Abstract
Obesity is a multifactorial disease resulting in excessive accumulation of adipose tissue. Over the last decade, growing evidence has identified the gut microbiota as a potential factor in the pathophysiology of both obesity and the related metabolic disorders. The gut microbiota is known to protect gastrointestinal mucosa permeability and to regulate the fermentation and absorption of dietary polysaccharides, perhaps explaining its importance in the regulation of fat accumulation and the resultant obesity. The proposed mechanisms by which the gut microbiota could contribute to the pathogenesis of obesity and the related metabolic diseases include: (a) a high abundance of bacteria that ferment carbohydrates, leading to increased rates of short-chain fatty acid (SCFA) biosynthesis, providing an extra source of energy for the host, that is eventually stored as lipids or glucose; (b) increased intestinal permeability to bacterial lipopolysaccharides (LPS), resulting in elevated systemic LPS levels that aggravate low-grade inflammation and insulin resistance; (c) increased activity of the gut endocannabinoid system. Fecal transplantation studies in germ-free mice have provided crucial insights into the potential causative role of the gut microbiota in the development of obesity and obesity-related disorders. Diet +/− bariatric surgery have been reported to modulate the gut microbiota, leading to lean host phenotype body composition. This review aims to report clinical evidence for a link of the gut microbiota with human obesity and obesity-related diseases, to provide molecular insights into these associations, and to address the effect of diet and bariatric surgery on the gut microbiota, including colonic microbiota, as a potential mechanism for promoting weight loss.
Introduction
The gut microbiota has recently been reported to have a role in the pathogenesis of cardiometabolic diseases. The component organisms contain more than 100 genes compared with the human genome [1]. One role of the gut bacteria is to protect and support the intestinal mucosa in close symbiotic relationships with the host’s body. This close relationship allows the gut microbiota bacteria to contribute to physiological homeostasis, and abnormalities of the gut microbiota are reported to contribute to the development of several diseases [2].
In metabolic disease, including obesity, the gut microbiota is altered so as to increase energy harvesting from the diet [2]. Indeed, the gut microbiota has an important role in the regulation of fat storage [3], increasing energy harvesting, and in modulating the formation of substrates for storable fat synthesis [4]; in addition, the gut microbiota produces compounds absorbed into the systemic circulation that are likely to contribute to the development of obesity-related complications, since they increase both tissue inflammatory damage and insulin resistance [5]. Changes of the gut microbiota in early life have also been reported to encourage the onset of obesity due to adverse effects on mechanisms regulating satiety/appetite [6, 7]. Overall, obesity is associated with the gut microbiota that have reduced the diversity of their composition, which, in turn, reduces metabolic energy consumption in comparison with that of the microbiota of lean people [8]; since microbiota facilitating efficient fermentation [digestion] of indigestible carbohydrates into short-chain fatty acids provide excess energy substrate to the host, that then contributes to the development of obesity, while low-efficiency gut bacterial breakdown of complex carbohydrates could contribute to avoidance of the development of obesity.
Based on this information, it has been hypothesized that modulation of the gut microbiota might provide a novel treatment target in obesity. Several environmental factors, including diet, as well as gut microbiota composition, influence host metabolism [9]; for example, chronic intake of the Mediterranean diet (MD) over 2 years can normalize gut microbiota actions, paralleling reductions in insulin resistance, in obese patients with coronary heart disease [10, 11]. Adherence to the MD has been associated with the healthy gut microbiota, as assessed by quantitative PCR and plate-count techniques in adults [12]. Bariatric surgery is increasingly used to treat obesity, and in morbid obesity, it may be the most effective treatment option for achieving weight loss [13] through various surgical techniques, sometimes reducing food entry into the stomach using a gastric band, sometimes removing a portion of the stomach, and sometimes by removing sections of the stomach and re-routing the remaining stomach “pouch” into the small intestine. It is reported that such surgery can affect the composition of the gut microbiota; subjects who have had gastric bypass have a different microbiota composition from that of lean or obese subjects, with increased contents of both Gammaproteobacteria (including Enterobacteriaceae), and Fusobacteriaceae, and with proportional decreases in Clostridiathus content [14]. These authors also suggested that changes in the duration of gut wall exposure to food, and the different pH distribution along the gut after bypass surgery, might contribute to changes in the gut microbiota [14].
Overall, the aims of this review are, first, to review current evidence on the association of obesity [and obesity-related complications] with the composition of the gut microbiota, and second, to review how various treatments of obesity (e.g., diet and bariatric surgery) might modulate the composition of the gut microbiota, so that it may contribute to the reduction of the risk of obesity and of its complications.
Obesity and the gut microbiota
The microbiota of people with obesity contains lower proportions of Bacteroidetes and higher proportions of Firmicutes than those from people without obesity [15], as is also seen in pregnancy. Bacteroidetes and S. aureus concentrations were also significantly higher in overweight than in normal weight women and higher proportions of Bacteroidetes were associated with excessive weight gain during pregnancy [16]. Obesity-related differences have also been identified in the contents of microbes, such as Clostridium innocuum, Eubacterium dolichum, Catenibacterium mitsuokai, Lactobacillus reuteri, Lactobacillus sakei, and Actinobacteria, and in numbers of rarer Archaea organisms such as Methanobrevibacter smithii [15, 17, 18]. This clinical evidence suggests that there is a tight association between the gut microbiota and body weight regulation, as is already reported in animal studies. In the 1980s, Wostman’s group observed that germ-free (GF) mice required higher energy intakes to reach and maintain target body mass than did wild mice [19].
These findings were clarified by Bached et al. who found a 47% larger total fat mass in wild than in GF mice, despite GF animals having higher energy intakes. In addition, when the gut of GF mice was colonized with cecum-derived microbiota from wild mice, those GF mice increased their body fat mass by 60% [3]. It has also been reported that GF mice fed with high-carbohydrate/high-fat Western diet for 8 weeks gained less weight than non-GF mice. Furthermore, GF mice did not experience derangements of glucose metabolism compared with wild-type mice [20].
Looking further at the gut microbiota, Ley et al. analyzed >5000 bacterial 16S rRNA sequences corresponding to the cecal microbiota seen in lean and in genetically obese animals. They found that the relative proportion of two predominant bacterial divisions differed between the mice homozygous for a leptin gene mutation, causing phenotypic obesity and controls, with 50% fewer Bacteroides and 50% more Firmicutes organisms in the microbiota of lean wild-type animals versus those with obesity.
Turnbaugh et al. later studied microbiota colonization of GF wild-type mice with the cecal microbiota from lean or obese donors; animals colonized with the microbiota from obese mice showed significantly greater percentage increases in body fat than did mice colonized with the microbiota from lean donors [4].
Several studies have investigated potential mechanisms underlying the associations between obesity and the microbiota (Fig. 1) that might account for the different adiposity phenotypes seen with a different microbiota composition, seeking to determine how microbiota composition might modulate host energy metabolism and whether the induced changes might be transmissible [4]. The abundance of Bacteroidetes in the gut microbiota has been directly associated with fecal concentrations of SCFAs, especially propionate, butyrate, and acetate fatty acids, which interact with G-protein-coupled receptors (GPCRs), and specifically with the GPR41 (free fatty acid receptor 3, or FFAR3) expressed in the gut, adipocytes, and the peripheral nervous system. This receptor binds primarily to Gi/Go proteins, while GPR43 (free fatty acid receptor 2, or FFAR2) mainly activates Gi/Go proteins in the gut and adipose tissue. Further data suggest that SCFA binding to GPR43 may modulate the immune response by specifically reducing the release of inflammatory cytokines [21], and by increasing hypothalamic sensitivity to leptin [22, 23].
Fig. 1.
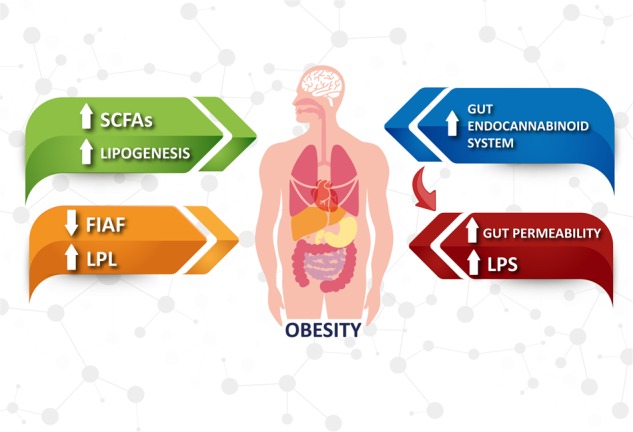
Mechanisms underlying the association between obesity. Obesity is associated with a high abundance of bacteria capable of fermenting carbohydrates that increases the rate of short-chain fatty acid (SCFA) biosynthesis, providing an extra source of energy for the host, which ends up being stored as lipids or glucose. The decreased expression of fasting-induced adipose factor (FIAF), a circulating lipoprotein lipase inhibitor (LPL), results in an increased fat storage in the white adipose tissue. Latter changes in the microbiota activate the endocannabinoid system in the gut. This mechanism contributes to increase gut permeability, which enhances plasma LPS levels and exacerbates gut barrier disruption. The increased endocannabinoid tone along with the increased LPS levels contribute to the increase of adipogenesis
Studies in Gpr41-deficient mice suggest that activation of GPR41 by SCFA stimulates the release of PYY, which induces satiety and reduces food intake; when expressed in the adipose tissue, GPR41 stimulates leptin secretion, adding to the induction of satiety [23].
In adipose tissue, GPR43-pathway activation suppresses insulin signaling, thereby inhibiting fat accumulation and stimulating lipolysis in adipocytes. Gut GPR43 promotes the secretion of glucagon-like peptide 1 (GLP-1), which in turn increases postprandial insulin release and decreases glucagon secretion [24, 25]. Supporting these observations, Gpr43-deficient mice fed with high-carbohydrate, high-fat diets develop lower body mass and higher lean mass than their wild-type siblings [26]. However, in comparison with those with normal weight, people with obesity have a greater abundance of bacteria capable of fermenting carbohydrates [and thus increasing the rate of SCFA biosynthesis], providing an extra energy substrate to the host, which is then stored as lipids or glucose. Besides these mechanisms, the changes in the composition of the gut microbiota seen in obesity decrease the expression of fasting-induced adipose factor (FIAF); since this is a circulating lipoprotein lipase inhibitor (LPL), this leads to increased white fat storage [3].
Changes in microbiota composition to a predominance of Gram-negative bacterial species may also alter the protein structure in gut endothelial “tight junctions”, leading to increased intestinal permeability, increasing the absorption of bacterial endotoxins such as lipopolysaccharides (LPS) into the circulation through CD14 receptor binding, which induces increased LPS-signaling cascades that lead to the production of pro-inflammatory cytokines, as seen with the development of obesity and increased insulin resistance [27].
Changes in the microbiota also activate the gut endocannabinoid system through stimulation of endothelial CB1 receptors, which contribute to increases in gut permeability, further increasing plasma LPS levels with increased gut barrier disruption and increased endocannabinoid system activity in both the gut and in adipose tissue, aggravating the initial disorders and leading to a vicious cycle [28].
The gut microbiota and obesity-related comorbidities
Type 2 diabetes
It is widely recognized that the gut microbiota is further modified in obesity-related comorbidities, such as type 2 diabetes (T2D) [27, 28]. Larsen et al. have shown that Firmicutes concentrations in the microbiota of adults with type 2 diabetes (T2D) are significantly increased versus those from comparable adults without diabetes [29]. Interestingly, those authors showed that plasma glucose (but not body mass index [BMI]) was positively correlated with the Bacteroidetes to Firmicutes ratio.
Faecalibacterium prausnitzii, from the phylum Firmicutes, is one of the most abundant organisms in the human gut microbiota, but is reduced in people with T2D in association with the increases in circulating levels of inflammatory markers [30]. Xiuyng Z et al. examined the relationship of the gut microbiota with the appearance of T2D in 121 individuals with normal glucose tolerance [NGT], prediabetes, or a newly diagnosed T2D, and found that butyrate-producing bacteria (e.g., Akkermansia muciniphila and Faecalibacterium prausnitzii) were most abundant in those with NGT, but that Bacteroides organism contents were halved in those with T2D versus those with either NGT or prediabetes [31]. Overweight and obesity are becoming more prevalent among people with type 1 diabetes (T1D) [32], and some recent data suggest a relationship between T1D and the gut microbiota, with microbial-regulated metabolites being modified in children before the development of either T1DM or of the relevant autoantibodies [33], in a population showing changes in the gut microbiota with disease progression [34] and showing less diversity with progression of the disease than in those without T1D, suggesting that some gut organisms (Bacteroides ovatus and Bacteroides fragilis) may exert some protection against progression to T1D [34]. The same pattern has been observed in T2D progression, being accompanied with increases in Bacteroidetes and decreases in Firmicutes in the microbiota, as compared with nondiabetics [29]. In particular, the gut microbiota in NOD mice deficient in MyD88 seems to be protective against T1D development, a protective effect blunted by oral antibiotic treatment [35]. The role of the gut microbiota in the development of autoimmunity and of T1D warrants further investigation.
Metabolic syndrome
Obesity, T2D, and metabolic syndrome are conditions sharing a range of phenotypes and with substantial numbers of interactions between genetic/epigenetic risk factors and environmental influences, recently including the gut microbiota. Pseudo-germ-free rodents have been used to evaluate causative links between the gut microbiota and obesity [36, 37]. Further work using toll-like receptor 5 (TLR5)-knockout mice and leptin-deficient(ob/ob) mice that develop hyperphagia-induced obesity and insulin resistance [38, 39] showed that eliminating the bulk of the gut microbiota by ampicillin–neomycin treatment induced reductions in inflammation and insulin resistance [36, 37], suggesting that the gut microbiota may contribute to the development of a metabolic syndrome. Such potentially causative relationships have also been investigated in a recent clinical trial of “transplantation” of the gut microbiota [40]; obese participants received fecal transplants from lean donors or, in controls of their own fecal microbiotas, showed that lean-microbiota receivers became more insulin-sensitive over 6 weeks, showing for the first time a causative role for the healthy gut microbiota in the control of abnormal insulin resistance in humans, providing also the first evidence of the gut microbiota as a “pathogen-like” factor satisfying Koch’s postulates as a causative agent for a specific condition. In addition, Ussar et al., using three inbred strains of mice exposed to long-term environmental intervention and short-term dietary challenge, showed that in mice with a permissive genetic background, modulation of the microbiota could reduce metabolic syndrome disorders; these data also suggest that there is an association between specific microbiontal bacterial composition and different metabolic phenotypes, suggesting complex interactions between the gut microbiota, host genetics, and environmental risk factors (diet/medication/lifestyle) [41].
Cardiovascular diseases
It is widely recognized that there is a close relationship between inflammatory and metabolic pathways in the etiology of atherosclerosis, as well as for the development of obesity and metabolic syndrome [42]. Gut microbiontal abnormalities may affect lipid metabolism and atheromatous plaque development through several inflammatory-dependent mechanisms. LPS, the major component of the outer membrane of Gram-negative bacteria [43], passes through the gut wall by two main mechanisms, “passive” chylomicron-associated transport, and leakage through faulty tight junctions (leaky gut). LPS, which acts through Toll-like receptors (TLRs) has been linked with insulin resistance and increased macrophage infiltration of adipose tissue. Since the gut microbiota is the main source of LPS in humans, it could influence host metabolic syndrome and cardiovascular risk through upregulation of inflammatory signaling pathways [44, 45].
TLR signaling-deficient mice are characterized by reduced adiposity and improved glycemia [37, 46–48], and ablation of the specific TLR5 gene in mice leads to hyperphagia and metabolic syndrome abnormalities [37].
Consistent differences in gut microbiota composition are seen with these phenotypes, and importantly, these phenotypes are transmissible through the transplantation of microbiota from TLR5-deficient to germ-free mice, with the development of obesity and increased insulin resistance [37]. These observations support the suggested connection between the immune system modulation and host metabolism through modifications of the gut microbiota.
More recent experimental and clinical studies have shown that the contribution of the gut microbiota to increased cardiovascular disease (CVD) risks involves compound molecules, which are synthesized by the intestinal microbiota [49]; such compounds also increase the risk of arterial thrombosis [50, 51] and worsen ischemic stroke outcomes [52]. Diet composition (particularly high-fat diets) seems to be a major factor influencing the effect of the gut microbiota on CVD risks [53], though gut microbiota dysbiosis can also contribute to the development of hypertension in mice [54] and humans [55]; specifically, high-fiber diets, which increased murine gut microbiota short-chain fatty acid (SCFA) production, reduced systolic and diastolic blood pressures in mouse models of hypertension [56], while SCFA acetate supplementation downregulated the renin–angiotensin system in overweight/obese pregnant women [57].
CVD has been associated in a dose-dependent manner with circulating levels of betaine, choline, and trimethylamine N-oxide (TMAO) in humans [58], and the gut microbiota produce trimethylamine (TMA), a bacterial choline metabolite formed after consumption of dietary phosphatidylcholine choline and L-carnitine; this TMA is absorbed into the portal circulation and converted in the liver into trimethylamine N-oxide (TMAO) [49, 58]. Koeth et al. found that high-plasma TMAO concentrations were significantly associated with incident CV events and with fasting plasma carnitine concentrations [49]; in addition, they showed that L-carnitine-supplemented ApoE−/− mice developed approximately double the aortic root atherosclerotic plaque seen in chow-fed control mice [49]. However, these effects were abolished by elimination of the commensal gut microbiota in the L-carnitine-supplemented ApoE−/− mice, also through a decrease of TMA and TMAO levels. The choline supplementation avoided foam cell infiltration in the plaque and contributed to decrease the plaque size in ApoE−/− mice [49].
Clinical studies have reported that TMAO levels are an independent predictor of mortality risk in coronary artery disease [59, 60], but this has not been confirmed in other studies [61]. Further studies are, therefore, required to determine whether microbiota-derived TMAO does induce atherosclerosis, especially in the context of overweight and obesity.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity and characterized by excessive fat infiltration in the liver, categorized histologically as either nonalcoholic fatty liver (NAFL) or as having progressed to nonalcoholic steatohepatitis (NASH) in about 25% of cases [62]. Thus, liver fat accumulation alone may not account for disease progression, and other factors may promote progression to NASH. The gut microbiota may be a contributory factor for several reasons, first, it is involved in carbohydrates, amino acids, and fatty acid metabolism, thereby influencing nutrient supplies [63]; second, it is involved in the modulation and development of host immunity [64], and specific types of microbiota can affect the development/progress of inflammation in the liver as in adipose tissue and the vasculature [as discussed above]. The microbiota transplantation from TLR5 knockout mice to wild-type germ-free mice resulted in weight gain, chronic inflammation, and glucose derangements, thus allowing to hypothesize an association between host immune activity and the gut microbiota [37]. In addition, the gut microbiota may modulate gut hormone secretion (e.g., glucagon-like peptide 1 (GLP-1)) [65]. For example, Dumas et al. showed that microbial modulation of phosphatidylcholine (PC) metabolism increased influential steatosis in mice susceptible to insulin resistance and NASH through their amplified microbial destruction of PC to form methylamines (as measured in the urine), since PC is necessary for VLDL synthesis. As impaired PC secretion leads to increased hepatic triglyceride deposition, those findings suggest a mechanistic link between the gut microbiota and progression from NAFL to NASH [66]. Observations in humans provide a possible link between choline-deficient diet-induced fatty liver and the gut microbiota, since on that diet, alterations of the gut microbiome paralleled fatty liver development, suggesting associations between diet, the gut microbiome, and liver health [67]. Increased risks of progression to NASH may also be associated with the gut microbiota through their secretion of ethanol, well known to have adverse effects on liver health, since alcohol-free food consumption induces increases in blood alcohol content, and the gut microbiota is a major source of endogenous alcohol production [68, 69]. Studies showing that mice with the abnormal gut microbiota had elevated breath alcohol levels that were reduced by oral neomycin treatment [70] support this hypothesis; these authors conclude that since individuals with NASH are usually obese and since the histology of NASH matches that of alcoholic liver disease, they may produce more endogenous alcohol than those without disease progression [70]. This hypothesis is supported by the finding that patients with NASH have higher blood ethanol concentrations than those with NAFLD (mean 0.12 µmol/µl vs. 0.09 µmol/µl, respectively) [71]. Interestingly, Zhu et al. in an alcohol-free population of children and adolescents confirmed a measureable blood ethanol concentration in obese subjects with, but not in those without, NASH, that was also associated with a significantly higher microbiome content of Escherichia genus bacteria in NASH+ subjects. Since Escherichia bacteria are known ethanol producers, these findings support the hypothesis that these microbiontal organisms could contribute to NAFDL progression to NASH and for the association of NASH with elevated blood ethanol levels [72].
Weight loss and the gut microbiota
Diet
The microbiota contains principally organisms from the Bacteroides and Firmicutes phyla. The Bacteriodes have a high capacity for digesting dietary polysaccharides, and those species are more abundant in lean people than in morbid obesity, where dysbiotic intestinal microbiota are common with increased Firmicutes phyla content versus that seen in lean subjects. Recent research has also documented that the dysbiosis seen in obesity could be changed by several months on a low-energy diet. Thus, different compositions of the gut microbiota are associated with differences in the degradation of various otherwise indigestible polysaccharides, which could contribute to the risks of developing obesity [73]. It has also been reported that the intake of some micronutrients, fatty acids, prebiotics, and probiotics could have an impact on gut microbiota composition and on the regulation of gene expression at the liver, muscle, and adipose tissue site [74].
Overall, the production and the activation of B and K vitamins are induced by the fermentation of short-chain fatty acids coming from carbohydrates, ammonia, and nitrogenous compounds by the gut microbiota. This results in an increased intake of energy from the food at gut level. The high intake of carbohydrates had an impact on gut microbiota composition, promoting the reduction of pH in the gut lumen that in turn modifies bacterial metabolism [75].
It has been reported that Firmicutes had an inhibitory effect on lipoprotein lipase (LPL) inhibitor release, thus resulting in an increased enzymatic activity and a tendency to enhance surplus energy accumulation as fat [76]. Furthermore, since obesity results from abnormal regulation of energy intake, expenditure and conservation and microbiontal microorganisms contribute to the regulation of energy accumulation from the diet; both abnormal microbiontal composition and activity and excessive dietary intake must contribute to adiposity through increasing the accumulation of body fat [75].
However, other studies have not confirmed findings on the contribution of various bacterial microbiota components to the development of obesity; in particular, the Bacteroides/Firmicutes ratio has not always been found to be a significant modulatory factor for obesity [77]. These discrepancies imply that further undetected variants in the gut microbiota may also contribute to obesity risks, or that unconsidered factors, dietary, genetic, or environmental are affecting microbiota function. Dietary carbohydrates, including structural polysaccharides, plant-based oligosaccharides, and starches resistant to digestion in the small intestine, are fermented by the large intestine microbiota, providing the main source of energy supporting microbial growth in the colon.
Fermentation releases energy as short-chain fatty acids (SCFAs), which is a energy source for host cells [78]. In turn, the decreased assumption of fermentable carbohydrates could have an effect on the composition of large gut bacterial phyla. The effect of a nutritional pattern characterized by a decreased carbohydrate intake on gut bacterial components of obese subjects has been investigated by Duncan et al., reporting that carbohydrate intake decreased, so that the total fecal SCFA was reduced. Furthermore, the decreased carbohydrate intake resulted in a decrease in the Roseburia spp., the Eubacterium rectale subgroups of cluster XIVa and bifidobacteria [78].
Since weight loss on diets rich in protein but low in fermentable carbohydrates alter colonic bacterial populations, with further implications for intestinal health and function, variations in the colonic microbiota might be one such factor [78], further studies in this area are clearly necessary.
In support of observations discussed in obesity, recent studies have shown that anorexia is characterized by an imbalance in Gram+/Gram− abundance in the gut microbiota with an increased abundance of Bacteroidetes and a reduced abundance of Firmicutes, which is the inverse of the findings seen in obesity. Furthermore, since the relative abundance of Firmicutes [including Ruminococcus, Roseburia, and Clostridium fermenting carbohydrates] is reduced in people with anorexia, the fecal butyrate concentration is lower in obesity than in anorexia [79], though it remains to be established whether this finding in anorexia is caused by dietary restriction or whether it has any specific causative role for weight loss.
To evaluate the temporal relationships between food intake, intestinal microbiota, and metabolic and inflammatory phenotypes, Cotillard et al. studied weight loss and weight stabilization following dietary restriction in people with obesity or overweight. They found that individuals with reduced microbial gene richness (by 40%) showed marked low-grade inflammation and showed increased metabolic syndrome abnormalities. Weight loss improved gene richness and clinical phenotype, but was less effective in reducing markers of inflammation in people with initially lower microbial gene richness, while low genetic richness seemed to have predictive potential for the effectiveness of dietary intervention [80].
Probiotics could have an important role in this regard, because resoring the tight junctions at gut level avoid the translocation of lipopolysaccharide in the bloodstream. This could contribute to prevent the onset of a chronic inflammatory milieu that it is well known to contribute to insulin resistance [81].
To investigate the modulatory effect of oolong tea polyphenols (OTP) on intestinal microbiota, the influence of OTP [prepared by column chromatography, on the composition of the intestinal flora has been evaluated by high-throughput gene sequencing in a mouse model of obesity induced by high-fat diet (HFD); large increases in gut bacterial biodiversity and in the abundance of butyrate and acetate-producing bacteria were found, with significant increases in Bacteroidetes and decreases in Firmicutes in the microbiota after 4 weeks administration of OTP [82]. The corresponding decrease in the Firmicutes/Bacteroidetes ratio reflected a positive modulating effect of OTP on the intestinal microbiota, and since OTP is rich in tea catechins, especially O-methylated derivatives, it may also have probiotic activity and be useful as a functional food component contributing to the prevention of obesity-related metabolic disorders through modification of the intestinal microbiota [82].
Few reported studies have examined the relationship between gut flora and bacterial metabolite production. One recent study found that galactooligosaccharide (GOS) probiotics that beneficially affect host intestinal microbiota and gut metabolite production, decreased the abundance of Ruminococcaceae and Oscillibacter in the mouse microbiota and decreased the production of ~31 metabolites, including oleic acid, arachidic acid, and behenic acid in the intestine as well as decreasing blood triglyceride content versus controls, suggesting that GOS could improve murine lipid metabolism. Also, on diets rich in GOS, mouse the abundance of Alloprevotella, Bacteroides, and Parasutterell in the microbiota were increased [83]. While control blood glucose concentrations were significantly higher than those of the GOS-fed animals, the gut contents of Coprococcus and Odoribacter, [butyrate-producing bacteria] were lower and butyrate, which reduces plasma glucose content, was significantly downregulated with potentially detrimental effects on host glycemia, as mentioned. However, this effect could optimize the effects of consumption of combinations of probiotics, some of which increase gluconeogenesis [83], methods of prebiotic integration and the development of preventive prebiotic interventions [83].
Bariatric surgery
Bariatric surgery is an increasingly popular therapeutic option for treating people with morbid obesity and achieves the largest BMI reductions [84]. Weight loss after bariatric surgery is associated with microbiota modifications as are seen with any surgical procedure [85]. Recent research on the effects of different bariatric surgical procedures, in particular Roux-en-Y gastric bypass (RYGB) suggests it increases Proteobacteria and decreases Firmicutes microbiota content; sleeve gastrectomy (LSG) leading to less marked gut microbiota alterations with increased anti-inflammatory Fecali bacterium prausnitzii abundance though alterations in microbiota Fecalibacterium species abundance seen after RYGB have been inconsistent [85]. Some work showing increased and decreased abundance of Gamma proteobacteria and Clostridia, respectively in the feces of obese subjects after bariatric surgery [86]. Other work has shown increased abundance of Bacteroides/Prevotella and Escherichia coli [a member of the Gammaproteobacteria class] after bariatric surgery, supporting earlier findings [32]. Liou et al. also demonstrated that alterations of the intestinal microbiota after Roux-en-Y gastric bypass (RYGB) were associated with postoperative reductions in both host body weight and percentage fat mass [87]. Furthermore, the transfer of the post-RYGB intestinal microbiota to germ-free mice induced weight loss, a finding in accordance with the recent finding that an obese phenotype could be induced in germ-free mice through the introduction of the obese subject gut microbiota [87].
Comparison of the impact of RYGB versus SG on post procedure intestinal microbiota in Sprague-Dawley rats (SD), showed that RYGB, but not SG, altered the intestinal microbiota, reducing the variety of microbiota organisms in the intestine, while Gammaproteobacteria abundance was negatively correlated with achieved body weight post surgery, so that an altered microbiota has the potential to be a factor contributing to the achievement of stable reductions in postoperative weight [88].
Conclusions
Overall, currently available evidence suggests that changes in the gut microbiota could contribute to the pathogenesis of obesity and to the development of obesity-related metabolic disorders, including type 2 diabetes, NAFLD, metabolic syndrome, and cardiovascular disease.
Obesity treatments such as calorie reduced diets and/or bariatric surgery modify the gut microbiota in ways that are associated with health benefits, supporting the hypothesis that changing gut microbiontal composition has the potential to provide an additional mechanism for achieving stable weight loss.
However, further studies are needed to better understand the mechanisms of the observed associations between gut microbiota and obesity, the role of the colonic microbiota, and to determine whether manipulation of the gut microbiota through diet, with or without increased intakes of pre/probiotics, could provide potential therapeutic options contributing to the prevention or treatment of human obesity.
Acknowledgements
Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group members served as collaborators and approved the final version of the paper: Annamaria Colao, Antonio Aversa, Barbara Altieri, Luigi Angrisani, Giuseppe Annunziata, Rocco Barazzoni, Luigi Barrea, Giuseppe Bellastella, Bernadette Biondi, Elena Cantone, Brunella Capaldo, Sara Cassarano, Rosario Cuomo, Luigi Di Luigi, Andrea Di Nisio, Carla Di Somma, Ludovico Docimo, Katherine Esposito, Carlo Foresta, Pietro Forestieri, Alessandra Gambineri, Francesco Garifalos, Cristiano Giardiello, Carla Giordano, Francesco Giorgino, Dario Giugliano, Daniela Laudisio, Davide Lauro, Andrea Lenzi, Silvia Magno, Paolo Macchia, MariaIda Maiorino, Emilio Manno, Chiara Marocco, Paolo Marzullo, Chiara Mele, Davide Menafra, Silvia Migliaccio, Marcello Monda, Filomena Morisco, Fabrizio Muratori, Giovanna Muscogiuri, Mario Musella, Gerardo Nardone, Claudia Oriolo, Uberto Pagotto, Pasquale Perrone Filardi, Luigi Piazza, Rosario Pivonello, Barbara Polese, Paolo Pozzilli, Giulia Puliani, Stefano Radellini, Gabriele Riccardi, Domenico Salvatore, Ferruccio Santini, Giovanni Sarnelli, Lorenzo Scappaticcio, Silvia Savastano, Bruno Trimarco, Dario Tuccinardi, Paola Vairano, Nunzia Verde, Roberto Vettor.
Funding
This article is published as part of a supplement funded by the Endocrinology Unit, Department of Clinical Medicine and Surgery, University Federico II, Naples, Italy.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Members of Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group are listed under Acknowledgements section.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92:286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 3.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 5.Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642. doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13:11–25. doi: 10.1038/nrendo.2016.150. [DOI] [PubMed] [Google Scholar]
- 7.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boroni Moreira AP, Fiche Salles Teixeira T, do CGPM, de Cassia Goncalves Alfenas R. Gut microbiota and the development of obesity. Nutr Hosp. 2012;27:1408–14. doi: 10.3305/nh.2012.27.5.5887. [DOI] [PubMed] [Google Scholar]
- 9.Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12:272–81. doi: 10.1111/j.1467-789X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 10.Haro Carmen, García-Carpintero Sonia, Rangel-Zúñiga Oriol A., Alcalá-Díaz Juan F., Landa Blanca B., Clemente José C., Pérez-Martínez Pablo, López-Miranda José, Pérez-Jiménez Francisco, Camargo Antonio. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Molecular Nutrition & Food Research. 2017;61(12):1700300. doi: 10.1002/mnfr.201700300. [DOI] [PubMed] [Google Scholar]
- 11.Haro C, Montes-Borrego M, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, et al. Two healthy diets modulate gut microbial community improving insulin sensitivity in a human obese population. J Clin Endocrinol Metab. 2016;101:233–42. doi: 10.1210/jc.2015-3351. [DOI] [PubMed] [Google Scholar]
- 12.Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117:1645–55. doi: 10.1017/S0007114517001593. [DOI] [PubMed] [Google Scholar]
- 13.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014;349:g3961. doi: 10.1136/bmj.g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 17.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 18.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–42. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 19.Wostmann BS, Larkin C, Moriarty A, Bruckner-Kardoss E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab Anim Sci. 1983;33:46–50. [PubMed] [Google Scholar]
- 20.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obesity Rev. 2013;14:232–44. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 22.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 23.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–72. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14:390–403. doi: 10.1038/s41574-018-0016-2. [DOI] [PubMed] [Google Scholar]
- 26.Bjursell M, Admyre T, Göransson M, Marley AE, Smith DM, Oscarsson J, et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab. 2011;300:E211–20. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 27.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22:277–82. doi: 10.1097/MED.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 33.Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205:2975–84. doi: 10.1084/jem.20081800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 37.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin HV, Frassetto A, Kowalik EJ, Jr., Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6 e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, et al. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–30. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–8. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 43.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–5. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 44.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8:923–34. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson GB, Riggs BL, Platt JL. A genetic basis for the “Adonis” phenotype of low adiposity and strong bones. FASEB J. 2004;18:1282–4. doi: 10.1096/fj.04-1572fje. [DOI] [PubMed] [Google Scholar]
- 47.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, et al. Statement of Retraction. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986–1998. Diabetes. 2016;65:1126–7. doi: 10.2337/db16-rt04a. [DOI] [PubMed] [Google Scholar]
- 48.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–29. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackel S, Kiouptsi K, Lillich M, Hendrikx T, Khandagale A, Kollar B, et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood. 2017;130:542–53. doi: 10.1182/blood-2016-11-754416. [DOI] [PubMed] [Google Scholar]
- 52.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–23. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drouin-Chartier JP, Cote JA, Labonte ME, Brassard D, Tessier-Grenier M, Desroches S, et al. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7:1041–51. doi: 10.3945/an.115.011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karbach SH, Schonfelder T, Brandao I, Wilms E, Hormann N, Jackel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 57.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, et al. Increased Systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 2016;68:974–81. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, et al. Plasma trimethylamine N-Oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620–8. doi: 10.1016/j.jacc.2016.03.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer KA, Benton TZ, Bennett BJ, Jacobs DR, Jr., Lloyd-Jones DM, Gross MD, et al. Microbiota-dependent metabolite trimethylamine N-oxide and coronary artery calcium in the coronary artery risk development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5 pii: e003970. [DOI] [PMC free article] [PubMed]
- 62.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 63.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noverr MC, Huffnagle GB. Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 2004;12:562–8. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Vrieze A, Holleman F, Zoetendal EG, de Vos WM, Hoekstra JB, Nieuwdorp M. The environment within: how gut microbiota may influence metabolism and body composition. Diabetologia. 2010;53:606–13. doi: 10.1007/s00125-010-1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park JS, Seo JH, Youn HS. Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:22–7. doi: 10.5223/pghn.2013.16.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarkola T, Eriksson CJ. Effect of 4-methylpyrazole on endogenous plasma ethanol and methanol levels in humans. Alcohol Clin Exp Res. 2001;25:513–6. [PubMed] [Google Scholar]
- 69.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- 70.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–7. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 71.Volynets V, Kuper MA, Strahl S, Maier IB, Spruss A, Wagnerberger S, et al. Nutrition, intestinal permeability, and blood ethanol levels are altered in patients with nonalcoholic fatty liver disease (NAFLD) Dig Dis Sci. 2012;57:1932–41. doi: 10.1007/s10620-012-2112-9. [DOI] [PubMed] [Google Scholar]
- 72.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 73.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 74.Stenvinkel P. Obesity--a disease with many aetiologies disguised in the same oversized phenotype: has the overeating theory failed? Nephrol Dial Transplant. 2015;30:1656–64. doi: 10.1093/ndt/gfu338. [DOI] [PubMed] [Google Scholar]
- 75.Resta SC. Effects of probiotics and commensals on intestinal epithelial physiology: implications for nutrient handling. J Physiol. 2009;587:4169–74. doi: 10.1113/jphysiol.2009.176370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dridi B, Raoult D, Drancourt M. Archaea as emerging organisms in complex human microbiomes. Anaerobe. 2011;17:56–63. doi: 10.1016/j.anaerobe.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Annalisa N, Alessio T, Claudette TD, Erald V, Antonino de L, Nicola DD. Gut microbioma population: an indicator really sensible to any change in age, diet, metabolic syndrome, and life-style. Mediators Inflamm. 2014;2014:901308. doi: 10.1155/2014/901308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–8. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, et al. Microbiota in anorexia nervosa: the triangle between bacterial species, metabolites and psychological tests. PLoS ONE. 2017;12:e0179739. doi: 10.1371/journal.pone.0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 81.Crovesy L, Ostrowski M, Ferreira DMTP, Rosado EL, Soares-Mota M. Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int J Obes (Lond) 2017;41:1607–14. doi: 10.1038/ijo.2017.161. [DOI] [PubMed] [Google Scholar]
- 82.Cheng M, Zhang X, Zhu J, Cheng L, Cao J, Wu Z, et al. A metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity mice fed with oolong tea polyphenols. Food Funct. 2018;9:1079–87. doi: 10.1039/c7fo01570d. [DOI] [PubMed] [Google Scholar]
- 83.Cheng W, Lu J, Lin W, Wei X, Li H, Zhao X, et al. Effects of a galacto oligosaccharide-rich diet on fecal microbiota and metabolite profiles in mice. Food Funct. 2018;9:1612–20. doi: 10.1039/c7fo01720k. [DOI] [PubMed] [Google Scholar]
- 84.Rajjo T, Mohammed K, Alsawas M, Ahmed AT, Farah W, Asi N, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102:763–75. doi: 10.1210/jc.2016-2574. [DOI] [PubMed] [Google Scholar]
- 85.Ejtahed HS, Angoorani P, Hasani-Ranjbar S, Siadat SD, Ghasemi N, Larijani B, et al. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: a systematic review. Microb Pathog. 2018;116:13–21. doi: 10.1016/j.micpath.2017.12.074. [DOI] [PubMed] [Google Scholar]
- 86.Zhang H, Di Baise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric by pass. J Proc Natl Acad Sci. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao Y, Ding R, Xu B, Hua R, Shen Q, He K, et al. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. Obes Surg. 2017;27:295–302. doi: 10.1007/s11695-016-2297-7. [DOI] [PubMed] [Google Scholar]


