Abstract
Epidemiological studies reported that vitamin D deficiency represents an increasingly widespread phenomenon in various populations. Vitamin D deficiency is considered a clinical syndrome determined by low circulating levels of 25-hydroxyvitamin D (25(OH)D), which is the biologically-inactive intermediate and represents the predominant circulating form. Different mechanisms have been hypothesized to explain the association between hypovitaminosis D and obesity, including lower dietary intake of vitamin D, lesser skin exposure to sunlight, due to less outdoor physical activity, decreased intestinal absorption, impaired hydroxylation in adipose tissue and 25(OH)D accumulation in fat. However, several studies speculated that vitamin D deficiency itself could cause obesity or prevent weight loss. The fat-solubility of vitamin D leads to the hypothesis that a sequestration process occurs in body fat depots, resulting in a lower bioavailability in the obese state. After investigating the clinical aspects of vitamin D deficiency and the proposed mechanisms for low 25(OH)D in obesity, in this manuscript we discuss the possible role of vitamin D replacement treatment, with different formulations, to restore normal levels in individuals affected by obesity, and evaluate potential positive effects on obesity itself and its metabolic consequences. Food-based prevention strategies for enhancement of vitamin D status and, therefore, lowering skeletal and extra-skeletal diseases risk have been widely proposed in the past decades; however pharmacological supplementation, namely cholecalciferol and calcifediol, is required in the treatment of vitamin D insufficiency and its comorbidities. In individuals affected by obesity, high doses of vitamin D are required to normalize serum vitamin D levels, but the different liposolubility of different supplements should be taken into account. Although the results are inconsistent, some studies reported that vitamin D supplementation may have some beneficial effects in people with obesity.
Sources and metabolism of vitamin D
Vitamin D is a lipophilic hormone playing a key role in bone metabolism and calcium homeostasis [1], mainly acting by binding the vitamin D receptor (VDR), whose distribution involves almost all human tissues and cells. Interestingly, recent data have also demonstrated potential modulation of extra-skeletal effects such as the immune system, cardiovascular diseases, insulin resistance, type 2 diabetes and cancer [2], conditions commonly linked with obesity. In order to achieve its biological function, pre-vitamin D3 (D3) must be transformed to its active form, 1,25-dihydroxyvitamin D3 (1,25(OH)D), calcitriol, by two sequential hydroxylation pathways promoted by 25-hydroxylase and 1a-hydroxylase [1].
Vitamin D is derived from two sources: exposure to sunlight and diet. In the first case, during sun exposure, ultraviolet radiation (UVB, wavelength 290 to 315 nm) converts 7—dehydrocholesterol in the skin to pre-vitamin D3 which is immediately converted to vitamin D3 [3]. In the second case, Vitamin D can also be acquired from the diet, but few foods (mainly oily fish) naturally contain vitamin D [1] and several foods, such as margarines and milk, are fortified with Vit D in many countries. After either cutaneous synthesis or dietary absorption, Vitamin D, in its inactive form, circulates bound to the vitamin D–binding protein, which transports it to the liver, where it is converted by vitamin D-25-hydroxylase to 25-hydroxyvitamin D [25(OH)D], the major circulating form of vitamin D used by clinicians to determine vitamin D status [2]. However, 25(OH)D is still biologically inactive, and needs a further hydroxylation process in the kidneys by 25-hydroxyvitamin D-1α- hydroxylase (CYP27B1), which converts 25(OH)D to the biologically active form - 1,25-dihydroxyvitamin D [1,25(OH)D] [3]. This process is tightly regulated by plasma parathyroid hormone levels and serum calcium and phosphorus levels [3]. The main biological function of 1,25(OH)D is the tight regulation of the absorption of renal calcium and of intestinal calcium and phosphorus, all of which are increased in the presence of 1,25(OH)D [3]. In a negative feedback control system, 1,25(OH)D is inactivated by the 25-hydroxyvitamin D-24-hydroxylase (CYP24), which is upregulated by 1,25(OH)D itself [3]. Further, 1,25(OH)D enhances intestinal calcium absorption in the small intestine by interacting with the vitamin D receptor–retinoic acid x-receptor complex (VDR-RXR) [3].
Skeletal and extra-skeletal effects of vitamin D
Nowadays, the biological effects of vitamin D are divided into skeletal and extra-skeletal, the latter being separate from the role in calcium and phosphorus metabolism [4].
Skeletal effects of vitamin D
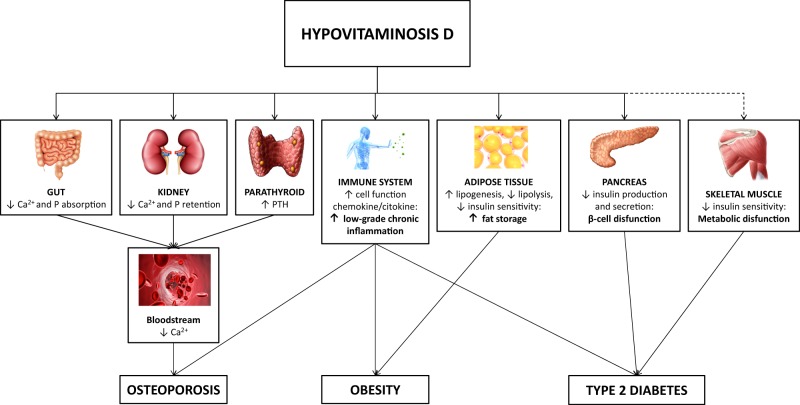
It is well known that Vitamin D plays a pivotal role for normal bone development both in utero and in childhood, leading to optimal skeletal health in adults [3]. Vitamin D fulfils its skeletal function acting directly on three target organs (Fig 1): intestine, stimulating dietary calcium and phosphorus absorption in a parathormone (PTH) independent manner; kidneys, where calcitriol with PTH increases the renal distal tubule reabsorption of calcium; bone, where both calcitriol and PTH stimulate osteoblasts to mobilize skeletal calcium stores [5].
Fig. 1.
Model of hypovitaminosis D-induced alterations in obesity. Potential mechanisms in the modulation of skeletal and extra-skeletal modification. Red lines: inhibition pathways. Dotted lines: not well established mechanisms. PHT: parathormone; Ca2+: calcium; P: phosphorus; ↓: decreased; ↑: increased
In the skeleton, the biological function of 1,25(OH)D is exerted by binding its receptor in osteoblasts, resulting in an increase in the expression of the receptor activator of nuclear factor-κB ligand (RANKL); RANK, the receptor for RANKL on preosteoclasts, binds RANKL, which induces the maturation of preosteoclasts in osteoclasts [4]. Mature osteoclasts keep calcium and phosphorus at normal levels in the blood, at the expense of bone [4]. Adequate calcium (Ca2+) and phosphorus (HPO42–) levels promote the mineralization of the skeleton [4]. Severe vitamin D deficiency causes two clinical syndromes: rickets in children and osteomalacia in adults [3].
The absorption of dietary calcium and phosphorus is reduced by 90 and 40% respectively when vitamin D is missing [5]. The binding of 1,25(OH)D with the VDR increases intestinal calcium absorption to 30–40% and phosphorus absorption to approximately 80% [5]. In a study on white, black, and Mexican-American men and women [6], 25(OH)D serum levels needed to be approximately 40 ng per millilitre in order to achieve optimal bone density,. When the level was 30 ng per milliliter or less, there was a significant decrease in intestinal calcium absorption [5] that was associated with increased PTH [4].The deposition of calcium in the skeleton is also altered during foetal development if low levels of calcium and vitamin D are present in utero [7]. If vitamin D deficiency persists, the parathyroid glands are maximally stimulated, leading to secondary hyperparathyroidism [4].
Extraskeletal effects of vitamin D
Calcitriol exerts multiple effects on muscle health: it is probably produced in situ in muscle cells, as indicated by the recent identification of 1α- hydroxylase [8] in muscle, which is thought to modulate muscle physiology via the VDR by regulating gene transcription and inducing de-novo protein synthesis [9]. The influence of this pathway on skeletal muscle physiology could explain why vitamin D deficiency is often associated with diffuse muscle pain and muscle weakness, possibly as a result of muscle atrophy of mainly type II muscle fibers and secondary hyperparathyroidism and its related hypophosphatemia [2, 10].
Furthermore, Vitamin D has important anti-inflammatory and immunoregulatory functions, namely the enhancement of the innate immune system and the inhibition of the adaptive immune responses [11]. Therefore, vitamin D seems to be involved in immune tolerance and, thus, might play a key role in the pathophysiology of various autoimmune diseases (i.e., type 1 diabetes mellitus, multiple sclerosis, Crohn’s disease, rheumatoid arthritis) [2, 11]. Calcitriol can modulate the immune responses in secondary lymphoid organs as well as in target organs through a number of actions: it regulates chemokine biosynthesis, it counteracts autoimmune inflammation and it induces differentiation of immune cells to promote self-tolerance [11]. Immune cells possess both CYP27B1 bioactivity and a VDR (Fig. 1), and this could explain why specific polymorphisms in the VDR gene may increase the risk for multiple autoimmune diseases, disease activity and time of onset [11, 12].
Moreover, due to the ability to modulate immune system, some activities have been historically ascribed to vitamin D, especially in fighting tuberculosis, influenza and viral upper respiratory tract infections [13]. These putative beneficial effect of vitamin D on the immune system have been ascribed to its capability to enhance the production of antimicrobial peptides like cathelicidin, to the induction of autophagy and the stimulation of the synthesis of reactive oxygen species by the (reduced) nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and potentially reactive nitrogen species by inducible nitric oxide synthase (iNOS) [2, 13], ultimately leading to a stimulation of the innate immune system.
Biological and rational prerequisites exist to consider vitamin D a key molecule for neuromodulation and neuroprotection, as a consequence of some observations: the wide and heterogeneous presence of VDR in specific areas of brain (both neurons and glia), vitamin D’s influence on synthesis of neurotransmitters and neurotrophins, its antioxidant properties and its ability to improve clearance of amyloid-β peptide [14]. Thus, hypovitaminosis D could be considered as a risk factor for cognitive decline, dementia, neuropsychiatric disorders [14, 15].
Interestingly, preclinical studies reported that Vitamin D has anticancer effects in vitro and in vivo through several mechanisms [16] including: the anti-proliferative action on cancer tissue by inducing cyclin-dependent kinase (CDK) inhibitor synthesis, and by interfering with different growth factors and their signalling pathways (e.g., IGF-1, TGF-β, NF-kB, Wnt/β-catenin, MAP kinase 5); several pro-apoptotic mechanisms (by upregulating the pro-apoptotic gene Bax and by downregulating the anti-apoptotic gene Bcl-2); the suppression of cancer angiogenesis (by regulating the expression of pivotal factors controlling the angiogenesis, i.e., prostaglandins); the suppression of cancer invasion and metastasis (i.e., by inducing E-cadherin expression, by reducing the activity of cell invasion-associated serine proteases and metalloproteinases) [16]. Vitamin D also appears to downregulate androgen and estrogen receptor signalling, thus inhibiting tumour growth in vitro of prostate and breast cancer, well known sex hormone-dependent tumours; [16] finally, it has also been shown to suppress the expression of aromatase in vitro, thereby hindering breast cancer growth [16].
Moreover, several animal model studies suggest that vitamin D has potential protective effects against type 2 diabetes development, especially when the damage of β-cell and insulin action is in its early stages (reviewed in [17]). The antidiabetic properties of vitamin D rely on the improvement of hepatic glucose and lipid metabolism through activation of Ca2 + /CaMKK/AMPK signalling (AMPK is even a therapeutic target of metformin) [17]; then, vitamin D shows a RAS-suppressing action that may benefit β-cell function.
It is interesting that a wide variety of potential cardiovascular benefits can be part of vitamin D’s pleiotropic profile, even as a consequence of direct positive actions on vascular smooth muscle cells, cardiomyocytes, renin-angiotensin-aldosterone system (RAAS) [18]; indeed, vitamin D seems to protect the cardiovascular system, (namely against atherosclerosis and both myocardial infarction and stroke) at least in part, by causing relaxation of vascular smooth muscle, decreasing the development of atherosclerotic forming foam cells, lowering production of renin [18].
Lastly, health span advantages (i.e., antiaging properties) may arise from Vitamin D induction of α-klotho, a renal hormone that is an enzyme/co-receptor that protects against osteopenia, hyperphosphatemia, endothelial damage, cognitive decline, neurodegenerative diseases, hearing loss, and skin atrophy [19].
Hypovitaminosis D and its related morbidities
Adipose tissue expresses VDR and 1α-hydroxylase locally converts 25(OH) cholecalciferol to calcitriol, and it is, thus, both a direct target of vitamin D and a site of local synthesis of calcitriol [20]; moreover, of interest, adipose tissue 1α-hydroxylase is not regulated by dietary calcium, cholecalciferol and other different factors (i.e., PTH, calcitonin) by contrast with renal 1α-hydroxylase [20]. Indeed, it appears that Vitamin D might play an anti-obesity effect by inhibiting the adipogenesis during early adipocyte differentiation and independently of PTH [20]. Furthermore, secondary hyperparathyroidism induced by a low vitamin D can cause the overflow of calcium into adipocytes, increasing lipogenesis and expression of fatty acid synthase (a key regulatory enzyme in the storage of lipids), and reducing lipolysis [21]. Despite the above biological assumptions and the apparent role of vitamin D in preventing excess body fatness, the precise nature of the common association between hypovitaminosis D and obesity [22] still constitutes a matter of open debate [23], as it cannot be excluded that a bidirectional causal relationship exists where a low vitamin D status represents a risk factor of adiposity excess.
Vitamin D receptors (VDRs) are expressed on different cell types including myocytes, cardiomyocytes, pancreatic beta-cells, vascular endothelial cells, neurons, immune cells and cells in a large variety of tissues, in particular brain, prostate, breast, and colon, which are able to respond to 1,25(OH)D stimulation [4]. In addition, some of these tissues and cells express the CYP27B1 enzyme, involved in the activation of 25(OH)D to 1,25(OH)D, the active form[2], which directly or indirectly regulates more than 200 genes involved in the regulation of cellular proliferation, differentiation, apoptosis, and angiogenesis [2]. In particular, 1,25(OH)D reduces cellular proliferation and induces terminal differentiation of both normal and cancer cells [2, 4]. Moreover, vitamin D deficiency seems to be associated with an increased risk of cardiovascular diseases (CVD) and non-skeletal major chronic diseases in general [24]. Hypovitaminosis D might predispose to hypertension, left ventricular hypertrophy, congestive heart failure, chronic vascular inflammation and increases the risk of major adverse cardiovascular events [2, 24]. Furthermore, cross-sectional studies showed that vitamin D deficiency is associated with an increased risk of developing metabolic syndrome and its related complications and clinical signs, i.e., reduced HDL-cholesterol levels [24–26].
Obesity
Vitamin D deficiency is a well-recognized common feature of people with obesity [27], suggesting that the adipose tissue might play a role in the low vitamin D serum levels. However a causal relationship between obesity and low levels of circulating 25(OH)D has not been completely elucidated yet. Behavioural factors have been proposed, such as reduced sunlight exposure and low dietary intake of vitamin D-enriched foods in people with obesity [28, 29]. Alternatively it has been proposed that vitamin D, being fat-soluble, could be sequestered in body fat depots, leading to lower bioavailability in the obese state [30], a hypothesis that was confirmed in humans and animal models receiving high doses of oral vitamin D [31]. A second hypothesis considers low serum 25(OH)D in people with obesity as a result of volumetric dilution of vitamin D in the large adipose stores [32], as recently confirmed in a small population of women with obesity and those with normal body weight [33].
Despite the well-established association between obesity and vitamin D deficiency, few experimental studies have investigated the biological bases involved in vitamin D metabolism in adipose tissue, with inconsistent results [34]. Moreover, the obesity-induced inflammation and insulin resistance of adipose tissue further complicates this scenario [23]. Indeed, resistance to the lipolytic effects of catecholamines and natriuretic peptide in both people with obesity [35] and those with insulin-resistance [36], mediated by a low density of β2-adrenergic receptors in adipocytes, might lead to a reduced release of vitamin D from adipose tissue. Moreover, both human and murine adipocytes express vitamin D-metabolizing enzymes [37] and 25-hydroxylation and 1α-hydroxylation are impaired in obese women [38], suggesting that adipose tissue not only passively accumulates vitamin D, leading to reduced bioavailability for 25-hydroxylation, but also changes its metabolism in obesity.
These recent advances suggest a sequestration process of fat-soluble vitamin D in body fat depots, leading to lower bioavailability in the obese state [30], which is further promoted by resistance to the stimulation of lipolysis by catecholamines and natriuretic peptide in both people with obesity [35] and in adipocytes taken from people with insulin-resistance [36]. Indeed, one of the factors contributing to the development of obesity may be an impaired ability to use fat (as demonstrated by resistance to the stimulation of lipolysis by catecholamines) that it has been reported to not change after weight loss, thus suggesting that this could be a factor leading to the development of overweight rather than a secondary factor resulting from the obese state [39–41]. Therefore, catecholamine resistance could be an important cause of obesity considering the corroborated evidence of different polymorphisms in genes encoding key proteins involved in the lipolytic pathway [42]. This derangement could ultimately lead to a reduced release of vitamin D from fat depots, given the fat-soluble secosteroid nature of vitamin D. Indeed, vitamin D release in the medium was reduced after catecholamine stimulation of insulin-resistant adipocytes, compared with controls [43], possibly due to a reduction of β2-adrenoceptor expression, as showed also in subcutaneous adipose tissue from people with obesity [43].
Hypovitaminosis D and obesity: clinical aspects from fetal imprinting until older age
Epidemiological data indicate that deficiency of vitamin D in various populations represents an increasingly widespread phenomenon [44]. Vitamin D deficiency is considered a clinical syndrome determined by low circulating levels of 25-hydroxyvitamin D (25(OH)D) [44]. Most studies considered different ranges of low vitamin D to define deficiency or insufficiency status [44]. Recently, attention has been focused on the pleiotropic effects exerted by vitamin D [4].
Therefore, hypovitaminosis D is thought to result in a number of negative consequences for health and may be correlated with the manifestation of specific civilization-linked diseases, including obesity [2, 4]. In fact, a major health issue linked to Vitamin D is the growing obesity rate. The World Health Organization stated that in 2016 more than 1.9 billion adults were overweight, of which 650 million were obese [45]. To explain the deficiency of this fat-soluble vitamin in people with an increased adipose tissue mass, the following possible mechanisms have been suggested: lower dietary intake; altered behaviour that reduces cutaneous synthesis, reduced synthetic capacity, reduced intestinal absorption, altered metabolism, and sequestration in adipose tissue [46].
An interesting association between hypovitaminosis D and obesity has been demonstrated in several studies. Based on evidence that people with obesity showed higher serum PTH concentrations, compared to people with a healthy weight, and considering that PTH levels are inversely associated with vitamin D levels, Bell and colleagues [47] investigated, for the first time, the role of obesity in influencing vitamin D status. Their study demonstrated that serum 25(OH)D was lower in people with obesity compared to individuals who were lean, probably due to a feedback inhibition of hepatic synthesis of the metabolite by increased circulating levels of 1,25-dihydroxycholecalciferol (1,25(OH)D) [47]. Subsequently several different studies reproduced these results both in adults and children [48, 49]. These studies demonstrated that each unit of increase of BMI was associated with a ∼1.00% decrease of 25(OH)D [50, 51] and confirmed that this inverse association persisted independently from variation in physical activity or vitamin D intake. Moreover, the authors showed a strong association between subcutaneous and visceral fat and low concentrations of 25(OH)D [52]. Arunabh et al. demonstrated that low levels of vitamin D are more strongly associated with total body fat measured using dual-energy x-ray absorptiometry (DXA) but not with BMI and concluded that body fat percentage is independently, inversely associated with serum 25(OH)D [53]. More recently, Brock colleagues confirmed this association both in men and in women [54], and González-Molero and colleagues hypothesized that lower 25-(OH)D values in people with obesity could be an independent predictor of obesity rather than being secondary to this condition [55]. Indeed, considering that low vitamin D status is able to induce secondary hyperparathyroidism, the consequent increase of intracellular calcium in adipocytes could be responsible for the increased expression of fatty acid synthase, a key regulatory enzyme in the deposition of lipids, and the decrease of lipolysis [46].
On the other hand, Ding and colleagues [48] demonstrated that adiposity parameters could be considered predictors of both increased incidence of vitamin D deficiency and reduced chances of recovering from it; the authors also suggested a link between markers of an obesity-associated chronic pro-inflammatory state, such as leptin and interleukin 6 (IL-6), and vitamin D deficiency in older adults [48]. Finally, hypovitaminosis D could be able to determine an increase in adiposity during childhood, with an important early metabolic impairment in these individuals [56].
Several different mechanisms could be considered as being causative for obesity-related vitamin D deficiency [51], including lower dietary intake of vitamin D, a scarce skin exposure to sunlight, due to less outdoor physical activity compared to lean individuals, decreased intestinal absorption, impaired hydroxylation in adipose tissue and accumulation of 25(OH)D in tissue mass [46, 50]. However, vitamin D deficiency itself could contribute to the development of obesity or interfere with an effective weight loss [50, 57]. Thus, hypovitaminosis D could contribute to adipose tissue accrual with a consequent negative impact on metabolic homeostasis leading to many comorbidities, including insulin resistance and type 2 diabetes mellitus [58].
Vitamin D status during pregnancy could have an epigenetic role since it is not only pivotal in maternal skeletal maintenance and fetal skeletal development, but could influence fetal “imprinting”, which can affect chronic disease susceptibility soon after birth [59]. Interestingly, maternal vitamin D deficiency during pregnancy was associated with impaired lung development in 6-year-old offspring, neurocognitive difficulties at the age of 10, increased risk of eating disorders in adolescence, and lower peak bone mass at the age of 20 [60]. Moreover, an optimal Vitamin D level is also essential from the adolescent years until old age since it is needed for several health benefits [61]. Serum Vitamin D levels decline with puberty onset, predisposing to a higher risk of obesity and insulin resistance (IR), in particular in patients with pre-pubertal suboptimal Vitamin D serum levels [62, 63].
In people with obesity, vitamin D deficiency was demonstrated to be independently linked to impaired glucose tolerance, IR and type 2diabetes mellitus, associated with increased fat tissue [64, 65]. Since vitamin D has anti-inflammatory properties [16, 66], it is not surprising that it can have beneficial effects on improving islet-cell functions, insulin release, and decreasing IR [67]. A seasonal variation in plasma glucose concentration was observed in people both with and without diabetes, leading to the hypothesis of an association between vitamin D status and diabetes mellitus in humans [67]. Starting from this evidence, cross-sectional studies in adults, children and adolescents demonstrated the correlation between low 25(OH)D concentrations and glucose intolerance, IR and the future risk of metabolic syndrome [58, 68, 69]. This inverse association with the metabolic syndrome risk seems to be partly driven by the association of vitamin D with glucose homeostasis [70].
Further, visceral obesity is known to be associated with Non-Alcoholic Fatty Liver disease (NAFLD) leading some authors to hypothesize that Vitamin D could also be linked to NAFLD. Seo and colleagues in 2013 confirmed the strong inverse correlation between vitamin D levels and NAFLD [71], demonstrating that low vitamin D levels were highly associated with NAFLD independently of visceral obesity in subjects with diabetes or IR.
Furthermore, low 25(OH)D concentrations seem to be involved in the pathogenesis of hypertriglyceridemia and atherogenic dyslipidemia through inflammation, both in adults and children [72]. In patients with type 2 diabetes, serum 25(OH)D levels were found to be inversely correlated with monocyte adhesion to endothelial cells [73]. Deficient or insufficient serum 25(OH)D levels have been documented in patients with diabetes and cardiovascular diseases (CVD) (i.e., myocardial infarction, stroke, heart failure, and peripheral arterial disease) [18]. The prospective observational Framingham Offspring Study, demonstrated an association between hypovitaminosis D and CVD in 1,739 subjects who were free of CVD at baseline [74]. In this population, followed up for a mean of 5.4 years, the rate of CVD end point (fatal or nonfatal MI, stroke or heart failure) was 53–80% higher in subjects with lower levels of vitamin D [74].
Moreover, studies from our group demonstrated decreased levels of vitamin D in people with obesity independently from gender [75], related to a lower bone mineral density, likely leading to an increased risk of osteoporosis and further suggesting a pivotal role of adipose tissue in the modulatory role of vitamin D level, instead of a role of sex steroids in regulating vitamin D status.
In addition, it has to be pointed out that epidemiological and animal studies have shown that low levels of vitamin D seem to be linked with an increased risk of cancer [76, 77]. Nevertheless, although known factors of cancer risk (i.e., obesity, unhealthy habits [78], smoking) may influence and even falsify this apparent relationship, to date it has not been proven whether there is a beneficial effect of vitamin D in decreasing the adverse repercussions of adiposity excess on cancer incidence [79]. Lastly, a potential and promising strategy for cancer prevention could be to target vitamin D insufficiency [79].
Although the effect of hypovitaminosis D in adults with obesity have been described and evaluated over several years, less is known regarding decreased levels in childhood and adolescence. In the United States (US) prevalence of hypovitaminosis D in children and adolescents is associated with the degree of adiposity and reaches about 35% in obese and 50% in severely obese individuals [80]. Interestingly, in this population, hypovitaminosis D seems to exacerbate the negative effects of fat accumulation alone on overall health [80, 81] in particular IR, a pro-inflammatory state, and reduced bone mineralization, predisposing to an increased future risk of osteoporosis, type 2 diabetes, and cardiovascular diseases [82]. Most of the studies in children and adolescents with obesity demonstrated a significant association between circulating 25(OH)D concentration and indices of glucose homeostasis [83]. However, to date the underlying biological mechanisms of this association are unknown, but Peterson and colleagues hypothesized an enhancement of peripheral/hepatic uptake of glucose, a regulation of insulin homeostasis and/or anti-inflammatory properties [83].
Regarding CVD risk, cross-sectional studies demonstrated a significant inverse association between 25(OH)D, systolic blood pressure, arterial stiffness and CVD risk factors in obese children [84].
NAFLD represents another condition related to obesity and metabolic complications in children with obesity. However, the association between NAFLD and vitamin D levels is still controversial. Different studies demonstrated that patients with NAFLD had lower serum concentrations of 25(OH)D compared to obese children and adolescents without NAFLD [85] and that lower 25(OH)D concentration is independently inversely associated with NAFLD [86]. On the other side, a report using data from NHANES, showed that vitamin D status is not independently associated with NAFLD [87]. Therefore, further studies are needed to determine whether vitamin D deficiency contributes to the risk of developing NAFLD.
As regards to the relationship between vitamin D status and bone homeostasis, the effect of obesity in influencing bone mineral accretion is not fully clarified. It is known that childhood obesity represents an important risk factor for low bone mass and consequently fractures [88]. For this reason, along with other factors, Vitamin D deficiency seems to promote an altered bone homeostasis in obese children through indirect (inflammation) and/or direct (calcium homeostasis/bone mineralization) mechanism at the skeletal level [83].
Puberty with its complex phenomena is able to influence and modify many of the metabolic patterns and conditions related to obesity. However, the effect of puberty in determining the correlation between 25(OH)D serum levels and IR is still debated. Two studies demonstrated that this association does not become significant after reaching puberty [89] and a cross-sectional analysis on children with obesity needed adjustments for puberty to reveal vitamin D status associations with HOMA-IR [90]. On the contrary, Creo and colleagues found no association between vitamin D status and IR in a cohort of children with obesity [91].
Vitamin D deficiency and bariatric surgery
Studies regarding calcium and phosphorus metabolism of patients who underwent bariatric surgery are limited, although negative energy balance and nutritional alterations related to different types of bariatric surgery are well-documented in the literature [92–95].
Bariatric surgery consists of a number of procedures, but the most commonly performed techniques are gastric ring (or AGB), the vertical sleeve gastrectomy (VSG), the Roux-en-Y Gastric Bypass (RYGB) and biliopancreatic diversion (BPD) [96]. An emerging complication related to bariatric surgery is mineral and vitamin D deficiency [96]. It is likely that at least five main mechanisms may explain vitamin D deficiency in patients who underwent bariatric surgery: (1) the pre-surgical hypovitaminosis D that characterised people with obesity; (2) inappropriate vitamin D supplementation during weight loss after bariatric surgery; (3) vitamin D malabsorption caused by bile salt deficiency linked to bariatric surgery; (4) vitamin D malabsorption due to intestinal bacterial overgrowth [97]; (5) the delayed blend of ingested nutrients with pancreatic enzymes and bile acids causing vitamin D malabsorption
Independently from the baseline vitamin D status, the AGB and the VSG techniques do not seem to influence PTH concentrations or serum vitamin D levels, with the latter remaining stable or increasing [98, 99].
As regards the RYGB procedure, some studies documented lower intestinal absorption of calcium and higher rates of vitamin D deficiency;[100] however, despite vitamin D supplementations, improvements in vitamin D status did not occur [100]. Moreover, in another study, albeit an increased vitamin D dose (200% of normal) was taken, vitamin concentrations remained stable [101]. Finally, Stein et al. performed a prospective study in women who underwent bariatric surgery between May 2009 and February 2011 prior to and 1 year after surgery. All the participants had normal vitamin D levels before surgery. Among the RYGB patients, 9 took ergocalciferol in doses of 50,000 IU weekly (mean duration of use 13 weeks), 2 received vitamin D in a multivitamin (as cholecalciferol; mean dose 550 IU/d, mean duration of use 18 months), whereas 3 did not take any vitamin D supplementation. Of those who had restrictive procedures, 3 were taking ergocalciferol in doses of 50,000 IU weekly (mean duration of use 5 wk), 4 were receiving cholecalciferol as part of a multivitamin, and 1 was not using any supplementation prior to surgery. After surgery vitamin D levels remained stable [102].
The BPD procedure, which is considered the most effective in reducing body weight, is associated with vitamin D deficiency in more than 50% of cases despite vitamin D supplementation [103], furthermore comparing post-surgical 25(OH)D levels, hypovitaminosis D was more prevalent in the BPD group than the RYGB group, and vitamin D supplementation dosage was always higher in the BPD group [104].
Vitamin D treatment
In adult and paediatric populations food-based strategies could improve vitamin D status and, therefore, reduce skeletal and extra-skeletal diseases risk [105, 106].
The right dose of vitamin D intake necessary to maintain an adequate serum concentration is still debated. Lee et al. demonstrated that a larger amount of vitamin D supplementation is required in people with obesity compared with those who are lean, suggesting that supplementation dosage should be modified on the basis of the degree of obesity [107]. Moreover, their data suggested that the higher supplementation doses of vitamin D also allow an increased conversion of 25(OH)D to 1,25(OH) D [107].
Therefore, Holick et al. concluded that in people with obesity higher doses of vitamin D3 are necessary to treat vitamin D deficiency and to maintain 25(OH)D levels > 30 ng/mL (two to three times higher, initially at least 6000–10,000 IU/d followed by maintenance therapy of 3000–6000 IU/d) [44].
As reported previously, while pre-vitamin D (cholecalciferol) is mainly found in fat depots (approx. 75%), 25(OH)D is more equally distributed (approx. 35% in fat) [32]; so, since cholecalciferol is predominantly stored in adipose tissue, when it is administered orally it is less effective in raising 25(OH)D levels in obese subjects [108, 109]. For this reason the supplementation with calcifediol (25(OH)D), rather than the more fat-soluble cholecalciferol, should be preferred in obese patients to avoid a massive accumulation of the latter in fat cells. Indeed, in a cohort of people with vitamin D-deficiency randomly assigned to two treatment regimens receiving either calcifediol or cholecalciferol, people with normal weight had comparable responses to either treatment. Conversely, the proportion of people with obesity achieving vitamin D sufficiency was significantly higher after 6 months of supplementation with calcifediol, suggesting a more rapid effect of this treatment in the obese state [43]; indeed, the authors [43] estimated vitamin D supplementation be 2 to 3 times higher for people with obesity and 1.5 times higher for those who were overweight relative to people with normal weight [110, 111]. Some studies reported that vitamin D supplementation may have beneficial effects in people with obesity [112, 113]. For example, in a double-blind, placebo-controlled, randomized clinical trial, Salehpour and colleagues [112] demonstrated that 12 weeks vitamin D supplementation (1000 IU per day as cholecalciferol) in women with overweight or obesity resulted in a significant decrease in the body fatness compared with those in the placebo group, with the extent of the response to vitamin D supplementation depending on baseline body weight. Moreover, after supplementation, the increase in serum 25(OH)D levels was lower in women with overweight or obesity than in those with normal BMI [113]. In women with obesity ∼2.5 IU/kg were required to obtain a unit (0.4 nmol/l) increment in 25(OH)D [32].
Regarding low vitamin status after a malabsorptive bariatric procedure, the Endocrine Society Clinical Practice Guidelines state that low vitamin D status should be treated with 50.000 IU once a week for 8 weeks or 6.000 IU/day of vitamin D3 to achieve a serum concentration of 25(OH)D3 > 30 ng/mL, followed by maintenance therapy of 1500–2000 IU/day [44, 114].
Finally, with regard to treatment of hypovitaminosis D for children with documented deficiency, the AAP guidelines recommend a daily dose of 5000 IU until stores are replenished [115].
Recommendation of Endocrine Society guidelines suggested with 50,000 IU once a week for 8 weeks or 6000 IU/day of vitamin D3 to achieve a serum concentration of 25(OH)D3 > 30 ng/mL, followed by maintenance therapy of 1500–2000 IU/day [44]. A vitamin D supplementation with 25,000 IU weekly in young people with obesity (8–18-year olds) was found to be well tolerated and restored a sufficient vitamin D status in more than 84% of individuals [116].
Conclusions
Vitamin D deficiency is a global and increasing health challenge. Serum 25(OH)D is not only a predictor of bone health, but it is also an independent predictor of other chronic diseases. In particular, low levels of 25(OH)D are highly frequent in obesity. Many explanations for this association have been proposed, such as altered vitamin D metabolism, behavioural factors leading to reduced sunlight exposure, or reduced intake of vitamin D-enriched foods in obesity, but the difference in 25(OH)D between people with or without obesity is most likely to be explained by the increased adipose mass that could act as a storage site for highly lipophilic hormones such as vitamin D. Thus, body size should be taken into account when prescribing vitamin D supplementation, as people with obesity may need larger dosages. Moreover, the biochemical properties of different supplements should also been considered, as pre-vitamin D3 (cholecalciferol) is more liposoluble than 25(OH)D (calcifediol), and therefore the latter should represent the preferred compound in clinical practice in obese patients. Finally, based on the results of recent trials, vitamin D supplementation shows some beneficial effects in the treatment of obesity and related comorbidities. In conclusion, we suggest that awareness of the relationship between obesity and vitamin D status is clinically relevant and the investigation of the pathogenic mechanisms could lead to improve the clinical approach to obesity by healthcare professionals such as dietitians and clinicians.
Acknowledgements
Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group members served as collaborators and approved the final version of the manuscript: Annamaria Colao, Antonio Aversa, Barbara Altieri, Luigi Angrisani, Giuseppe Annunziata, Rocco Barazzoni, Luigi Barrea, Giuseppe Bellastella, Bernadette Biondi, Elena Cantone, Brunella Capaldo, Sara Cassarano, Rosario Cuomo, Luigi Di Luigi, Andrea Di Nisio, Carla Di Somma, Ludovico Docimo, Katherine Esposito, Carlo Foresta, Pietro Forestieri, Alessandra Gambineri, Francesco Garifalos, Cristiano Giardiello, Carla /Giordano, Francesco Giorgino, Dario Giugliano, Daniela Laudisio, Davide Lauro, Andrea Lenzi, Silvia Magno, Paolo Macchia, MariaIda Maiorino, Emilio Manno, Chiara Marocco, Paolo Marzullo, Chiara Mele, Davide Menafra, Silvia Migliaccio, Marcello Monda, Filomena Morisco, Fabrizio Muratori, Giovanna Muscogiuri, Mario Musella, Gerardo Nardone, Claudia Oriolo, Uberto Pagotto, Pasquale Perrone Filardi, Luigi Piazza, Rosario Pivonello, Barbara Polese, Paolo Pozzilli, Giulia Puliani, Stefano Radellini, Gabriele Riccardi, Domenico Salvatore, Ferruccio Santini, Giovanni Sarnelli, Lorenzo Scappaticcio, Silvia Savastano, Bruno Trimarco, Dario Tuccinardi, Paola Vairano, Nunzia Verde, Roberto Vettor.
Funding
This article is published as part of a supplement funded by Endocrinology Unit, Department of Clinical Medicine and Surgery, University Federico II, Naples, Italy.
Compliance with ethical standards
Conflict of interest
SM received consulting fees from Aegerion, Shire, and Eli Lilly. The remaining authors declare that they have no conflict of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Battault S, Whiting SJ, Peltier SL, Sadrin S, Gerber G, Maixent JM. Vitamin D metabolism, functions and needs: from science to health claims. Eur J Nutr. 2013;52:429–41. doi: 10.1007/s00394-012-0430-5. [DOI] [PubMed] [Google Scholar]
- 2.Wacker M, Holick MF. Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;10:111–48. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–72. doi: 10.1172/JCI29449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96:365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 7.Cooper C, Javaid K, Westlake S, Harvey N, Dennison E. Developmental origins of osteoporotic fracture: the role of maternal vitamin D insufficiency. J Nutr. 2005;135:2728S–34S. doi: 10.1093/jn/135.11.2728S. [DOI] [PubMed] [Google Scholar]
- 8.Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303:C396–C405. doi: 10.1152/ajpcell.00014.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari H. Relevance of vitamin D in muscle health. Rev Endocr Metab Disord. 2012;13:71–77. doi: 10.1007/s11154-011-9200-6. [DOI] [PubMed] [Google Scholar]
- 10.Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 11.Antico A, Tampoia M, Tozzoli R, Bizzaro N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev. 2012;12:127–36. doi: 10.1016/j.autrev.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Bellastella G, Maiorino MI, Petrizzo M, De Bellis A, Capuano A, Esposito K, et al. Vitamin D and autoimmunity: what happens in autoimmune polyendocrine syndromes? J Endocrinol Invest. 2015;38:629–33. doi: 10.1007/s40618-014-0233-z. [DOI] [PubMed] [Google Scholar]
- 13.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: Modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Eyles DW, Burne THJ, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2012;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Annweiler C, Dursun E, Feron F, Gezen-Ak D, Kalueff AV, Littlejohns T, et al. Vitamin D and cognition in older adults: updated international recommendations. J Intern Med. 2015;277:45–57. doi: 10.1111/joim.12279. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–36. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 17.Leung PS. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients. 2016;5;8:147. doi: 10.3390/nu8030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muscogiuri G, Annweiler C, Duval G, Karras S, Tirabassi G, Salvio G, et al. Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke. Int J Cardiol. 2017;230:577–84. doi: 10.1016/j.ijcard.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. 2015;36:174–93. doi: 10.1210/er.2013-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas MA. Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165(Pt B):369–38. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Zemel MB. Regulation of adiposity and obesity risk by dietary calcium: Mechanisms and implications. J Am Coll Nutr. 2002;21:146S–51S. doi: 10.1080/07315724.2002.10719212. [DOI] [PubMed] [Google Scholar]
- 22.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham heart study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamendola CA, Ariel D, Feldman D, Reaven GM. Relations between obesity, insulin resistance, and 25-hydroxyvitamin D. Am J Clin Nutr. 2012;95:1055–9. doi: 10.3945/ajcn.111.032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Ma J, Manson JE, Buring JE, Gaziano JM, Sesso HD. A prospective study of plasma vitamin D metabolites, vitamin D receptor gene polymorphisms, and risk of hypertension in men. Eur J Nutr. 2013;52:1771–9. doi: 10.1007/s00394-012-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraser A, Williams D, Lawlor DA. Associations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: analysis of 3 NHANES cycles (2001–6) PLoS ONE. 2010;9;5:e13882. doi: 10.1371/journal.pone.0013882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;11:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 27.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Ober Rev. 2015;16:341–9. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 28.Hyppönen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 29.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34:2359–63. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]
- 30.Need AG, Morris HA, Horowitz M, Nordin C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–5. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 31.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 32.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20:1444–8. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 33.Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res. 2016;32:237–42. doi: 10.1002/jbmr.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, Cole L, et al. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J Steroid Biochem Mol Biol. 2014;144(Pt A):232–6. doi: 10.1016/j.jsbmb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 35.Ryden M, Backdahl J, Petrus P, Thorell A, Gao H, Coue M, et al. Impaired atrial natriuretic peptide-mediated lipolysis in obesity. Int J Obes. 2016;40:714–20. doi: 10.1038/ijo.2015.222. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–73. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 37.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–23. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 38.Wamberg L, Christiansen T, Paulsen SK, Fisker S, Rask P, Rejnmark L, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue—the effect of obesity and diet-induced weight loss. Int J Obes. 2012;37:651–7. doi: 10.1038/ijo.2012.112. [DOI] [PubMed] [Google Scholar]
- 39.Blaak EE, Van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH. beta-Adrenergic stimulation of skeletal muscle metabolism in relation to weight reduction in obese men. Am J Physiol Endocrinol Metab. 1994;267::E316–22. doi: 10.1152/ajpendo.1994.267.2.E316. [DOI] [PubMed] [Google Scholar]
- 40.Bougnères P, Stunff CL, Pecqueur C, Pinglier E, Adnot P, Ricquier D. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. J Clin Invest. 1997;99:2568–73. doi: 10.1172/JCI119444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellström L, Langin D, Reynisdottir S, Dauzats M, Arner P. Adipocyte lipolysis in normal weight subjects with obesity among first-degree relatives. Diabetologia. 1996;39:921–8. doi: 10.1007/BF00403911. [DOI] [PubMed] [Google Scholar]
- 42.Jocken JWE, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. 2008;94:219–30. doi: 10.1016/j.physbeh.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Di Nisio A, De Toni L, Sabovic I, Rocca MS, De Filippis V, Opocher G, et al. Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. J Clin Endocrinol Metab. 2017;102:2564–74. doi: 10.1210/jc.2016-3591. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 45.WHO—World Health Organization. Obesity and Overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 1 March 2018.
- 46.Vanlint S. Vitamin D and obesity. Nutrients. 2013;5:949–56. doi: 10.3390/nu5030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J. Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest. 1985;76:470–3. doi: 10.1172/JCI111995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding C, Parameswaran V, Blizzard L, Burgess J, Jones G. Not a simple fat-soluble vitamin: Changes in serum 25-(OH)D levels are predicted by adiposity and adipocytokines in older adults. J Intern Med. 2010;268:501–10. doi: 10.1111/j.1365-2796.2010.02267.x. [DOI] [PubMed] [Google Scholar]
- 49.Scragg R, Camargo CA., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;15;168:577–86. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, Orio F, et al. Low vitamin D status and obesity: role of nutritionist. Rev Endocr Metab Disord. 2017;18:215–25. doi: 10.1007/s11154-017-9410-7. [DOI] [PubMed] [Google Scholar]
- 51.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e100138. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–61. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 54.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, Stolzenberg-Solomon R, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121:462–6. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.González-Molero I, Rojo-Martínez G, Morcillo S, Gutierrez C, Rubio E, Pérez-Valero V, et al. Hypovitaminosis D and incidence of obesity: a prospective study. Eur J Clin Nutr. 2013;67:680–2. doi: 10.1038/ejcn.2013.48. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert-Diamond D, Baylin A, Mora-Plazas M, Marin C, Arsenault JE, Hughes MD, et al. Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr. 2010;92:1446–51. doi: 10.3945/ajcn.2010.29746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes. 2012;36:387–96. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 58.Khan H, Kunutsor S, Franco OH, Chowdhury R. Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc Nutr Soc. 2013;72:89–97. doi: 10.1017/S0029665112002765. [DOI] [PubMed] [Google Scholar]
- 59.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88:720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart PH, Lucas RM, Walsh JP, Zosky GR, Whitehouse AJ, Zhu K, et al. Vitamin D in fetal development: findings from a birth cohort study. Pediatrics. 2015;135:e167–73. doi: 10.1542/peds.2014-1860. [DOI] [PubMed] [Google Scholar]
- 61.Mehmood ZH, Papandreou D. An updated mini review of vitamin D and obesity: adipogenesis and inflammation state. Open Access Maced J Med Sci. 2016;4:526–32. doi: 10.3889/oamjms.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cediel G, Corvalán C, Aguirre C, de Roma-a D, Uauy R. Serum 25-Hydroxyvitamin D associated with indicators of body fat and insulin resistance in prepubertal chilean children. Int J Obes. 2015;40:147–52. doi: 10.1038/ijo.2015.148. [DOI] [PubMed] [Google Scholar]
- 63.Cediel G, Corvalan C, Lopez de Romana D, Mericq V, Uauy R. Prepubertal adiposity, vitamin D status, and insulin resistance. Pediatrics. 2016;138:e20160076–e20160076. doi: 10.1542/peds.2016-0076. [DOI] [PubMed] [Google Scholar]
- 64.Al-Shoumer KA, Al-Essa TM. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? World J Diabetes. 2015;6:1057–64. doi: 10.4239/wjd.v6.i8.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wimalawansa SJ. Associations of vitamin D with insulin resistance, obesity, type 2 diabetes, and metabolic syndrome. J Steroid Biochem Mol Biol. 2018;175:177–89. doi: 10.1016/j.jsbmb.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Chagas CE, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4:52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pilz S, Kienreich K, Rutters F, de Jongh R, van Ballegooijen AJ, Grübler M, et al. Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr Diab Rep. 2013;13:261–70. doi: 10.1007/s11892-012-0358-4. [DOI] [PubMed] [Google Scholar]
- 68.Chung SJ, Lee YA, Hong H, Kang MJ, Kwon HJ, Shin CH, et al. Inverse relationship between vitamin D status and insulin resistance and the risk of impaired fasting glucose in Korean children and adolescents: the Korean National Health and Nutrition Examination Survey (KNHANES) 2009–10. Public Health Nutr. 2014;17:795–802. doi: 10.1017/S1368980013002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olson M, Maalouf N, Oden J, White P, Hutchison M. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab. 2012;97:279–28. doi: 10.1210/jc.2011-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kayaniyil S, Harris SB, Retnakaran R, Vieth R, Knight JA, Gerstein HC, et al. Prospective association of 25(OH)D with metabolic syndrome. Clin Endocrinol. 2014;80:502–5075. doi: 10.1111/cen.12190. [DOI] [PubMed] [Google Scholar]
- 71.Seo JA, Eun CR, Cho H, Lee SK, Yoo HJ, Kim SG, et al. Low vitamin D status is associated with nonalcoholic Fatty liver disease independent of visceral obesity in Korean adults. PLoS ONE. 2013;9:e75197. doi: 10.1371/journal.pone.0075197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rusconi RE, De Cosmi V, Gianluca G, Giavoli C, Agostoni C. Vitamin D insufficiency in obese children and relation with lipid profile. Int J Food Sci Nutr. 2015;66:132–4. doi: 10.3109/09637486.2014.959902. [DOI] [PubMed] [Google Scholar]
- 73.Riek AE, Oh J, Sprague JE, Timpson A, de las Fuentes L, Bernal-Mizrachi L, et al. Vitamin D suppression of endoplasmic reticulum stress promotes an antiatherogenic monocyte/macrophage phenotype in type 2 diabetic patients. J Biol Chem. 2012;287:38482–94. doi: 10.1074/jbc.M112.386912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fornari R, Francomano D, Greco EA, Marocco C, Lubrano C, Wannenes F, et al. Lean mass in obese adult subjects correlates with higher levels of vitamin D, insulin sensitivity and lower inflammation. J Endocrinol Invest. 2015;38:367–72. doi: 10.1007/s40618-014-0189-z. [DOI] [PubMed] [Google Scholar]
- 76.Ordóñez Mena JM, Brenner H. Vitamin D and cancer: an overview on epidemiological studies. Adv Exp Med Biol. 2014;359:17–32. [PubMed] [Google Scholar]
- 77.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;359:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 78.Scappaticcio L, Maiorino MI, Bellastella G, Giugliano D, Esposito K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine. 2017;56:231–9. doi: 10.1007/s12020-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 79.Manousaki D, Richards JB. Low vitamin D levels as a risk factor for cancer. BMJ. 2017;31:359–j4952. doi: 10.1136/bmj.j4952. [DOI] [PubMed] [Google Scholar]
- 80.Turer CB, Lin H, Flores G. Prevalence of vitamin D deficiency among overweight and obese US children. Pediatrics. 2013;131:e152–e161. doi: 10.1542/peds.2012-1711. [DOI] [PubMed] [Google Scholar]
- 81.Bellone S, Esposito S, Giglione E, et al. Vitamin D levels in a paediatric population of normal weight and obese subjects. J Endocrinol Invest. 2014;37:805–9. doi: 10.1007/s40618-014-0108-3. [DOI] [PubMed] [Google Scholar]
- 82.Peterson C. Vitamin D deficiency and childhood obesity: interactions, implications, and recommendations. Nutr Dietary Suppl. 2015;7:29–3. [Google Scholar]
- 83.Peterson CA, Tosh AK, Belenchia AM. Vitamin D insufficiency and insulin resistance in obese adolescents. Ther Adv Clin Endocrinol Metab. 2014;5:166–89. doi: 10.1177/2042018814547205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dolinsky DH, Armstrong S, Mangarelli C, Kemper AR. The association between vitamin D and cardiometabolic risk factors in children: a systematic review. Clin Pediatr. 2013;52:210–23. doi: 10.1177/0009922812470742. [DOI] [PubMed] [Google Scholar]
- 85.Yildiz I, Erol OB, Toprak S, et al. Role of vitamin D in children with hepatosteatosis. J Pediatr Gastroenterol Nutr. 2014;59:106–11. doi: 10.1097/MPG.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 86.Black LJ, Jacoby P, She Ping-Delfos WC, et al. Low serum 25-hydroxyvitamin D concentrations associate with non-alcoholic fatty liver disease in adolescents independent of adiposity. J Gastroenterol Hepatol. 2014;29:1215–22. doi: 10.1111/jgh.12541. [DOI] [PubMed] [Google Scholar]
- 87.Katz K, Brar PC, Parekh N, Liu YH, Weitzman M. Suspected nonalcoholic fatty liver disease is not associated with vitamin d status in adolescents after adjustment for obesity. J Obes. 2010;2010:496829. doi: 10.1155/2010/496829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.NIH—National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 2000;17:1–45 [PubMed]
- 89.Buyukinan M, Ozen S, Kokkun S, Saz EU. The relation of vitamin D deficiency with puberty and insulin resistance in obese children and adolescents. J Pediatr Endocrinol Metab. 2012;25:83–87. doi: 10.1515/jpem-2011-0426. [DOI] [PubMed] [Google Scholar]
- 90.Kelly A, Brooks LJ, Dougherty S, Carlow DC, Zemel BS. A cross-sectional study of vitamin D and insulin resistance in children. Arch Dis Child. 2011;96:447–52. doi: 10.1136/adc.2010.187591. [DOI] [PubMed] [Google Scholar]
- 91.Creo AL, Rosen JS, Ariza AJ, Hidaka KM, Binns HJ. Vitamin D levels, insulin resistance, and cardiovascular risks in very young obese children. J Pediatr Endocrinol Metab. 2013;26:97–104. doi: 10.1515/jpem-2012-0244. [DOI] [PubMed] [Google Scholar]
- 92.Soleymani T, Tejavanija S, Morgan S. Obesity, bariatric surgery, and bone. Curr Opin Rheumatol. 2011;23:396–405. doi: 10.1097/BOR.0b013e328346f832. [DOI] [PubMed] [Google Scholar]
- 93.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–18. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JA. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14:52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 95.Stein EM, Silverberg S. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diab Endocrinol. 2014;2:165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lespessailles E, Toumi H. Vitamin D alteration associated with obesity and bariatric surgery. Exp Biol Med. 2017;242:1086–94. doi: 10.1177/1535370216688567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koch TR, Finelli FC. Postoperative metabolic and nutritional complications of bariatric surgery. Gastroenterol Clin North Am. 2010;39:109–24. doi: 10.1016/j.gtc.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Giusti V, Gasteyger C, Suter M, Heraief E, Gaillard RC, Burkhardt P. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: Potential role of serum C telopeptides for follow-up. Int J Obes. 2005;29:1429–35. doi: 10.1038/sj.ijo.0803040. [DOI] [PubMed] [Google Scholar]
- 99.Nogués X, Goday A, Peña MJ, Benaiges D, de Ramón M, Crous X, et al. Bone mass loss after sleeve gastrectomy: a prospective comparative study with gastric bypass. Cir Esp. 2010;88:103–9. doi: 10.1016/j.ciresp.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 100.De Prisco C, Levine SN. Metabolic bone disease after gastric bypass surgery for obesity. Am J Med Sci. 2005;329:57–61. doi: 10.1097/00000441-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Fleisher J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98:541–49. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hewitt S, Sovik TT, Aasheim ET, Kristinsson J, Jahnsen J, Birketvedt GS, et al. Secondary hyperparathyroidism, vitamin D sufficiency, and serum calcium 5 years after gastric bypass and duodenal switch. Obes Surg. 2013;23:384–90. doi: 10.1007/s11695-012-0772-3. [DOI] [PubMed] [Google Scholar]
- 104.Aasheim ET, Hofso D, Sovik TT. Vitamin supplements after bariatric surgery. Clin Endocrinol. 2010;72:134–5. doi: 10.1111/j.1365-2265.2009.03611.x. [DOI] [PubMed] [Google Scholar]
- 105.Cashman KD. A review of vitamin D status and CVD. Proc Nutr Soc. 2014;73:65–72. doi: 10.1017/S0029665113003595. [DOI] [PubMed] [Google Scholar]
- 106.Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001–6. Am J Clin Nutr. 2011;94:225–33. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- 107.Lee P, Greenfield JR, Seibel MJ, Eisman JA, Center JR. Adequacy of vitamin D replacement in severe deficiency is dependent on body mass index. Am J Med. 2009;122:1056–60. doi: 10.1016/j.amjmed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Camozzi V, Frigo AC, Zaninotto M, Sanguin F, Plebani M, Boscaro M, et al. 25-Hydroxycholecalciferol response to single oral cholecalciferol loading in the normal weight, overweight, and obese. Osteoporos Int. 2016;27:2593–602. doi: 10.1007/s00198-016-3574-y. [DOI] [PubMed] [Google Scholar]
- 109.Didriksen A, Burild A, Jakobsen J, Fuskevag OM, Jorde R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3. Eur J Endocrinol. 2015;172:235–41. doi: 10.1530/EJE-14-0870. [DOI] [PubMed] [Google Scholar]
- 110.Cashman KD, Seamans KM, Lucey AJ, Sto E, Weber P, Kiely M, et al. Relative effectiveness of oral 25-hydroxyvitamin D 3 and vitamin D 3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95:1350–6. doi: 10.3945/ajcn.111.031427. [DOI] [PubMed] [Google Scholar]
- 111.Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ, Ebeling P, et al. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS ONE. 2014;9:e111265. doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J Steroid Biochem Mol Biol. 2013;136:195–200. doi: 10.1016/j.jsbmb.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 116.Radhakishun NN, van Vliet M, Poland DC, et al. Efficacy and tolerability of a high loading dose (25,000 IU weekly) vitamin D3 supplementation in obese children with vitamin D insufficiency/deficiency. Horm Res Paediatr. 2014;82:103–6. doi: 10.1159/000362236. [DOI] [PubMed] [Google Scholar]