Abstract
Objective:
Left ventricle (LV) geometry and dyssynchrony are associated with LV remodeling after acute myocardial infarction (AMI). The aim of this prospective study was to assess the diagnostic value of new three-dimensional echocardiography (3DE) parameters [sphericity (SI) and systolic dyssynchrony indexes (SDI)] for the prediction of LV remodeling after AMI and to compare them with two-dimensional echocardiography (2DE) parameters.
Methods:
2DE and 3DE were performed in 75 patients with AMI within 3 days from the onset of MI and 6 months later. LV remodeling was defined as a ≥15% increase in the LV end-diastolic volume (EDV) at follow-up. 3D SI was calculated by dividing EDV by the volume of a sphere whose diameter was derived from the major end-diastolic LV long axis. SDI was considered as a standard deviation of the time from cardiac cycle onset to minimum systolic volume in 16 LV segments.
Results:
LV remodeling was identified in 34 (45%) patients using the 2DE method and in 22 (29%) patients using the 3DE method. Evaluated 3DE parameters, such as EDV [area under the receiver operating characteristic (ROC) curve (AUC) 0.742, sensitivity 71%, specificity 79%], end-systolic volume (AUC 0.729, sensitivity 69%, specificity 78%), SDI (AUC 0.777, sensitivity 73%, specificity 77%), and SI, had significant prognostic value for LV remodeling. According to the AUC, the highest predictive value had 3D SI (AUC 0.957, sensitivity 90%, specificity 91%).
Conclusion:
3DE parameters, especially 3D SI and SDI, play important roles in the prediction of LV remodeling after AMI and can be used in clinical practice.
Keywords: 3D echocardiography, systolic dyssynchrony index, sphericity index, left ventricular remodeling, myocardial infarction
Introduction
Cardiovascular diseases continue to be the leading cause of death worldwide (1). Heart failure (HF) remains one of the most severe complications following acute myocardial infarction (AMI) that highly affects morbidity and mortality (2). Early detection of patients with AMI at risk of development of HF is necessary to avoid the evolution of left ventricular (LV) dysfunction (1-4). LV dilation, hypertrophy, and infarct expansion are the main expressions of LV remodeling due to AMI (4). The size of ventricular geometric modification and ventricular systolic and diastolic volumes measured early after AMI can predict clinical outcomes to patients late after AMI. The most important prognostic factors for the extent of LV remodeling [including mass, volumes, and ejection fraction (EF)] have been evaluated using two-dimensional echocardiography (2DE) due to wide availability (5-8). However, the limitations of this method are related to the functional analysis of the cardiac chamber volumes and their functions because most of the three-dimensional echocardiography (3DE) endpoints (e.g., LV volumes and EF) can only be derived from 2DE acquisitions by making geometrical assumptions. The advent of 3DE is arguably the most important technical advancement in ultrasound imaging over the past two decades. One of the most powerful features of the new 3DE systems is their ability to visualize cardiac anatomy and function from any number of spatial view planes. As a result of that, the quantification of LV geometry is improving (9). The use of 3DE extends beyond cardiac morphology, and the practice to evaluate cardiac function (ventricular systolic and diastolic volumes and EF) is increasing rapidly (10). One of the most important adaptations of 3DE is to provide the analysis of the geometric modification of the LV through the gauging of the 3D sphericity index (SI). While it has been many years since the SI was first shown to be an earlier and more accurate predictor of LV remodeling after AMI than many other clinical and echocardiographic variables (4), the currently installed base 3DE systems now open the door to more widespread application of this valuable metric. While it has been over a decade since it was first shown that LV dyssynchrony early after AMI in patients predicts subsequent LV remodeling (11), recent refinements in 3DE systems and image analysis software, in combination with their widespread availability, similarly open the door for more widespread application of this valuable metric.
The main goal of the present study was to evaluate the accuracy of the new 3DE parameters for the prediction of LV remodeling after AMI and to compare with standard 2DE parameters. By documenting the advantages of these 3DE techniques over conventional 2DE, it is our hope that the results of the current study will help to advance the standard of care for echocardiographic patient management in the future, particularly with regard to prognostication early after AMI.
Methods
Study population
This was a prospective cohort study. A total of 75 patients with AMI who underwent 2DE and 3DE at baseline (within the first 3 days from the onset of MI) and at 6 months after were included in the study. Inclusion criteria were patients with ST-elevation AMI, as evidenced by symptoms, electrocardiogram (ECG) changes, and serial troponin I concentrations. Primary percutaneous coronary intervention was performed, and thrombolysis in myocardial infarction-3 flow was achieved for all the patients. Complete revascularization was achieved in 44 (58.7%) patients at 3 days post-AMI. Fourteen (18.7%) patients required a staged revascularization procedure in 1 month due to ischemic symptoms repetition. According to the latest scientific research and approach (12), LV remodeling was defined as a ≥15% increase in the LV end-diastolic volume (EDV) at follow-up compared with the baseline by 3DE and 2DE methods (12-14). Patients with poor acoustic windows, atrial fibrillation or other significant arrhythmias, hemodynamic instability, valvular heart disease, cardiomyopathy, previously known MI and/or coronary artery bypass grafting, and severe non-cardiovascular disease were excluded from the study. The study was approved by the institutional bioethics committee. Written informed consent was obtained from all of the patients before undergoing 2DE and 3DE.
Clinical, laboratory, and echocardiographic data
Serial troponin I tests were assessed every 6 h after admission until peak values were achieved. The turbidimetric method (Verfen, normal values 0–7.5 mg/L) was performed for the measurement of C-reactive protein, whereas enzyme immunoassay was used for troponin I (Interlux, normal values 0–0.04 µg/L). The ECG data were used to evaluate ST segment and other ECG changes. Baseline 2DE and 3DE were recorded within 3±2 days after AMI and repeated at 6±1 months of follow-up using 1.5–4.6 MHz transducer, which was connected to Vivid7 (GE Healthcare, Horten, Norway). This echocardiographic imaging analysis was performed by EchoPac 4.0 software, where 3D SDI was measured automatically by TomTec LV 4D software. For imaging of the ventricles, a wide-angle acquisition in the apical window (4-chamber) was used. While performing the acquisition, the patient was asked to hold their breath for 10 s. Therefore, by repetition of holding breath, multi-beat dataset was collected. For the following analysis, the dynamic pyramidal 3DE database was collected, including the total acquisition time. The dataset was measured and evaluated by semi-automated tracking down the endocardium in nine equidistant long axes. However, note that the operator maintained the option of manually adjusting the initial automated selection of the endocardial border. Basic parameters, such as LV EDV, end-systolic volume (ESV), volumes indexed to body surface area, and EF, were obtained according to the current recommendations (15). The 3D SI was calculated by dividing EDV by the volume of a sphere whose diameter (D) was derived from the major end-diastolic LV long axis: 3D SI=[4/3×µ× (D/2)3]. The LV long axis was obtained from the 3DE dataset as the longest distance between the center of the mitral annulus and the endocardial apex (4). 3D SDI is a standard deviation of the time from cardiac cycle onset to minimum systolic volume in 16 LV segments (the technique of standard deviation of measured times was performed according to the recommendations) (16). LV remodeling was defined as an increase of EDV >15% at 6 months after MI in relation to baseline. The analysis of LV volumes, together with the measurement of 3DE SI and SDI, was approximately 15–25 min.
Interobserver variability was measured by the analysis of 15 patients (selected randomly from the 75 enrollees) by 2 experienced independent blinded observers. Intraobserver variability was measured by the analysis of 15 patients by the same observers at two different time points. Interobserver and intraobserver variabilities were calculated and expressed as percentage (%).
Statistical analysis
Statistical analysis was performed using IBM SPSS 23.0 software package (IBM Corp., Armonk, New York, USA). Qualitative variables were described based on their frequency and relative frequency rate (%). Qualitative variables homogeneous distribution was evaluated by chi-square test or Fisher’s exact test, in case of small expected values. Mann–Whitney U test and Student’s t-test were used for comparison of quantitative variables. Independent samples t-test was used to compare normally distributed variables. Normally distributed data were presented as mean±SD. Mann–Whitney U test was used to compare non-normally distributed variables. Non-normally distributed data were presented as median (interquartile range). The SI, EDV, SDI, and other parameters as remodeling indicators were evaluated by using the receiver operating characteristic (ROC) curves. A subject was assessed as positive or negative according to whether the parameter value was greater than, less than, or equal to a given cut-off value. Associated with any cut-off value was the probability of a true positive (sensitivity) and a true negative (specificity). The most commonly used index of accuracy is the area under the ROC curve (AUC), with values close to 1.0 indicating high diagnostic accuracy. Pearson coefficients of correlation and their associated probability (p) were used to evaluate the relationship between 3DE and 2DE parameters. A p-value <0.05 was considered as statistically significant.
Results
A total of 82 consecutive patients with AMI were screened initially. Of the 82 patients, 7 (8.5%) were excluded because of poor image quality or incomplete follow-up. Baseline clinical, echocardiographic, and laboratory characteristics of the study population are shown in Table 1. Events of anterior MI and history of smoking were significantly more common among patients with LV remodeling. Other clinical characteristics had no significant difference. There were no significant differences in the use of beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin II antagonists, and calcium channel blockers. Even though troponin I showed a tendency to be higher in the remodeling group (p=0.048), other inflammatory markers did not differ between the study groups. Echocardiographic parameter analyses showed that 3DE parameters, such as EDV, ESV, SI, and SDI, were significantly higher in the remodeling group (EDV p=0.024, ESV p=0.030, EDV index p=0.038, ESV index p=0.045, SI p=0.009, and SDI p=0.013). Other 3DE parameters, such as EF, had no significant difference (p>0.05). 2DE parameters did not differ significantly between the groups. The results of the Mann–Whitney U and Student’s t-test analyses revealed that measured by 3DE EDV, ESV, EDV index, and ESV index were higher, and that EF was lower than measured by 2DE (Table 2).
Table 1.
Baseline clinical, echocardiographic, and laboratory characteristics of the study population
| Characteristics | All patients (n=75) | Remodeling group (n=22) | Without remodeling group (n=53) | P-value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Gender, male, n (%) | 48 (64.0) | 18 (37.5) | 30 (62.5) | 0.090 |
| Age (year), mean (±SD) | 56.8±10.8 | 57.5±10.3 | 56.51±11.1 | 0.769 |
| History of hypertension, n (%) | 59 (78.7) | 22 (37.3) | 37 (62.7) | 0.166 |
| History of smoking, n (%) | 29 (38.7) | 18 (62.1) | 11 (37.9) | 0.002 |
| Obesity, n (%) | 18 (24) | 8 (44.4) | 10 (55.6) | 0.965 |
| Diabetes, n (%) | 6 (8) | 3 (50) | 3 (50) | 0.758 |
| Anterior infarction, n (%) | 32 (42.7) | 20 (62.5) | 12 (37.5) | 0.005 |
| Inferior infarction, n (%) | 43 (57.3) | 2 (4.7) | 41 (95.3) | <0.001 |
| Medication (at hospital discharge) | ||||
| Beta-blockers, n (%) | 75 (100.0) | 22 (29.3) | 53 (70.7) | 0.105 |
| ACE inhibitors/angiotensin II antagonists, n (%) | 67 (89.3) | 21(31.3) | 46 (68.7) | 0.209 |
| Calcium blockers, n (%) | 3 (5.5) | 2 (66.7) | 1 (33.3) | 0.408 |
| Heart rate, mean (±SD), (bpm) | 75.7±14.5 | 76.8±11.5 | 75.2±15.7 | 0.549 |
| 2D echocardiography | ||||
| End-diastolic volume (mL) | 95.1±12.7 | 102.3±13.5 | 89.4±14.4 | 0.092 |
| End-systolic volume (mL) | 51.2±8.0 | 56.1±7.1 | 49.7±5.2 | 0.088 |
| End-diastolic volume index (mL/m2) | 58.2 ± 6.7 | 65.3±7.9 | 54.8±8.0 | 0.125 |
| End-systolic volume index (mL/m2) | 33.2±6.9 | 39.7±8.5 | 31.4±6.8 | 0.233 |
| Ejection fraction (%) | 50 (5) | 48 (11) | 54 (7) | 0.245 |
| 3D echocardiography | ||||
| End-diastolic volume (mL) | 114.7±18.3 | 120.9±21.3 | 103.2±17.6 | 0.024 |
| End-systolic volume (mL) | 62.0±10.5 | 69.0±11.2 | 58.9±7.9 | 0.030 |
| End-diastolic volume index (mL/m2) | 68.3±15.2 | 75.4±17.0 | 62.1±10.3 | 0.038 |
| End-systolic volume index (mL/m2) | 41.8±9.6 | 46.9±8.4 | 38.1±11.0 | 0.045 |
| Ejection fraction (%) | 46 (7) | 44 (6) | 52 (6) | 0.092 |
| Systolic dyssynchrony index (%) | 3.9±0.3 | 4.3±0.2 | 3.4±0.2 | 0.013 |
| 3D sphericity index | 0.35±0.03 | 0.38±0.04 | 0.29±0.03 | 0.009 |
| Laboratory parameters | ||||
| Troponin I (mcg/L) | 11.2±2.1 | 20.6±3.1 | 6.8±0.3 | 0.048 |
| CRP (mg/L) | 9.7±1.8 | 12.5±1.6 | 8.0±1.4 | 0.283 |
| Leukocytes (*109/L) | 10.8±2.4 | 11.7±1.9 | 10.6±1.1 | 0.191 |
ACE - angiotensin-converting enzyme; CRP - C-reactive protein
Table 2.
Echocardiographic parameters compared by 2D and 3D echocardiography
| Parameters | 2D echocardiography (n=75) | 3D echocardiography (n=75) | P-value |
|---|---|---|---|
| End-diastolic volume (mL) | 95.1±12.7 | 114.7±18.3 | 0.021 |
| End-systolic volume (mL) | 51.2±8.0 | 62.0±10.5 | 0.028 |
| End-diastolic volume index (mL/m2) | 58.2±6.7 | 68.3±15.2 | 0.044 |
| End-systolic volume index (mL/m2) | 33.2±6.9 | 41.8±9.6 | 0.042 |
| Ejection fraction (%) | 50±5 | 46±7 | 0.134 |
At 6 months, analysis results showed that 3DE parameters were significantly higher than baseline parameters (EDV p=0.011, ESV p=0.035, EDV index p=0.043, ESV index p=0.040, SI p=0.008, and SDI p=0.036) and remained higher in the remodeling group, except LV EF, which had no significant difference. The same tendency was found in 2DE parameters (EDV p=0.038, ESV p=0.045, EDV index p=0.042, ESV index p=0.039, and EF p=0.156), but did not differ significantly between the groups as at baseline (Table 3). LV remodeling was identified in 34 (45%) patients using the 2DE method and in 22 (29%) patients using the 3D method. It was found that remodeling evaluation by 2DE and 3DE had a significant difference (p=0.007).
Table 3.
Comparison of 2D and 3D echocardiography parameters evaluated 6 months later in different groups: with and without LV remodeling
| Parameters | Remodeling group (n=22) | Without remodeling group (n=53) | P-value |
|---|---|---|---|
| 3D parameters | |||
| End-diastolic volume (mL) | 142.9±31.3 | 115.2±17.6 | 0.006 |
| End-systolic volume (mL) | 89.5±11.2 | 66.9±9.9 | 0.003 |
| End-diastolic volume index (mL/m2) | 87.4±17.0 | 72.1±10.3 | 0.030 |
| End-systolic volume index (mL/m2) | 55.9±8.1 | 41.1±10.2 | 0.045 |
| Ejection fraction (%) | 40 (7) | 46 (8) | 0.301 |
| Systolic dyssynchrony index (%) | 5.6±0.21 | 3.8±0.13 | 0.034 |
| 3D sphericity index | 0.53±0.03 | 0.36±0.02 | 0.001 |
| 2D parameters | |||
| End-diastolic volume (mL) | 125.3±23.5 | 109.4±24.4 | 0.311 |
| End-systolic volume (mL) | 72.1±16.1 | 63.7±12.2 | 0.088 |
| End-diastolic volume index (mL/m2) | 76.9±11.7 | 69.9±10.5 | 0.424 |
| End-systolic volume index (mL/m2) | 46.8±9.5 | 43.4±7.8 | 0.553 |
| Ejection fraction (%) | 43 (5) | 47 (7) | 0.449 |
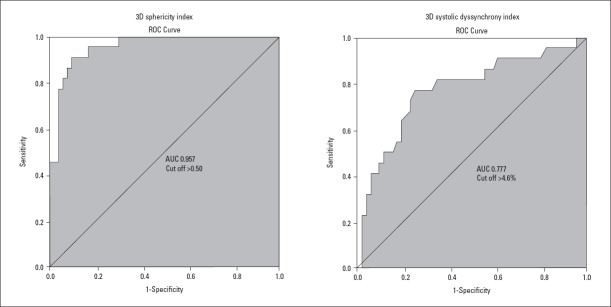
The diagnostic value of 3DE and 2DE parameters for LV remodeling was assessed by ROC curves. EDV (AUC 0.742, sensitivity 71%, specificity 79%), ESV (AUC 0.729, sensitivity 69%, specificity 78%), SDI (AUC 0.777, sensitivity 73%, specificity 77%), and SI measured by 3DE had statistically significant prognostic value for LV remodeling (Fig. 1, Table 4). According to the AUC, 3D SI had the strongest predictive value for LV remodeling (AUC 0.957, sensitivity 90%, specificity 91%). EF had no significant predictive value (p>0.05). EDV, ESV, EF, and LV end-diastolic diameter measured by 2DE had no significant prognostic value for LV remodeling (p>0.05) (Table 4).
Figure 1.
ROC curves of 3D sphericity index and systolic dyssynchrony index. Cut-off values are shown
Table 4.
ROC curve analysis of 3D and 2D echocardiography selected parameters for the prediction of LV remodeling
| Parameters | Cut-off value | Sensitivity | Specificity | AUC | P-value |
|---|---|---|---|---|---|
| 3D parameters | |||||
| End-diastolic volume (ml) | >115.3 | 71 | 79 | 0.742 | 0.028 |
| End-systolic volume (ml) | >69.8 | 69 | 78 | 0.729 | 0.042 |
| Ejection fraction (%) | <39 | 44 | 82 | 0.609 | 0.248 |
| Systolic dyssynchrony index (%) | >4.6 | 73 | 82 | 0.777 | <0.001 |
| 3D sphericity index | >0.50 | 90 | 91 | 0.957 | <0.001 |
| 2D parameters | |||||
| End-diastolic volume (mL) | >96.5 | 44 | 78 | 0.588 | 0.181 |
| End-systolic volume (mL) | >46.5 | 39 | 74 | 0.485 | 0.056 |
| Ejection fraction (%) | <44 | 31 | 55 | 0.421 | 0.802 |
| Laboratory parameters | |||||
| Troponin I (µg/L) | >7.7 | 67 | 77 | 0.705 | 0.032 |
| CRP (mg/L) | >13.9 | 48 | 78 | 0.601 | 0.108 |
| Leukocytes (×109/L) | >13.4 | 28 | 61 | 0.497 | 0.567 |
AUC – area under the ROC curve; CRP - C-reactive protein
Additional diagnostic value of combining 3DE and laboratory parameters for the assessment of LV remodeling was evaluated. According to ROC analysis, the combination of SI, SDI, and troponin I increased the prognostic value (SI+troponin I–AUC 0.961 and SDI+troponin I–AUC 0.802). However, the difference between the combination and separate parameters was not significant (AUC 0.957 vs. 0.961, p=0.633 and AUC 0.777 vs. 0.802, p=0.324, respectively).
3DE parameters, such as EDV, ESV, and EF, significantly correlated with LV remodeling as follows: r=0.489, r=0.402, and r=0.40 (p<0.001, for all).
Interobserver and intraobserver agreements were 95% and 96% for the assessment of 3D SI and 97% and 98% for 3D SDI, respectively.
Discussion
The results of our study showed that predominance of the anterior MI, being a smoker, higher troponin I level, increased LV volume, 3D SI, and SDI play important roles in LV remodeling following AMI. Patients who develop LV dilatation and reduction of EF following AMI have significantly reduced survival rate (17). Morphologically, MI creates a response to LV in which stretched and dilated infarcted tissue increases LV volume with a combined volume and pressure load on non-infarcted areas (18). Furthermore, Gaudron et al. (19) demonstrated that LV dilatation following AMI precedes the deterioration of exercise performance and plays an active role in the development of chronic HF. It confirms the importance of our research-the prediction of LV remodeling after AMI may help to prevent possible HF and unfavorable outcomes.
Zaliaduonyte-Peksiene et al. (20) showed the same tendency in higher number of anterior wall MI, increased LV volume, and higher troponin I concentration in LV remodeling group patients and described them as important predictors. Several previous studies revealed that one of the main determinants of LV remodeling is the infarct size, and that the left anterior descending artery as a culprit artery is the most common in patients with LV remodeling (21-23). These findings agree with our study and show the importance of coronary artery lesion localization as a clinical predictor for LV remodeling.
Our study demonstrates two main echocardiographic findings. First, our study results indicate that 3DE parameters are much more accurate than 2DE parameters in detecting LV remodeling after AMI. Konstam et al. (24) reported that 3DE has emerged as a clinically feasible method for quantifying ventricular volume and mass, and that the quantification of ventricular volumes and EF can be performed rapidly. Furthermore, Yang et al. (25) supported 3DE significance for detecting myocardial structural changes, as well as it suggests LV end-systolic volume index as the most accurate parameter observed in their study. Moreover, this method avoids the geometric assumptions and problems of image plane position that are associated with 2DE. Several previous studies have observed that 3D echocardiographic assessments of LV volumes, mass, and EF correlate favorably with cardiac magnetic resonance (24, 26). The results of our research confirmed this data-EDV and ESV evaluated by 3DE were significantly higher in the LV remodeling group at baseline and were significant predictors of LV remodeling after 6 months. LV volumes evaluated by 2DE showed no significant difference between the groups and were not significant predictors of early LV remodeling. Similarly, Vieira et al. (27) also found no association between the 2DE LV volumes and SI and the LV remodeling at 6 months of follow-up after AMI.
Second, our study results demonstrated that 3D SDI and SI are the most sensitive and specific parameters predicting early LV remodeling after AMI. Moreover, these two 3DE parameters are still new for the evaluation of LV remodeling. Only a few studies have tried to evaluate the accuracy of these measurements for identifying early LV remodeling after AMI. The first study, published in 2004, revealed that 3D SI measured at baseline is far more sensitive than 3D LV volumes. This small study showed that 3D SI sensitivity and specificity are 100% and 90%, respectively, and are in agreement with our data (4).
Previous investigations in this field allow the studies about the dyssynchrony index appear (11, 28, 29). The analysis suggested prognostic value of dyssynchrony index with a sensitivity of 82% and a specificity of 95%. However, to the best of our knowledge, this is the first study showing the role of 3D SDI as an important predictor of LV remodeling after AMI with a 73% sensitivity and 82% specificity. Zhou et al. (30) in a cross-sectional study investigated LV mechanical dyssynchrony in patients with HF after MI by real-time 3DE and found that LV dyssynchrony occurs more often in patients with cardiac dysfunction after MI. The other previous study that evaluated ventricular dyssynchrony by tissue Doppler imaging in predicting LV remodeling after ST-elevation MI found that LV dyssynchrony is a strong predictor of LV remodeling after AMI with a 72.7% sensitivity and 83.3% specificity (31). 3D SDI measured in our study had a similar accuracy. Studies suggest that the techniques of performing 3D SDI are reproducible and have been implemented at many institutions worldwide.
Our study reveals the importance of 3DE SI and SDI assessments at baseline after AMI as independent predictors of LV remodeling. 3DE SI and SDI, measured early after the onset of AMI, had significant incremental value over the 2DE parameters and 3DE LV volumes. This technique is simple and not time-consuming with the possibility to perform it in almost all echocardiography laboratories. Early measurement of 3DE SI and SDI after AMI can provide important information about cardiac mechanics changes after MI and may suggest some therapeutical implications. Owing to this, it could be used in daily clinical practice for the assessment of early LV remodeling after AMI.
Study limitations
Several limitations should be considered. First, LV remodeling in our study was evaluated at baseline and at 6 months after AMI. However, the evaluation of LV parameters after 12 and 24 months can provide additional important information about 3D SDI and SI predictive values. Second, the image quality of 3DE is also an important limitation if we want to use SI and SDI in daily clinical practice. Finally, a relatively small sample size is an important limitation. Therefore, these findings should be repeated in a larger population with AMI.
Conclusion
The present study showed that anterior MI, smoking habits, elevated troponin I at admission, and high SI and SDI values evaluated by 3DE play important roles in LV remodeling prediction after AMI. Moreover, 3D SI and SDI have the highest predictive values for LV remodeling following AMI and can be used in clinical practice as simple, cost-effective measurements. 3DE, as innovative technique, should become the main echocardiography technique for the assessment and follow-up after AMI.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally and internally peer-reviewed.
Authorship contributions: Concept – A.K., E. R., J. J. V., D.Z.P.; Design – A.K., R.J., R.Z.; Supervision – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Fundings – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Materials – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Data collection &/or processing – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Analysis &/or interpretation – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Literature search – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Writing – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.; Critical review – A.K., E.R., D.V., T.P., G.M., E.K., V.Z., R.J., J.J.V., R.Z., D.Z.P.
References
- 1.Updated May 2017. Available from:URL: http://www.who.int/mediacentre/factsheets/fs317/en/
- 2.Abuomara HZA, Hassan OM, Rashid T, Baraka M. Myocardial performance index as an echocardiographic predictor of early in-hospital heart failure during first acute anterior ST-elevation myocardial infarction. Egypt Heart J. 2018;70:71–5. doi: 10.1016/j.ehj.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography:a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112:992–1000. doi: 10.1161/CIRCULATIONAHA.104.474445. [DOI] [PubMed] [Google Scholar]
- 4.Mannaerts HF, van der Heide JA, Kamp O, Stoel MG, Twisk J, Visser CA. Early identification of left ventricular remodelling after myocardial infarction, assessed by transthoracic 3D echocardiography. Eur Heart J. 2004;25:680–7. doi: 10.1016/j.ehj.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 6.Gopal AS, Shen Z, Sapin PM, Keller AM, Schnellbaecher MJ, Leibowitz DW, et al. Assessment of cardiac function by three-dimensional echocardiography compared with conventional noninvasive methods. Circulation. 1995;92:842–53. doi: 10.1161/01.cir.92.4.842. [DOI] [PubMed] [Google Scholar]
- 7.King DL, Harrison MR, King DL, Jr, Gopal AS, Martin RP, DeMaria AN. Improved reproducibility of left atrial and left ventricular measurements by guided three-dimensional echocardiography. J Am Coll Cardiol. 1992;20:1238–45. doi: 10.1016/0735-1097(92)90383-x. [DOI] [PubMed] [Google Scholar]
- 8.Sugeng L, Mor-Avi V, Lang RM. Three-dimensional echocardiography:coming of age. Heart. 2008;94:1123–5. doi: 10.1136/hrt.2007.133702. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Tsang W, Weinert L, Mor-Avi V, Chandra S. Valvular heart disease. The value of 3-dimensional echocardiography. J Am Coll Cardiol. 2011;58:1933–44. doi: 10.1016/j.jacc.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JM, Miller O. Three-dimensional echocardiography in congenital heart disease. Arch Cardiovasc Dis. 2011;104:45–56. doi: 10.1016/j.acvd.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Mollema SA, Su San Liem, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, et al. Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol. 2007;50:1532–40. doi: 10.1016/j.jacc.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Li XC, Jin FL, Jing C, Xiao Q, Liu Y, Ran ZS, et al. Predictive value of left ventricular remodeling by area strain based on three-dimensional wall-motion tracking after PCI in patients with recent NSTEMI. Ultrasound Med Biol. 2012;38:1491–501. doi: 10.1016/j.ultrasmedbio.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Rizzello V, Poldermans D, Boersma E, Biagini E, Schinkel AF, Krenning B, et al. Opposite patterns of left ventricular remodeling after coronary revascularization in patients with ischemic cardiomyopathy:role of myocardial viability. Circulation. 2004;110:2383–8. doi: 10.1161/01.CIR.0000145115.29952.14. [DOI] [PubMed] [Google Scholar]
- 14.Sarai M, Biswas S, Toyama H, Yamada A, Motoyama S, Iwase M, et al. Ventricular remodeling following acute myocardial infarction and its correlation with discordant perfusion and fatty acid metabolism. J Nucl Med. 2009;50(Suppl 2):1136. [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- 17.Visser CA. Left ventricular remodelling after myocardial infarction:importance of residual myocardial viability and ischaemia. Heart. 2003;89:1121–2. doi: 10.1136/heart.89.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opie LH, Commenford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodeling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 19.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87:755–63. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 20.Zaliaduonyte-Peksiene D, Vaskelyte JJ, Mizariene V, Jurkevicius R, Zaliunas R. Does longitudinal strain predict left ventricular remodeling after myocardial infarction? Echocardiography. 2012;29:419–27. doi: 10.1111/j.1540-8175.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 21.Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, et al. Left ventricular remodeling after primary coronary angioplasty:Patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–7. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 22.Pirolo JS, Hutchins GM, Moore GW. Infarct expansion:pathologic analysis of 204 patients with a single myocardial infarct. J Am Coll Cardiol. 1986;7:349–54. doi: 10.1016/s0735-1097(86)80504-6. [DOI] [PubMed] [Google Scholar]
- 23.Lund GK, Stork A, Muellerleile K, Barmeyer AA, Bansmann MP, Knefel M, et al. Prediction of left ventricular remodeling and analysis of infarct resorption in patients with reperfused myocardial infarcts by using contrast-enhanced MR imaging. Radiology. 2007;245:95–102. doi: 10.1148/radiol.2451061219. [DOI] [PubMed] [Google Scholar]
- 24.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure:current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Yang NI, Hung MJ, Cherng WJ, Wang CH, Cheng CW, Kuo LT. Analysis of left ventricular changes after acute myocardial infarction using transthoracic real-time three-dimensional echocardiography. Angiology. 2008-2009;59:688–94. doi: 10.1177/0003319708316006. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi M, Nishikage T, Mor-Avi V, Sugeng L, Weinert L, Nakai H, et al. Measurement of left ventricular mass by real time three-dimensional echocardiography:validation against magnetic resonance and comparison with two-dimensional and m-mode measurements. J Am Soc Echocardiogr. 2008;21:1001–5. doi: 10.1016/j.echo.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Vieira ML, Oliveira WA, Cordovil A, Rodrigues AC, Mônaco CG, Afonso T, et al. 3D Echo pilot study of geometric left ventricular changes after acute myocardial infarction. Arq Bras Cardiol. 2013;101:43–51. doi: 10.5935/abc.20130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nucifora G, Bertini M, Marsan NA, Delgado V, Scholte AJ, Ng AC, et al. Impact of left ventricular dyssynchrony early on left ventricular function after first acute myocardial infarction. Am J Cardiol. 2010;105:306–11. doi: 10.1016/j.amjcard.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Nucifora G, Bertini M, Ajmone Marsan N, Scholte AJ, Siebelink HM, Holman ER, et al. Temporal evolution of left ventricular dyssynchrony after myocardial infarction:relation with changes in left ventricular systolic function. Eur Heart J Cardiovasc Imaging. 2012;13:1041–6. doi: 10.1093/ehjci/jes095. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Deng Q, Huang J, Chen JL, Hu B, Guo RQ. Evaluation of left ventricular mechanical dyssynchrony in patients with heart failure after myocardial infarction by real-time three-dimensional echocardiography. Saudi Med J. 2012;33:256–61. [PubMed] [Google Scholar]
- 31.Turan B, Yilmaz F, Karaahmet T, Tigen K, Mutlu B, Basaran Y. Role of left ventricular dyssynchrony in predicting remodeling after ST elevation myocardial infarction. Echocardiography. 2012;29:165–72. doi: 10.1111/j.1540-8175.2011.01574.x. [DOI] [PubMed] [Google Scholar]