Abstract
To determine the impact of delay between surgery and radiotherapy on overall survival (OS) in temozolomide treatmented patients with the incorporation of O6-methylguanine–DNA methyltransferase (MGMT). From 2000 to 2012, 345 consecutive glioblastoma patients were treated with surgery, radiotherapy, and temozolomide at our institution. A Cox-regression model was constructed using significant univariate parameters, known prognostic factors including MGMT, and the interval from surgery to radio-therapy (≤2, 2–5, and ≥6 weeks). Survival rates were calculated by Kaplan–Meier methods. Cox-regression was utilized to calculate adjusted hazard ratios (HR). The median survival for the entire cohort was 12.2 months. The 1 year actuarial OS was 43.1 %, 53.3 %, and 64.3 % (p = 0.11), for intervals from surgery to radiotherapy of ≤2, 2–5, and ≥6 weeks, respectively. Patients radiated within 2 weeks post-surgery were more likely to have older age (p = 0.03), treated with 2D techniques (p < 0.001) and dose <36 Gy (p < 0.001), undergo a biopsy only (p < 0.001), KPS of<70 (p < 0.001), severe pre-radiotherapy neurologic symptoms (p = 0.04), and bilateral disease (p = 0.02). Multivariate analysis including MGMT status demonstrated a significant detriment in delaying radiotherapy (≤2 weeks as reference); 3–5 weeks (HR 2.80 [0.72–10.89], p = 0.14), and>6 weeks (HR 3.76 [1.01–14.57], p = 0.05). We report the first analysis on the survival impact of delaying post-operative radiotherapy for temozolomide treated glioblastoma patients with MGMT information. Our data does not support the OS benefit previously seen in delayed RT when correcting for important covariates. We demonstrate a survival detriment with delaying RT post-surgery greater than 6 weeks on multivariate analysis.
Keywords: Glioblastoma, Radiotherapy, Surgery, Temozolomide, Timing
Introduction
In 2013 an estimated 23,120 new primary central nervous system tumors will be diagnosed in the United States [1]. Glioblastoma, the most common adult brain tumor, accounts for approximately 15 % of brain tumors in adults between 45 and 70 years of age. The single largest advance for the treatment of glioblastoma in the last three decades was gained from the results of the EORTC/NCIC 26981 trial demonstrating a median survival benefit of 2.5 months with the addition of temozolomide to radiotherapy [2, 3]. Since the initial design of the EORTC/NCIC trial, unprecedented progress though epigenetic and whole genome sequencing has provided insights into powerful predictive and prognostic markers, such as O6-methylguanine–DNA methyltransferase (MGMT) and isocitrate dehydrogenase-1 (IDH-1), respectively [4, 5]. However, despite the advances in prognostication and treatment, the 5-year survival rates remain a devastating 9.8 % [2].
Given the paucity of effective treatments and poor outcomes even with standard of care therapy, research has emerged on how to optimize the current treatment regimen of surgery followed by post-operative radiotherapy. One such highly controllable variable is the time interval between surgical intervention and the start of radiation therapy. In current clinical practice, the interval from surgery to start of radiation therapy is influenced by a multitude of patient, physician, and infrastructure-related factors, and may not begin until after 4 weeks post-operatively [6]. However, the mean tumor volume doubling time for glioblastoma is estimated to be 24 days, suggesting that a delay in initiating radiotherapy could potentially have an adverse effect on outcome [7].
The impact of this delay has been studied in multiple contexts, including retrospective analyses of RTOG clinical trials [8], SEER-Medicare analyses [9], and single[10–12] and multi-institutional studies [6]. A pooled analysis of four of these studies in 2011 suggested an overall survival benefit of delaying radiotherapy up to 6 weeks [13]. Furthermore, the largest series reported from Blumenthal et al. on 2,855 patients found a 3.3 month benefit in survival from delaying radiotherapy from ≤2 weeks to >4 weeks [8].
Nearly all of the aforementioned studies were conducted in the pre-temozolomide era, and the use of MGMT was not available or in routine use. For this reason, we attempted to gain insight into the impact of the timing of radiotherapy post-operatively in a well-characterized population of patients treated with the current standard of care of surgery, radiotherapy, and temozolomide for glioblastoma.
Methods
Institutional Review Board (IRB) approval was obtained to conduct this clinical study. An electronic query of all patients with primary brain tumors treated at our institution with surgery, radiotherapy and temozolomide was performed. Patients were screened from 2000 (during the accrual period of the EORTC/NCIC 26981 trial) until 2012; the initial screen identified 610 patients. Criteria were implemented on this cohort to exclude any patient with non-WHO IV glioblastoma tumor, any form of neoadjuvant therapy before surgery, prior radiotherapy to the brain, pediatric status (<21 years old), treatment with less than 24 Gy of radiotherapy, or any radiotherapy performed at an outside institution. In total, 345 consecutive patients met criteria and form the study cohort. Institutional pathology review was performed on all patients. Clinical information for this cohort was entered into a centralized database retrospectively.
Staging, treatment, and follow-up
In general, patients were diagnosed by biopsy or proceeded directly to definitive surgery. Patients routinely had a post-operative MRI (unless contraindicated) to determine extent of resection. Timing of post-operative radiotherapy was determined by subjective evaluation of clinical urgency and the planning time required for the complexity of technique chosen to treat the patient (2-dimensional, 3D conformal, or IMRT). Temozolomide dosing generally was 75 mg/m2 given concurrently with radiotherapy, and 150–200 mg/m2 adjuvantly thereafter. Duration of adjuvant therapy was highly variable, as patients often had signs of progression and/or died during the standard 6–12 month adjuvant phase (median survival of our cohort was 12.2 months). Patients were followed post-radiotherapy every 2 months with a serial MRI (unless contraindicated) and clinical neurological assessment.
Study outcomes
Overall survival (OS) was calculated from the time of completion of radiotherapy until the date of death or last follow-up. Dose cutoffs were chosen based on clinically relevant cut-offs (whole brain radiotherapy with conventional fractionation, fractionation based on Roa et al. [14] or hypofractionation, or standard full dose conventional RT based on Stupp et al. [3], typically to 60 Gy). Extent of surgical resection was determined based on the surgeon’s operative note and review of the post-operative MRI, generally performed within 48 h of surgery. For consistency if discordance was present, if the post-operative MRI visualized residual disease, then the patient was classified as having a subtotal resection (STR). Interval from surgery to radiotherapy was segregated into 3 groups, ≤2 weeks, 3–5 weeks, and ≥6 weeks.
The previously reported validated RPA based on the EORTC 26981/NCIC trial for patients treated with temozolomide was used [15]. As RPA stage V includes mini-mental status exam (MMSE)<27, we used clinical records in conjunction with neurologic status and KPS as a surrogate.
Statistical analysis
To compare baseline characteristics between groups based on timing of surgery to radiotherapy, contingency tables were generated. The Fisher’s exact test was used for categorical variables, and the Wilcoxon rank-sum test was used for continuous variables.
Actuarial curves for OS were calculated using the Kaplan–Meier method, and comparisons were performed using a log-rank test. To determine the impact of time from surgery to initiation of radiotherapy, a Cox-regression model was created based on significant univariate proportional hazard ratios (HR) and clinically relevant variables such as MGMT status. A confirmatory analysis excluding MGMT was performed to include the entire cohort of patients, which yielded a similar trend in results to our model with MGMT status (Supplementary data). Using the Cox regression method, adjusted Kaplan–Meier curves were generated [16]. For all statistical analyses, two-tailed P values of ≤0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS version 21.0.0.0 (SPSS Inc., Chicago, IL USA).
Results
Baseline and treatment characteristics
Baseline characteristics are shown in Table 1. The median age was 60 years old (IQR 61–68) and 56.2 % (n = 194) of the cohort were male. A total of 66.7 % (n = 230) were RPA stage IV, 81.2 % (n = 280) had unilateral tumors, and 88.4 % (n = 305) had a KPS ≥ 70. Most patients were treated with IMRT, 83.8 % (n = 289), and the median dose of RT was 60 Gy. STR was performed in 52.5 % (n = 181), gross total resection (GTR) in 30.4 % (n = 105), and biopsy alone in 17.1 % (n = 59). MGMT testing was performed in 45.8 % patients (n = 158) and 10.1 % (n = 35) had IDH mutational testing.
Table 1.
Baseline and treatment characteristics
| Total | Interval from surgery to radiotherapy |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
1–2 weeks |
3–5 weeks |
≥6 weeks |
P value | |||||
| n = 345 | % 100 | n = 21 | % 6.1 | n = 215 | % 62.3 | n = 109 | % 31.6 | ||
| Age | 0.027 | ||||||||
| Median (IQR) (years) | 60 | (51–68) | 62 | (55–73) | 61 | (51–68) | 56 | (48–65) | |
| Gender | 0.79 | ||||||||
| Male | 194 | 56.2 | 11 | 52.4 | 119 | 55.3 | 64 | 58.7 | |
| Female | 151 | 43.8 | 10 | 47.6 | 96 | 44.7 | 45 | 41.3 | |
| RPA | 0.001 | ||||||||
| III | 48 | 13.9 | 0 | 0 | 29 | 13.5 | 19 | 17.4 | |
| IV | 230 | 66.7 | 10 | 47.6 | 144 | 67 | 76 | 69.7 | |
| V | 67 | 19.4 | 11 | 52.4 | 42 | 19.5 | 14 | 12.8 | |
| Type of RT | 0.0003 | ||||||||
| 2D | 18 | 5.2 | 6 | 28.6 | 11 | 5.1 | 1 | 0.9 | |
| 3D | 38 | 11 | 4 | 19 | 20 | 9.3 | 14 | 12.8 | |
| IMRT | 289 | 83.8 | 11 | 52.4 | 184 | 85.6 | 94 | 86.2 | |
| Dose of RT | 0.0001 | ||||||||
| <36 Gy | 11 | 3.2 | 5 | 23.8 | 5 | 2.3 | 1 | 0.9 | |
| 36–54 Gy | 93 | 27 | 10 | 47.6 | 57 | 26.5 | 26 | 23.9 | |
| >54 Gy | 241 | 69.9 | 6 | 28.6 | 153 | 71.2 | 82 | 75.2 | |
| Type of surgery | 0.002 | ||||||||
| Biopsy | 59 | 17.1 | 8 | 38.1 | 36 | 16.7 | 15 | 13.8 | |
| STR | 181 | 52.5 | 12 | 57.1 | 119 | 55.3 | 50 | 45.9 | |
| GTR | 105 | 30.4 | 1 | 4.8 | 60 | 27.9 | 44 | 40.4 | |
| KPS | 0.0003 | ||||||||
| ≥90 | 150 | 43.5 | 2 | 9.5 | 95 | 44.2 | 53 | 48.6 | |
| 70–89 | 155 | 44.9 | 11 | 52.4 | 94 | 43.7 | 50 | 45.9 | |
| >70 | 40 | 11.6 | 8 | 38.1 | 26 | 12.1 | 6 | 5.5 | |
| MGMT | 0.22 | ||||||||
| Hyper-methylated | 49 | 31 | 3 | 60 | 27 | 27.3 | 19 | 35.2 | |
| Wild Type | 109 | 69 | 2 | 40 | 72 | 72.7 | 35 | 64.8 | |
| IDH | 0.58 | ||||||||
| Mutated | 3 | 8.6 | 0 | 0 | 1 | 5 | 2 | 14.3 | |
| Wild type | 32 | 91.4 | 1 | 100 | 19 | 95 | 12 | 85.7 | |
| Neurologic symtoms pre-RT | 0.04 | ||||||||
| None | 180 | 52.2 | 5 | 23.8 | 115 | 53.5 | 60 | 55 | |
| Seizure | 25 | 7.2 | 4 | 19 | 12 | 5.6 | 9 | 8.3 | |
| Sensory/motor deficit | 131 | 38 | 10 | 47.6 | 83 | 38.6 | 38 | 34.9 | |
| Paralysis | 9 | 2.6 | 2 | 9.5 | 5 | 2.3 | 2 | 1.8 | |
| Laterality | 0.02 | ||||||||
| Unilateral | 280 | 81.2 | 12 | 57.1 | 174 | 80.9 | 94 | 86.2 | |
| Bilateral | 65 | 18.8 | 9 | 42.9 | 41 | 19.1 | 15 | 13.8 | |
| Smoking | 0.13 | ||||||||
| Yes | 134 | 38.8 | 4 | 19 | 87 | 40.5 | 43 | 39.4 | |
| No | 210 | 60.9 | 17 | 81 | 127 | 59.1 | 66 | 60.6 | |
2D 2-dimensional, 3D 3-dimensional, GTR gross total resection, IDH isocitrate dehydrogenase, IMRT intensity modulated radiotherapy, IQR interquartile range, KPS Karnofsky Performance Status, MGMT O-6-methylguanine-DNA methyltransferase, RPA recursive partitioning analysis, RT radiotherapy, STR subtotal resection
When stratifying by interval from surgery to RT, 6.1 % (n = 21), 62.3 % (n = 215), and 31.6 % (n = 109) patients were irradiated ≤2 weeks, 3–5 weeks, and ≥6 weeks postsurgery. The median time from surgery to initiation of radiotherapy was 31 days. Patients treated within 2 weeks of surgery were more likely to be RPA stage V (p = 0.001), treated with 2D techniques (p < 0.001) and received less than 36 Gy (p < 0.001), undergo a biopsy only (p < 0.001), have a KPS < 70 (p < 0.001), have severe neurologic symptoms pre-radiotherapy (p = 0.04), and have bilateral disease (p = 0.02). There were no significant differences in MGMT (p = 0.22) or IDH mutational status (p = 0.58) between groups.
Temporal analyses on overall survival
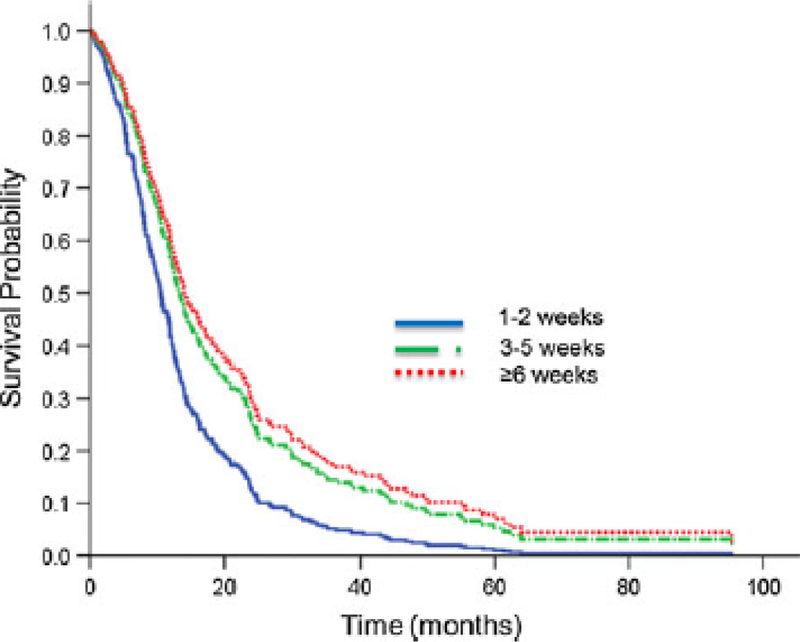
The median survival for the whole cohort was 12.2 months (IQR 7.4–22.6 months). The 1-year actuarial OS by interval from surgery to RT was 43.1, 53.3, and 64.3 % (log-rank, p = 0.11), for the ≤2 weeks, 2–5 weeks, and ≥6 weeks groups, respectively (Fig. 1).
Fig. 1.
Unadjusted Kaplan–Meier curve for overall survival stratified by time interval delay from surgery to initiating radiotherapy
Table 2 displays the significant co-variables on univariate analysis for OS. RPA stage IV (HR 1.48, p = 0.03), and stage V (HR 3.15, p < 0.001) had worse OS then stage III (reference). Higher doses of RT, use of IMRT, and a greater extent of surgery were all significantly associated with improved OS (p < 0.001). MGMT status trended for improved OS (HR 0.67 [95 %CI 0.44–1.02], p = 0.06). Patients with an interval of ≥6 weeks from surgery to radiation had improved OS in univariate analysis (HR 0.59, p = 0.04).
Table 2.
Univariate unadjusted analysis
| Variable | HR | 95 % CI |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (continuous) | 1.02 | 1.01 | 1.03 | 0.0000007 |
| Gender (male vs. female) | 1.12 | 0.88 | 1.42 | 0.35 |
| RPA | ||||
| III | 1 | – | – | Reference |
| IV | 1.48 | 1.03 | 2.13 | 0.034 |
| V | 3.15 | 2.07 | 4.82 | 0.0000001 |
| Interval surgery to RT | ||||
| 1–2 weeks | 1 | – | – | Reference |
| 3–5 weeks | 0.65 | 0.4 | 1.06 | 0.085 |
| ≥6 weeks | 0.59 | 0.35 | 0.98 | 0.041 |
| Type of RT | ||||
| 2D | 1 | – | – | Reference |
| 3D | 0.55 | 0.31 | 0.97 | 0.039 |
| IMRT | 0.44 | 0.27 | 0.71 | 0.0009 |
| Dose of RT | ||||
| <36 Gy | 1 | – | – | Reference |
| 36–54 Gy | 0.55 | 0.28 | 1.06 | 0.072 |
| >54 Gy | 0.34 | 0.18 | 0.64 | 0.0009 |
| Type of surgery | ||||
| Biopsy | 1 | – | – | Reference |
| STR | 0.64 | 0.47 | 0.88 | 0.006 |
| GTR | 0.33 | 0.23 | 0.48 | 0.000000003 |
| MGMT (Mutated vs WT) | 0.67 | 0.44 | 1.02 | 0.062 |
| KPS | ||||
| ≥90 | 1 | – | – | Reference |
| 70–89 | 1.43 | 1.11 | 1.85 | 0.005 |
| <70 | 3.37 | 2.31 | 4.93 | 3E – 10 |
| Neurologic symtoms pre-RT (continuous) | 1.32 | 1.17 | 1.48 | 0.000003 |
| Smoking (yes vs. no) | 1.18 | 0.93 | 1.5 | 0.179 |
| Laterality (bilateral vs. unilateral) | 1.68 | 1.25 | 2.24 | 0.00049 |
2D 2-dimensional, 3D 3-dimensional, CI confidence interval, GTR gross total resection, HR hazard ratio, IMRT intensity modulated radiotherapy, IQR interquartile range, KPS Karnofsky Performance Status, MGMT O-6-methylguanine-DNA methyltransferase, RT radiotherapy, STR subtotal resection
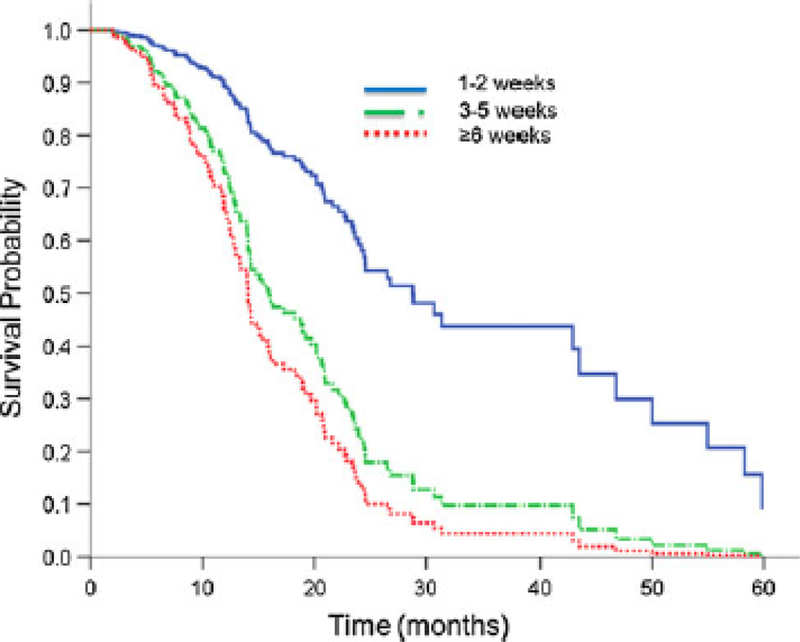
The following significant variables on univariate analysis were included in the multivariate model; age, technique of radiotherapy, dose, extent of surgery, KPS, neurological symptoms pre-radiotherapy, and extent of disease (unilateral vs bilateral disease) (p-values all < 0.001). MGMT was included despite borderline significance (p = 0.06) given that it is a known predictor of outcome for patients with glioblastoma treated with temozolomide. The Cox regression multivariate model demonstrated a significant detriment in delaying post-operative RT (≤2 week group as reference); 3–5 weeks (HR 2.80 [0.72–10.89], p = 0.14), and>6 weeks (HR 3.76 [1.01–14.57], p = 0.05) (Table 3). As shown in Fig. 2, a significant detriment in survival with an increasing interval from surgery to radiation was noted after adjusting for known prognostic factors.
Table 3.
Multivariate adjusted analysis
| Variable | HR | 95 % CI |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age (continuous) | 1.02 | 1.01 | 1.04 | 0.004 |
| Interval surgery to RT | ||||
| 1–2 weeks | 1 | – | – | Reference |
| 3–5 weeks | 2.8 | 0.72 | 10.89 | 0.14 |
| ≥6 weeks | 3.76 | 1.01 | 14.57 | 0.05 |
| Type of RT | ||||
| 2D | 1 | – | – | Reference |
| 3D | 5.33 | 1.03 | 27.64 | 0.046 |
| IMRT | 2.08 | 0.62 | 6.95 | 0.24 |
| Dose of RT | ||||
| <36 Gy | 1 | – | – | Reference |
| 36–54 Gy | 1.4 | 0.24 | 3.21 | 0.87 |
| >54 Gy | 1.32 | 0.54 | 2.89 | 0.88 |
| Type of surgery | ||||
| Biopsy | 1 | – | – | Reference |
| STR | 0.35 | 0.18 | 0.67 | 0.0014 |
| GTR | 0.24 | 0.12 | 0.49 | 0.000071 |
| MGMT (Mutated vs WT) | 0.42 | 0.27 | 0.67 | 0.00024 |
| KPS | ||||
| ≥90 | 1 | – | – | Reference |
| 70–89 | 1.47 | 0.94 | 2.3 | 0.093 |
| <70 | 6.51 | 3.18 | 13.36 | 0.00000032 |
| Neurologic symtoms pre-RT (continuous) | 1.15 | 0.93 | 1.42 | 0.21 |
| Laterality (bilateral vs unilateral) | 3.42 | 1.91 | 6.11 | 0.000034 |
2D 2-dimensional, 3D 3-dimensional, CI confidence interval, GTR gross total resection, HR hazard ratio, IMRT intensity modulated radiotherapy, IQR interquartile range, KPS Karnofsky Performance Status, MGMT O-6-methylguanine-DNA methyltransferase, RT radiotherapy, STR subtotal resection
Fig. 2.
Adjusted Kaplan–Meier curve for overall survival stratified by time interval delay from surgery to initiating radiotherapy. Adjusted for age, technique of radiotherapy, dose of radiotherapy, extent of surgical resection, MGMT status, KPS, neurological symptoms pre-radiotherapy, and laterality of disease
Discussion
The widespread implementation of more complex treatment planning techniques such as IMRT has increased the amount of time necessary to generate and initiate delivery of radiation treatment plans. Currently, the optimal timing of postoperative radiation therapy is still a point of debate. At our institution, definitive treatments planned to a full dose of 60 Gy are rarely simulated, planned, and initiated within 2 weeks after surgery. Several studies have suggested no impact [6, 9], or even a survival benefit [8, 13], with delaying radiotherapy, which has been used to justify the logistical delays from surgery and start of radiotherapy.
When interpreting the outcomes of prior retrospective studies examining the temporal relationship between surgery and radiotherapy on clinical outcome, it is of pivotal importance to consider the impact of imbalances in confounding variables with respect to the timing of radiotherapy, given that this is neither a stratified or randomized variable in any study to date. For example, in our study, patients undergoing radiotherapy within 2 weeks of surgery were more likely to have more unfavorable characteristics in virtually every known prognostic factor, including RPA class, extent of surgery, KPS, pre-radiotherapy neurologic symptoms, and bilateral tumor invasion. Given these imbalances and the lack of randomized data examining the timing of radiotherapy, to understand the independent effect of delaying post-operative radiotherapy a rigorous attempt to correct for all confounding variables is required. However, studies are limited to what is known at the time of being conducted, as new prognostic variables (MGMT) and new treatments modalities (temozolomide) may alter the initial findings.
Although three smaller retrospective single institution studies have noted a detriment in survival with the delay of radiotherapy [10–12], fitting the model of tumor doubling times [7], three much larger multi-center studies have shown either no effect of delaying radiotherapy [6, 9], or in fact a benefit in overall survival [8]. For example, Blumenthal et al. [8] reported a secondary analysis from 16 RTOG trials enrolling 2,855 patients, the largest series in the pre-temozolomide era on the impact of radiotherapy timing for glioblastoma patients. The authors identified a surprising median overall survival benefit of 3.3 months from delaying radiotherapy beyond 4 weeks after surgery. By comparison, this survival benefit was greater than the 2.5 month improvement in overall survival resulting from the addition of temozolomide to radiotherapy in the EO-RTC/NCIC trial [3]. Many of the RTOG trials used in the Blumental paper were the trials that actually helped create the infamous RTOG RPA, which remains one of the most powerful prognostic variable. For this reason likely only RPA class was adjusted for as a confounding variable in their analysis (furthermore MGMT was not even recognized as a prognostic marker when the RTOG trials were conducted). Blumenthal et al. [8] with impressive foresight understood that their findings of delaying radiotherapy improving survival seemed inconsistent and should not be taken as a definitive conclusion. As we and others have since demonstrated, the RPA is only one of numerous factors that significantly impact survival outcomes in glioblastoma. Similarly, when we corrected solely for RPA in our multivariate analysis, a prolonged interval from surgery to radiotherapy (≥6 weeks) was associated with a trend towards improved survival in comparison to radiotherapy within 2 weeks of surgery, although this was not statistically significant (Supplementary Data). However, when correcting for additional confounding variables (listed in Table 3), including MGMT status, many of which were not available from RTOG trials, our analysis supported the opposite conclusion, showing a significant survival detriment in delaying radiotherapy.
Lai et al. [9] reported the second largest analysis on the impact of timing of post-operative radiotherapy. The authors performed a SEER-Medicare analysis comprised of 1,375 elderly patients (>65 years old), and concluded that “although effort should be made to initiate radiotherapy as soon as possible…a brief delay similar to that experienced by our cohort does not have a significant impact on survival.” They reported that no difference could be identified by delaying radiotherapy. The authors corrected for a unique series of potential confounding factors, including age, race, socioeconomic status, distance from residence to treatment facility, comorbidity score, extent of surgery, and use of chemotherapy, the SEER-Medicare dataset unfortunately lacks information regarding variables such as KPS, neurologic symptoms, or MGMT status. However, as all patients in their study were>65 years of age it is unknown if MGMT status or the use of temozolomide would alter their findings.
The most recent analysis reported from France also failed to demonstrate an impact of treatment delay on survival [6]. This report was unique from previously published work in that 322 patients received concurrent temozolomide. Despite similar patient size and the use of temozolomiade to our series, our conclusions appear contradictory. However, several differences in our multivariate model should be considered. In the study from Noel et al. [6] their expedited radiotherapy group was combined to ≤4 weeks, while our expedited radiotherapy group initiated treatment ≤2 weeks post-operatively. Noel et al. [6] did not incorporate KPS, neurologic status, technique of radiotherapy, or an indicator of extent of disease (i.e., laterality or tumor volume). Lastly, we were fortunately able to collect MGMT status for a subset of our patients, which may explain the difference in part in our results.
Despite our criticism of the previously reported work, our present study also has limitations worthy of mention. Timing of radiotherapy was not a randomized variable and is subject to bias. The retrospective methodology limits the understanding of all underlying confounding variables, as subjective and difficult-to-quantify patient and physician variables may not have been identified. Variables including socio-economic status and distance from residence to our institution were not captured, and hence not corrected for. Additionally, only 45.8 % of our cohort had MGMT methylation status, and only 10.1 % had IDH mutational status known; due to the small patient numbers IDH status was not able to be incorporated into our analyses. We cannot rule out the possibility that imbalances in these factors may have had an impact on our conclusions.
Conclusions
This is the first analysis on the temporal relationship of surgery and radiotherapy on oncologic outcome for glioblastoma patients to incorporate MGMT status, and the largest temozolomide-treated cohort to evaluate the clinical impact of timing of post-operative radiotherapy. Our data does not support the OS benefit previously seen in delayed RT after correcting for important additional covariates that are now known. In fact, we demonstrate a survival detriment on multivariate analysis with delaying RT greater than 6 weeks from surgery. Larger pooled analyses with thorough reporting of possible confounding variables will be necessary to further understand the clinical impact of the temporal relationship of surgery and radiotherapy, given the low likelihood that a prospective trial will be performed in the future to address this question.
Supplementary Material
Acknowledgments
Dr. Yamada is a Consultant for Varian Medical Systems, Inc. and a member of the Speakers Bureau for the Institute for Medical Education; the other authors have no financial disclosures or conflicts of interest to report.
Footnotes
This study in part will be presented as an oral presentation at the annual American Society for Radiation Oncology (ASTRO) conference in Atlanta, GA in September 2013.
Electronic supplementary material The online version of this article (doi:10.1007/s11060-013-1302-4) contains supplementary material, which is available to authorized users.
Contributor Information
Daniel E. Spratt, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA
Michael Folkert, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA.
Zachary S. Zumsteg, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA
Timothy A. Chan, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA
Kathryn Beal, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA.
Philip H. Gutin, Department of Neurosurgery, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Elena Pentsova, Department of Medical Oncology, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Yoshiya Yamada, Department of Radiation Oncology, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, Box 22, New York, NY 10065, USA.
References
- 1.Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1):11–30 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K et al. (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466 [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The N Engl j med 352(10):987–996 [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al. (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. The N Engl J Med 352(10):997–1003 [DOI] [PubMed] [Google Scholar]
- 5.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS et al. (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483(7390):479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noel G, Huchet A, Feuvret L, Maire JP, Verrelle P, Le Rhun E, Aumont M, Thillays F, Sunyach MP, Henzen C et al. (2012) Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: a French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J Neurooncol 109(1):167–175 [DOI] [PubMed] [Google Scholar]
- 7.Burnet NG, Jena R, Jefferies SJ, Stenning SP, Kirkby NF (2006) Mathematical modelling of survival of glioblastoma patients suggests a role for radiotherapy dose escalation and predicts poorer outcome after delay to start treatment. Clin oncol 18(2):93–103 [DOI] [PubMed] [Google Scholar]
- 8.Blumenthal DT, Won M, Mehta MP, Curran WJ, Souhami L, Michalski JM, Rogers CL, Corn BW (2009) Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol 27(5):733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai R, Hershman DL, Doan T, Neugut AI (2010) The timing of cranial radiation in elderly patients with newly diagnosed glioblastoma multiforme. Neuro-oncology 12(2):190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin C, Hunn M, Purdie G, Hamilton D (2007) Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol 85(3):339–343 [DOI] [PubMed] [Google Scholar]
- 11.Do V, Gebski V, Barton MB (2000) The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol 57(2):131–136 [DOI] [PubMed] [Google Scholar]
- 12.Valduvieco I, Verger E, Bruna J, Caral L, Pujol T, Ribalta T, Boget T, Oleaga L, Pineda E, Graus F (2013) Impact of radiotherapy delay on survival in glioblastoma. Clin Transl Oncol 15(4):278–282 [DOI] [PubMed] [Google Scholar]
- 13.Lawrence YR, Blumenthal DT, Matceyevsky D, Kanner AA, Bokstein F, Corn BW (2011) Delayed initiation of radiotherapy for glioblastoma: how important is it to push to the front (or the back) of the line? J Neurooncol 105(1):1–7 [DOI] [PubMed] [Google Scholar]
- 14.Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C et al. (2004) Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22(9):1583–1588 [DOI] [PubMed] [Google Scholar]
- 15.Mirimanoff RO, Gorlia T, Mason W, Van den Bent MJ, Kortmann RD, Fisher B, Reni M, Brandes AA, Curschmann J, Villa S et al. (2006) Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 24(16):2563–2569 [DOI] [PubMed] [Google Scholar]
- 16.Nieto FJ, Coresh J (1996) Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol 143(10):1059–1068 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.