Abstract
Aims
Hip fractures are associated with high morbidity, mortality, and costs. One strategy for improving outcomes is to incentivize hospitals to provide better quality of care. We aimed to determine whether a pay-for-performance initiative affected hip fracture outcomes in England by using Scotland, which did not participate in the scheme, as a control.
Materials and Methods
We undertook an interrupted time series study with data from all patients aged more than 60 years with a hip fracture in England (2000 to 2018) using the Hospital Episode Statistics Admitted Patient Care (HES APC) data set linked to national death registrations. Difference-in-differences (DID) analysis incorporating equivalent data from the Scottish Morbidity Record was used to control for secular trends. The outcomes were 30-day and 365-day mortality, 30-day re-admission, time to operation, and acute length of stay.
Results
There were 1 037 860 patients with a hip fracture in England and 116 594 in Scotland. Both 30-day (DID -1.7%; 95% confidence interval (CI) -2.0 to -1.2) and 365-day (-1.9%; 95% CI -2.5 to -1.3) mortality fell in England post-intervention when compared with outcomes in Scotland. There were 7600 fewer deaths between 2010 and 2016 that could be attributed to interventions driven by pay-for-performance. A pre-existing annual trend towards increased 30-day re-admissions in England was halted post-intervention. Significant reductions were observed in the time to operation and length of stay.
Conclusion
This study provides evidence that a pay-for-performance programme improved the outcomes after a hip fracture in England.
Cite this article: Bone Joint J 2019;101-B:1015–1023.
Keywords: Hip fracture, Best practice tariff, Pay-for-performance
Hip fracture is a leading cause of death and disability among the elderly worldwide.1,2 The incidence is rising as populations age, and there are now over 1.6 million hip fractures globally each year.1 In the United Kingdom alone, there are 70 000 cases annually at a cost of £2 billion.3
Pay-for-performance initiatives are increasingly used to improve outcomes.4-6 These schemes link healthcare payments to quality metrics in order to incentivize providers to improve the quality or efficiency of care.7 There is mixed evidence about whether these initiatives can truly drive improvements in healthcare.4,6 There is evidence that they can modestly improve care. However, few pay-for-performance schemes have been shown to positively affect outcomes.6
A national clinical audit was established in England and Wales in 2007 with the aim of improving hip fracture outcomes.8 This programme included a National Hip Fracture Database (NHFD) and support for local clinical teams to improve the quality of care provided to elderly patients with a hip fracture. In 2010, the NHFD was the basis for a pay-for-performance initiative, called the ‘Best Practice Tariff’ (BPT). The BPT scheme paid hospitals a supplement for each patient whose care satisfied six clinical standards, such as surgery within 36 hours.9 Cases satisfying these standards, which have evolved over time (Supplementary Table i), were identified from data submitted to the NHFD. Importantly, Scottish hospitals did not participate in the NHFD and were not subject to the BPT.
This study aimed: first to quantify any effect of the NHFD and BPT on the outcomes of hip fractures in England using data from Scotland to control for secular trends; and second to estimate the effect of introducing pay-for-performance for hip fractures in Scotland.
Materials and Methods
This study was a natural experiment using interrupted time series10 and difference-in-differences (DID) analysis.11 It relied on national data from two sources in order to conduct quasi-experimental modelling of temporal trends. Changes in England, where the NHFD/BPT was introduced, were analyzed as an ‘exposed’ group and those in Scotland as a ‘control’. Given the countries’ geographical proximity, cultural similarities, and common political union within the United Kingdom, it was anticipated that secular changes in Scotland would closely mimic those in England had the NHFD/BPT not been implemented.12
Data for England were abstracted from the Hospital Episode Statistics Admitted Patient Care (HES APC) data set13 linked to Office for National Statistics (ONS) death certificate registrations. Data for Scotland were abstracted from Scottish Morbidity Records (SMR01).14
The HES APC data set is managed by NHS Digital and collects data on all admissions to National Health Service (NHS) hospitals, as well as those treated in private hospitals but funded by the NHS.13 Approximately 98% of hospital activity in England is funded by the NHS.15 It is unlikely that many elderly patients with a hip fracture were treated in the private sector during the study period. The HES APC data does not include information about Emergency Department attendances that do not lead to admission.13
The ONS holds data on all deaths registered in England and Wales. All English deaths should be captured, although registration could be delayed in cases referred to a coroner for post-mortem or inquest. In 2016, upwards of 96% of deaths were registered within the year that they occurred.16
The SMR collects administrative data on episodes of inpatient care provided by all hospitals in Scotland. It is managed by the Information Services Division (ISD) for NHS National Services Scotland and is linked directly to Scottish death certificate data. ISD Scotland estimate that the SMR01 captures 99% of admissions to hospitals in Scotland.17
The United Kingdom is a unitary state composed of four countries: England, Scotland, Wales, and Northern Ireland. Comprehensive publicly funded healthcare is freely available throughout the United Kingdom under the NHS. Provision of healthcare under the auspices of NHS Scotland was devolved to the Scottish Parliament by the Scotland Act 1998.18
All adults aged more than 60 years were included in the analysis if they were treated for a hip fracture in England or Scotland with inpatient admission dates between January 2000 and December 2016 and had complete follow-up information for a period of one year following admission (2000 to 2017). No additional exclusion criteria were applied. Patients had to present with a primary International Classification for Diseases, Tenth Revision (ICD-10) diagnostic code19 on admission consistent with: S72.0 (“fracture of neck of femur”), S71.1 (“pertrochanteric fracture”), or S72.2 (“subtrochanteric fracture”).
The principal aim of the study was to determine the effect of introducing a pay-for-performance initiative on outcomes for elderly patients with a hip fracture. However, the Hip Fracture BPT was only feasible once a framework had been established for capturing high-quality clinical audit data. This framework was provided by the National Hip Fracture Database (NHFD), which was launched three years previously. We therefore examined the effect: first of the introduction of the NHFD from January 2007; second of the introduction of the Hip Fracture BPT from April 2010; and third of the combined effect of the NHFD/BPT intervention.
The NHFD was launched in 2007 and captures data on most adults aged more than 60 years with a hip fracture treated in England, Wales, and Northern Ireland.8 All acute hospitals treating hip fractures in England, Wales, and Northern Ireland contribute data to the NHFD.8 In addition to publicly reporting hospital-level outcomes in an annual report, the NHFD provides an online platform, through which clinical teams can visualize their outcomes and performance and compare them with national clinical standards.9 These standards have changed over time (Supplementary Table i).
All NHS hospitals are reimbursed by a system of tariffs based on an adjusted formula applied to the “reference costs” returned by NHS organizations that estimate the cost of treating patients the previous year. In order to achieve the Hip Fracture BPT, hospitals must satisfy all the criteria shown in Supplementary Table i. The NHFD reports patient-level compliance with the national standards to the local Clinical Commissioning Group (CCG),20 which makes a quarterly correction payment to individual hospitals.
The primary outcome was 30-day mortality. Secondary outcomes included 60-, 90-, and 365-day mortality as well as 30-, 60-, and 90-day re-admission, time to operation (defined as binary early time to the operating room of less than or more than two days), and acute length of stay (LOS) in days.
Statistical analysis
Differences in demographic and clinical variables were compared between countries before and after the introduction of the NHFD and BPT in 2007 to 2010, respectively, in order to visualize potential differences between groups. The ‘pre-intervention’ period was defined as 1 January 2000 to 31 December 2006 and the ‘post-intervention’ period was defined as 1 May 2010 to 1 February 2018. Patients admitted in the period between 1 January 2007 and 30 April 2010 were incorporated into stepwise analyses examining changes before and after establishment of the NHFD and the BPT. Covariate information, presented in Table I, was largely consistent between groups with subtle time-consistent differences in admitted hip fracture patients in England and Scotland.
Table I.
Differences in demographic parameters before and after implementation in England and Scotland
| Overall | Pre-intervention: January 2000 to December 2006 | Post-intervention: April 2010 to December 2016 | ||||
|---|---|---|---|---|---|---|
| Variable | England | Scotland | England | Scotland | England | Scotland |
| Hip fractures, n (%) | 1 037 860 (89.9) | 116 594 (10.1) | 391 697 (89.4) | 46 404 (10.6) | 446 098 (90.3) | 47 730 (9.7) |
| Age, n (%) | ||||||
| 60 to 64 yrs | 30 454 (2.9) | 5071 (4.3) | 10 225 (2.6) | 1889 (4.1) | 13 692 (3.1) | 2115 (4.4) |
| 65 to 69 yrs | 49 178 (4.7) | 7646 (6.6) | 17 336 (4.4) | 2978 (6.4) | 23 178 (5.2) | 3176 (6.7) |
| 70 to 74 yrs | 83 750 (8.1) | 12 308 (10.6) | 33 538 (8.6) | 5236 (11.3) | 34 752 (7.8) | 4767 (10.0) |
| 75 to 79 yrs | 152 778 (14.7) | 19 502 (16.7) | 63 205 (16.1) | 8170 (17.6) | 60 507 (13.6) | 7605 (15.9) |
| 80 to 84 yrs | 240 553 (23.2) | 26 397 (22.6) | 96 160 (24.5) | 10 642 (22.9) | 97 993 (22.0) | 10 691 (22.4) |
| 85 to 89 yrs | 259 565 (25.0) | 25 767 (22.1) | 92 498 (23.6) | 9813 (21.1) | 113 854 (25.5) | 10 825 (22.7) |
| ≥ 90 yrs | 221 582 (21.3) | 19 903 (17.1) | 78 735 (20.1) | 7676 (16.5) | 102 122 (22.9) | 8551 (17.9) |
| Sex, n (%) | ||||||
| Male | 256 703 (24.7) | 29 141 (25.0) | 84 411 (21.6) | 10 426 (22.5) | 112 404 (25.2) | 12 927 (27.1) |
| Female | 781 157 (75.3) | 87 453 (75.0) | 307 286 (78.4) | 35 978 (77.5) | 323 694 (72.6) | 34 803 (72.9) |
| Multiple deprivation index, n (%) | ||||||
| Least deprived 10% | 94 045 (9.1) | N/A | 32 649 (8.3) | N/A | 42 881 (9.6) | N/A |
| Less deprived 10% to 20% | 105 647 (10.2) | N/A | 38 843 (9.9) | N/A | 46 275 (10.4) | N/A |
| Less deprived 20% to 30% | 109 502 (10.6) | N/A | 40 488 (10.3) | N/A | 48 010 (10.8) | N/A |
| Less deprived 30% to 40% | 113 209 (10.9) | N/A | 41 918 (10.7) | N/A | 49 311 (11.1) | N/A |
| Less deprived 40% to 50% | 115 604 (11.1) | N/A | 43 150 (11.0) | N/A | 50 517 (11.3) | N/A |
| More deprived 40% to 50% | 112 110 (10.8) | N/A | 42 733 (10.9) | N/A | 48 052 (10.8) | N/A |
| More deprived 30% to 40% | 104 486 (10.1) | N/A | 39 906 (10.2) | N/A | 44 443 (10.0) | N/A |
| More deprived 20% to 30% | 99 736 (9.6) | N/A | 38 543 (9.8) | N/A | 41 805 (9.4) | N/A |
| More deprived 10% to 20% | 92 699 (8.9) | N/A | 36 962 (9.4) | N/A | 37 803 (8.5) | N/A |
| Most deprived 10% | 90 822 (8.8) | N/A | 36 505 (9.3) | N/A | 37 001 (8.3) | N/A |
| Charlson Comorbidity Index, n (%) | One-year look back | Hospital reported | One-year look back | Hospital reported | One-year look back | Hospital reported |
| 0 | 465 976 (44.9) | 102 874 (88.2) | 229 389 (58.6) | 40 917 (88.2) | 143 451 (32.2) | 42 178 (88.4) |
| 1 | 309 067 (29.8) | 9331 (8.0) | 104 123 (26.6) | 3805 (8.2) | 140 969 (31.6) | 3774 (7.9) |
| 2 | 139 411 (13.4) | 3583 (3.1) | 36 722 (9.4) | 1387 (3.0) | 77 399 (17.4) | 1444 (3.0) |
| ≥ 3 | 123 406 (11.9) | 806 (0.7) | 21 463 (5.5) | 295 (0.6) | 84 279 (18.9) | 334 (0.7) |
N/A, not applicable
Changes in hip fracture outcomes in England and Scotland were first visualized graphically by month in order to detect obvious changes and ensure the existence of pre-intervention parallel trends, which is a requirement of quasi-experimental DID analysis. These visualizations included scatter plots with locally weighted smoothing (LOWESS) lines, which are smooth lines created using regression analysis to help visualize trends over time.
The quantitative assessment of before-and-after changes was also undertaken for English data using interrupted time series analysis (ITSA). ITSA was used to contextualize the main DID results for mortality and to account for changes in English outcomes not reported in Scottish data, such as time to operation. ITSA functions by fitting linear regression models to observations from the pre- and post-intervention periods. For the purposes of ITSA analysis, longitudinal patient-level data were aggregated into monthly bins for each month of the year and were plotted by month as the proportion (or indicated quantile of initial LOS) of each outcome of interest. Analyses were based on 84 pre-intervention points (patients admitted between January 2000 and December 2006) and 81 post-intervention points (patients admitted between April 2010 and December 2016). Models estimated the pre-intervention trend (‘pre-intervention annual change’), change in level immediately following the intervention (‘instant change’) and change in post-intervention trend (‘post-intervention annual change’). The presence of autocorrelation was tested using the Durbin–Watson test. The extent to which the intercept of the post-intervention model deviates from the anticipated pre-intervention trend is assumed to represent an instantaneous causal effect of the intervention taking place during the same period of time. Ongoing changes during the post-intervention period, the post-intervention slope, can sometimes be observed as a marked and maintained change from pre-intervention trends.
Differences in outcomes for mortality for England and Scotland were further compared using DID regression. This quasi-experimental technique functions by fitting linear models to temporally aggregated data from the pre- and post-intervention periods. It includes coefficients for intervention group (e.g. England vs Scotland), time period (e.g. pre- vs post-intervention) and an interaction term between an intervention group and a period of time. The magnitude and direction of the interaction term (the so-called DID between temporal changes within each country) is assumed to represent the causal effect.12
Software: StataIC v.15.0 (StataCorp, College Station, Texas) was used for all statistical analyses. Panel data for ITSA were constructed from the admission-level master data set using collapse commands. They were analyzed using the ITSA module21 in Stata. The DIFF module22 was used for linear DID regression in order to obtain p-values and country-specific and time period-specific tabulations for tables; 95% confidence intervals (CI) were obtained by manually fitted versions of the same models.
The use of HES data for this project was approved by the NHS Digital Independent Group Advising on the Release of Data. NHS Digital undertook the linkage to ONS data and created mortality flags at defined timepoints. Pseudo-anonymized data were then transmitted to researchers at the University of Oxford. The Information Services Division of National Services Scotland provided pseudo-anonymized records from the Scottish Morbidity Record Scheme. Ethical approval was not sought in line with GAFReC guidance.23 Personal data were processed under Articles 6(1)(f) and 9(2)(f) of the General Data Protection Regulation (EU) 2016/679.
This research was undertaken independently of the authors’ funding bodies, which did not have any influence on the study design, analysis, data interpretation, or decision to publish.
Results
A total of 1 037 860 adults aged more than 60 years were admitted between 2000 and 2016 with a hip fracture in England, and 116 594 in Scotland. The demographic characteristics of these groups are shown in Table I. Table II presents the ITSA results of English data, and Table III shows the more detailed DID analyses for mortality that include Scotland as a comparison group.
Table II.
Interrupted time-series analysis (ITSA) results before and after implementation among adults aged more than 60 years in England, 2000 to 2016. ITSA compared differences in English temporal trends before (January 2000 to December 2006) and after (April 2010 to December 2016) combined policy implementation in 2007 to 2010
| Annual trend pre-implementation | 95% CI | Instant change | 95% CI | p-value | Annual trend post-implementation | 95% CI | |
|---|---|---|---|---|---|---|---|
| Mortality, % | |||||||
| 30-day | 0.0 | -0.1 to 0.2 | 2.6 | -3.4 to -1.7 | < 0.001 | - 0.2 | -0.2 to -0.1 |
| 60-day | 0.1 | 0.0 to 0.3 | - 4.3 | -5.5 to -3.1 | < 0.001 | - 0.2 | -0.4 to -0.1 |
| 90-day | 0.2 | 0.0 to 0.4 | - 5.4 | -6.8 to -4.1 | < 0.001 | - 0.2 | -0.4 to -0.1 |
| 365-day | 0.2 | 0.1 to 0.4 | - 5.3 | -6.3 to -4.2 | < 0.001 | - 0.1 | -0.2 to 0.0 |
| Re-admission, % | |||||||
| 30-day | 0.4 | 0.3 to 0.5 | - 1.3 | -2.2 to -0.5 | 0.003 | - 0.2 | -0.4 to -0.1 |
| 60-day | 0.7 | 0.5 to 0.8 | - 1.4 | -2.3 to -0.5 | 0.002 | - 0.1 | -0.1 to 0.0 |
| 90-day | 0.8 | 0.6 to 0.9 | - 1.2 | -2.1 to -0.2 | 0.015 | 0.0 | -0.1 to 0.1 |
| Length of stay | |||||||
| 50th percentile (median) | -0.1 | -0.2 to 0.0 | -2.8 | -3.5 to -2.1 | < 0.001 | -0.4 | -0.5 to -0.3 |
| 60th percentile | -0.1 | -0.2 to 0.0 | -3.8 | -4.6 to -3.1 | < 0.001 | -0.5 | -0.7 to -0.4 |
| 70th percentile | -0.1 | -0.3 to 0.0 | -5.4 | -6.4 to -0.3 | < 0.001 | -0.7 | -0.8 to -0.5 |
| 80th percentile | -0.3 | -0.5 to -0.1 | -7.3 | -8.8 to -5.8 | < 0.001 | -1.0 | -1.2 to -0.8 |
| Early time to theatre, % | - 0.6 | -0.9 to -0.4 | 15.4 | 13.7 to 17.0 | < 0.001 | 0.7 | 0.5 to 0.8 |
CI, confidence interval
Table III.
Difference-in-difference (DID) results before and after Best Practice Tariff (BPT) implementation among adults aged more than 60 years, 2000 to 2016. DID compared differences in mortality trends before (January 2000 to December 2006) and after (April 2010 to December 2016) combined policy implementation in 2007 to 2010 in England versus Scotland, ‘intervention overall’. They also broke down contributions to differences in mortality before (January 2000 to December 2016) and after (January 2008 to March 2010) introduction of the National Hip Fracture Database (NHFD) and clinical audit alone in 2007, ‘NHFD introduced’; and before (January 2008 to March 2010) and after (April 2010 to December 2016) formal introduction of payment penalties under the Best Practice Tariff alone in 2010, ‘BPT introduced’
| Scotland | England | NHFD introduced | BPT introduced | Intervention overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 to 2006 | 2007 to 2009 | 2010 to 2016 | 2000 to 2006 | 2007 to 2009 | 2010 to 2016 | DID | 95% CI | p-value | DID | 95% CI | p-value | DID | 95% CI | p-value | |
| Mortality, % | |||||||||||||||
| 30-day | 9.8 | 8.8 | 8.5 | 9.8 | 8.7 | 6.8 | -0.1 | 0.5 to 0.5 | 0.987 | 1.6 | 2.1 to 1.2 | < 0.001 | 1.7 | 2.0 to 1.2 | < 0.001 |
| 60-day | 15.4 | 13.8 | 13.1 | 15.6 | 14.0 | 11.4 | 0.0 | 0.6 to 0.6 | 0.994 | 1.9 | 2.5 to 1.3 | < 0.001 | 1.9 | 2.3 to 1.4 | < 0.001 |
| 90-day | 18.9 | 17.3 | 16.4 | 19.4 | 17.6 | 14.7 | -0.2 | 0.9 to 0.5 | 0.581 | 2.0 | 2.6 to 1.3 | < 0.001 | 2.2 | 2.6 to 1.6 | < 0.001 |
| 365-day | 32.0 | 30.4 | 29.8 | 31.9 | 30.2 | 27.8 | -0.1 | 1.0 to 0.6 | 0.688 | 1.8 | 2.5 to 1.0 | < 0.001 | 1.9 | 2.5 to 1.3 | < 0.001 |
| Re-admission, % | |||||||||||||||
| 30-day | 11.1 | 11.9 | 12.2 | 20.5 | 20.0 | 21.0 | -1.3 | 1.9 to 0.6 | < 0.001 | 0.7 | 0.1 to 1.4 | 0.051 | 0.6% | 1.1 to 0.1 | 0.038 |
| Length of stay | |||||||||||||||
| 50th percentile | 20.0 | 21.0 | 20.0 | 17.0 | 14.0 | 12.0 | -4.0 | -4.4 to -3.6 | < 0.001 | -1.0 | -1.4 to -0.6 | < 0.001 | -5.0 | -5.3 to -4.7 | < 0.001 |
| 60th percentile | 28.0 | 29.0 | 26.0 | 21.0 | 18.0 | 15.0 | -4.0 | -4.4 to -3.6 | < 0.001 | 0.0 | -0.1 to 0.1 | 0.999 | -4.0 | -4.2 to -3.8 | < 0.001 |
| 70th percentile | 38.0 | 39.0 | 36.0 | 28.0 | 22.0 | 19.0 | -7.0 | -7.5 to -6.5 | < 0.001 | 0.0 | -0.2 to 0.2 | 0.999 | -7.0 | -7.2 to -6.8 | < 0.001 |
| 80th percentile | 53.0 | 54.0 | 50.0 | 37.0 | 30.0 | 25.0 | -8.0 | -9.1 to -6.9 | < 0.001 | -1.0 | -1.7 to -0.3 | 0.008 | -9.0 | -9.7 to -8.3 | < 0.001 |
CI, confidence interval
Figure 1a shows that the pre-intervention trends in 30-day mortality were the same in England and Scotland; 30-day mortality trended downwards in both countries after the launch of the NHFD, although the decline was more pronounced in England. The diverging lines became more obvious after April 2010 and this continued until the data were censored in December 2016 (ITSA instant change following combined policy implementation -2.6 percentage points (95% CI -3.4 to -1.7); annual trend post-implementation -0.2 (-0.2 to -0.1). DID analysis corroborated these findings, suggesting an overall reduction in 30-day mortality in England relative to Scotland of -1.7 percentage-points (95% CI -2.0 to -1.2). When stratified by each component of the intervention alone, the results suggest a modest reduction in 30-day mortality following NHFD introduction that did not reach significance (-0.1 (-0.5 to +0.5) percentage-points; p = 0.987) and a larger significant change of -1.6 percentage-points (-2.1 to -1.2) following introduction of the BPT. Between 2010 and 2016 in England (Fig. 1a), there were 7600 fewer deaths than expected within 30 days, following implementation of the BPT.
Fig. 1.
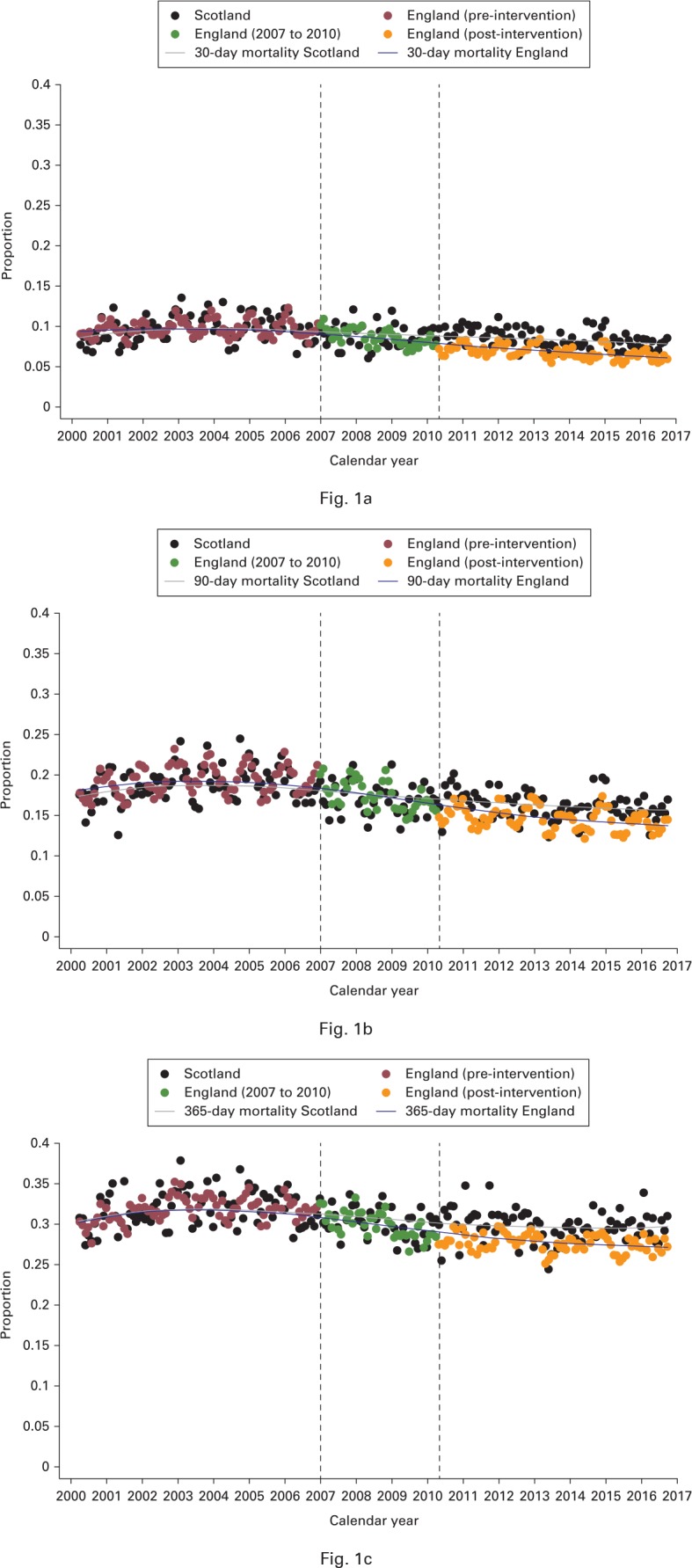
Charts showing monthly changes in a) 30-, b) 90- and c) 365-day mortality among adults aged more than 60 years, 2000 to 2016. Dashed lines represent introduction of the National Hip Fracture Database in January 2007 and the Best Practice Tariff in April 2010.
The effects on 60-day mortality showed the same direction and magnitude as on 30-day mortality (Fig. 1a). Figure 1b shows similar findings for 90-day mortality, although the effect of the BPT was more apparent at this time. DID analysis suggested that the combined intervention was associated with a change of -2.2 percentage-points (95% CI -2.6 to -1.6). However, this appeared to be driven entirely by the BPT: NHFD DID -0.2 percentage-points (95% CI -0.9 to -0.5) and BPT -2.0 (-2.6 to -1.3)).
Figure 1c shows that the effect on mortality at 365 days was similar to that for mortality at 30 and 60 days. Although a small (non-significant; p = 0.688) improvement was observed when the NHFD was introduced (DID -0.1 percentage point; 95% CI -1.0 to 0.6), the BPT was associated with a significant fall in 365-day mortality (-1.8; 95% CI -2.5 to -1.0; p < 0.001). The effect of the combined intervention on 365-day mortality was a change of -1.9 percentage-points (95% CI -2.5 to -1.3). Projection modelling (presented in Supplementary Figure a) suggests that were the BPT to be implemented in Scotland in 2019, upwards of 115 deaths could be prevented each year – a number totaling more than 1377 deaths by 2030.
Table II shows that re-admissions at all times (30, 60, and 90 days) were increasing steadily in England in the pre-implementation phase. The annual trend towards increasing 30-day re-admissions (0.4 percentage-points; 95% CI 0.3 to 0.5) was, however, reversed on implementation of the BPT (instant change -1.3 percentage points; 95% CI -2.2 to -0.5) and this decline continued each year subsequently (annual trend post-implementation -0.2 percentage points; 95% CI -0.4 to -0.1). Similar findings were observed for 60- and 90-day re-admissions, although the annual trend post-implementation did not change after the sudden fall associated with the BPT at these timepoints.
There was an annual trend towards fewer patients undergoing surgery within 36 hours in the pre-intervention period (annual trend -0.6 percentage-points; 95% CI -0.9 to -0.4). However, in the year following introduction of the NHFD/BPT, the proportion of patients reaching the operating theatre within this timeframe increased by an absolute value of 15.4 percentage-points (95% CI 13.7 to 17.0; Table II). This positive trend continued to increase by 0.7 percentage-points (95% CI 0.5 to 0.8) each year thereafter. Projection modelling (presented in Supplementary Figure a) suggests that were the BPT to be implemented in Scotland starting in 2019, upwards of 220 fewer re-admissions within 30 days would be expected among Scottish hip fracture patients by 2030.
The median LOS was declining modestly (annual trend -0.6 days; 95% CI -0.2 to 0.0) in the pre-intervention period. This reduction increased following implementation of the NHFD/BPT (instant change following policy implementation -2.8 days; 95% CI -3.5 to -2.1; annual trend post-policy implementation: -0.4 days; 95% CI -0.5 to -0.3) The magnitude of these reductions increased in a stepwise manner with each ascending quantile (e.g. 60th, 70th, and 80th) so that the largest reductions were observed amongst the patients with greatest initial LOS (80th percentile instant change: -7.3 days; 95% CI -8.8 to -5.8; annual trend post-implementation -1.0 days, 95% CI -1.2 to -0.8).
Discussion
This study provides evidence that the BPT drove changes in practice that reduced mortality for elderly patients with a hip fracture in England by as many as 7600 fewer deaths within 30 days between 2010 and 2016. It also suggests that the BPT increased the proportion of patients receiving an operation within 36 hours, shortened LOS, and reduced re-admissions within 30, 60, and 90 days.
A number of small studies have reported improved compliance with measures that were associated with introduction of the NHFD24 and BPT.25-27 The NHFD annual reports have also shown that English hospitals are increasingly achieving the hip fracture national clinical standards.8 One national study reported that mortality fell from 10.9% before the NHFD was launched to 8.5% afterwards.9 However, this study did not include a control population or analyze data after the BPT came into effect. Our data suggest that there was a gradual trend towards reduced mortality between 2007 and 2010 but that this was also apparent, albeit to a lesser extent, in Scotland, which did not participate in the NHFD. DID results restricted to the influence of the NHFD suggest that the differential trend between the two countries was not statistically significant following introduction of the NHFD in isolation. There was, however, a significant change leading up to full BPT implementation in 2010. DID results comparing BPT implementation alone between England and Scotland revealed a 2.0 percentage-point reduction in 90-day mortality (8.7% to 6.8% in England) that accounted for 90.9% of the overall effect (DID -2.2 percentage-points; 9.8% to 6.8% in England).
There are a number of changes that could account for improved outcomes over time across the United Kingdom, including publication of the BOA/BGS guidelines,28 increasing recognition of the need for early surgery and postoperative rehabilitation,29 and the emergence of orthogeriatrics as a medical subspecialty dedicated to caring for elderly patients with a fracture.30,31 It therefore seems unlikely that the NHFD alone accounted for the fall in mortality reported by Neuburger et al.9 Although our findings suggest that the NHFD might have had a small positive effect on English hip fracture outcomes, this was not statistically significant. However, implementation of the BPT was associated with a marked and sustained improvement in outcomes. It is nevertheless worth noting that the NHFD was a prerequisite for the choice of hip fracture outcomes as a target for pay-for-performance in England and so these two interventions are fundamentally linked.32 However, our data suggest that a system for rewarding best practice can improve outcomes beyond that of a voluntary audit of national clinical standards.
The improvements in LOS and re-admission suggest substantial resource savings attributable to the BPT in addition to reduced mortality.4,6 Importantly, the BPT itself did not require a substantial investment. It was initially set up as a payment of £445 ($570), which was based on an estimate of the cost that an average hospital was likely to incur to provide additional operating capacity. However, the base tariff was reduced initially to adjust for compliance with the BPT criteria that was already present throughout the NHS. As a consequence of the falling base tariff, the BPT has accounted for a greater proportion of the overall payment to hospitals each year – from £445 ($570) in 2010/11 to £890 ($1141) in 2011/12, £1335 ($1712) in 2012/13, and £1353 ($1735) in 2016/17.32 As 100% compliance with the standards has not been achieved, the overall payment nationally by CCGs changed little during the first three years. Although we have not presented a formal health economic analysis, it is likely that the BPT delivered improved hip fracture care at reduced cost to NHS commissioners. There is a rectification process in the NHS of hospital trusts returning reference costs, for the delivery of care, to ensure alignment between tariff price and the average cost of delivery.
Previous evaluations of pay-for-performance initiatives have reported mixed findings.4,6 Many early studies focused on ‘Value-Based Purchasing’ (VBP), which is a strategy used by the Centers for Medicare and Medicaid Services (CMS) in the United States. The VBP programme withholds 2% of annual Medicare payments and allocates these to hospitals based on the quality of care, compliance with best clinical practice, and patient experience.33 However, few studies have been able to demonstrate improvements in mortality or re-admissions that may be attributable to VBP.34-36 A similar scheme in the northwest region of England (‘Advancing Quality’) was found to have no long-term effect on 30-day mortality.37 A number of explanations have been proposed for this finding.38 First, the financial impact of the VBP is small (average $213 000 bonus and $1 200 000 penalty per hospital in 2015),39 which might be insufficient to motivate changes in clinical pathways given that only a proportion of patients in the United States are funded through CMS. Second, there are 21 individual measures and improving these in isolation is unlikely to improve a hospital’s overall score.40 Third, the financial reward for improvement is unclear until the end of the performance period because the scheme is designed to be cost-neutral and to transfer payments from low- to high-performers.38 By contrast, the Hip Fracture BPT overcomes many of these criticisms as it has a simple design, focuses on a small number of high-value measures and carries a financial incentive that may be sufficient to motivate changes.41 An alternative explanation for the success of the BPT is that it was part of a more complex intervention that began with the national clinical audit and NHFD. There is evidence that some hospitals engaged with the NHFD from 2007, by designing quality improvement processes, aimed at improving their performance using data provided through online visual dashboards and in publicly accessible reports.8,24 It is possible that the BPT provided additional impetus that helped clinicians and hospital leaders to create business cases that justified local investment in hip fracture services. Our study provides evidence that, despite concerns about the success of other schemes, it is possible to improve hip fracture outcomes through pay-for-performance. Further work should aim to identify the features that distinguish programmes that can demonstrably improve outcomes.
The apparent success of the Hip Fracture BPT in England could have policy implications for a number of countries. First, although there are 36 national clinical audits that are operated by the Healthcare Quality Improvement Program (HQIP)42 in England, there are only 21 BPTs that are used by NHS England to refine healthcare payments.43 This suggests that there are further opportunities to extend pay-for-performance to other groups of patients in England. Second, our findings raise the possibility that the introduction of a comparable pay-for-performance initiative might reduce mortality following hip fracture in Scotland. Finally, this study might encourage policy makers outside the United Kingdom to consider implementing pay-for-performance programmes to improve hip fracture outcomes. For example, although the CMS coordinates health payments for most patients aged more than 65 years in the United States, the VBP programme does not yet extend to hip fractures. There are more than 200 000 hip fractures in the United States each year44,45 with a reported mortality of 5.2% at 30 days.44 If the estimated benefits of the BPT in England were generalizable to the United States, the CMS expansion of pay-for-performance to elderly patients with a hip fracture could prevent as many as 3600 deaths per year.
The strengths of this study are the use of comprehensive national cohorts linked to death certificate registrations and a ‘control’ region, which overcame the limitations of earlier before-and-after studies. There are, however, a number of possible limitations to this approach. First, our study would still be vulnerable to confounding factors if another event had occurred at the same time as the NHFD/BPT but only affected outcomes in either England or Scotland.12 We are not aware of any such events and the factors that are thought to have driven recent trends towards improved hip fracture outcomes, such as the rise of orthogeriatrics as a medical subspecialty,31 would be expected to have applied across the whole of the United Kingdom. Although there was a reconfiguration of major trauma services in England (but not Scotland) from April 2012, this did not have a measurable effect on the quality of hip fracture care.46 Second, we did not have access to some variables, such as LOS, in Scottish data and so were limited to undertaking ITSA without a control region for these outcomes. As discussed above, the absence of a control can result in erroneous attribution of change to a single intervention.12 It is, however, reassuring that the findings from ITSA for the other outcomes were consistent with those of the DID analyses. A lack of variables also restricted our ability to present baseline characteristics for hip fracture patients in England and Scotland. Some variables from SMR01, such as age, were categorized by the data owners to preserve the anonymity of patients and others, such as index of multiple deprivation (IMD), were available for England but not Scotland. However, this limitation is partly accounted for by the study design as there is no obvious reason why differences in patient characteristics should have changed between the pre-intervention period (when outcome trends were parallel) and post-intervention period (when trends diverged). Third, we selected outcomes that could be readily quantified using administrative data. Although mortality and re-admissions are important quality metrics, other outcomes such as pain, mobility, and health-related quality of life might be more important to patients.47 Finally, we focused on hip fracture outcomes and so could not determine whether the BPT had an effect on other groups of patients. Unintended consequences of the BPT could include the deprioritization of other elderly patients with lower limb injuries, such as of the distal femur or ankle, who share many vulnerabilities as those with a hip fracture.48,49 Alternatively, further benefits might extend to such patients (a so-called ‘halo effect’) as hospitals are likely to have invested in orthogeriatricians and dedicated trauma operating theatres in order to achieve the BPT. The effect of pay-for-performance on related groups patients should be a focus for future research.
In conclusion, this study provides evidence that the Hip Fracture BPT improved hip fracture outcomes in England. It is therefore possible that BPTs could improve outcomes and reduce costs in other disease groups. Policymakers and clinicians should support the controlled expansion of the BPT model to other clinical areas and health policy environments.
Take home message
- The Hip Fracture Best Practice Tariff (BPT) was associated with reduced mortality for elderly patients with a hip fracture in England.
- The BPT may also have driven changes that increased the proportion of patients receiving prompt surgery, shortened length of stay, and reduced hospital re-admissions.
Author contributions
D. Metcalfe: Designed the study, Analyzed and interpreted the data, Wrote the manuscript.
C. K. Zogg: Designed the study, Analyzed and interpreted the data, Edited the manuscript.
A. Judge: Designed the study, Interpreted the data, Edited the manuscript.
D. C. Perry: Designed the study, Interpreted the data, Edited the manuscript.
B. Gabbe: Designed the study, Interpreted the data, Edited the manuscript.
K. Willett: Designed the study, Interpreted the data, Edited the manuscript.
M. L. Costa: Designed the study, Interpreted the data, Edited the manuscript.
D. Metcalfe and C. K. Zogg contributed equally and should be considered joint first authors.
Funding statement
D. Metcalfe is funded by an Oxford-UCB Prize Fellowship in Biomedical Research (which funded this paper’s open access status) and a Royal College of Surgeons of Edinburgh Small Pump Priming Grant. C. K. Zogg is supported by a National Institutes of Health (NIH) Medical Scientist Training Program Training Grant T32GM007205. C. K. Zogg is the principal investigator of a grant from the Emergency Medical Foundation and American College of Emergency Physicians entitled, ‘Understanding Emergency Medicine Providers’ Perceptions of the ACA in a Renewed Era of Healthcare Reform: National Survey and Qualitative Mixed-Methods Approach’. A. Judge is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. D. Perry is supported by a NIHR Clinician Scientist Fellowship (NIHR/CS/2014/14/012). All authors carried out this research independently of the funding bodies. The views expressed in this publication are those of the authors and do not necessarily reflect those of the NHS, the National Institute for Health Research, or the Department of Health and Social Care.
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgements
We are grateful to NHS Digital and the Information Services Division (ISD) Scotland for providing the data used for this study.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
This article was primary edited by J. Scott.
Supplementary material
A table summarizing eligibility for payment of the Best Practice Tariff, as well as a figure showing the projected reductions in 30-day mortality, 365-day mortality, and 30-day re-admission were the Best Practice Tariff to be introduced in Scotland in 2019.
Follow D. Metcalfe @TraumaDataDoc
Follow C. K. Zogg @CherylZogg
Follow A. Judge @andyjudgeox
Follow D. C. Perry @MrDanPerry
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–1733. [DOI] [PubMed] [Google Scholar]
- 2.Rapp K, Buchele G, Dreinhofer K, et al. Epidemiology of hip fractures: systematic literature review of German data and an overview of the international literature. Z Gerontol Geriatr 2018;52,10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U. The cost of osteoporotic fractures in the UK: projections for 2000–2010. J Med Econ 2001;4:51–62. [Google Scholar]
- 4.Maynard A. The powers and pitfalls of payment for performance. Health Econ 2012;21:3–12. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor RJ, Neumann VC. Payment by results or payment by outcome? The history of measuring medicine. J R Soc Med 2006;99:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogundeji YK, Bland JM, Sheldon TA. The effectiveness of payment for performance in health care: A meta-analysis and exploration of variation in outcomes. Health Policy 2016;120:1141–1150. [DOI] [PubMed] [Google Scholar]
- 7.Marshall L, Charlesworth A, Hurst J. The NHS payment system: evolving policy and emerging evidence. Nuffield Trust. 2014. https://www.nuffieldtrust.org.uk/research/the-nhs-payment-system-evolving-policy-and-emerging-evidence (date last accessed 2 April 2019).
- 8.No authors listed Falls and Fragility Fracture Audit Programme (FFFAP). National Hip Fracture Database (NHFD) Annual Report 2017. National Hip Fracture Database; 2017. https://www.nhfd.co.uk/2017report (date last accessed 2 April 2019). [Google Scholar]
- 9.Neuburger J, Currie C, Wakeman R, et al. The impact of a national clinician-led audit initiative on care and mortality after hip fracture in England: an external evaluation using time trends in non-audit data. Med Care 2015;53:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health 2012;66:1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015;350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014;312:2401–2402. [DOI] [PubMed] [Google Scholar]
- 13.Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data Resource Profile: Hospital Episode Statistics Admitted Patient Care (HES APC). Int J Epidemiol 2017;46:1093–1093i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.No authors listed SD Scotland (2010). Secondary care information collection. https://www.isdscotland.org/Products-and-Services/Terminology-Services/Information-for-Clinicians/Secondary-Care-Information (date last accessed 24 May 2019).
- 15.No authors listed Healthcare across the UK: a comparison of the NHS in England, Scotland, Wales and Northern Ireland. National Audit Office. 2012. https://www.nao.org.uk/report/healthcare-across-the-uk-a-comparison-of-the-nhs-in-england-scotland-wales-and-northern-ireland (date last accessed 2 April 2019).
- 16.No authors listed Impact of registration delays on mortality statistics. Office for National Statistics. 2016. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/impactofregistrationdelaysonmortalitystatistics (date last accessed 2 April 2019).
- 17.No authors listed SMR Completeness Estimates. Information Services Division (ISD) Scotland. 2018. https://www.isdscotland.org/Products-and-Services/Data-Support-and-Monitoring/SMR-Completeness (date last accessed 2 April 2019).
- 18.Robson K. The National Health Service in Scotland 2016. The Scottish Parliament. 2016. http://www.parliament.scot/ResearchBriefingsAndFactsheets/S5/SB_16–100_The_National_Health_Service_in_Scotland.pdf (date last accessed 23 April 2019).
- 19.World Health Organization The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. WHO: Geneva: https://www.who.int/classifications/icd/en/bluebook.pdf (Date last accessed 24 May 2019). [Google Scholar]
- 20.No authors listed 2019/20 National Tariff Payment System – A Consultation Notice: Annex DtD. NHS England and NHS Improvement. https://improvement.nhs.uk/documents/484/Annex_DtD_Best_practice_tariffs.pdf (date last accessed 24 May 2019).
- 21.Linden A. ITSA: Stata module to perform interrupted time series analysis for single and multiple groups. 2017; Statistical Software Components, Boston College Department of Economics, Massachusetts: https://ideas.repec.org/c/boc/bocode/s457793.html (date last accessed 24 May 2019). [Google Scholar]
- 22.Villa JM. DIFF: Stata module to perform differences in differences estimatation. 2018; Statistical Software Components, Boston College Department of Economics, Massachusetts, USA: https://ideas.repec.org/c/boc/bocode/s457083.html (date last accessed 24 May 2019). [Google Scholar]
- 23.No authors listed Governance arrangements for research ethics committees. NHS Health Research Authority. 2018. https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/governance-arrangement-research-ethics-committees (date last accessed 2 April 2019).
- 24.Patel NK, Sarraf KM, Joseph S, Lee C, Middleton FR. Implementing the National Hip Fracture Database: An audit of care. Injury 2013;44:1934–1939. [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain M, Pugh H. Improving inpatient care with the introduction of a hip fracture pathway. BMJ Qual Improv Rep 2015;4.u204075.w2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisk R, Yeong K. Reducing mortality from hip fractures: a systematic quality improvement programme. BMJ Qual Improv Rep 2014;3:u205006.w2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oakley B, Nightingale J, Moran CG, Moppett IK. Does achieving the best practice tariff improve outcomes in hip fracture patients? An observational cohort study. BMJ Open 2017;7:e014190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.No authors listed The care of patients with fragility fracture (Blue Book). British Geriatrics Society. 2007. https://www.bgs.org.uk/resources/care-of-patients-with-fragility-fracture-blue-book (date last accessed 2 April 2019).
- 29.Bretherton CP, Parker MJ. Early surgery for patients with a fracture of the hip decreases 30-day mortality. Bone Joint J 2015;97-B:104–108. [DOI] [PubMed] [Google Scholar]
- 30.Hawley S, Javaid MK, Prieto-Alhambra D, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing 2016;45:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahota O, Currie C. Hip fracture care: all change. Age Ageing 2008;37:128–129. [DOI] [PubMed] [Google Scholar]
- 32.Gerschlick B. Best Practice Tariffs. Country Background Note: United Kingdom (England). 2016. https://www.oecd.org/els/health-systems/Better-Ways-to-Pay-for-Health-Care-Background-Note-England-Best-practice-tariffs.pdf (date last accessed 24 May 2019).
- 33.No authors listed Hospital Value-Based Purchasing. Centers for Medicare and Medicaid Services. 2017. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf (date last accessed 2 April 2019).
- 34.Chee TT, Ryan AM, Wasfy JH, Borden WB. Current state of Value-Based Purchasing programs. Circulation 2016;133:2197–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueroa JF, Tsugawa Y, Zheng J, Orav EJ, Jha AK. Association between the Value-Based Purchasing pay for performance program and patient mortality in US hospitals: observational study. BMJ 2016;353:i2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan AM, Krinsky S, Maurer KA, Dimick JB. Changes in Hospital quality associated with hospital value-based purchasing. N Engl J Med 2017;376:2358–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kristensen SR, Meacock R, Turner AJ, et al. Long-term effect of hospital pay for performance on mortality in England. N Engl J Med 2014;371:540–548. [DOI] [PubMed] [Google Scholar]
- 38.Dalzell MD. Now might be the right time to kill hospital Value-Based Purchasing program. Manag Care 2017;26:14–16. [PubMed] [Google Scholar]
- 39.Rau J. 1,700 hospitals win quality bonuses from Medicare, but most will never collect. Kaiser Health News. 22 January 2015 https://khn.org/news/1700-hospitals-win-quality-bonuses-from-medicare-but-most-will-never-collect (date last accessed 24 May 2019).
- 40.No authors listed Growth of population-based payments is not associated with a decerease in market-level cost growth, yet. Leavitt Partners. 2018. https://leavittpartners.com/whitepaper/growth-of-population-based-payments-is-not-associated-with-a-decrease-in-market-level-cost-growth-yet/ (last accessed 2 April 2019).
- 41.Jha AK. Time to get serious about pay for performance. JAMA 2013;309:347–348. [DOI] [PubMed] [Google Scholar]
- 42.No authors listed A-Z of National Clinical Audits. Healthcare Quality Improvement Partnership. 2018. https://www.hqip.org.uk/a-z-of-nca (last accessed 2 April 2019).
- 43.No authors listed 2017/18 and 2018/19 National Tariff Payment System. NHS England and NHS Improvement. 2016 https://improvement.nhs.uk/resources/national-tariff-1719/ (last accessed 2 April 2019). [Google Scholar]
- 44.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009;302:1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michael Lewiecki E, Wright NC, Curtis JR, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int 2018;29:717–722. [DOI] [PubMed] [Google Scholar]
- 46.Metcalfe D, Gabbe BJ, Perry DC, et al. Quality of care for patients with a fracture of the hip in major trauma centres: a national observational study. Bone Joint J 2016;98-B:414–419. [DOI] [PubMed] [Google Scholar]
- 47.Haywood KL, Griffin XL, Achten J, Costa ML. Developing a core outcome set for hip fracture trials. Bone Joint J 2014;96-B:1016–1023. [DOI] [PubMed] [Google Scholar]
- 48.Lester HE, Hannon KL, Campbell SM. Identifying unintended consequences of quality indicators: a qualitative study. BMJ Qual Saf 2011;20:1057–1061. [DOI] [PubMed] [Google Scholar]
- 49.Smith JR, Halliday R, Aquilina AL, Collaborative-Orthopaedic Trauma Society (OTS) . Distal femoral fractures: the need to review the standard of care. Injury 2015;46:1084–1088. [DOI] [PubMed] [Google Scholar]


