Abstract
We aimed to compare the efficacy of percutaneous transhepatic biliary stenting (PTBS) and PTBS combined with 125I particles implantation in the treatment of advanced extrahepatic cholangiocarcinoma (EHC). A total of 184 advanced EHC patients, who received PTBS (PTBS group) or PTBS combined with 125I particles implantation (PTBS + 125I group) from January 2012 to April 2017 in our department, were retrospectively reviewed. The improvement of jaundice and liver function was observed in both groups. The postoperative complications, risk of biliary re-obstruction, and overall survival (OS) were compared between the two groups. Amongst, 71 cases received PTBS and 113 had the additional implantation of 125I particles. The jaundice and liver function were significantly improved in all patients, especially in PTBS + 125I group. There was no significant difference in the risk of postoperative complications between the two groups. However, the risk of biliary re-obstruction significantly reduced in PTBS + 125I group (19.5% vs. 35.2%, p = 0.017). Kaplan Meier analysis showed that patients in PTBS + 125I group had a significantly better OS, both for hilar and distal cholangiocarcinoma. Univariate analysis demonstrated that preoperative levels of carbohydrate antigen 19-9 (CA19-9), total bilirubin, neutrophil count, lymphocyte count, and different therapeutic method were significant factors affecting OS. Multivariate analysis further identified the treatment of PTBS combined with 125I particles implantation as an independent protective prognostic factor (HR = 0.26, 95% CI: 0.17–0.39, p < 0.001). In conclusion, for patients with advanced EHC, PTBS combined with 125I particles implantation is superior to PTBS alone in improving liver function, inhibiting biliary re-obstruction, and prolonging survival time.
Subject terms: Bile duct cancer, Radiotherapy, Surgical oncology
Introduction
Cholangiocarcinoma (CCA) is an epithelial cell malignancy with features of cholangiocyte differentiation. The incidence of CCA has increased globally over the past few decades1. The most contemporary classification based on anatomic location includes intrahepatic cholangiocarcinoma (IHC) and extrahepatic cholangiocarcinoma (EHC). Notably, the two types do not only differ in anatomical origin, but also in clinicopathological characteristics. Of the two types, EHC, which consists of hilar cholangiocarcinoma (HCCA) and distal cholangiocarcinoma (DCCA), contributes to 90% of all CCA cases2.
Because of the diagnostic difficulty and limited treatment options, the prognosis of EHC is unsatisfactory, with a 5-year survival rate of less than 20%3. Currently, surgical resection and liver transplantation are primary radical treatments for early-stage EHC4,5. However, the majority of patients with EHC are already at the advanced stage and lose the opportunity of surgery at the time of diagnosis6. Chemotherapy with gemcitabine and cisplatin is the standard treatment for inoperable EHC, but the efficacy is poor6,7. Radiotherapy is also considered as an effective local modality for unresectable EHC8. Biliary drainage and biliary stent implantation have been gradually applied in advanced EHC due to the efficacy of improving biliary obstruction and relieving symptoms. However, because of the rapid tumor development, biliary stenting alone may easily cause the re-obstruction of biliary tract9. In contrast, the additional implantation of 125I particles can effectively suppress tumor growth through the release of continuous γ rays10.
Recent studies have shown that biliary stenting combined with 125I particles implantation is a safe and feasible palliative treatment for advanced EHC11,12. However, to date, few reports have compared the efficacy of biliary stenting alone with additional implantation of 125I particles. Here, we summarized 184 cases of advanced EHC who underwent percutaneous transhepatic biliary stenting (PTBS) or PTBS combined with 125I particles implantation in our department. The efficacy, complications, risk of biliary re-obstruction, and overall survival (OS) were compared between the two groups.
Methods
Patients
Advanced EHC patients who were admitted to our department from January 2012 to April 2017 were retrospectively analyzed. Inclusion criteria: (1) pathologically or clinically diagnosed as HCCA or DCCA; (2) unresectable or unwilling to surgery; (3) received PTBS (PTBS group) or PTBS combined with 125I particles implantation (PTBS + 125I group) for the first time. Exclusion criteria: (1) benign biliary stenosis; (2) distant metastasis; (3) previously received surgery, endoscopic stenting, or chemotherapy for EHC; (4) incomplete follow-up data. We reported this study in compliance with STrengthening the Reporting of OBservational studies in Epidemiology (STROBE). Our study was complied with Helsinki Declaration13, and was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. All patients signed informed consent prior to treatment.
Surgical procedures
Percutaneous transhepatic cholangial drainage (PTCD) was performed in advance for patients who had obstructive jaundice or biliary tract infection. About one week later, PTBS with or without 125I particles implantation were performed under digital subtraction angiography. The process has been described previously14,15. The dose delivered by 125I particles implantation was approximately at 1 cm from the center of the radioactive source.
Data collection and follow-up
The electronic medical records were reviewed to collect the following information: patient’s age, gender, tumor location, and preoperative serological examinations, including total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), albumin (ALB), c-reactive protein (CRP), neutrophil count (NC), lymphocyte count (LC), platelet count (PLT), prothrombin time-international normalized ratio (PT-INR), carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA).
All patients were followed-up until September 2017 or until the death. The follow-up contents included biochemical routine, tumor markers, abdomen ultrasound, and/or CT. Changes of serum ALT, AST, TBIL, DBIL, ALP, and ALB were observed at 1, 3, and 6 months postoperatively. When “P” type tube shifted, appropriate adjustment was performed. As 125I particles have almost no anti-tumor effect at about 6 months after operation, re-implantation of new 125I particles was performed every 6 months. Discarded 125I particles were sent to Nuclear Medicine Center for centralized disposal.
Statistical analysis
We used SPSS 22.0 software for statistical analysis. Continuous variables with normal distribution were expressed as mean ± standard deviation and differences between groups were compared with t test. Or else, median (min-max) with Wilcoxon test was used. Postoperative changes of liver function were analyzed by using paired t test. The optimal cutoff value of continuous variable is determined by the largest value of Youden Index (sensitivity + specificity −1). The primary outcome was OS, which was assessed by Kaplan Meier curves compared with log-rank test. Variables with P value less than 0.05 in the univariate analysis were entered into a multivariate Cox regression model. P < 0.05 indicates statistically significant difference.
Results
Baseline information
A total of 238 advanced EHC patients were admitted to our department from January 2012 to April 2017. Finally, 184 patients met selection criteria and were included in this study. Baseline data of the 184 patients are shown in Table 1. 44 (62.0%) out of the 71 cases in PTBS group versus 73 (64.6%) out of the 113 cases in PTBS + 125I group were pathologically or clinically diagnosed as HCCA. There were no significant differences in gender, age, tumor location, serum levels of CA19-9, CEA, ALB, PT-INR, NC, LC, and CRP between the two groups (all p > 0.05). However, patients in the PTBS + 125I group had significantly higher preoperative levels of ALT, AST, ALP, GGT, and TBIL (p < 0.05). The length of hospital stay was slightly longer in the PTBS + 125I group, while the difference was not statistically significant (median: 15 vs. 13 days, p = 0.058). Moreover, hospital costs were relatively higher in PTBS + 125I group compared with PTBS group (median: 36,631 vs. 28,821 CNY, p < 0.001).
Table 1.
Basic characteristics of the included patients.
| Variables | Overall | PTBS group (n = 71) | PTBS + 125I group (n = 113) | p value |
|---|---|---|---|---|
| Gender (male/female) | 118/66 | 43/28 | 75/38 | 0.424 |
| Age (y) | 68.9 ± 12.3 | 68.8 ± 14.1 | 68.9 ± 11.0 | 0.819 |
| Location (HCCA/DCCA) | 117/67 | 44/27 | 73/40 | 0.718 |
| CA19-9 (ng/ml) | 650.0 (0.6–2068) | 886 (1.1–1967) | 509.2 (0.6–2068) | 0.358 |
| CEA (ng/ml) | 4.4 (0.9–490.5) | 4.4 (1.0–490.5) | 4.4 (0.9–490.5) | 0.510 |
| ALT (U/L) | 131 (18–542) | 106 (18–542) | 149 (22–513) | 0.001 |
| AST (U/L) | 134 (19–793) | 98 (19–793) | 158 (28–541) | <0.001 |
| ALP (U/L) | 487 (39–2556) | 404 (102–1990) | 553 (39–2556) | 0.013 |
| GGT (U/L) | 484(4.1–2528) | 406(47–1837) | 534 (4.1–2528) | 0.047 |
| TBIL (µmol/L) | 224.7 (5.8–706.2) | 158 (5.8–551.5) | 249.7 (14.1–706.2) | 0.003 |
| DBIL (µmol/L) | 179.3 (3.0–649.0) | 150 (3–424) | 201.4 (5–649) | 0.006 |
| ALB (g/L) | 34.3 (23.6–45.3) | 34.6 (25.1–43.6) | 34.2 (23.6–45.3) | 0.669 |
| PT-INR | 1.03 (0.63–16.5) | 1.1 (0.6–1.6) | 1.0 (0.7–16.5) | 0.234 |
| NC (109/L) | 4.5 (1.6–35.6) | 4.8 (1.7–34.8) | 4.3 (1.6–35.6) | 0.236 |
| LC (109/L) | 1.3 (0.3–4.2) | 1.2 (0.3–2.8) | 1.4(0.4–4.2) | 0.152 |
| PLT (109/L) | 241 (30–644) | 252 (30–644) | 237 (54–533) | 0.817 |
| CRP (mg/L) | 16.4 (0.6–270.0) | 16.3 (1.5–270.0) | 16.4 (0.6–205.6) | 0.332 |
| The length of stay (d) | 14 (3–47) | 13 (3–47) | 15 (4–34) | 0.058 |
| Hospital costs (CNY) | 34,368 (5,533–64,267) | 28,821 (14,442–64,267) | 36,631 (5,533–61,928) | <0.001 |
| Survival time (m) | 9 (1–30) | 7 (1–13) | 12 (1–30) | <0.001 |
PTBS percutaneous transhepatic biliary stenting, HCCA hilar cholangiocarcinoma, DCCA distal cholangiocarcinoma, CA19-9 carbohydrate antigen 19-9, CEA carcino-embryonic antigen, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma-glutamyl transpeptidase, TBIL total bilirubin, DBIL direct bilirubin, ALB albumin, PT-INR prothrombin time-international normalized ratio, NC neutrophil count, LC lymphocyte count, PLT platelet count, CRP c-reactive protein.
Evaluation of clinical efficacy
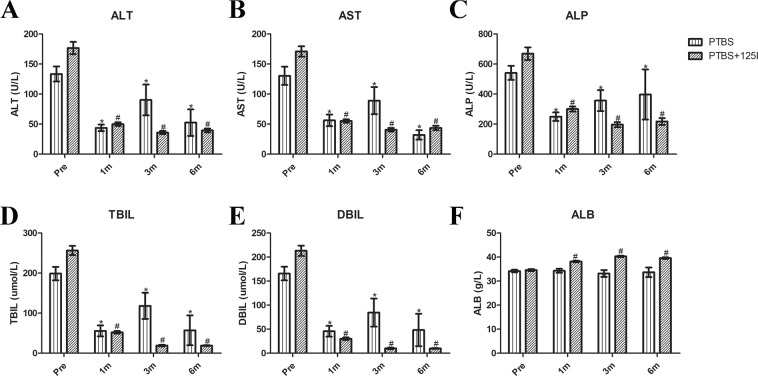
All patients had significantly improved clinical symptoms (itching, fever, etc.) in both groups. In the PTBS group, the serum levels of ALT, AST, ALP, TBIL, and DBIL significantly decreased at 1, 3, and 6 months postoperatively, compared with preoperative levels (Fig. 1, all p < 0.05). However, no remarkable change of ALB was observed after operation in the PTBS group (p > 0.05). In contrast, the changes of all the above serum indices (including ALB) in the PTBS + 125I group were more significant than that in the PTBS group.
Figure 1.
Changes of liver function after operation in the PTBS and PTBS + 125I groups. Changes of ALT (A), AST (B), ALP (C), TBIL (D), DBIL (E), and ALB (F) at 1 month, 3 months and 6 months post-operatively (*p < 0.05 compared with preoperative values in the PTBS group; #p < 0.05 compared with preoperative values in the PTBS + 125I group).
Comparisons of complications and risk of biliary re-obstruction
After operation, a total of 9 (12.7%) patients in the PTBS group experienced complications, 6 of which had hyperamylasemia and 3 had biliary tract infection. In contrast, there were complications in 15 (13.3%) patients in the PTBS + 125I group. Amongst, 10 cases had hyperamylasemia and 5 had biliary tract infection. No serious complications, radiation-related complications, or perioperative deaths were observed in both groups.
There was no statistically significant difference in overall complications between the two groups. However, compared with PTBS alone, the addition of 125I particles reduced the risk of biliary re-obstruction (19.5% vs. 35.2%, p = 0.017).
Predictors of OS
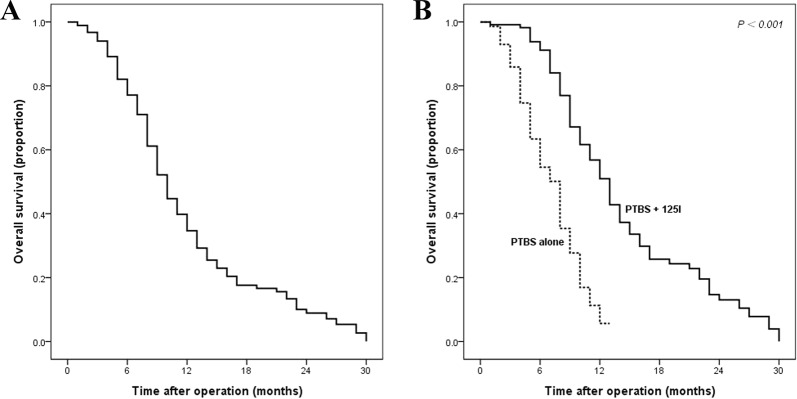
During the follow-up period, 150 (81.5%) patients died. As shown in Fig. 2, the OS was significantly better in the PTBS + 125I group than that in the PTBS group (median: 13 vs. 8 months, 1-year survival rate: 56.8% vs. 11.3%, p < 0.001).
Figure 2.
The survival curves of advanced EHC patients. Overall survival curves for the whole cohort (A), and stratified according to treatment method (B).
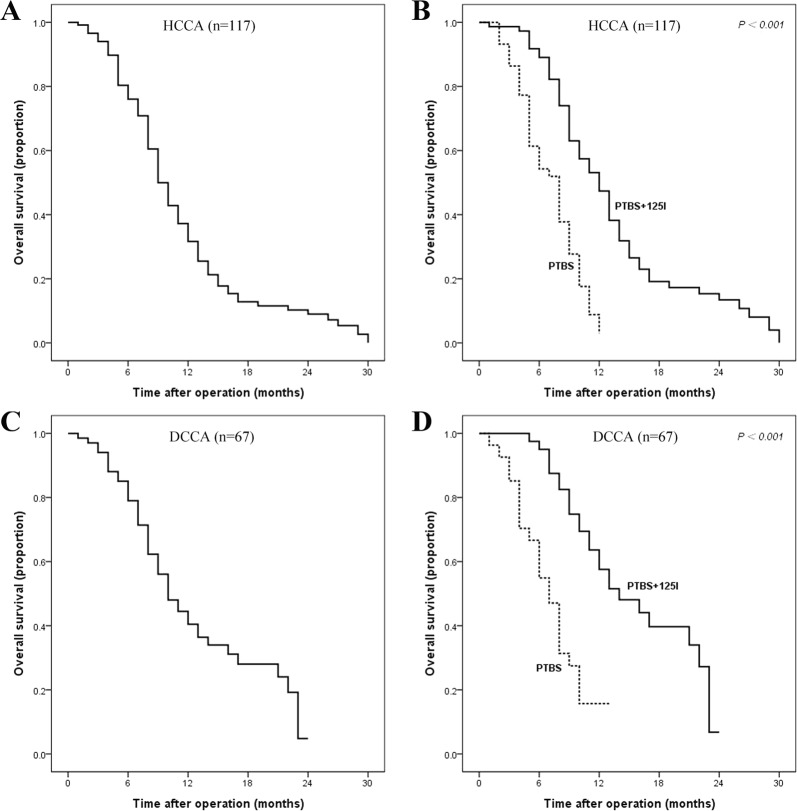
The comparison of OS was further stratified according to tumor location. The median survival time was 9 months versus 10 months and the one-year survival rate was 37.2% versus 44.5% for patients with advanced HCCA and DCCA, respectively. Compared with PTBS alone, the addition of 125I particles can significantly improve OS both in HCCA and in DCCA (Fig. 3, both p < 0.001).
Figure 3.
The survival curves of advanced HCCA and DCCA patients. Overall survival curves for HCCA patients (A) and stratified according to treatment method in HCCA (B); Overall survival curves for DCCA patients (C) and stratified according to treatment method in DCCA (D).
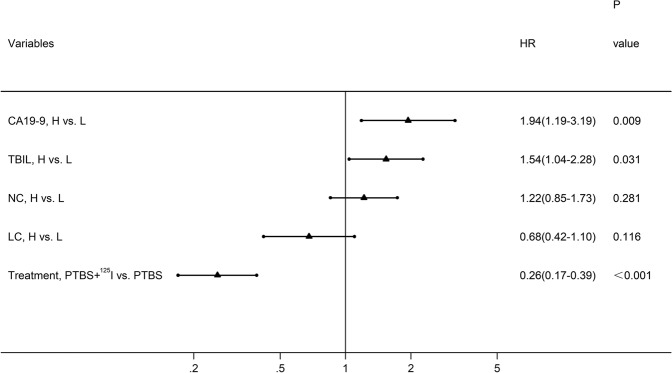
Univariate analysis showed that preoperative levels of CA19-9 (HR = 1.99, 95% CI:1.23–3.23, p = 0.005), TBIL (HR = 1.91, 95% CI: 1.33–2.75, p < 0.001), NC (HR = 1.44, 95% CI: 1.03–2.00, p = 0.032), LC (HR = 0.61, 95% CI: 0.38–0.98, p = 0.043), and treatment method (HR = 0.28, 95% CI: 0.19–0.41, p < 0.001 were significant prognostic factor affecting OS (Table 2). Subsequently, the above factors were entered into the Cox multivariate regression model. Higher preoperative levels of CA19-9 (HR = 1.94, 95% CI: 1.19–3.19, p = 0.009) and TBIL (HR = 1.54, 95% CI: 1.04–2.28, p = 0.031) were identified as independent adverse prognostic factors, and the treatment of PTBS plus 125I implantation was an independent favorable prognostic factor (HR = 0.26, 95% CI: 0.17–0.39, p < 0.001) (Fig. 4).
Table 2.
Univariate analysis of factors associated with OS of EHC patients.
| Variables | Univariate analysis | |
|---|---|---|
| HR (95% CI) | p value | |
| Gender (male vs. female) | 1.027 (0.736–1.433) | 0.876 |
| Age (high vs. low) | 1.049 (0.759–1.449) | 0.771 |
| Location (DCCA vs. HCCA) | 0.826 (0.585–1.168) | 0.279 |
| CA19-9 (high vs. low) | 1.991 (1.225–3.234) | 0.005 |
| CEA (high vs. low) | 1.131 (0.804–1.591) | 0.478 |
| ALT (high vs. low) | 1.225 (0.782–1.921) | 0.376 |
| AST (high vs. low) | 1.188 (0.831–1.697) | 0.345 |
| ALP (high vs. low) | 1.229 (0.785–1.923) | 0.368 |
| GGT (high vs. low) | 1.401 (0.791–2.483) | 0.248 |
| TBIL (high vs. low) | 1.914 (1.334–2.747) | <0.001 |
| DBIL (high vs. low) | 1.370 (0.976–1.924) | 0.069 |
| ALB (high vs. low) | 0.764 (0.550–1.063) | 0.110 |
| PT-INR (high vs. low) | 1.182 (0.826–1.691) | 0.360 |
| NC (high vs. low) | 1.438 (1.032–2.003) | 0.032 |
| LC (high vs. low) | 0.612 (0.381–0.984) | 0.043 |
| PLT (high vs. low) | 0.878 (0.594–1.298) | 0.514 |
| CRP (high vs. low) | 1.189 (0.823–1.718) | 0.356 |
| PTBS + 125I vs. PTBS | 0.280 (0.193–0.406) | <0.001 |
HCCA hilar cholangiocarcinoma, DCCA distal cholangiocarcinoma, CA19-9 carbohydrate antigen 19-9, CEA carcino-embryonic antigen, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma-glutamyl transpeptidase, TBIL total bilirubin, DBIL direct bilirubin, ALB albumin, PT-INR prothrombin time-international normalized ratio, NC neutrophil count, LC lymphocyte count, PLT platelet count, CRP c-reactive protein, PTBS percutaneous transhepatic biliary stenting.
Figure 4.
Forest plot based on the results of multivariate analysis of the factors associated with overall survival of EHC patients.
Discussion
In recent years, the incidence of EHC has increased year by year worldwide. Currently, there are limited treatment options for advanced EHC. It is reported that the 5-year survival rate of advanced EHC is less than 5%3,16. Therefore, the exploration of more effective therapeutic methods is critical to improve the outcomes of advanced EHC.
It is generally acknowledged that local progression or metastasis is considered advanced and unresectable for EHC. PTCD can improve the quality of life and has become one of the main treatment for advanced EHC3. Compared to palliative surgery, biliary stenting reduces postoperative complications and shortens length of hospital stay9. However, due to the rapid growth of tumor, biliary stent alone can easily cause re-obstruction of biliary tract and re-implantation of stent may be needed. In addition, biliary stenting alone shows a limited efficacy in improving survival of EHC9.
Radiotherapy is one of the effective treatments for advanced EHC. Recent study has shown that, compared with biliary stenting alone, the addition of external radiotherapy can extend stent patency time (median: 326 vs. 196 days, P = 0.022) and improve survival time (median: 367 vs. 267 days, p = 0.025)17. The implantation of radioactive particles is an effective form of brachytherapy11,18. Compared with external radiation, the implanted particles can directly act on tumor, and the radioactivity in the tumor is much higher than that in surrounding normal tissues. In addition, brachytherapy can significantly reduce radiation-induced complications such as peptic ulcers, hemorrhage, and radiation enteritis.
Since 2012, PTBS with or without 125I particles implantation has been routinely applied for advanced EHC in our department. In this study, we summarized the relevant clinical data of 184 cases with advanced EHC. We found that, compared with PTBS alone, the addition of 125I particles significantly reduced the risk of biliary re-obstruction and improved survival, while it did not increase the risk of postoperative complications. No radiation-related complications, such as duodenal ulcer and enteritis, were observed in the PTBS + 125I group. It is suggested that, the brachytherapy treatment is safe and may be not limited by some external considerations such as the proximity to the duodenal wall. However, the technique still has several disadvantages. Firstly, as the “P” type tube contained 125I particles is implanted in the intra-cavity of biliary stent, 125I particles implantation will be performed selectively (usually several days later) provided the stent expansion is insufficient even with balloon assisted dilation. Secondly, the tube contained 125I particles may slide or even remove from the stent due to inappropriate postoperative activity.
Multivariate analysis further showed that treatment of PTBS combined with 125I particles were an independent favorable factor of OS, and could reduce the risk of death by 74%. Preoperative TBIL and CA19-9 were also identified as important factors affecting the prognosis of EHC, which are consistent with previous reports by Cai et al. and Waghray et al.19,20. However, the effects of postoperative levels as well as dynamic changes of TBIL and CA19-9 in the prognosis of EHC need further research.
The reasons of the addition of 125I particles significantly improves outcome in advanced EHC may be manifold. It is reported that 125I particles can continuously release X and γ rays to effectively kill tumor cells and inhibit tumorigenesis10. Recently, Du et al. and Kubo et al. showed that, after the implantation of radioactive 125I particles, the percentages of CD3 + T, CD4 + T, natural killer (NK), and regulatory T cells significantly increased in peripheral blood of tumor patients21,22. In addition, the concentrations of IgM, IgG, and IgA, and complements C3 and C4 also increased, indicating that 125I particles may stimulate not only cellular immunity but also humoral immunity21.
There are several limitations in the current study. Firstly, because of the relatively small sample size and single-center retrospective design, larger multi-center prospective studies are still required to validate our findings. Secondly, as sampling poses a huge challenge for EHC, the pathological diagnosis is impossible in the majority of cases.
Chemotherapy and radiotherapy are the two major options for advanced EHC. The combination of 125I particles brachytherapy with chemotherapy (or chemoradiation) is a promising treatment and the safety and efficacy need to be further studied.
Conclusions
In conclusion, for patients with advanced EHC, PTBS combined with 125I particles implantation is superior to PTBS alone in improving liver function, inhibiting biliary re-obstruction and prolonging survival time.
Ethical considerations
This study was complied with Helsinki Declaration and was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. All patients signed informed consent prior to treatment.
Acknowledgements
This work was supported by the Science and Technology Innovation Fund project of Anhui Province (Grant No. 1501041155) and Academic and Technical Leaders Fund project of Anhui Province (Grant No. 2018D194).
Author Contributions
H.L. and H.J. designed the study and revised the manuscript; L.Z., W.W. and Z.Q. performed the clinical data collection and extraction; Q.P. performed clinical data extraction, analysis, and revised the manuscript; Y.W. and Z.M. performed data analysis; X.H. and S.Y. wrote the manuscript; All authors read and approved the final manuscript.
Data Availability
The raw datasets generated during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing Pang, Lei Zhou and Xiao-Si Hu contributed equally.
Contributor Information
Hao Jin, Email: jinhaogandan@126.com.
Hui-Chun Liu, Email: liuhcdoctor@126.com.
References
- 1.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Squadroni M, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11–31. doi: 10.1016/j.critrevonc.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y, et al. The current management of cholangiocarcinoma: A comparison of current guidelines. Biosci Trends. 2016;10:92–102. doi: 10.5582/bst.2016.01048. [DOI] [PubMed] [Google Scholar]
- 5.Zamora-Valdes D, Heimbach JK. Liver Transplant for Cholangiocarcinoma. Gastroenterol Clin North Am. 2018;47:267–280. doi: 10.1016/j.gtc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Lewis HL, Rahnemai-Azar AA, Dillhoff M, Schmidt CR, Pawlik TM. Current Management of Perihilar Cholangiocarcinoma and Future Perspectives. Chirurgia (Bucur). 2017;112:193–207. doi: 10.21614/chirurgia.112.3.193. [DOI] [PubMed] [Google Scholar]
- 7.Park K, Kim KP, Park S, Chang HM. Comparison of gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer. Asia Pac J Clin Oncol. 2017;13:13–20. doi: 10.1111/ajco.12592. [DOI] [PubMed] [Google Scholar]
- 8.Kim YI, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat Oncol. 2013;8:292. doi: 10.1186/1748-717X-8-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indar AA, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol. 2003;15:915–919. doi: 10.1097/00042737-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Qiu H, et al. The Efficacy and Safety of Iodine-125 Brachytherapy Combined with Chemotherapy in Treatment of Advanced Lung Cancer: A Meta-Analysis. J Coll Physicians Surg Pak. 2017;27:237–245. [PubMed] [Google Scholar]
- 11.Wang T, et al. Clinical Study on Using 125I Seeds Articles Combined with Biliary Stent Implantation in the Treatment of Malignant Obstructive Jaundice. Anticancer Res. 2017;37:4649–4653. doi: 10.21873/anticanres.11556. [DOI] [PubMed] [Google Scholar]
- 12.Zhu HD, et al. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol. 2012;56:1104–1111. doi: 10.1016/j.jhep.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 13.World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 14.Cui P, et al. Nutritional prognostic scores in patients with hilar cholangiocarcinoma treated by percutaneous transhepatic biliary stenting combined with 125I seed intracavitary irradiation: A retrospective observational study. Medicine (Baltimore). 2018;97:e11000. doi: 10.1097/MD.0000000000011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, et al. Prognostic value of inflammation-based markers in patients with recurrent malignant obstructive jaundice treated by reimplantation of biliary metal stents: A retrospective observational study. Medicine (Baltimore). 2017;96:e5895. doi: 10.1097/MD.0000000000005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cidon EU. Resectable Cholangiocarcinoma: Reviewing the Role of Adjuvant Strategies. Clin Med Insights Oncol. 2016;10:43–48. doi: 10.4137/CMO.S32821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan Y, Zhu JY, Qiu BA, Xia NX, Wang JH. Percutaneous biliary stenting combined with radiotherapy as a treatment for unresectable hilar cholangiocarcinoma. Oncol Lett. 2015;10:2537–2542. doi: 10.3892/ol.2015.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattiucci GC, et al. A Phase I study of high-dose-rate intraluminal brachytherapy as palliative treatment in extrahepatic biliary tract cancer. Brachytherapy. 2015;14:401–404. doi: 10.1016/j.brachy.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Cai WK, et al. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol. 2014;7:7890–7898. [PMC free article] [PubMed] [Google Scholar]
- 20.Waghray A, et al. Serum albumin predicts survival in patients with hilar cholangiocarcinoma. Gastroenterol Rep (Oxf). 2017;5:62–66. doi: 10.1093/gastro/gow021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du E, et al. Analysis of immune status after iodine-125 permanent brachytherapy in prostate cancer. Onco Targets Ther. 2017;10:2561–2567. doi: 10.2147/OTT.S137491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo M, et al. Enhanced activated T cell subsets in prostate cancer patients receiving iodine-125 low-dose-rate prostate brachytherapy. Oncol Rep. 2018;39:417–424. doi: 10.3892/or.2017.6095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets generated during the current study are available from the corresponding author on reasonable request.