angew. Chem. 2001. , 113, 2112–2115. [DOI] [PubMed] [Google Scholar]
- 58. Stroot J., Beckhaus R., Saak W., Haase D., Lützen A., Eur. J. Inorg. Chem. 2002, 1729–1737. [Google Scholar]
- 59. Stroot J., Saak W., Haase D., Beckhaus R., Z. Anorg. Allg. Chem. 2002, 628, 755–761. [Google Scholar]
- 60. Manßen M., Lauterbach N., Woriescheck T., Schmidtmann M., Beckhaus R., Organometallics 2017, 36, 867–876. [Google Scholar]
- 61. Oswald T., Gelert T., Lasar C., Schmidtmann M., Klüner T., Beckhaus R., Angew. Chem. Int. Ed. 2017, 129, 12297–12301. [DOI] [PubMed] [Google Scholar]
- 62. Fischer M., Jaugstetter M., Schaper R., Schmidtmann M., Beckhaus R., Eur. J. Inorg. Chem. 2018, 5146–5159. [Google Scholar]
- 63. Gessner V. H., Tannaci J. F., Miller A. D., Tilley T. D., Acc. Chem. Res. 2011, 44, 435–446. [DOI] [PubMed] [Google Scholar]
- 64. Schafer L. L., Nitschke J. R., Mao S. S. H., Liu F.-Q., Harder G., Haufe M., Don Tilley T., Chem. Eur. J. 2002, 8, 74–83. [DOI] [PubMed] [Google Scholar]
- 65. Nitschke J. R., Don Tilley T., J. Am. Chem. Soc. 2001, 123, 10183–10190. [DOI] [PubMed] [Google Scholar]
- 66. Nitschke J. R., Don Tilley T., Angew. Chem. 2001, 113, 2200; [Google Scholar]; Angew. Chem. Int. Ed. 2001, 40, 2142–2145. [Google Scholar]
- 67.
- 67a. Kiel G. R., Ziegler M. S., Tilley T. D., Angew. Chem. 2017, 129, 4917–4922; [Google Scholar]; Angew. Chem. Int. Ed. 2017, 56, 4839–4922; [Google Scholar]
- 67b. Kiel G. R., Patel S. C., Smith P. W., Levine D. S., Don Tilley T., J. Am. Chem. Soc. 2017, 139, 18456–18459. [DOI] [PubMed] [Google Scholar]
- 68. Becker L., Arndt P., Jiao H., Spannenberg A., Rosenthal U., Angew. Chem. 2013, 125, 11607–11611; [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2013, 52, 11396–11400. [DOI] [PubMed] [Google Scholar]
- 69. Becker L., Strehler F., Korb M., Arndt P., Spannenberg A., Baumann W., Lang H., Rosenthal U., Chem. Eur. J. 2014, 20, 3061–3068. [DOI] [PubMed] [Google Scholar]
- 70.
- 70a. Becker L., Arndt P., Spannenberg A., Jiao H., Rosenthal U., Angew. Chem. 2015, 127, 5614–5617; [Google Scholar]; Angew. Chem. Int. Ed. 2015, 54, 5523–5526; [DOI] [PubMed] [Google Scholar]
- 70b. Becker L., Spannenberg A., Arndt P., Rosenthal U., Acta Crystallogr. 2015, E71, m219–m220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tomaschun G., Altenburger K., Reiß F., Becker L., Spannenberg A., Arndt P., Jiao H., Eur. J. Inorg. Chem. 2016, 272–280. [Google Scholar]
- 72.
- 72a. Becker L., Reiß F., Altenburger K., Spannenberg A., Arndt P., Jiao H., Rosenthal U., Chem. Eur. J. 2016, 22, 10826–10838; [DOI] [PubMed] [Google Scholar]
- 72b. Reiß M., Reiß F., Spannenberg A., Arndt P., Beweries T., Organometallics 2018, 37, 4415–4423. [Google Scholar]
- 73. Kiel G., Samkian A. E., Nicolay A., Witzke R. J., Don Tilley T., J. Am. Chem. Soc. 2018, 140, 2450–2454. [DOI] [PubMed] [Google Scholar]
- 74. Linshoeft J., Baum E. J., Hussain A., Gates P. J., Näther Ch., Staubitz A., Angew. Chem. Int. Ed. 2014, 53, 12916–12920. [DOI] [PubMed] [Google Scholar]
- 75.
- 75a. Urrego-Riveros S., Ramirez y Medina I.-M., Hoffmann J., Hoffmann A., Staubitz A., Chem. Eur. J. 2018, 24, 5680–5696; [DOI] [PubMed] [Google Scholar]
- 75b. Ramirez y Medina I.-M., Rohdenburg M., Mostaghimi F., Grabowsky S., Swiderek P., Beckmann J., Dorcet V., Hissler M., Staubitz A., Inorg. Chem. 2018, 57, 12562–12575; [DOI] [PubMed] [Google Scholar]
- 75c. Urrego-Riveros S., Ramirez y Medina I.-M., Sönnichsen F. D., Staubitz A., Chem. Eur. J. 2019, 25, DOI 10.1002/chem.201902255; [Google Scholar]; Heitkemper D., Sindlinger C. P., Chem. Eur. J. 2019, 25, 6628–6637. [DOI] [PubMed] [Google Scholar]
- 76. Kraft S., Beckhaus R., Haase D., Saak W., Angew. Chem. Int. Ed. 2004, 43, 1583–1587. [DOI] [PubMed] [Google Scholar]
- 77. Kraft S., Hannuschek E., Beckhaus R., Haase D., Saak W., Chem. Eur. J. 2005, 11, 969–978. [DOI] [PubMed] [Google Scholar]
- 78. Piglosiewicz I. M., Beckhaus R., Haase W. Saak. D., J. Am. Chem. Soc. 2015, 127, 14190–14191. [DOI] [PubMed] [Google Scholar]
- 79. Piglosiewicz I. M., Beckhaus R., Wittstock G., Haase W. Saak. D., Inorg. Chem. 2007, 46, 7610–7620. [DOI] [PubMed] [Google Scholar]
- 80. Jung T., Beckhaus R., Klüner T., Höfener S., Klopper W., J. Chem. Theory Comput. 2009, 5, 2044–2949. [DOI] [PubMed] [Google Scholar]
- 81. Theilmann O., Saak W., Haase D., Beckhaus R., Organometallics 2009, 28, 2799–2807. [Google Scholar]
- 82. Wolff C., Gottschlich A., England J., Wieghardt K., Haase W. Saak. D., Beckhaus R., Inorg. Chem. 2015, 54, 4811–4820. [DOI] [PubMed] [Google Scholar]
- 83. Sander P., Markovic A., Schmidtmann M., Janka O., Wittstock G., Beckhaus R., Inorg. Chem. 2018, 57, 1165–1174. [DOI] [PubMed] [Google Scholar]
- 84. Sander P., Geibel I., Witte C., Hüller S., Schmidtmann M., Beckhaus R., Eur. J. Inorg. Chem. 2018, 3717–3724. [Google Scholar]


